Maternal Serum Inhibin-A Augments the Value of Maternal Serum PlGF and of sFlt-1/PlGF Ratio in the Prediction of Preeclampsia and/or FGR Near Delivery—A Secondary Analysis
Abstract
:1. Introduction
2. Sample and Methods
2.1. Sample
2.2. Biochemical and Biophysical Markers
2.3. Outcome Measures
2.4. Statistical Analyses
3. Results
3.1. Cohort Characteristics
3.2. Median Marker Levels in the Outcome Groups
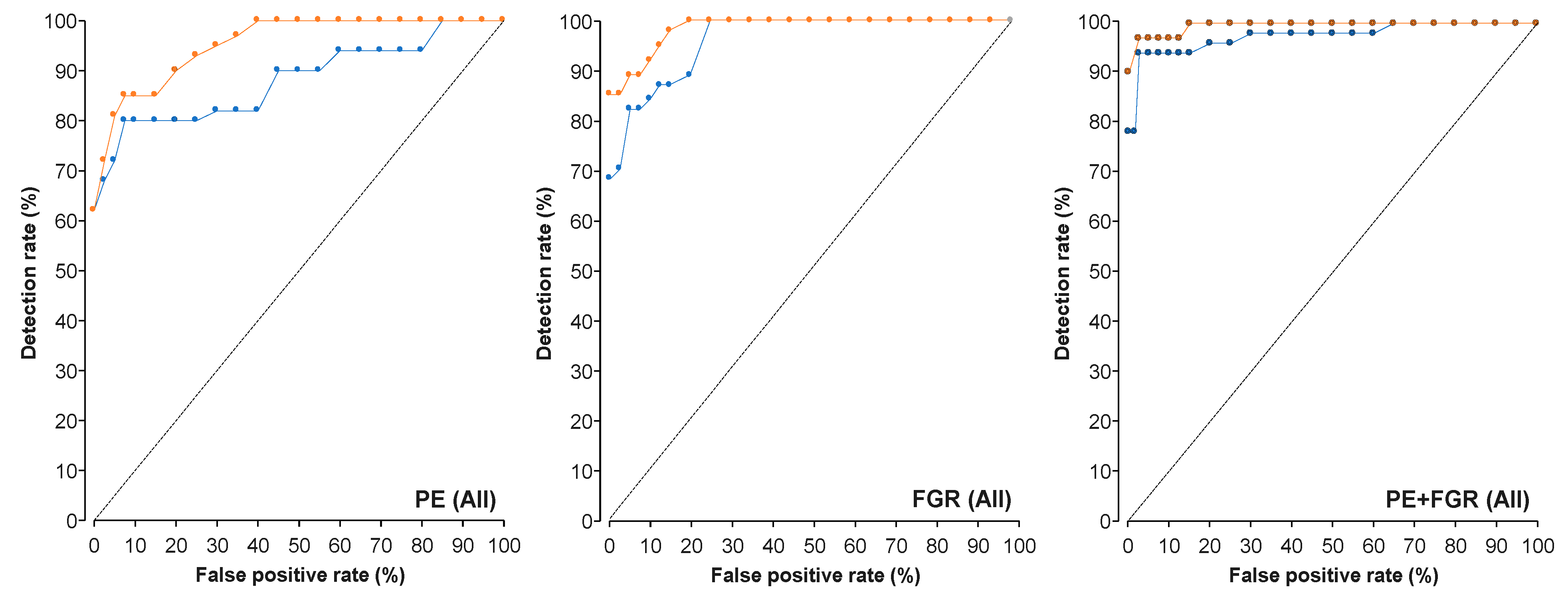
3.3. AUC Analysis
4. Discussion
4.1. Main Findings
4.2. Interpretation of Results and Comparison with Findings of Previous Studies
4.3. Implications for Clinical Practice
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACOG | American College of Obstetrics and Gynecology |
| AUC | Area under the curve of the receiver operation characteristic curve |
| BMI | Body mass Index |
| BP | Blood pressure |
| dBP | Diastolic blood pressure |
| DR | Detection rate (sensitivity) |
| FMF | Fetal Medicine Foundation |
| FGR | Fetal growth restriction |
| FPR | False positive rate (1-specificity) |
| ISSHP | International Society for the Study of Hypertension disorders of Pregnancy |
| ISUOG | International Society of ultrasound in Obstetrics and Gynecology |
| IVF | In vitro fertilization |
| MAP | Mean arterial blood pressure |
| NPV | Negative predictive value |
| PE | Preeclampsia |
| PlGF | Placenta growth factor |
| PPV | Positive predictive value |
| PTD | Preterm delivery |
| ROC | Receiver operation characteristic curve |
| UTPI | Uterine Artery Pulsatility Index |
| sBP | Systolic blood pressure |
| sFlt-1 | Soluble Fms-like tyrosine kinase 1 |
| 95% CI | 95% Confidence Interval |
References
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018, 13, 291–310. [Google Scholar] [PubMed]
- American College of Obstetricians and Gynecologists. Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol. 2020, 135, e237–e260. [CrossRef] [PubMed]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, challenges, and perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef] [PubMed]
- Sibai, B.; Dekker, G.; Kupferminc, M. Pre-eclampsia. Lancet 2005, 365, 785–799. [Google Scholar] [CrossRef]
- Zhang, J.; Klebanoff, M.A.; Roberts, J.M. Prediction of adverse outcomes by common definitions of hypertension in pregnancy. Obs. Gynecol. 2001, 97, 261–267. [Google Scholar]
- Huppertz, B. “Molecular and Cellular Mechanisms of Preeclampsia”-Editorial. Huppertz, B. Int. J. Mol. Sci. 2020, 21, 4801. [Google Scholar] [CrossRef]
- Women and Families. Available online: preeclampsia.org (accessed on 29 January 2020).
- Awuah, S.P.; Okai, I.; Ntim, E.A.; Bedu-Addo, K. Prevalence, placenta development, and perinatal outcomes of women with hypertensive disorders of pregnancy at Komfo Anokye Teaching Hospital. PLoS ONE 2020, 15, e0233817. [Google Scholar] [CrossRef]
- WHO Managing Complications in Pregnancy and Childbirth: A Guide for Midwives and Doctors. 2019. Available online: http://apps.who.int/iris/bitstream/handle/10665/255760/9789241565493-eng.pdf;jsessionid=2BF1F3D8022566FA91FE021D76D50256?sequence=1 (accessed on 1 July 2019).
- NICE Guidelines, Hypertension in Pregnancy. 2010. Available online: https://www.nice.org.uk/guidance/cg107/resources/hypertension-in-pregnancy-diagnosis-and-management-pdf-35109334011877 (accessed on 25 June 2019).
- Salomon, L.; Alfirevic, Z.; Costa, F.D.S.; Deter, R.; Figueras, F.; Ghi, T.; Glanc, P.; Khalil, A.; Lee, W.; Napolitano, R.; et al. ISUOG Practice Guidelines: Ultrasound assessment of fetal biometry and growth. Ultrasound Obs. Gynecol. 2019, 53, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Gordijn, S.J.; Beune, I.M.; Wynia, K.; Ganzevoort, W.; Figueras, F.; Kingdom, J.; Marlow, N.; Papageorghiou, A.; Sebire, N.; et al. Essential variables for reporting research studies on fetal growth restriction: A Delphi consensus. Ultrasound Obs. Gynecol. 2019, 53, 609–614. [Google Scholar] [CrossRef] [Green Version]
- Figueras, F.; Gratacos, E. An integrated approach to fetal growth restriction. Best Pr. Res. Clin. Obs. Gynaecol. 2017, 38, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. 2008. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Romero, R.; Dey, S.K.; Fisher, S.J. Preterm labor: One syndrome, many causes. Science 2014, 345, 760–765. [Google Scholar] [CrossRef] [Green Version]
- Levine, R.J.; Maynard, S.E.; Qian, C.; Lim, K.-H.; England, L.J.; Yu, K.F.; Schisterman, E.F.; Thadhani, R.; Sachs, B.P.; Epstein, F.H.; et al. Circulating Angiogenic Factors and the Risk of Preeclampsia. N. Engl. J. Med. 2004, 350, 672–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, R.J.; Lam, C.; Qian, C.; Yu, K.F.; Maynard, S.E.; Sachs, B.P.; Sibai, B.M.; Epstein, F.H.; Romero, R.; Thadhani, R.; et al. Soluble Endoglin and Other Circulating Antiangiogenic Factors in Preeclampsia. N. Engl. J. Med. 2006, 355, 992–1005. [Google Scholar] [CrossRef]
- Saleh, L.; Vergouwe, Y.; van den Meiracker, A.H.; Verdonk, K.; Russcher, H.; Bremer, A.; Versendaal, H.J.; Steegers, E.A.P.; Danser, A.H.J.; Visser, W. Angiogenic Markers Predict Pregnancy Complications and Prolongation in Preeclampsia: Continuous Versus Cutoff Values. Hypertension 2017, 70, 1025–1033. [Google Scholar] [CrossRef] [Green Version]
- Zeisler, H.; Llurba, E.; Chantraine, F.; Vatish, M.; Staff, A.C.; Sennström, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H.; Allegranza, D.; et al. Predictive Value of the sFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. N. Engl. J. Med. 2016, 374, 13–22. [Google Scholar] [CrossRef]
- Panaitescu, A.M.; Ciobanu, A.; Syngelaki, A.; Wright, A.; Wright, D.; Nicolaides, K.H. Screening for pre-eclampsia at 35–37 weeks’ gestation. Ultrasound Obs. Gynecol. 2018, 52, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Dragan, I.; Wright, D.; Fiolna, M.; Leipold, G.; Nicolaides, K.H. Development of pre-eclampsia within 4 weeks of sFlt-1/PlGF ratio > 38: Comparison of performance at 31–34 vs 35–37 weeks’ gestation. Ultrasound Obs. Gynecol. 2017, 49, 209–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frampton, G.K.; Jones, J.; Rose, M.; Payne, L. Placental growth factor (alone or in combination with soluble fms-like tyrosine kinase 1) as an aid to the assessment of women with suspected pre-eclampsia: Systematic review and economic analysis. Health Technol. Assess. 2016, 20, 1–160. [Google Scholar] [CrossRef] [Green Version]
- Sharabi-Nov, A.; Kumar, K.; Vodušek, V.F.; Sršen, T.P.; Tul, N.; Fabjan, T.; Meiri, H.; Nicolaides, K.H.; Osredkar, J. Establishing a Differential Marker Profile for Pregnancy Complications Near Delivery. Fetal Diagn. 2019, 47, 471–484. [Google Scholar] [CrossRef]
- Kumer, K.; Premru-Srsen, T.; Fabjan-Vodušek, V.; Tul, N.; Fabjan, T.; Osredkar, J. Peripheral arterial tonometry and angiogenic biomarkers in preeclampsia. Hypertens. Pregnancy 2018, 37, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Kumer, K.; Sharabi-Nov, A.; Fabjan Vodušek, V.; Premru Sršen, T.; Tul, N.; Fabjan, T.; Meiri; Nicolaides, K.H.; Osredkar, J. Pro-and-anti-angiogenic markers as clinical tool for suspected preeclampsia with and without FGR near delivery. Reprod. Med. 2021, 2, 12–25. [Google Scholar] [CrossRef]
- Phupong, V.; Paiwattananupant, K.; Honsawek, S. Inhibin A levels and severity of preeclampsia. Arch. Gynecol. Obs. 2008, 280, 183–186. [Google Scholar] [CrossRef]
- Paiwattananupant, K.; Phupong, V. Serum Inhibin A Level in Preeclampsia and Normotensive Pregnancy. Hypertens. Pregnancy 2008, 27, 337–343. [Google Scholar] [CrossRef]
- Hadlock, F.P.; Shah, Y.P.; Kanon, D.J.; Lindsey, J.V. Fetal crown-rump length: Reevaluation of relation to menstrual age (5-18 weeks) with high-resolution real-time US. Radiology 1992, 182, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Poon, L.; Kametas, N.A.; Valencia, C.; Chelemen, T.; Nicolaides, K.H. Hypertensive Disorders in Pregnancy: Screening by Systolic Diastolic and Mean Arterial Pressure at 11–13 Weeks. Hypertens. Pregnancy 2010, 30, 93–107. [Google Scholar] [CrossRef]
- Arnaldo Cassia, M.; Daminelli, G.; Zambon, M.; Cardellicchio, M.; Cetin, I.; Gallieni, M. Proteinuria in pregnancy: Clinically driven considerations. Nephrol. Point Care 2008, 4, 1–5. [Google Scholar]
- Jodkowska, A.; Martynowicz, H.; Kaczmarek-Wdowiak, B.; Mazur, G. Trombocytopenia in pregnancy—Pathogenesis and diagnostic approach. Postepy Hig. Med. Dosw. 2015, 69, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Burwick, R.M.; Rincon, M.; Beeraka, S.S.; Gupta, M.; Feinberg, B.B. Evaluation of Hemolysis as a Severe Feature of Preeclampsia. Hypertension 2018, 72, 460–465. [Google Scholar] [CrossRef]
- Ekun, O.A.; Olawumi, O.M.; Makwe, C.C.; Ogidi, N.O. Biochemical Assessment of Renal and Liver Function among Preeclamptics in Lagos Metropolis. Int. J. Reprod. Med. 2018, 2018, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadlock, F.P.; Harrist, R.B.; Martinez-Poyer, J. In utero analysis of fetal growth: A sonographic weight standard. Radiology 1991, 181, 129–133. [Google Scholar] [CrossRef]
- Hadlock, F.P.; Harrist, R.; Sharman, R.S.; Deter, R.L.; Park, S.K. Estimation of fetal weight with the use of head, body, and femur measurements—A prospective study. Am. J. Obs. Gynecol. 1985, 151, 333–337. [Google Scholar] [CrossRef]
- Salomon, L.J.; Alfirevic, Z.; Berghella, V.; Bilardo, C.; Hernandez-Andrade, E.; Johnsen, S.L.; Kalache, K.; Leung, K.-Y.; Malinger, G.; Munoz, H.; et al. Practice guidelines for performance of the routine mid-trimester fetal ultrasound scan. Ultrasound Obs. Gynecol. 2010, 37, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Farina, A.; Park, J.H.; Kim, S.H.; Kim, J.Y.; Rizzo, N.; Elmakky, A.; Jun, H.S.; Hahn, W.B.; Cha, D.H. Down syndrome biochemical markers and screening for preeclampsia at first and second trimester: Correlation with the week of onset and the severity. Prenat. Diagn. 2008, 28, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Ree, P.H.; Hahn, W.B.; Chang, S.W.; Jung, S.H.; Kang, J.H.; Cha, D.H.; Kang, M.S.; Huh, J.Y. Early Detection of Preeclampsia Using Inhibin A and Other Second-Trimester Serum Markers. Fetal Diagn. 2011, 29, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.J.; Riley, S.C.; Roberts, S.A.; Shearing, C.H.; Groome, N.P.; Martin, C.W. Maternal serum activin, inhibin, human chorionic gonadotrophin and alpha-fetoprotein as second trimester predictors of pre-eclampsia. Bjog Int. J. Obs. Gynaecol. 2003, 110, 46–52. [Google Scholar] [CrossRef]
- Muttukrishna, S.; North, R.; Morris, J.; Schellenberg, J.-C.; Taylor, R.; Asselin, J.; Ledger, W.; Groome, N.; Redman, C. Serum inhibin A and activin A are elevated prior to the onset of pre-eclampsia. Hum. Reprod. 2000, 15, 1640–1645. [Google Scholar] [CrossRef] [Green Version]
- Muttukrishna, S.; Hyett, J.; Paine, M.; Moodley, J.; Groome, N.; Rodeck, C. Uterine vein and maternal urinary levels of activin A and inhibin A in pre-eclampsia patients. Clin. Endocrinol. 2006, 64, 469–473. [Google Scholar] [CrossRef]
- Neuman, R.I.; Van Der Meer, M.M.A.; Nieboer, D.; Saleh, L.; Verdonk, K.; Kalra, B.; Kumar, A.; Alpadi, K.; Meiracker, A.H.V.D.; Visser, W.; et al. PAPP-A2 and Inhibin A as Novel Predictors for Pregnancy Complications in Women with Suspected or Confirmed Preeclampsia. J. Am. Hear. Assoc. 2020, 9, 018219. [Google Scholar] [CrossRef]
- Rana, S.; Burke, S.D.; Karumanchi, S.A. Imbalances in circulating angiogenic factors in the pathophysiology of preeclampsia and related disorders. Am. J. Obs. Gynecol. 2020, 20. [Google Scholar] [CrossRef]
- Verlohren, S.; Dröge, L.-A. The diagnostic value of angiogenic and antiangiogenic factors in differential diagnosis of preeclampsia. Am. J. Obs. Gynecol. 2020, 28. [Google Scholar] [CrossRef]
- Suresh, S.; Mueller, A.; Salahuddin, S.; Naseem, H.; Dhir, R.; Rana, S. Evaluation of angiogenic factors in the decision to admit women with suspected preeclampsia. Pregnancy Hypertens. 2020, 21, 124–131. [Google Scholar] [CrossRef]
- Agrawal, S.; Shinar, S.; Cerdeira, A.S.; Redman, C.; Vatish, M. Predictive Performance of PlGF (Placental Growth Factor) for Screening Preeclampsia in Asymptomatic Women: A Systematic Review and Meta-Analysis. Hypertension 2019, 74, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Barton, J.R.; Woelkers, D.A.; Newman, R.B.; Combs, C.A.; How, H.Y.; Boggess, K.A.; Martin, J.N., Jr.; Kupfer, K.; Sibai, B.M.; PETRA (Preeclampsia Triage by Rapid Assay) Trial. Placental growth factor predicts time to delivery in women with signs or symptoms of early preterm preeclampsia: A prospective multicenter study. Am. J. Obs. Gynecol. 2020, 222, 259.e1–259.e11. [Google Scholar] [CrossRef] [PubMed]
- Saleh, L.; Meiracker, A.H.V.D.; Geensen, R.; Kaya, A.; Van Lennep, J.E.R.; Duvekot, J.J.; Verdonk, K.; Steegers, E.A.; Russcher, H.; Danser, A.J.; et al. Soluble fms-like tyrosine kinase-1 and placental growth factor kinetics during and after pregnancy in women with suspected or confirmed pre-eclampsia. Ultrasound Obs. Gynecol. 2018, 51, 751–757. [Google Scholar] [CrossRef]
- Grafka, A.; Maciej Łopucki, M.; Pęksa1, B.; Surdacka, A.; Grywalska, E.; Roliński, G.; Elżbieta Starosławska, E. PLGF and sFlt-1 in clinical diagnosis of preeclampsia. Diagn. Lab. 2014, 50, 339–344. [Google Scholar]
- Yu, J.; Shixia, C.Z.; Wu, Y.; Duan, T. Inhibin A, activin A, placental growth factor and uterine artery Doppler pulsatility index in the prediction of pre-eclampsia. Ultrasound Obs. Gynecol. 2011, 37, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Thissier-Levy, S.; Wu, Y.; Wei, S.Q.; Luo, Z.-C.; Delvin, E.; Fraser, W.D.; Audibert, F.; Boucoiran, I.; The MIROS Study Group. Risks for Preeclampsia and Small for Gestational Age: Predictive Values of Placental Growth Factor, Soluble fms-like Tyrosine Kinase-1, and Inhibin A in Singleton and Multiple-Gestation Pregnancies. Am. J. Perinatol. 2012, 30, 607–612. [Google Scholar] [CrossRef]


| Characteristic | Term Delivery | Birth < 37 Weeks | Preeclampsia (PE) | Fetal Growth Restriction (FGR) | PE+FGR | p-Value |
|---|---|---|---|---|---|---|
| All Participants | ||||||
| Number of Patients | 21 | 15 | 31 | 16 | 42 | |
| Gestational Age at Enrollment (weeks) | 34.0 [32.0–35.9] | 31.2 [29.4–32.9] * | 33.9 [32.3–35.6] | 31.4 [29.1–33.6] * | 31.8 [30.7–32.8] * | 0.027 |
| Maternal Age (years) | 31.6 [29.5–33.8] | 31.3 [29.7–32.9] | 32.0 [29.9–34.1] | 31.7 [29.7–33.7] | 32.9 [31.1–34.7] | 0.792 |
| Body Mass Index (kg/m2) | 25.8 [23.7–27.9] | 24.6 [22.9–26.4] | 29.5 [26.5–32.6] * | 27.6 [24.2–31.0] | 29.6 [26.9–32.4] * | 0.011 |
| Previous PE (%) | 4.8 | 6.7 | 6.5 | 6.3 | 9.5 | 0.965 |
| Chronic Hypertension (%) | 0 | 0 | 19.4 * | 0 | 16.7 * | 0.032 |
| Diabetes (%) | 0 | 0 | 3.2 | 0 | 4.8 | 0.787 |
| Polycystic Ovary (%) | 0 | 0 | 0 | 0 | 7.1 * | 0.204 |
| Parity | 1.7 [1.3–2.0] | 1.6 [1.2–2.1] | 1.4 [1.0–1.8] | 1.5 [1.1–1.9] | 1.5 [1.2–1.8] | 0.806 |
| Conception by IVF (%) | 4.8 | 0 | 6.5 | 0 | 11.9 * | 0.361 |
| MAP (mm HG) | 85 [80–90] | 90 [82–98] | 113 [109–116] ** | 97 [93–101] * | 113 [109–116] ** | <0.001 |
| UTPI | 0.68 [0.66–0.70] | 0.70 [0.64–0.61] | 0.80 [0.60–1.17] * | 1.35 [1.05–1.66] ** | 1.42 [1.25–1.56] ** | <0.001 |
| Inhibin-A (pg/mL) | 724 [491–904] | 330 [261–928] | 2097 [1546–2660] * | 1269 [760–2348] * | 1876 [1239–2295] * | <0.001 |
| sFlt-1/PlGF | 5 [3–31] | 6 [2–9] | 177 [106–301] * | 195 [55–310] * | 265 [168–382] * | <0.001 |
| PlGF (pg/mL) | 524 [223–681] | 693 [308–980] | 101 [69–153] * | 76 [43–117] * | 62 [48–87] * | <0.001 |
| Inhibin-A/PlGF | 3.1 [0.7–3.6] | 1.2 [0.2–1.2] | 41.0 [10.2–39.4] ** | 36.3 [11.2–50.8] ** | 45.0 [19.5–44.1] ** | 0.001 |
| Gestational Age at Delivery (weeks) | 39.1 [38.5–39.7] | 33.8 [32.1–35.5] * | 34.2 [32.6–35.9] * | 31.7 [29.4–34.0] ** | 32.0 [31.0–33.1] ** | <0.001 |
| Delivery by C-Section (%) | 23.8 | 30.8 | 54.8 ** | 60.0 * | 83.4 ** | <0.001 |
| Baby’s Birthweight (grams) | 3330 [3133–3528] | 2207 [1872–2542] * | 2306 [1906–2705] * | 1306 [834–1778] ** | 1449 [1247–1651] ** | <0.001 |
| Birth < 34 weeks | ||||||
| Number of Patients | 6 | 10 | 12 | 28 | ||
| Gestationa Age at Enrollment (wks) | 29.2 [26.8–31.6] | 29.9 [27.5–32.3] | 29.3 [27.7–30.8] | 29.9 [28.9–30.9] | 0.805 | |
| Maternal Age (years) | 31.3 [27.8–34.8] | 33.8 [33.0–37.7] | 31.5 [29.2–33.8] | 33.1 [30.7–35.5] | 0.668 | |
| Body Mass Iindex (kg/meter2) | 24.7 [21.0–28.4] | 30.7 [26.2–35.2] | 26.3 [23.9–28.8] | 29.7 [26.1–33.4] | 0.123 | |
| Previous PE (%) | 0 | 0 | 0 | 7.1 | 0.591 | |
| Chronic Hypertension (%) | 0 | 7.7 | 0 | 21.4* | 0.146 | |
| Diabetes (%) | 0 | 0 | 0 | 3.6 | 0.771 | |
| Polycystic Ovary (%) | 0 | 0 | 0 | 3.6 | 0.771 | |
| Parity | 1.8 [1.0–2.6] | 1.5 [0.7–2.4] | 1.3 [0.9–1.7] | 1.6 [1.2–2.0] | 0.807 | |
| Conception by IVF (%) | 0 | 15.4 * | 0 | 10.7 | 0.498 | |
| MAP (mmHg) | 87 [70–103] | 114 [107–121] | 96 [91–101] | 115 [110–119] * | >0.001 | |
| UTPI | 0.69 [0.57–0.80] | 1.20 [0.83–1.57] * | 1.62 [1.35–1.90] ** | 1.43 [1.27–1.58] ** | >0.001 | |
| Inhibin-A (pg/mL) | 457 [0–1015] | 3216 [2212–4220] ** | 1503 [1019–1987] * | 238 [1711–3057] ** | 0.003 | |
| sFlt-1/PlGF | 6 [0–13] | 521 [246–796] * | 307 [174–439] * | 460 [273–647 * | 0.050 | |
| PlGF (pg/mL) | 762 [182–1343] | 215 [0–479] * | 70 [27–113] ** | 103 [39–167] ** | >0.001 | |
| Inhibin-A/PlGF | 1.1 [0.2–3.5] | 75.0 [17.1–114.2] ** | 44.8 [11.2–70.3] * | 57.7 [19.8–66.0] ** | 0.049 | |
| GA at delivery (wks) | 31.0 [28.0–34.0] | 30.2 [27.8–32.6] | 29.5 [28.0–31.1] | 30.2 [29.2–31.2] | 0.805 | |
| Delivery by C-Section (%) | 20.0 | 77.9 * | 72.7 * | 92.6 ** | 0.003 | |
| Baby Birthweight (grams) | 1669 [1318–2020] | 1276 [923–1628] * | 874 [627–1121] ** | 1171 [995–1346] * | 0.018 | |
| Condition | Marker | Continuous Model | Cutoff Model | |||||
|---|---|---|---|---|---|---|---|---|
| AUC (95% CI) | DR at 10% FPR | Cutoff | AUC (95% CI) | DR at 10% FPR | PPV | NPV | ||
| All PE (n = 31) | Inhibin-A | 0.91 (0.84–0.98) | 72 | 1000 pg/mL | 0.80 (0.69–0.92) | 42 | 79 | 81 |
| PlGF | 0.85 (0.75–0.95) | 53 | 200 pg/mL | 0.82 (0.71–0.93) | 43 | 82 | 83 | |
| sFlt-1/PlGF | 0.89 (0.80–0.97) | 79 | 38 | 0.85 (0.74–0.96) | 68 | 85 | 83 | |
| Inhibin-A/PlGF | 0.92 (0.85–0.99) | 79 | 7 | 0.83 (0.72–0.94) | 73 | 91 | 79 | |
| All FGR (n = 16) | Inhibin-A | 0.82 (0.70–0.95) | 50 | 1000 pg/mL | 0.75 (0.59–0.91) | 36 | 65 | 84 |
| PlGF | 0.95 (0.89–1.00) | 77 | 200 pg/mL | 0.86 (0.75–0.98) | 68 | 74 | 94 | |
| sFlt-1/PlGF | 0.97 (0.92–1.00) | 81 | 38 | 0.86 (0.73–0.99) | 69 | 76 | 91 | |
| Inhibin-A/PlGF | 0.94 (0.88–1.00) | 75 | 7 | 0.84 (0.71–0.98) | 76 | 86 | 88 | |
| All PE+FGR (n = 42) | Inhibin-A | 0.87 (0.78–0.95) | 68 | 1000 pg/mL | 0.80 (0.69–0.90) | 41 | 84 | 74 |
| PlGF | 0.92 (0.86–0.98) | 71 | 200 pg/mL | 0.87 (0.78–0.96) | 71 | 88 | 85 | |
| sFlt-1/PlGF | 0.97 (0.93–1.00) | 93 | 38 | 0.92 (0.84–0.99) | 80 | 90 | 91 | |
| Inhibin-A/PlGF | 0.94 (0.88–1.00) | 85 | 7 | 0.90 (0.82–0.98) | 86 | 95 | 83 | |
| PE < 34 w (n = 10) | Inhibin-A | 0.98 (0.93–1.00) | 91 | 400 pg/mL | 0.90 (0.68–1.00) | 49 | 92 | 100 |
| PlGF | 0.89 (0.73–1.00) | 60 | 300 pg/mL | 0.91 (0.76–1.00) | 53 | 100 | 71 | |
| sFlt-1/PlGF | 0.93 (0.80–1.00) | 82 | 120 | 0.91 (0.76–1.00) | 82 | 100 | 71 | |
| Inhibin-A/PlGF | 0.96 (0.88–1.00) | 91 | 2 | 0.86 (0.62–1.00) | 45 | 100 | 71 | |
| FGR < 34 w (n = 12) | Inhibin-A | 0.90 (0.71–1.00) | 50 | 400 pg/mL | 0.90 (0.68–1.00) | 50 | 92 | 100 |
| PlGF | 1.00 (1.00–1.00) | 100 | 300 pg/mL | 1.00 (1.00–1.00) | 100 | 100 | 100 | |
| sFlt-1/PlGF | 1.00 (1.00–1.00) | 100 | 120 | 0.92 (0.78–1.00) | 85 | 100 | 71 | |
| Inhibin-A/PlGF | 0.98 (0.93–1.00) | 92 | 2 | 0.90 (0.68–1.00) | 50 | 100 | 71 | |
| PE+FGR < 34 w (n = 28) | Inhibin-A | 0.93 (0.80–1.00) | 67 | 400 pg/mL | 0.88 (0.67–1.00) | 100 | 100 | 80 |
| PlGF | 0.96 (0.90–1.00) | 100 | 300 pg/mL | 0.96 (0.90–1.00) | 100 | 100 | 71 | |
| sFlt-1/PlGF | 1.00 (1.00–1.00) | 100 | 120 | 0.91 (0.81–1.00) | 83 | 100 | 50 | |
| Inhibin-A/PlGF | 0.99 (0.97–1.00) | 96 | 2 | 0.88 (0.67–1.00) | 47 | 100 | 63 | |
| Condition | Marker | AUC (95% CI) | p | DR at 10% FPR |
|---|---|---|---|---|
| All PE (n = 31) | PlGF | 0.85 (0.75–0.95) | <0.001 | 53 |
| PlGF + Inhibin-A | 0.98 (0.90–1.00) | 0.006 | 94 | |
| sFlt-1/PlGF | 0.89 (0.80–0.97) | <0.001 | 79 | |
| sFlt-1/PlGF + Inhibin-A | 0.95 (0.91–0.99) | 0.003 | 87 | |
| All FGR (n = 16) | PlGF | 0.95 (0.89–1.00) | <0.001 | 77 |
| PlGF + Inhibin-A | 0.98 (0.87–1.00) | 0.002 | 93 | |
| sFlt-1/PlGF | 0.97 (0.92–1.00) | <0.001 | 81 | |
| sFlt-1/PlGF + Inhibin-A | 0.99 (0.94–1.00) | <0.001 | 90 | |
| All PE+FGR (n = 42) | PlGF | 0.92 (0.86–0.98) | <0.001 | 71 |
| PlGF + Inhibin-A | 0.98 (0.91–0.99) | <0.001 | 90 | |
| sFlt-1/PlGF | 0.97 (0.93–1.00) | <0.001 | 93 | |
| sFlt-1/PlGF + Inhibin-A | 0.99 (0.93–1.00 | 0.004 | 95 | |
| PE < 34 w (n = 10) | PlGF | 0.89 (0.73–1.00) | 0.015 | 60 |
| PlGF + Inhibin-A | 0.99 (0.91–1.00) | <0.001 | 98 | |
| sFlt-1/PlGF | 0.93 (0.80–1.00) | 0.008 | 82 | |
| sFlt-1/PlGF +Inhibin-A | 0.99 (0.89–1.00) | 0.009 | 98 | |
| FGR < 34 w (n = 12) | PlGF | 1.00 (1.00–1.00) | <0.001 | 100 |
| PlGF + Inhibin-A | No added value | No added value | ||
| sFlt-1/PlGF | 1.00 (1.00–1.00) | <0.001 | 100 | |
| sFlt-1/PlGF + Inhibin-A | No added value | No added value | ||
| PE+FGR < 34 w (n = 28) | PlGF | 0.96 (0.90–1.00) | <0.001 | 100 |
| PlGF + Inhibin-A | No added value | No added value | ||
| sFlt-1/PlGF | 1.00 (1.00–1.00) | <0.001 | 100 | |
| sFlt-1/PlGF + Inhibin-A | No added value | No added value |
| Inhibin-A | PlGF | sFlt-1/PlGF | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | B | S.E. | β | B | S.E. | β | B | S.E. | β |
| GA (weeks) | 104.06 | 88.83 | 0.34 | −27.88 | 24.77 | −0.29 | −16.05 | 12.57 | −1.28 |
| BW (g) | −0.61 | 0.39 | −0.51 | 0.17 | 0.11 | 0.47 | −0.05 | 0.06 | −0.24 |
| MAP (mm HG) | −16.04 | 14.16 | −0.16 | 0.79 | 3.86 | 0.26 | −6.08 | 1.96 | −0.33 ** |
| PE | 1692 | 493 | 0.49 ** | −401 | 135 | −0.37 ** | 240 | 69 | 0.39 ** |
| FGR | 661 | 568 | 0.19 | −424 | 157 | −0.40 ** | 118 | 80 | 0.19 |
| FGR+PE | 1166 | 506 | 0.39 * | −381 | 139 | −0.41 ** | 210 | 70 | 0.39 ** |
| F(6,63) | 3.63 ** | 6.40 *** | 11.22 *** | ||||||
| R2 | 0.28 | 0.40 | 0.54 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharabi-Nov, A.; Premru Sršen, T.; Kumer, K.; Fabjan Vodušek, V.; Fabjan, T.; Tul, N.; Meiri, H.; Nicolaides, K.H.; Osredkar, J. Maternal Serum Inhibin-A Augments the Value of Maternal Serum PlGF and of sFlt-1/PlGF Ratio in the Prediction of Preeclampsia and/or FGR Near Delivery—A Secondary Analysis. Reprod. Med. 2021, 2, 35-49. https://doi.org/10.3390/reprodmed2010005
Sharabi-Nov A, Premru Sršen T, Kumer K, Fabjan Vodušek V, Fabjan T, Tul N, Meiri H, Nicolaides KH, Osredkar J. Maternal Serum Inhibin-A Augments the Value of Maternal Serum PlGF and of sFlt-1/PlGF Ratio in the Prediction of Preeclampsia and/or FGR Near Delivery—A Secondary Analysis. Reproductive Medicine. 2021; 2(1):35-49. https://doi.org/10.3390/reprodmed2010005
Chicago/Turabian StyleSharabi-Nov, Adi, Tanja Premru Sršen, Kristina Kumer, Vesna Fabjan Vodušek, Teja Fabjan, Nataša Tul, Hamutal Meiri, Kypros H. Nicolaides, and Joško Osredkar. 2021. "Maternal Serum Inhibin-A Augments the Value of Maternal Serum PlGF and of sFlt-1/PlGF Ratio in the Prediction of Preeclampsia and/or FGR Near Delivery—A Secondary Analysis" Reproductive Medicine 2, no. 1: 35-49. https://doi.org/10.3390/reprodmed2010005
APA StyleSharabi-Nov, A., Premru Sršen, T., Kumer, K., Fabjan Vodušek, V., Fabjan, T., Tul, N., Meiri, H., Nicolaides, K. H., & Osredkar, J. (2021). Maternal Serum Inhibin-A Augments the Value of Maternal Serum PlGF and of sFlt-1/PlGF Ratio in the Prediction of Preeclampsia and/or FGR Near Delivery—A Secondary Analysis. Reproductive Medicine, 2(1), 35-49. https://doi.org/10.3390/reprodmed2010005






