Comparison of Decidual Vasculopathy in Central and Peripheral Regions of Placenta with Implication of Lateral Growth and Spiral Artery Remodeling
Abstract
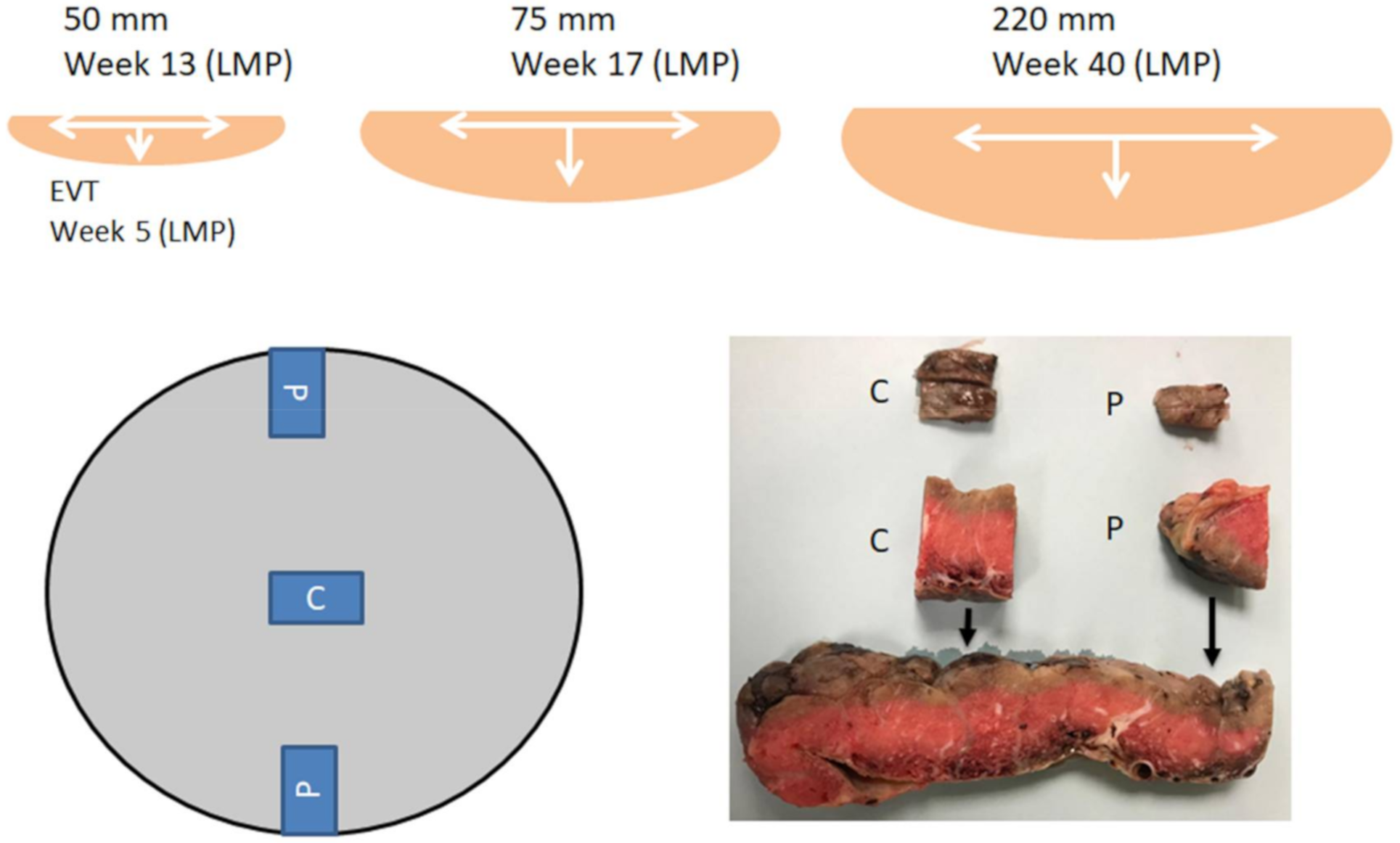
1. Introduction
2. Material and Methods
3. Result
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Benirschke, K.; Burton, G.J.; Baergen, R.N. Pathology of the Human Placenta, 6th ed.; Springer: New York, NY, USA, 2012. [Google Scholar]
- Hutcheon, J.A.; McNamara, H.; Platt, R.W.; Benjamin, A.; Kramer, M.S. Placental weight for gestational age and adverse perinatal outcomes. Obstet. Gynecol. 2012, 119, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Salafia, C.M.; Charles, A.K.; Maas, E.M. Placenta and fetal growth restriction. Clin. Obstet. Gynecol. 2006, 49, 236–256. [Google Scholar] [CrossRef] [PubMed]
- Salafia, C.M.; Maas, E.; Thorp, J.M.; Eucker, B.; Pezzullo, J.C.; Savitz, D.A. Measures of placental growth in relation to birth weight and gestational age. Am. J. Epidemiol. 2005, 162, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Salafia, C.M.; Pezzullo, J.C.; Charles, A.K.; Ernst, L.M.; Maas, E.M.; Gross, B.; Pijnenborg, R. Morphometry of the basal plate superficial uteroplacental vasculature in normal midtrimester and at term. Pediatr. Dev. Pathol. 2005, 8, 639–646. [Google Scholar] [CrossRef]
- Bonds, D.R.; Gabbe, S.G.; Kumar, S.; Taylor, T. Fetal weight/placental weight ratio and perinatal outcome. Am. J. Obstet. Gynecol. 1984, 149, 195–200. [Google Scholar] [CrossRef]
- Bonds, D.R.; Mwape, B.; Kumar, S.; Gabbe, S.G. Human fetal weight and placental weight growth curves. A mathematical analysis from a population at sea level. Biol. Neonate 1984, 45, 261–274. [Google Scholar] [CrossRef]
- Raff, M.C.; Durand, B.; Gao, F.B. Cell number control and timing in animal development: The oligodendrocyte cell lineage. Int. J. Dev. Biol. 1998, 42, 263–267. [Google Scholar]
- Conlon, I.J.; Dunn, G.A.; Mudge, A.W.; Raff, M.C. Extracellular control of cell size. Nat. Cell Biol. 2001, 3, 918–921. [Google Scholar] [CrossRef]
- Raff, M.C. Size control: The regulation of cell numbers in animal development. Cell 1996, 86, 173–175. [Google Scholar] [CrossRef]
- Craven, C.M.; Zhao, L.; Ward, K. Lateral placental growth occurs by trophoblast cell invasion of decidual veins. Placenta 2000, 21, 160–169. [Google Scholar] [CrossRef]
- Pijnenborg, R.; Dixon, G.; Robertson, W.B.; Brosens, I. Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta 1980, 1, 3–19. [Google Scholar] [CrossRef]
- Pijnenborg, R.; Vercruysse, L.; Hanssens, M. The uterine spiral arteries in human pregnancy: Facts and controversies. Placenta 2006, 27, 939–958. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, J.E.; Fraser, R.; Leslie, K.; Wallace, A.E.; James, J.L. Remodelling at the maternal-fetal interface: Relevance to human pregnancy disorders. Reproduction 2010, 140, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Robertson, W.B.; Khong, T.Y.; Brosens, I.; De Wolf, F.; Sheppard, B.L.; Bonnar, J. The placental bed biopsy: Review from three European centers. Am. J. Obstet. Gynecol. 1986, 155, 401–412. [Google Scholar] [CrossRef]
- Khong, T.Y.; Chambers, H.M. Alternative method of sampling placentas for the assessment of uteroplacental vasculature. J. Clin. Pathol. 1992, 45, 925–927. [Google Scholar] [CrossRef] [PubMed]
- Khong, T.Y.; Mooney, E.E.; Ariel, I.; Balmus, N.C.; Boyd, T.K.; Brundler, M.A.; Derricott, H.; Evans, M.J.; Faye-Petersen, O.M.; Gillan, J.E.; et al. Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch. Pathol. Lab. Med. 2016, 140, 698–713. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P. Phenotypic Switch of Endovascular Trophoblasts in Decidual Vasculopathy with Implication for Preeclampsia and Other Pregnancy Complications. Fetal. Pediatr. Pathol. 2020, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Hertig, A. Vascular pathology in the hypertensive albuminuric toxemias of pregnancy. Clinics 1945, 4, 602–614. [Google Scholar]
- Zhang, P. Expression of Wilm’s Tumor Gene in Endometrium with Potential Link to Gestational Vascular Transformation. Reprod. Med. 2020, 1, 17–31. [Google Scholar] [CrossRef]
- Hastie, N.D. Wilms’ tumour 1 (WT1) in development, homeostasis and disease. Development 2017, 144, 2862–2872. [Google Scholar] [CrossRef]
- Zhang, P. Decidual Vasculopathy in Preeclampsia and Spiral Artery Remodeling Revisited: Shallow Invasion versus Failure of Involution. AJP Rep. 2018, 8, e241–e246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P. Decidual vasculopathy and spiral artery remodeling revisited II: Relations to trophoblastic dependent and independent vascular transformation. J. Matern. Fetal Neonatal Med. 2020, 1–7. [Google Scholar] [CrossRef]
- Martínez-Estrada, O.M.; Lettice, L.A.; Essafi, A.; Guadix, J.A.; Slight, J.; Velecela, V.; Hall, E.; Reichmann, J.; Devenney, P.S.; Hohenstein, P.; et al. Wt1 is required for cardiovascular progenitor cell formation through transcriptional control of Snail and E-cadherin. Nat. Genet. 2010, 42, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Chau, Y.Y.; Hastie, N. Wt1, the mesothelium and the origins and heterogeneity of visceral fat progenitors. Adipocyte 2015, 4, 217–221. [Google Scholar] [CrossRef]
- Small, T.W.; Penalva, L.O.; Pickering, J.G. Vascular biology and the sex of flies: Regulation of vascular smooth muscle cell proliferation by wilms’ tumor 1-associating protein. Trends Cardiovasc. Med. 2007, 17, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Small, T.W.; Bolender, Z.; Bueno, C.; O’Neil, C.; Nong, Z.; Rushlow, W.; Rajakumar, N.; Kandel, C.; Strong, J.; Madrenas, J.; et al. Wilms’ tumor 1-associating protein regulates the proliferation of vascular smooth muscle cells. Circ. Res. 2006, 99, 1338–1346. [Google Scholar] [CrossRef] [PubMed]
- Brosens, I.; Puttemans, P.; Benagiano, G. Placental bed research: I. The placental bed: From spiral arteries remodeling to the great obstetrical syndromes. Am. J. Obstet. Gynecol. 2019, 221, 437–456. [Google Scholar] [CrossRef]




| Groups | Classic Type (n = 87) | Mixed Type (n = 18) | p-Value |
|---|---|---|---|
| Delivery | 0.492 | ||
| −C-section | 33 (37.9%) | 9 (50.0%) | |
| −Vaginal | 54 (62.1%) | 9 (50.0%) | |
| PIH | 0.002 | ||
| 0 | 80 (92.0%) | 11 (61.1%) | |
| 1 | 7 (8.0%) | 7 (38.9%) | |
| Weight | 456.8 ± 122.0 | 345.3 ± 106.6 | 0.000 |
| Gestational age (W) | 40.0 [38.5; 40.0] | 39.0 [36.0; 40.0] | 0.025 |
| Infarcts | 0.585 | ||
| 0 | 75 (86.2%) | 14 (77.8%) | |
| 1 | 12 (13.8%) | 4 (22.2%) | |
| Chorioamnionitis | 0.381 | ||
| 0 | 31 (35.6%) | 9 (50.0%) | |
| 1 | 56 (64.4%) | 9 (50.0%) | |
| Meconium | 0.584 | ||
| 0 | 49 (56.3%) | 12 (66.7%) | |
| 1 | 38 (43.7%) | 6 (33.3%) | |
| Thrombosis | 1.000 | ||
| 0 | 65 (74.7%) | 13 (72.2%) | |
| 1 | 22 (25.3%) | 5 (27.8%) | |
| GDM2 | 0.603 | ||
| 0 | 79 (90.8%) | 15 (83.3%) | |
| 1 | 8 (9.2%) | 3 (16.7%) | |
| Category 2 tracing | 0.037 | ||
| 0 | 65 (74.7%) | 18 (100.0%) | |
| 1 | 22 (25.3%) | 0 (0.0%) | |
| Villitis | 0.371 | ||
| 0 | 72 (82.8%) | 17 (94.4%) | |
| 1 | 15 (17.2%) | 1 (5.6%) | |
| Abruption | 0.126 | ||
| 0 | 86 (98.9%) | 16 (88.9%) | |
| 1 | 1 (1.1%) | 2 (11.1%) | |
| IUGR | 0.038 | ||
| 0 | 83 (95.4%) | 14 (77.8%) | |
| 1 | 4 (4.6%) | 4 (22.2%) | |
| Cord issues | 0.755 | ||
| 0 | 82 (94.3%) | 16 (88.9%) | |
| 1 | 5 (5.7%) | 2 (11.1%) | |
| Others | 1.000 | ||
| 0 | 80 (92.0%) | 16 (88.9%) | |
| 1 | 7 (8.0%) | 2 (11.1%) | |
| Cord coiling | 3.0 [1.0; 4.0] | 3.5 [2.0; 5.0] | 0.225 |
| Oligohydramnios | 1.000 | ||
| 0 | 85 (97.7%) | 17 (94.4%) | |
| 1 | 2 (2.3%) | 1 (5.6%) | |
| Central | 0.930 | ||
| 0 | 28 (32.2%) | 5 (27.8%) | |
| 1 | 59 (67.8%) | 13 (72.2%) | |
| Peripheral | 1.000 | ||
| 0 | 14 (16.1%) | 3 (16.7%) | |
| 1 | 73 (83.9%) | 15 (83.3%) | |
| Both C + P (DV) | 0.64 | ||
| 0 | 42 (48.3%) | 7 (38.9%) | |
| 1 | 45 (51.7%) | 11 (61.1%) | |
| Recovery (Central) | 0.695 | ||
| 0 | 46 (52.9%) | 8 (44.4%) | |
| 1 | 41 (47.1%) | 10 (55.6%) | |
| Recovery (Peripheral) | 1.000 | ||
| 0 | 68 (78.2%) | 14 (77.8%) | |
| 1 | 19 (21.8%) | 4 (22.2%) | |
| Both (C + P) PRR | 1.000 | ||
| 0 | 76 (87.4%) | 16 (88.9%) | |
| 1 | 11 (12.6%) | 2 (11.1%) | |
| Both (C + P) ARR | 0.982 | ||
| 0 | 50 (57.5%) | 11 (61.1%) | |
| 1 | 37 (42.5% | 7 (38.9%) |
| Decidual Vasculopathy | Central | Peripheral | Both (P) | Recovery (C) | Recovery (P) | Both (R) | p-Value |
|---|---|---|---|---|---|---|---|
| Total | 72 | 88 | 56 | 51 | 23 | 13 | <0.0001 |
| Classic | 59 | 73 | 45 | 41 | 19 | 11 | <0.0001 |
| Mixed | 13 | 15 | 11 | 10 | 4 | 2 | =0.118 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P. Comparison of Decidual Vasculopathy in Central and Peripheral Regions of Placenta with Implication of Lateral Growth and Spiral Artery Remodeling. Reprod. Med. 2020, 1, 158-168. https://doi.org/10.3390/reprodmed1030012
Zhang P. Comparison of Decidual Vasculopathy in Central and Peripheral Regions of Placenta with Implication of Lateral Growth and Spiral Artery Remodeling. Reproductive Medicine. 2020; 1(3):158-168. https://doi.org/10.3390/reprodmed1030012
Chicago/Turabian StyleZhang, Peilin. 2020. "Comparison of Decidual Vasculopathy in Central and Peripheral Regions of Placenta with Implication of Lateral Growth and Spiral Artery Remodeling" Reproductive Medicine 1, no. 3: 158-168. https://doi.org/10.3390/reprodmed1030012
APA StyleZhang, P. (2020). Comparison of Decidual Vasculopathy in Central and Peripheral Regions of Placenta with Implication of Lateral Growth and Spiral Artery Remodeling. Reproductive Medicine, 1(3), 158-168. https://doi.org/10.3390/reprodmed1030012





