Antenatal Secondhand Smoke (SHS) Exposure and the Receptor for Advanced Glycation End-Products (RAGE)
Abstract
:1. Introduction
2. Methods
2.1. Animal Housing and Tissue Collection
2.2. Secondhand Smoke (SHS) Exposure
2.3. Blood Pressure and Heart Rate
2.4. Western Blotting
2.5. Histology
2.6. Immunofluorescence (IF)
2.7. Lung Mitochondrial Respiration Analysis
2.8. Statistics
3. Results
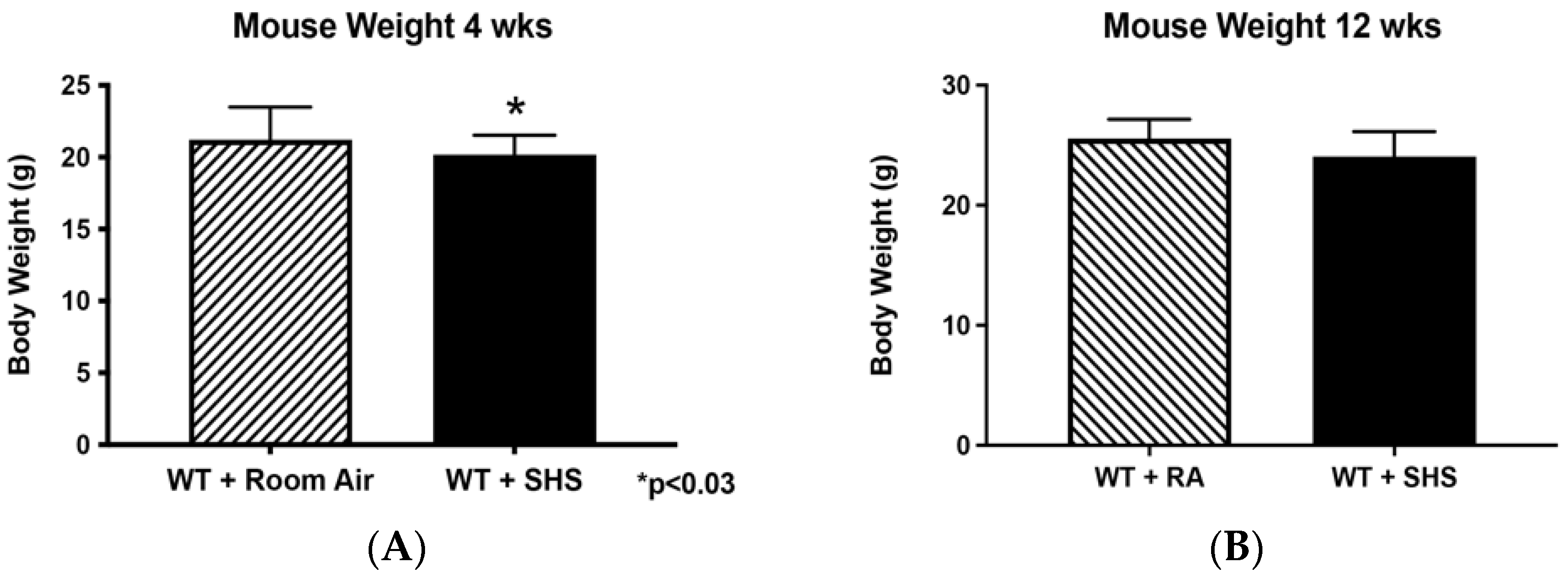
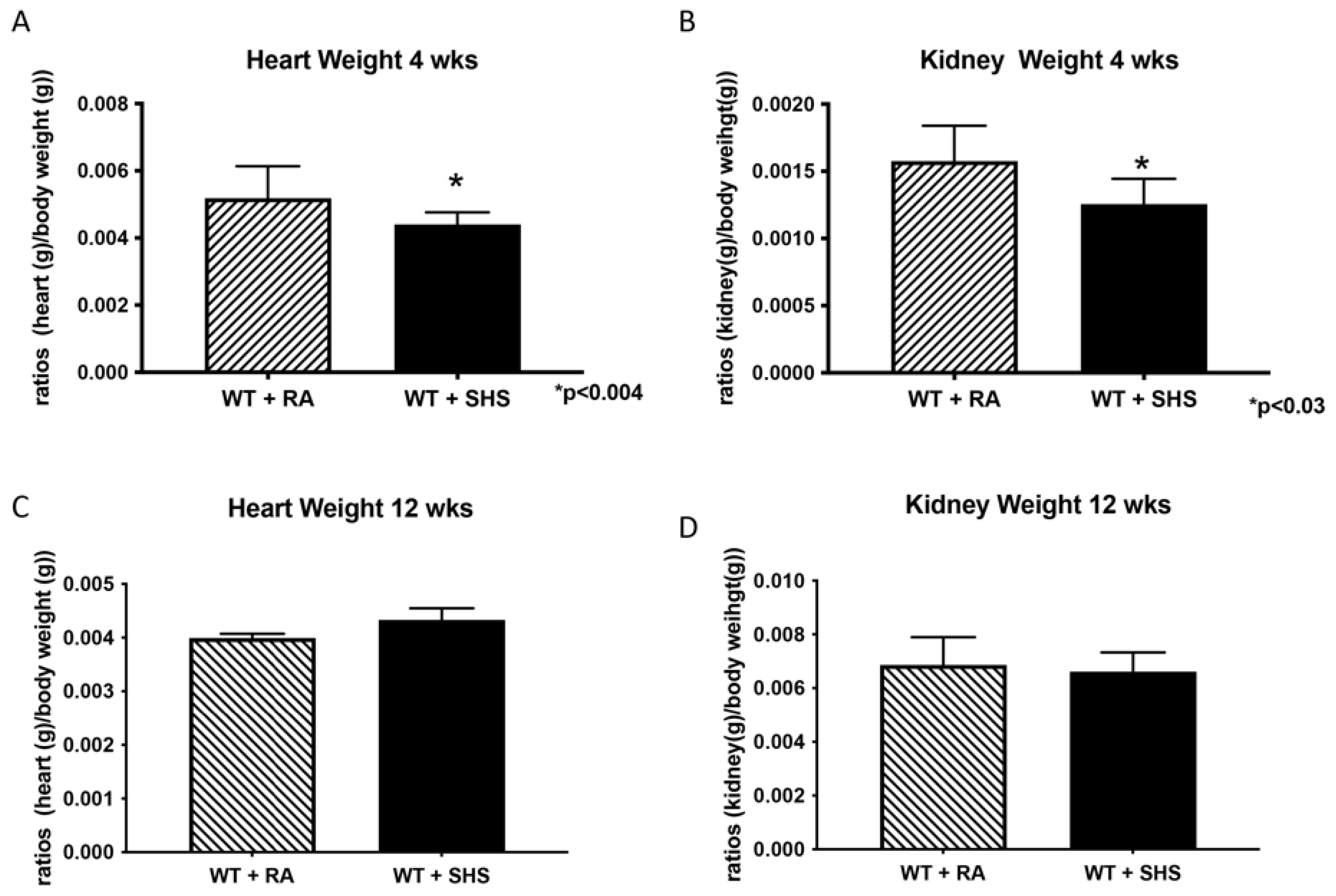
3.1. Body and Organ Weights
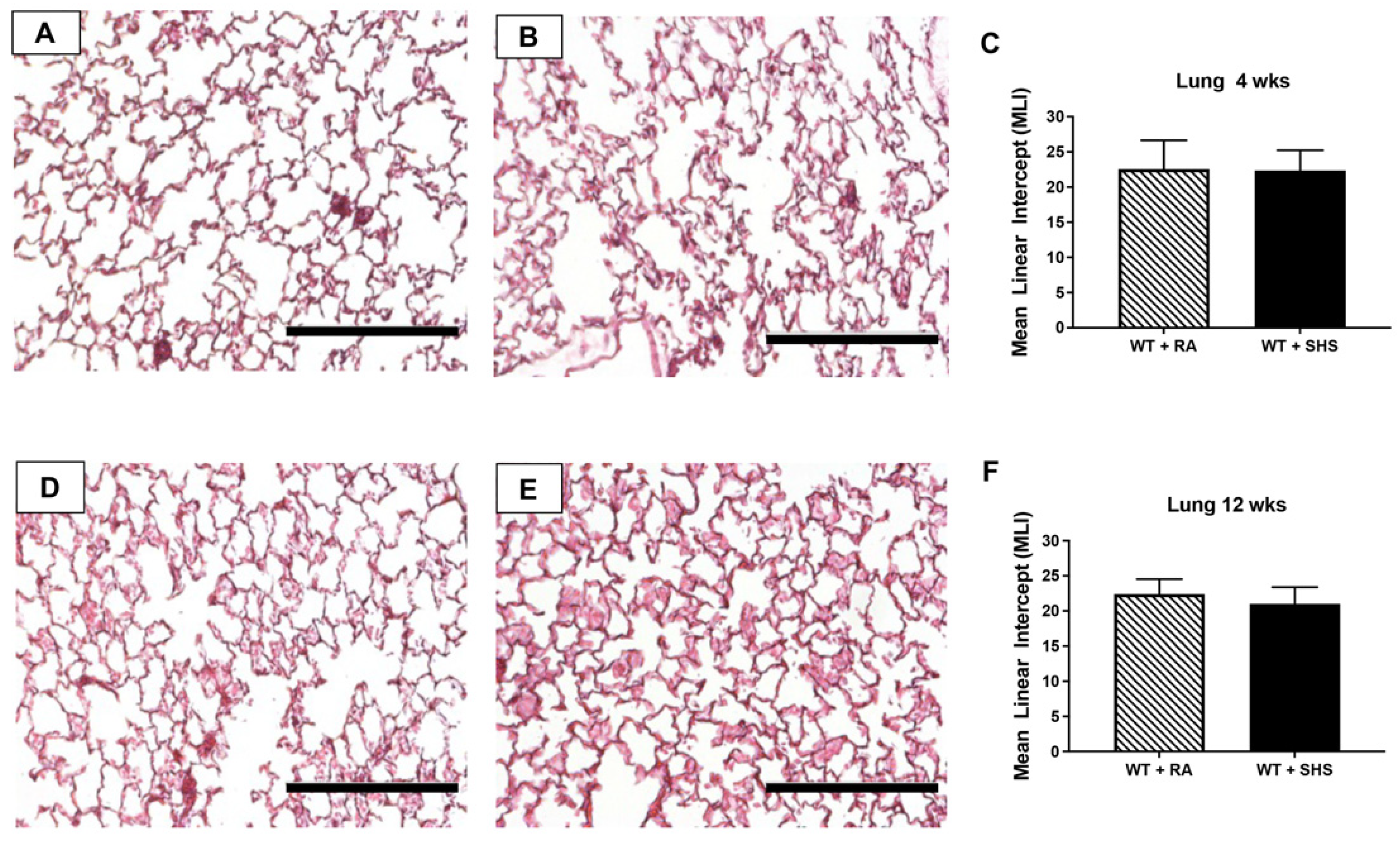
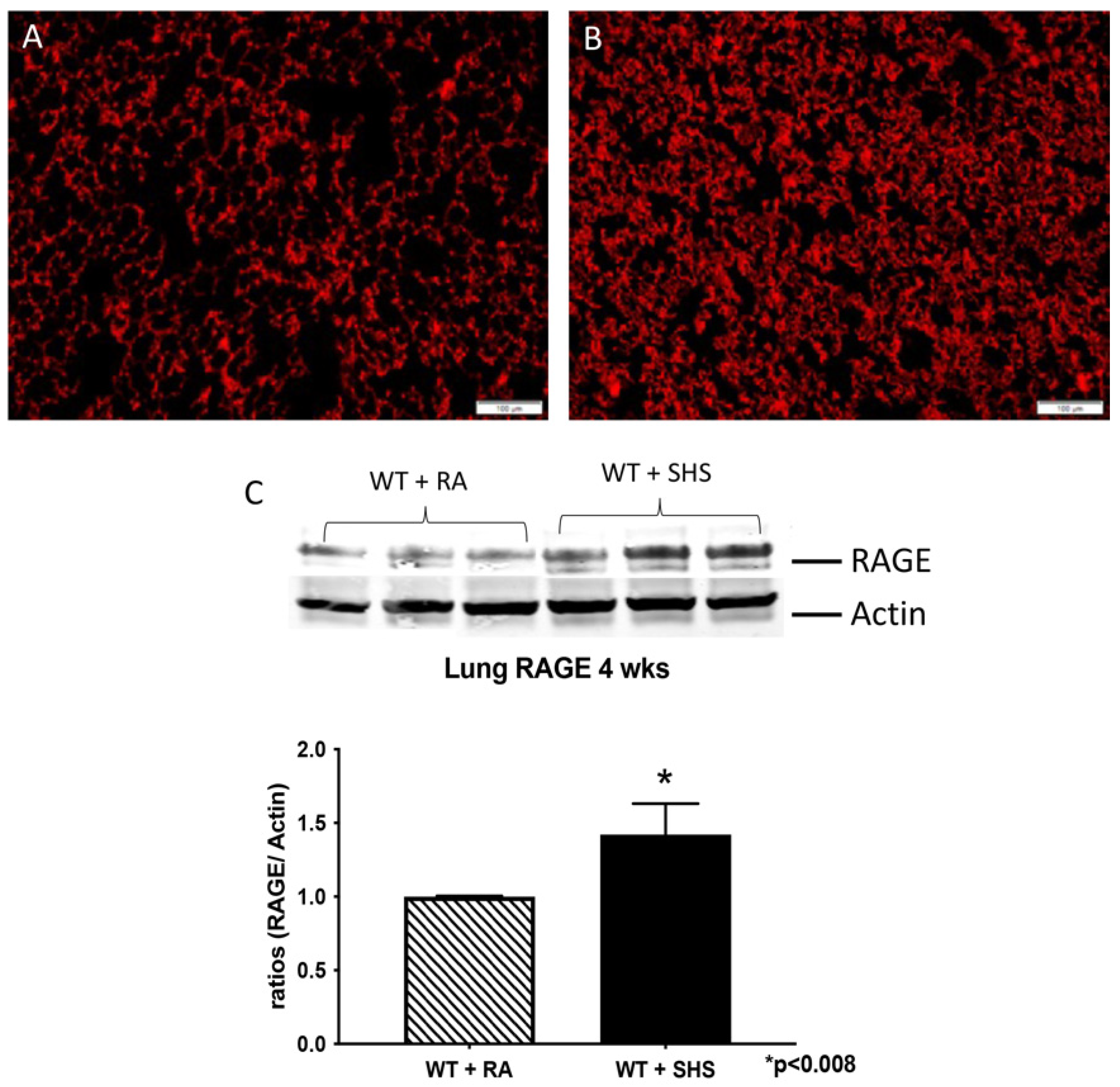
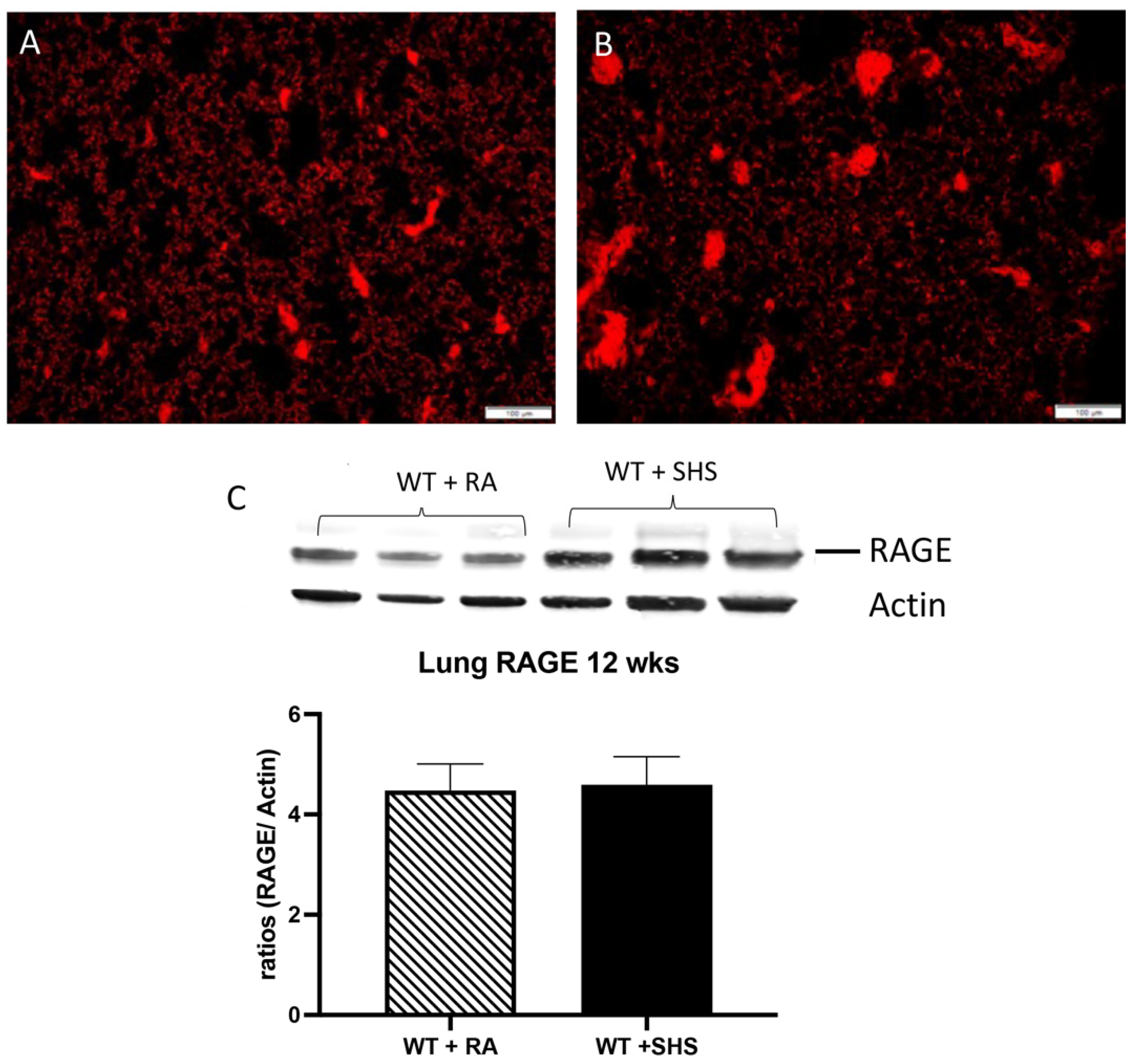
3.2. Pulmonary Structure and RAGE Expression
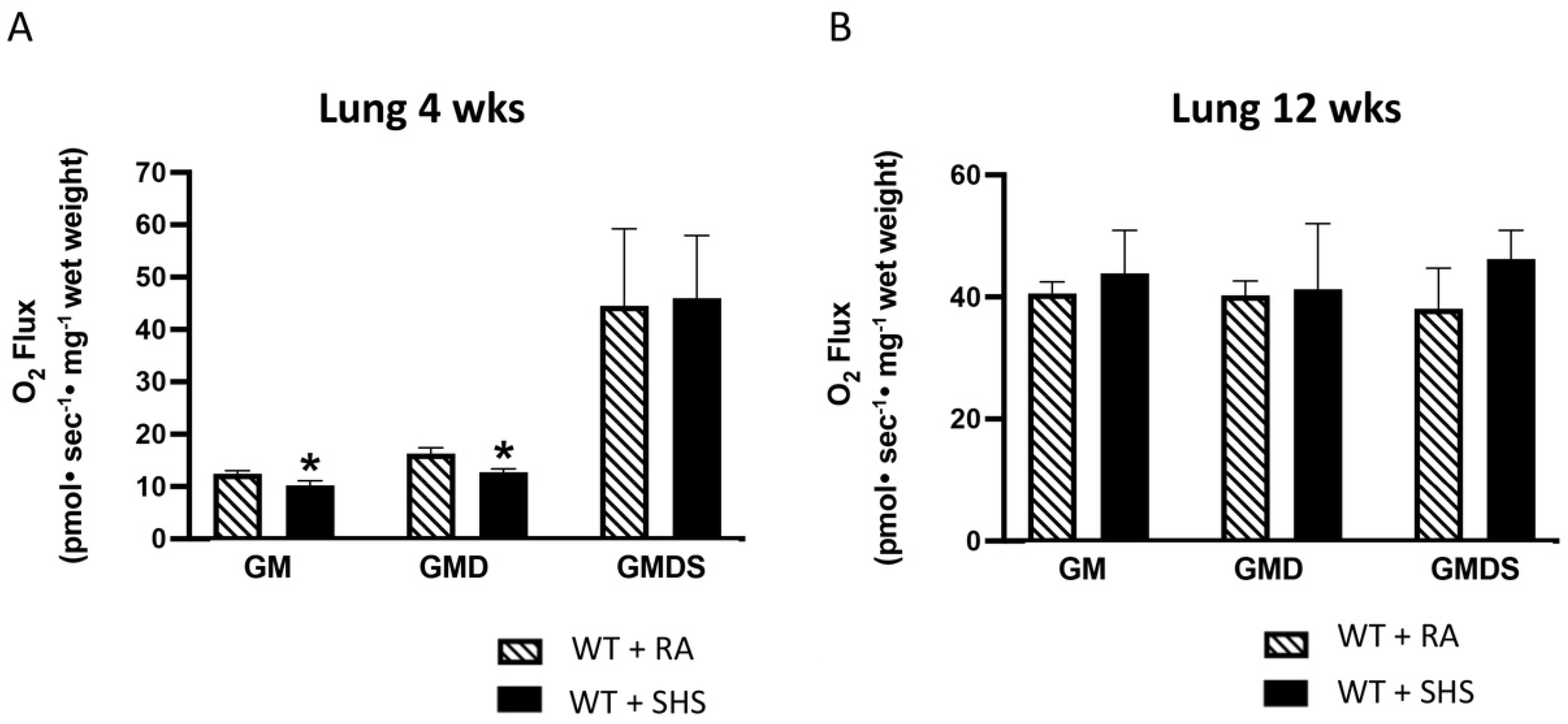
3.3. Lung Mitochondrial Respiration Analysis
3.4. Blood Pressure and Heart Rate
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abraham, M.; Alramadhan, S.; Iniguez, C.; Duijts, L.; Jaddoe, V.W.; Den Dekker, H.T.; Crozier, S.; Godfrey, K.M.; Hindmarsh, P.; Vik, T.; et al. A systematic review of maternal smoking during pregnancy and fetal measurements with meta-analysis. PLoS ONE 2017, 12, e0170946. [Google Scholar] [CrossRef]
- Lewis, J.B.; Mejia, C.; Jordan, C.; Monson, T.D.; Bodine, J.S.; Dunaway, T.M.; Egbert, K.M.; Lewis, A.L.; Wright, T.J.; Ogden, K.C.; et al. Inhibition of the receptor for advanced glycation end-products (RAGE) protects from secondhand smoke (SHS)-induced intrauterine growth restriction IUGR in mice. Cell Tissue Res. 2017, 370, 513–521. [Google Scholar] [CrossRef]
- Andres, R.L.; Day, M.C. Perinatal complications associated with maternal tobacco use. Semin. Neonatol. 2000, 5, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Reeves, S.; Bernstein, I. Effects of maternal tobacco-smoke exposure on fetal growth and neonatal size. Expert Rev. Obstet. Gynecol. 2008, 3, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Windham, G.C.; Hopkins, B.; Fenster, L.; Swan, S.H. Prenatal active or passive tobacco smoke exposure and the risk of preterm delivery or low birth weight. Epidemiology 2000, 11, 427–433. [Google Scholar] [CrossRef]
- Badlissi, D.; Guillemette, A.; Fadin, A. Prematurite et petit poids ala naissance: Les effets du tabagisme actif et passif durant la grossesse. [Prematurity and low birth weight: Effects of active and passive smoking during pregnancy]. Can. J. Public Health 2001, 92, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Salihu, H.M.; Sharma, P.P.; Getahun, D.; Hedayatzadeh, M.; Peters, S.; Kirby, R.S.; Alio, A.P.; Gaafer-Ahmed, H. Prenatal tobacco use and risk of stillbirth: A case-control and bidirectional case-crossover study. Nicotine Tob. Res. 2008, 10, 159–166. [Google Scholar] [CrossRef]
- Taal, H.R.; Geelhoed, J.J.M.; Steegers, E.A.P.; Hofman, A.; Moll, H.A.; Lequin, M.; van der Heijden, A.J.; Jaddoe, V.W.V. Maternal smoking during pregnancy and kidney volume in the offspring: The Generation R Study. Pediatr. Nephrol. 2011, 26, 1275–1283. [Google Scholar] [CrossRef]
- Anblagan, D.; Jones, N.W.; Costigan, C.; Parker, A.J.J.; Allcock, K.; Aleong, R.; Coyne, L.H.; Deshpande, R.; Raine-Fenning, N.; Bugg, G.; et al. Maternal smoking during pregnancy and fetal organ growth: A magnetic resonance imaging study. PLoS ONE 2013, 8, e67223. [Google Scholar] [CrossRef]
- Block, D.B.; Mesquita, F.F.; de Lima, I.P.; Boer, P.A.; Gontijo, J.A. Fetal kidney programming by maternal smoking exposure: Effects on kidney structure, blood pressure and urinary sodium excretion in adult offspring. Nephron 2015, 129, 283–292. [Google Scholar] [CrossRef]
- Edstedt Bonamy, A.K.; Bengtsson, J.; Nagy, Z.; De Keyzer, H.; Norman, M. Preterm birth and maternal smoking in pregnancy are strong risk factors for aortic narrowing in adolescence. Acta Paediatr. 2008, 97, 1080–1085. [Google Scholar] [CrossRef]
- Malik, S.; Cleves, M.A.; Honein, M.A.; Romitti, P.A.; Botto, L.D.; Yang, S.; Hobbs, C.A.; the National Birth Defects Prevention Study. Maternal smoking and congenital heart defects. Pediatrics 2008, 121, e810–e816. [Google Scholar] [CrossRef]
- Gutvirtz, G.; Wainstock, T.; Landau, D.; Sheiner, E. Maternal smoking during pregnancy and long-term neurological morbidity of the offspring. Addict. Behav. 2019, 88, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Sowan, N.A.; Stember, M.L. Effect of maternal prenatal smoking on infant growth and development of obesity. J. Perinat. Educ. 2000, 9, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Peters, H.; Gama, A.; Carvalhal, M.I.M.; Nogueira, H.G.M.; Rosado-Marques, V.; Padez, C. Maternal smoking in pregnancy association with childhood adiposity and blood pressure. Pediatr. Obes. 2016, 11, 202–209. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, C.T.; Spindel, E.R. Pulmonary Effects of Maternal Smoking on the Fetus and Child: Effects on Lung Development, Respiratory Morbidities, and Life Long Lung Health. Paediatr. Respir. Rev. 2017, 21, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Spindel, E.R.; McEvoy, C.T. The Role of Nicotine in the Effects of Maternal Smoking during Pregnancy on Lung Development and Childhood Respiratory Disease. Implications for Dangers of E-Cigarettes. Am. J. Respir. Crit. Care Med. 2016, 193, 486–494. [Google Scholar] [CrossRef]
- Falkner, B. Maternal and gestational influences on childhood blood pressure. Pediatr. Nephrol. 2020, 35, 1409–1418. [Google Scholar] [CrossRef]
- Yang, J.; Hashemi, S.; Han, W.; Song, Y.; Lim, Y. Exposure and Risk Assessment of Second- and Third-Hand Tobacco Smoke Using Urinary Cotinine Levels in South Korea. Int. J. Environ. Res. Public Health 2022, 19, 3746. [Google Scholar] [CrossRef]
- Yang, J.; Hashemi, S.; Kim, T.; Park, J.; Park, M.; Han, W.; Park, D.; Lim, Y. Risk assessment and estimation of controlling safe distance for exposure to particulate matter from outdoor secondhand tobacco smoke. Air Qual. Atmos. Health 2023, 17, 139–154. [Google Scholar] [CrossRef]
- Winden, D.R.; Barton, D.B.; Betteridge, B.C.; Bodine, J.S.; Jones, C.M.; Rogers, G.D.; Chavarria, M.; Wright, A.J.; Jergensen, Z.R.; Jimenez, F.R.; et al. Antenatal exposure of maternal secondhand smoke (SHS) increases fetal lung expression of RAGE and induces RAGE-mediated pulmonary inflammation. Respir. Res. 2014, 15, 129. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, A.; Blood, D.C.; del Toro, G.; Canet, A.; Lee, D.C.; Qu, W.; Tanji, N.; Lu, Y.; Lalla, E.; Fu, C.; et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature 2000, 405, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.A.; Drury, S.; Fu, C.; Qu, W.; Taguchi, A.; Lu, Y.; Avila, C.; Kambham, N.; Bierhaus, A.; Nawroth, P.; et al. RAGE mediates a novel proinflammatory axis: A central cell surface receptor for S100/calgranulin polypeptides. Cell 1999, 97, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.M.; Sahagan, B.; Nelson, R.B.; Selmer, J.; Rothlein, R.; Bell, J.M. The role of RAGE in amyloid-beta peptide-mediated pathology in Alzheimer’s disease. Curr. Opin. Investig. Drugs 2009, 10, 672–680. [Google Scholar] [PubMed]
- Demling, N.; Ehrhardt, C.; Kasper, M.; Laue, M.; Knels, L.; Rieber, E.P. Promotion of cell adherence and spreading: A novel function of RAGE, the highly selective differentiation marker of human alveolar epithelial type I cells. Cell Tissue Res. 2006, 323, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, P.R.; Kasteler, S.D.; Cosio, M.G.; Sturrock, A.; Huecksteadt, T.; Hoidal, J.R. RAGE: Developmental expression and positive feedback regulation by Egr-1 during cigarette smoke exposure in pulmonary epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294, L1094–L1101. [Google Scholar] [CrossRef]
- Wolf, L.; Herr, C.; Niederstrasser, J.; Beisswenger, C.; Bals, R. Receptor for advanced glycation endproducts (RAGE) maintains pulmonary structure and regulates the response to cigarette smoke. PLoS ONE 2017, 12, e0180092. [Google Scholar] [CrossRef]
- Sparvero, L.J.; Asafu-Adjei, D.; Kang, R.; Tang, D.; Amin, N.; Im, J.; Rutledge, R.; Lin, B.; Amoscato, A.A.; Zeh, H.J.; et al. RAGE (Receptor for Advanced Glycation Endproducts), RAGE ligands, and their role in cancer and inflammation. J. Transl. Med. 2009, 7, 17. [Google Scholar] [CrossRef]
- Robinson, A.B.; Stogsdill, J.A.; Lewis, J.B.; Wood, T.T.; Reynolds, P.R. RAGE and tobacco smoke: Insights into modeling chronic obstructive pulmonary disease. Front. Physiol. 2012, 3, 301. [Google Scholar] [CrossRef]
- Nelson, M.B.; Swensen, A.C.; Winden, D.R.; Bodine, J.S.; Bikman, B.T.; Reynolds, P.R. Cardiomyocyte mitochondrial respiration is reduced by receptor for advanced glycation end-product signaling in a ceramide-dependent manner. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H63–H69. [Google Scholar] [CrossRef]
- Lubitz, I.; Ricny, J.; Atrakchi-Baranes, D.; Shemesh, C.; Kravitz, E.; Liraz-Zaltsman, S.; Maksin-Matveev, A.; Cooper, I.; Leibowitz, A.; Uribarri, J.; et al. High dietary advanced glycation end products are associated with poorer spatial learning and accelerated Abeta deposition in an Alzheimer mouse model. Aging Cell 2016, 15, 309–316. [Google Scholar] [CrossRef]
- Belmokhtar, K.; Robert, T.; Ortillon, J.; Braconnier, A.; Vuiblet, V.; Boulagnon-Rombi, C.; Diebold, M.D.; Pietrement, C.; Schmidt, A.M.; Rieu, P.; et al. Signaling of Serum Amyloid A Through Receptor for Advanced Glycation End Products as a Possible Mechanism for Uremia-Related Atherosclerosis. Arter. Thromb. Vasc. Biol. 2016, 36, 800–809. [Google Scholar] [CrossRef]
- Reynolds, P.R.; Kasteler, S.D.; Schmitt, R.E.; Hoidal, J.R. Receptor for advanced glycation end-products signals through Ras during tobacco smoke-induced pulmonary inflammation. Am. J. Respir. Cell Mol. Biol. 2011, 45, 411–418. [Google Scholar] [CrossRef]
- Reynolds, P.R.; Stogsdill, J.A.; Stogsdill, M.P.; Heimann, N.B. Up-regulation of receptors for advanced glycation end-products by alveolar epithelium influences cytodifferentiation and causes severe lung hypoplasia. Am. J. Respir. Cell Mol. Biol. 2011, 45, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Hirschi, K.M.; Tsai, K.Y.F.; Davis, T.; Clark, J.C.; Knowlton, M.N.; Bikman, B.T.; Reynolds, P.R.; A Arroyo, J. Growth arrest-specific protein-6/AXL signaling induces preeclampsia in ratsdagger. Biol. Reprod. 2020, 102, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Challis, J.R.; Lockwood, C.J.; Myatt, L.; Norman, J.E.; Strauss, J.F., 3rd; Petraglia, F. Inflammation and pregnancy. Reprod. Sci. 2009, 16, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Pilling, E.L.; Elder, C.J.; Gibson, A.T. Growth patterns in the growth-retarded premature infant. Best Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Delpisheh, A.; Brabin, L.; Attia, E.; Brabin, B.J. Pregnancy late in life: A hospital-based study of birth outcomes. J. Women’s Health 2008, 17, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Mandruzzato, G.; Antsaklis, A.; Botet, F.; Chervenak, F.A.; Figueras, F.; Grunebaum, A.; Puerto, B.; Skupski, D.; Stanojevic, M. Intrauterine restriction (IUGR). J. Perinat. Med. 2008, 36, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Yang, X.; Wu, D.; Wang, X.; Li, T.; Liu, H.; Guo, C.; Wang, J.; Hu, X.; Yu, G.; et al. Insights into infancy weight gain patterns for term small-for-gestational-age babies. Nutr. J. 2018, 17, 97. [Google Scholar] [CrossRef]
- Crume, T.L.; Scherzinger, A.; Stamm, E.; McDuffie, R.; Bischoff, K.J.; Hamman, R.F.; Dabelea, D. The long-term impact of intrauterine growth restriction in a diverse U.S. cohort of children: The EPOCH study. Obesity 2014, 22, 608–615. [Google Scholar] [CrossRef]
- Chakraborty, S.; Joseph, D.V.; Bankart, M.J.; Petersen, S.A.; Wailoo, M.P. Fetal growth restriction: Relation to growth and obesity at the age of 9 years. Arch. Dis. Child.-Fetal Neonatal Ed. 2007, 92, F479–F483. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kaur, S.; Sarkar, M.; Sarin, B.C.; Changotra, H. The AGE-RAGE Axis and RAGE Genetics in Chronic Obstructive Pulmonary Disease. Clin. Rev. Allergy Immunol. 2021, 60, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Yonchuk, J.G.; Silverman, E.K.; Bowler, R.P.; Agustí, A.; Lomas, D.A.; Miller, B.E.; Tal-Singer, R.; Mayer, R.J. Circulating soluble receptor for advanced glycation end products (sRAGE) as a biomarker of emphysema and the RAGE axis in the lung. Am. J. Respir. Crit. Care Med. 2015, 192, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Maremanda, K.P.; Sundar, I.K.; Rahman, I. Protective role of mesenchymal stem cells and mesenchymal stem cell-derived exosomes in cigarette smoke-induced mitochondrial dysfunction in mice. Toxicol. Appl. Pharmacol. 2019, 385, 114788. [Google Scholar] [CrossRef] [PubMed]
- Lugg, S.T.; Scott, A.; Parekh, D.; Naidu, B.; Thickett, D.R. Cigarette smoke exposure and alveolar macrophages: Mechanisms for lung disease. Thorax 2022, 77, 94–101. [Google Scholar] [CrossRef]
- Cohen, G.; Vella, S.; Jeffery, H.; Lagercrantz, H.; Katz-Salamon, M. Cardiovascular stress hyperreactivity in babies of smokers and in babies born preterm. Circulation 2008, 118, 1848–1853. [Google Scholar] [CrossRef]
- Parada-Ricart, E.; Luque, V.; Zaragoza, M.; Ferre, N.; Closa-Monasterolo, R.; Koletzko, B.; Grote, V.; Gruszfeld, D.; Verduci, E.; Xhonneux, A. Effect of maternal smoking during pregnancy on child blood pressure in a European cohort. Sci. Rep. 2022, 12, 17308. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curtis, K.L.; Hirshi, K.M.; Tsai, K.; Clark, E.T.; Stapley, B.M.; Bikman, B.T.; Reynolds, P.R.; Arroyo, J. Antenatal Secondhand Smoke (SHS) Exposure and the Receptor for Advanced Glycation End-Products (RAGE). Reprod. Med. 2024, 5, 1-11. https://doi.org/10.3390/reprodmed5010001
Curtis KL, Hirshi KM, Tsai K, Clark ET, Stapley BM, Bikman BT, Reynolds PR, Arroyo J. Antenatal Secondhand Smoke (SHS) Exposure and the Receptor for Advanced Glycation End-Products (RAGE). Reproductive Medicine. 2024; 5(1):1-11. https://doi.org/10.3390/reprodmed5010001
Chicago/Turabian StyleCurtis, Katrina L., Kelsey M. Hirshi, Kary Tsai, Evan T. Clark, Brendan M. Stapley, Benjamin T. Bikman, Paul R. Reynolds, and Juan Arroyo. 2024. "Antenatal Secondhand Smoke (SHS) Exposure and the Receptor for Advanced Glycation End-Products (RAGE)" Reproductive Medicine 5, no. 1: 1-11. https://doi.org/10.3390/reprodmed5010001
APA StyleCurtis, K. L., Hirshi, K. M., Tsai, K., Clark, E. T., Stapley, B. M., Bikman, B. T., Reynolds, P. R., & Arroyo, J. (2024). Antenatal Secondhand Smoke (SHS) Exposure and the Receptor for Advanced Glycation End-Products (RAGE). Reproductive Medicine, 5(1), 1-11. https://doi.org/10.3390/reprodmed5010001







