Abstract
Background: Outpatient operative hysteroscopy is a minimally invasive procedure widely used for the diagnosis and treatment of intrauterine pathologies, including intrauterine adhesions (IUAs), which significantly affect fertility. Despite its therapeutic potential, the procedure itself may predispose patients to de novo adhesion formation. This review evaluates the effectiveness of anti-adhesion gels, particularly hyaluronic-acid-based formulations, in preventing IUAs and improving reproductive outcomes after outpatient operative hysteroscopy. Materials and Methods: A systematic search was performed in PubMed, CINAHL, Embase, and Web of Science for studies published between January 2020 and May 2025. Inclusion and exclusion criteria were defined using PICO guidelines. Relevant studies were screened and selected by two independent reviewers. Results: Anti-adhesion gels, especially hyaluronic acid and its derivatives, were associated with a lower recurrence of IUAs and improved reproductive outcomes. Combination therapies, such as hyaluronic acid gel with intrauterine devices (IUDs), showed better efficacy than monotherapy. Several studies also reported increased endometrial thickness, higher implantation rates, and improved pregnancy outcomes, although live birth rates remained inconsistent. Conclusions: Hyaluronic-acid-based anti-adhesion gels appear effective in reducing postoperative adhesion formation and enhancing reproductive outcomes in outpatient hysteroscopy. The best results are seen with multimodal preventive strategies. However, heterogeneity across studies highlights the need for standardized, prospective, randomized controlled trials to establish optimal clinical use.
1. Introduction
Hysteroscopy is currently considered the gold standard for the diagnosis and treatment of intrauterine pathologies [1]. Specifically, the outpatient hysteroscopic procedures are performed in healthcare facilities including hospitals, community clinics, or freestanding surgical centers, where conscious sedation and pain control up to level 3 (a) (oral or inhalational medications with sedative effects) can be administered [2]. Consequently, the use of outpatient hysteroscopy has been steadily increasing, particularly in women presenting with abnormal uterine bleeding (AUB), due to its high diagnostic accuracy and minimal invasiveness [3].
The International Federation of Gynecology and Obstetrics (FIGO) recommends classifying AUB causes using the PALM-COEIN system, which separates the structural etiologies Polyp, Adenomyosis, Leiomyoma, and Malignancy and hyperplasia from the non-structural causes Coagulopathy, Ovulatory dysfunction, Endometrial disorders, Iatrogenic, and Not yet classified [4]. Notably, many of these conditions are not only linked to AUB but are also frequently associated with infertility. Indeed, intrauterine abnormalities are identified in approximately 40–50% of infertile women and are considered contributing factors to subfertility [5,6,7]. Among these abnormalities, intrauterine adhesions (IUAs), also known as Asherman’s syndrome, represent a particularly relevant clinical concern [8,9].
IUAs are characterized by the formation of fibrous scar tissue within the endometrial cavity, often resulting in partial or complete obliteration of the uterine space. Such adhesions may consequently cause a spectrum of reproductive consequences, including irregular menstruation, infertility, recurrent pregnancy loss, adverse pregnancy outcomes, and impaired embryo implantation [10,11]. Therefore, IUAs exert a substantial negative impact on women’s reproductive health and fertility potential. Although the overall prevalence of IUAs among infertile patients is estimated to be around 4.6%, certain clinical scenarios present a markedly higher risk. For instance, Hooker et al. reported an incidence of 21.2% following first-trimester surgical terminations and 16.1% after second-trimester procedures [12].
Although hysteroscopic adhesiolysis remains the most effective approach for both the diagnosis and treatment of IUAs, the procedure itself may paradoxically induce new adhesions due to mechanical trauma inflicted on the endometrial lining. In the past few years, growing attention has been directed toward the prevention of postoperative adhesion formation. Various strategies have been proposed, including systemic pharmacologic agents, intrauterine devices, biologics, and anti-adhesion gels. Among these, hyaluronic acid (HA)-based gels have emerged as one of the most widely studied and clinically adopted options. HA is a naturally occurring, biocompatible polymer that acts as a temporary physical barrier between the endometrial surfaces during the healing phase [13]. Importantly, beyond its mechanical properties, HA exhibits anti-inflammatory, fibrinolytic, and pro-angiogenic effects, which may further contribute to reducing adhesion formation [14,15]. In a review [16], the authors highlight the gel’s biocompatibility, biodegradability, viscoelasticity, and mucoadhesive properties, enabling diverse dosage forms (gels, drops, fillers) and targeted delivery. They also report that HA modulates inflammation through CD44-mediated pathways, exerts free-radical scavenging activity within connective tissues, and interacts with TSG-6 to preserve extracellular matrix integrity, mechanisms shown to support tissue repair and regeneration in preclinical and clinical settings.
Many studies have confirmed the efficacy of HA in adhesion prevention. A recent systematic review and meta-analysis [17] demonstrated that HA gel, either alone or combined with an intrauterine device, significantly reduces the incidence and severity of adhesion recurrence after intrauterine surgery. Similarly, another meta-analysis [18] reported a consistent decrease in adhesion formation and improved cavity restoration when HA-based barriers were used. Furthermore, a cost-effectiveness analysis [19] indicated that HA gels and similar barriers are economically advantageous, reducing the need for repeat procedures and associated complications. Moreover, a recent pharmacoeconomic analysis demonstrated that conformable anti-adhesion barriers can significantly reduce healthcare costs by approximately USD 2905 per patient, primarily due to the decreased incidence of preterm birth and related complications [20]. This is particularly relevant given that preterm delivery represents the most expensive adverse outcome in the model, with an estimated cost of USD 90,016 per event [20]. Similarly, outpatient hysteroscopic removal of endometrial polyps prior to IVF has been shown to be cost-effective, improving reproductive outcomes while lowering the overall financial burden of fertility treatment [21].
Therefore, this review aims to evaluate the effectiveness of anti-adhesion gel in the context of outpatient hysteroscopy, with a focus on its role in reducing the formation of intrauterine adhesions and improving reproductive outcomes.
The primary outcome of this review was the recurrence of intrauterine adhesions following outpatient operative hysteroscopy with anti-adhesion gel application. Secondary outcomes included reproductive endpoints such as pregnancy rates, live birth rates, and miscarriage rates, as well as surrogate markers of endometrial receptivity (e.g., endometrial thickness).
2. Materials and Methods
2.1. Search Strategy
The search was conducted in four main electronic databases—PubMed, CINAHL, Embase, and Web of Science—covering the period from January 2020 to May 2025, in order to include the most up-to-date publications. The primary aim of the selected studies was to examine the effectiveness of anti-adhesion gel in preventing the formation of adhesions following outpatient operative hysteroscopy. The search was limited to articles published in English in peer-reviewed journals. In collaboration with an expert librarian, the main search terms and Medical Subject Heading (MeSH) keywords were defined, including “Anti-Adhesion Agents” and “Hysteroscopy”. Additionally, the reference lists of previously published reviews were manually examined. A narrative review was conducted, and the literature search process was guided by the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) framework [22], as illustrated in the PRISMA flow diagram (Figure 1).
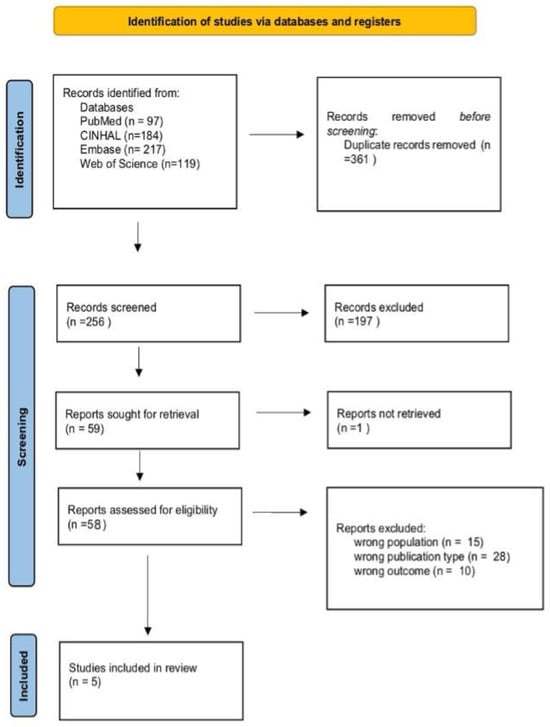
Figure 1.
PRISMA 2020 flow diagram of evaluated studies.
2.2. Inclusion Criteria
Based on the population–intervention–comparison–outcome (PICO) guidelines, the related data were collected and organized as follows: (i) Population: adult women undergoing outpatient operative hysteroscopy for the treatment of intrauterine adhesions. For the purposes of this review, “outpatient” was defined in accordance with the European Society of Gynaecological Endoscopy (ESGE) and Royal College of Obstetricians and Gynaecologists guidelines, as procedures performed in a non-inpatient setting, with use of small-diameter hysteroscopes and minimal or conscious sedation, allowing same-day discharge. We included studies where IV sedation was administered if the intervention took place in an outpatient facility and did not require overnight hospitalization, as these still fall within the scope of ambulatory operative hysteroscopy in current clinical practice.
In accordance with European Society of Gynaecological Endoscopy (ESGE) and Royal College of Obstetricians and Gynaecologist guidelines [21,22,23] and supported by the operative hysteroscopy nomenclature established by Carugno et al. [2], procedures were considered outpatient if they met technical criteria such as use of small-diameter hysteroscopes and minimal anesthesia; (ii) Intervention: the application of anti-adhesion gel during outpatient operative hysteroscopy. This included various formulations of anti-adhesion gels, such as hyaluronic-acid-based gel or other similar agents; (iii) Comparison: studies that did not use any gel or used a placebo, or those that compared it with other methods of adhesion prevention were included; (iv) Outcome: the beneficial and adverse effects of using anti-adhesion gel, including the prevention of intrauterine adhesions, reproductive outcomes and comparison with other treatments. Adhesion recurrence was designated as the primary outcome of interest. Secondary outcomes comprised reproductive outcomes (clinical pregnancy, live birth, miscarriage) and surrogate markers (endometrial thickness, cavity volume).
2.3. Exclusion Criteria
The exclusion criteria for this review were as follows: (i) studies involving patients who underwent hysteroscopy under general anesthesia in an inpatient setting; (ii) studies that utilized treatment methods other than anti-adhesion gel for the prevention of intrauterine adhesions; (iii) studies that were either study protocols or conference abstracts.
2.4. Data Extraction
The literature search resulted in a total of 617 articles sampled for screening. The study selection process was conducted by two authors (S.El, A.M.), including the preliminary search, systematic search in the databases, screening of titles and abstracts, and evaluation of full-text articles. Article management was performed using the Rayyan database [24]. After removing duplicates, titles and abstracts were screened based on the inclusion and exclusion criteria. Finally, two authors (S.El, A.M.) reviewed the full texts of the selected articles, and in case of discrepancies, a discussion allowed for a final consensus to be reached.
2.5. Risk of Bias and Quality Assessment
We performed a basic qualitative appraisal of the methodological quality of the five included studies. While the narrative nature of this review precluded the use of formal scoring tools, study design, sample size, and reported methodological limitations were considered. Randomized controlled trials (RCTs) were regarded as providing the highest level of evidence, followed by prospective cohort studies. Among the included studies, one was an RCT, two were prospective cohorts, and two were retrospective cohorts. Strengths observed across the literature included clearly defined inclusion criteria, standardized hysteroscopic techniques, and clinically relevant outcome measures such as adhesion recurrence and reproductive endpoints. Common limitations were the absence of blinding, relatively small sample sizes in some studies, single-center settings, heterogeneity of intervention protocols, and short follow-up in several cases. A summary of this appraisal is presented in Table 1 (Qualitative appraisal of included studies).

Table 1.
Qualitative appraisal of included studies.
3. Results
The review included five studies conducted in China [25,28], Spain [26], Vietnam [29], and Italy [27], involving a total of 1155 patients (Table 2, Study characteristics). The types of gels used included hyaluronic acid (HA) gel, autocrosslinked hyaluronic acid gel (ACP, such as Hyalobarrier®, Nordic Pharma, San Felice (MIilan), Italy, carboxymethylcellulose (CMC) gel, and crosslinked hyaluronic acid (cHA, such as MateRegen®, Kebomed UK, Swaines Hill Manor, Alton, Hampshire, UK). The follow-up duration varied across studies, ranging from 2 months in the study by Trinh et al. [29], 6 months in Dan et al. [28], 24 months in Esteban Manchado et al. [26], and 60 months in Di Spiezio Sardo et al. [27] to intermediate timepoints in Mao et al. [25].

Table 2.
Study characteristics.
The results are presented below, organized by outcome.
3.1. Prevention of Intrauterine Adhesions
The studies reviewed have all evaluated the effectiveness of hyaluronic acid (HA) gel in preventing postoperative adhesions, with variable results. The study by Trinh et al. [29] showed that the group treated with HA gel + IUD significantly reduced the recurrence of intrauterine adhesions compared to the other groups, with a recurrence rate of 10% in the combined group, compared to 32.2% in the HA group and 35.6% in the IUD group (p = 0.02). Additionally, this treatment significantly improved American Fertility Society (AFS) scores compared to the other groups (p = 0.02 compared to the HA group and p = 0.01 compared to the IUD group) [29]. Consistent with the findings of Dan et al. and Trinh et al., combination strategies appeared more effective than monotherapy in reducing adhesion recurrence, although reproductive benefits were less consistent [28,29]. This suggests that while HA gel alone provides some protective effect, its efficacy may be enhanced when combined with other interventions, although the translation of adhesion reduction into improved fertility outcomes remains uncertain.
3.2. Reproductive Outcomes
Reproductive outcomes, including pregnancy rates and term birth rates, have been analyzed in three studies. The study by Di Spiezio Sardo et al. [27] reported a clinical pregnancy rate of 72.9% with a miscarriage rate of 19.9% and a term birth rate of 86.4% with use of gel (either carboxymethylcellulose (CMC) gel or an auto-crosslinked hyaluronic acid (ACP) gel, depending on the case). This study also documented a significant improvement in the uterine cavity volume, from 1.42 cm3 to 2.09 cm3 (p < 0.0001), suggesting that the intervention with anti-adhesion gel (CMC or HA) may improve the anatomical quality of the uterus, thus increasing the likelihood of conception, even for women who had previously failed IVF/ICSI cycles [27]. These findings underline a potential structural benefit of HA-based gels, which may indirectly contribute to improved fertility. The study by Berta Esteban Manchado et al. [26], reported a clinical pregnancy rate of 78.9% with a 63.2% live birth rate, with most pregnancies carried to term (70%) and a miscarriage rate of 20%. No severe complications—such as postpartum hemorrhage or uterine rupture—emerged, suggesting that the use of gel and other preventive strategies may reduce risks for patients [26]. This supports the safety profile of HA gels in reproductive surgery, an important factor for their clinical applicability. Mao et al. [25], also investigated the effectiveness of cross-linked HA gel (cHA) in patients with moderate to severe intrauterine adhesions undergoing operative hysteroscopy followed by embryo transfer. In this study, the cHA-treated group showed significantly better reproductive outcomes compared to the control group. Clinical pregnancy rates, chemical pregnancy rates, and implantation rates were higher (all p < 0.05), highlighting a potential positive effect of cHA on endometrial receptivity. Additionally, the treated group showed a significant increase in endometrial thickness (from 6.35 ± 0.92 mm to 7.97 ± 1.37 mm, p < 0.001), a lower incidence of canceled embryo transfers due to thin endometrium, and a higher endometrial thickness on the day of transfer (p < 0.001). Although singleton pregnancy rates did not significantly differ between the groups, the improved endometrial quality may have positively influenced the implantation rate. Histologically, there was a trend towards an increase in tubular glands in the cHA group (15.1 ± 13.2 vs. 28.8 ± 30.4, p = 0.166) and a greater amount of preoperative fibrotic tissue, suggesting a possible regenerative effect of the gel on endometrial functionality. Together, these findings indicate that HA gels may exert a dual role, both improving the endometrial environment and enhancing implantation success. Similarly, Dan et al. [28] analyzed pregnancy outcomes in women with intrauterine adhesions treated with hysteroscopic adhesiolysis followed by HA gel application. In this study, the HA group achieved a pregnancy rate of 36%, which was significantly higher than the expectant group (21%, p = 0.0077), although lower than the YD group (52%, p = 0.0161). This suggests that HA gel alone offers measurable reproductive benefits compared with no treatment, but may be less effective than alternative medical approaches such as Yangmo decoction.
3.3. Comparison Between Treatments
As mentioned above, two studies compared the effectiveness of hyaluronic acid (HA) gel with other therapeutic options for the prevention of postoperative adhesions, such as the intrauterine device (IUD) or the combined treatment of HA gel + IUD. The results show significant differences in the effectiveness of these treatments. The study by Trinh et al. [29] compared three groups: the HA-gel-treated group, the IUD-treated group, and the HA gel + IUD-treated group. The recurrence of adhesions at 2 months, assessed by hysterosalpingography, was 32.2% in the HA group (n = 121), 35.6% in the IUD group (n = 59), and 10.0% in the HA gel + IUD group (n = 20). As a result, the HA gel + IUD treatment reduced recurrence by 22.2% compared to the HA group (p = 0.02), and by 25.6% compared to the IUD group alone (p = 0.01), suggesting that the combination of HA gel and IUD may be more effective in reducing adhesion formation compared to the single treatments. Moreover, multivariate analysis confirmed that the combined use of HA gel + IUD significantly reduced the recurrence of adhesions compared to both the HA-only treatment (aOR 0.19; 95% CI, 0.03–0.88) and the IUD-only treatment (aOR 0.13; 95% CI, 0.02–0.67). This underscores the efficacy of the combined treatments in improving adhesion prevention [29]. These results emphasize the potential synergistic effect of combining mechanical and biochemical barriers, although the evidence is limited by the short follow-up duration and relatively small subgroup sample sizes. However, it is important to note that, although the HA gel + IUD combination was superior, no significant differences in pregnancy rates emerged between the groups. The HA gel + IUD group showed an ongoing pregnancy rate of 61.2%, with no significant differences compared to the HA group (63.5%) or the IUD group (59.3%). This result suggests that, although adhesion prevention may be improved with the combination of treatments, the effects on conception may not be as pronounced [29]. This discrepancy indicates that adhesion reduction does not necessarily translate into proportional reproductive gains, highlighting the multifactorial nature of infertility in women with intrauterine adhesions. Dan et al. [28] explored a different approach to adhesion prevention by comparing the efficacy of HA gel with Yangmo decoction (YD), proving how, after six months, the recurrence of adhesions was significantly lower in the YD group (14%) compared to the groups treated with HA gel (32%) or with no additional treatment (EP, 35%). This comparison demonstrates a positive effect of HA gel, although lower than other therapeutic options. Additionally, pregnancy rates were significantly higher in the YD group (52%) compared to the HA (36%) and EP (21%) groups [27,28]. As a result, while HA gel treatment led to a reduction in adhesions compared to the no-treatment group, the YD treatment showed superiority in both adhesion prevention and reproductive outcomes [27,28]. Overall, these findings suggest that while HA gel remains a valid option for preventing adhesions, the use of combined treatments, such as HA gel + IUD, or alternatives like the YD treatment, may offer additional advantages, particularly in terms of adhesion prevention and improving pregnancy rates. However, the differences in pregnancy rates between the groups indicate that other factors, beyond adhesion prevention, influence reproductive outcomes [27,28]. These findings suggest that, although HA gel is a valuable option, its relative efficacy may depend on the comparator, and further high-quality studies are needed to clarify whether combination or alternative treatments should be prioritized in clinical practice.
4. Discussion
The choice of preventive measures for IUAs following office hysteroscopic procedures remains a controversial issue. Patients undergoing procedures within the endometrial cavity in an outpatient setting may benefit from the use of anti-adhesion gels, both in terms of improved reproductive outcomes and reduced incidence of adhesions.
A notable advantage of office polypectomy, for example, is the significant reduction in costs, owing to the elimination of hospital stays and the use of operating rooms [30]. Moreover, the outpatient setting is associated with a lower risk of serious complications compared to inpatient hysteroscopy [31,32].
In this review, we sought to explore the use of hyaluronic-acid-based gel in the setting of outpatient hysteroscopy. Despite the heterogeneity of the included studies both in terms of experimental design and population characteristics, the use of anti-adhesion gel appears promising in preventing IUAs and improving reproductive outcomes.
Our findings regarding the use of gel in combination with IUDs are supported by the study of Vitale et al. [33], in which the combination of HA + IUD achieved the highest Surface Under the Cumulative Ranking (SUCRA) score (49.9%) in terms of preventive efficacy.
Similarly, Wu et al. [34] highlighted that the combination of HA, IUD, and estrogen led to significant improvements in menstrual patterns, endometrial growth, and the severity of adhesions, as measured by AFS score. In particular, the E + HA and E + IUD + HA groups showed higher pregnancy rates (up to 90.23%), a lower incidence of spontaneous abortion (13.79%), and the highest full-term delivery rate (67.82%). These results suggest that multimodal therapeutic strategies may be more effective than hyaluronic acid alone [35].
The study by Pan et al. [36] supports our findings on the use of gel in combination with alternative Chinese medicine treatments. The authors report improved reproductive outcomes, suggesting that uterine cavity perfusion with an herbal decoction may enhance endometrial blood circulation and improve menstrual patterns following surgical management of IUAs. An important aspect is the growing role of operative hysteroscopy in the context of assisted reproductive technology (ART).
It allows for early diagnosis and timely treatment of intrauterine pathologies, optimizing the uterine environment in patients undergoing techniques such as in vitro fertilization and embryo transfer (IVF-ET) [37]. In these cases, the presence of endometrial polyps, submucosal fibroids, adhesions, or uterine anomalies may significantly hinder embryo implantation.
The use of anti-adhesion gel in this patient group thus appears reasonable to reduce the risk of IUAs and improve reproductive outcomes [38].
Recent literature has also highlighted the usefulness of the gel following early obstetric events. In a study by George et al. [39], office hysteroscopy enabled the identification of retained products of conception in a significant proportion of patients with normal or inconclusive ultrasound scans, thereby avoiding subsequent interventions under general anesthesia. In this context, the gel emerges as an effective preventive strategy. It is also important to consider the possible bidirectional relationship between IUAs and recurrent pregnancy loss (RPL), with mutual negative effects on reproductive outcomes [40]. Endometrial thickness (EMT), a key indicator of uterine receptivity, is often reduced in the presence of adhesions [41].
Mao et al. [25] demonstrated that gel application can promote greater EMT at the time of embryo transfer and reduce IVF-ET cycle cancellation rates. Although no statistically significant differences were observed in pregnancy rates between groups, it is plausible that improved endometrial quality had a positive effect on implantation.
Finally, data from post-abortion studies suggest that the use of self-crosslinking HA gel after uterine aspiration reduces the incidence of IUAs (8.06% versus 19.35% in the control group), especially in patients undergoing multiple cannula insertions [42,43]. Sroussi et al. [38] demonstrated that the application of hyaluronic-acid-based gel after dilation and curettage (D and C) halves the incidence of IUAs, representing a significant benefit. The data also indicated a lower need for subsequent surgical interventions in the gel-treated group.
Although fertility at 12 months was slightly higher in the treated group, the difference did not reach statistical significance. The use of the gel also appears to promote better anatomical and functional recovery of the uterus [38]. The study by Guo et al. [43] assessed the effectiveness of ACP gel in the secondary prevention of IUAs following hysteroscopic adhesiolysis in women with mild or moderate adhesions. The results showed that the addition of ACP gel, compared to standard care (intrauterine balloon and estrogen therapy), did not significantly reduce either the incidence or severity of adhesion recurrence, suggesting that the effectiveness of the treatment may depend on the initial severity of IUAs and the therapeutic protocol used.
However, the use of the gel as a standalone intervention presents certain limitations. Although HA is a biocompatible and biodegradable agent, its efficacy is limited mainly due to its tendency to leak from the uterine cavity due to gravity. For this reason, it is not recommended as the sole preventive measure following adhesiolysis [36]. Another critical issue is the heterogeneity of therapeutic protocols, variability of the gels used, and diversity of the outcomes analyzed, which limit the ability to draw definitive conclusions. In this regard, Torres-de la Roche et al. [44] highlighted that Hyalobarrier®, compared to MetaRegen®, has a higher HA concentration, greater rupture resistance, and better viscoelastic properties.
The volume of gel applied may also influence the results. Dou et al. [45] suggest that favorable short-term outcomes may be achieved in patients when ≥5 mL of HA gel is applied. Although anti-adhesion agents have been widely studied in various surgical contexts, particularly in inpatient hysteroscopy or other types of pelvic surgery, their effectiveness and specific outcomes in the setting of outpatient operative hysteroscopy remain underexplored in the current literature.
The results have been presented stratified by intervention type to improve clarity, distinguishing between hyaluronic acid gel alone, combination treatments (e.g., HA gel + IUD), and comparisons with alternative preventive strategies. Nevertheless, the synthesis of evidence was notably impacted by heterogeneity in patient characteristics, surgical techniques, adjunctive therapies, outcome definitions, and follow-up durations. These differences reduce the comparability of results across studies and limit the strength of pooled conclusions, underscoring the need for standardized study protocols in future research.
From a clinical perspective, our synthesis supports the consideration of anti-adhesion strategies, particularly hyaluronic acid gel, as an adjunct following outpatient operative hysteroscopy in women at high risk of intrauterine adhesion recurrence. This approach aligns with current AAGL and ESGE practice recommendations, which advocate for preventive measures in selected cases. Incorporating such interventions into routine clinical protocols may help improve reproductive outcomes while minimizing the need for repeat procedures. Further high-quality randomized controlled trials are warranted to refine patient selection criteria and optimize intervention protocols.
5. Conclusions
This review highlights the promising potential of hyaluronic-acid-based gel in preventing IUAs in an outpatient setting. Despite the heterogeneity of the studies analyzed, there is a favorable trend toward the use of multimodal therapeutic strategies combining the gel with other treatments in order to optimize reproductive outcomes. However, some limitations persist, such as variability in therapeutic protocols highlighting the need for further research to strengthen the current evidence. As a result, prospective and randomized studies are required to better define the efficacy and optimal use of anti-adhesion gel in the context of office hysteroscopy.
Author Contributions
Conceptualization, A.M. and S.E.M.; validation, A.M. and S.E.M.; formal analysis, A.M., S.E.M. and A.L.; investigation, A.M., S.E.M. and A.L.; writing—original draft preparation, A.M., S.E.M. and A.L.; writing—review and editing, I.G., L.L., B.M., V.R. and F.S.; supervision L.L. and B.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data was generated or manipulated.
Acknowledgments
Biella Biomedical Library—3Bi Foundation for research support.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| RCT | Randomized controlled trials |
| AFS | American Fertility Society |
| ACP | Auto-crosslinked hyaluronic acid |
| IUA | Intrauterine adhesion |
| NR | Not reported |
| HA | Hyaluronic acid gel |
| YD | Yangmo decoction |
| CMC | Sodium carboxymethylcellulose gel |
| CHC | Oral combined hormonal contraception |
| CHA | Cross-linked hyaluronan gel |
References
- Gkrozou, F.; Dimakopoulos, G.; Vrekoussis, T.; Lavasidis, L.; Koutlas, A.; Navrozoglou, I.; Stefos, T.; Paschopoulos, M. Hysteroscopy in Women with Abnormal Uterine Bleeding: A Meta-Analysis on Four Major Endometrial Pathologies. Arch. Gynecol. Obstet. 2015, 291, 1347–1354. [Google Scholar] [CrossRef]
- Carugno, J.; Grimbizis, G.; Franchini, M.; Alonso, L.; Bradley, L.; Campo, R.; Catena, U.; Carlo, D.A.; Attilio, D.S.S.; Martin, F.; et al. International Consensus Statement for Recommended Terminology Describing Hysteroscopic Procedures. J. Minim. Invasive Gynecol. 2022, 29, 385–391. [Google Scholar] [CrossRef]
- Nash, R.; Saidi, S. Outpatient Hysteroscopy: Suitable for All? A Retrospective Cohort Study of Safety, Success and Acceptability in Australia. Aust. N. Z. J. Obstet. Gynaecol. 2024, 64, 475–481. [Google Scholar] [CrossRef]
- Töz, E.; Sancı, M.; Özcan, A.; Beyan, E.; İnan, A.H. Comparison of Classic Terminology with the FIGO PALM-COEIN System for Classification of the Underlying Causes of Abnormal Uterine Bleeding. Int. J. Gynecol. Obstet. 2016, 133, 325–328. [Google Scholar] [CrossRef]
- Pounikar, M.; Shrivastava, D.; Sharma, S.; Tadghare, J. Role of Hysteroscopy in Patients with Previous In Vitro Fertilization Failure: An Institutional Experience in Rural Population. J. Obstet. Gynaecol. India 2023, 73, 77–82. [Google Scholar] [CrossRef]
- Genovese, F.; Di Guardo, F.; Monteleone, M.M.; D’Urso, V.; Colaleo, F.M.; Leanza, V.; Palumbo, M. Hysteroscopy as an Investigational Operative Procedure in Primary and Secondary Infertility: A Systematic Review. Int. J. Fertil. Steril. 2021, 15, 80–87. [Google Scholar] [CrossRef]
- Di Spiezio Sardo, A.; Iorio, G.G.; Guerra, S.; Isaacson, K.; Kafetzis, D.; Conforti, A.; De Angelis, M.C.; Zizolfi, B.; Alviggi, C. The Role of Hysteroscopy in Patients with Adenomyosis and Infertility: Bringing out the Submerged. Fertil. Steril. 2025, 123, 1140–1142. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Wong, Y.-M.; Cheong, Y.; Xia, E.; Li, T.-C. Asherman Syndrome—One Century Later. Fertil. Steril. 2008, 89, 759–779. [Google Scholar] [CrossRef] [PubMed]
- March, C.M. Management of Asherman’s Syndrome. Reprod. Biomed. Online 2011, 23, 63–76. [Google Scholar] [CrossRef]
- Hooker, A.; De Leeuw, R.; Twisk, J.; Huirne, J. Reproductive Performance Following Application of Hyaluronic Acid Gel after Dilatation and Curettage for Miscarriage in Women with at Least One Previous Curettage. Hum. Reprod. 2020, 35, i137. [Google Scholar]
- Ma, J.; Gao, W.; Li, D. Recurrent Implantation Failure: A Comprehensive Summary from Etiology to Treatment. Front. Endocrinol. 2022, 13, 1061766. [Google Scholar] [CrossRef]
- Hooker, A.B.; de Leeuw, R.; van de Ven, P.M.; Bakkum, E.A.; Thurkow, A.L.; Vogel, N.E.A.; van Vliet, H.A.A.M.; Bongers, M.Y.; Emanuel, M.H.; Verdonkschot, A.E.M.; et al. Prevalence of Intrauterine Adhesions after the Application of Hyaluronic Acid Gel after Dilatation and Curettage in Women with at Least One Previous Curettage: Short-Term Outcomes of a Multicenter, Prospective Randomized Controlled Trial. Fertil. Steril. 2017, 107, 1223–1231.e3. [Google Scholar] [CrossRef]
- Capella-Monsonís, H.; Kearns, S.; Kelly, J.; Zeugolis, D.I. Battling Adhesions: From Understanding to Prevention. BMC Biomed. Eng. 2019, 1, 5. [Google Scholar] [CrossRef]
- Liu, Y.-R.; Liu, B.; Yang, B.-P.; Lan, Y.; Chi, Y.-G. Efficacy of Hyaluronic Acid on the Prevention of Intrauterine Adhesion and the Improvement of Fertility: A Meta-Analysis of Randomized Trials. Complement. Ther. Clin. Pract. 2022, 47, 101575. [Google Scholar] [CrossRef]
- Anvari-Yazdi, A.F.; Badea, I.; Chen, X. Biomaterials in Postoperative Adhesion Barriers and Uterine Tissue Engineering. Gels 2025, 11, 441. [Google Scholar] [CrossRef]
- Vasvani, S.; Kulkarni, P.; Rawtani, D. Hyaluronic Acid: A Review on Its Biology, Aspects of Drug Delivery, Route of Administrations and a Special Emphasis on its Approved Marketed Products and Recent Clinical Studies. Int. J. Biol. Macromol. 2020, 151, 1012–1029. [Google Scholar] [CrossRef]
- Unanyan, A.; Pivazyan, L.; Krylova, E.; Obosyan, L.; Ishchenko, A. Comparison of Effectiveness of Hyaluronan Gel, Intrauterine Device and Their Combination for Prevention Adhesions in Patients after Intrauterine Surgery: Systematic Review and Meta-Analysis. J. Gynecol. Obstet. Hum. Reprod. 2022, 51, 102334. [Google Scholar] [CrossRef]
- Zheng, F.; Xin, X.; He, F.; Liu, J.; Cui, Y. Meta-Analysis on the Use of Hyaluronic Acid Gel to Prevent Intrauterine Adhesion after Intrauterine Operations. Exp. Ther. Med. 2020, 19, 2672–2678. [Google Scholar] [CrossRef]
- Schmerold, L.; Martin, C.; Mehta, A.; Sobti, D.; Jaiswal, A.K.; Kumar, J.; Feldberg, I.; Munro, M.G.; Lee, W.C. A Cost-Effectiveness Analysis of Intrauterine Spacers Used to Prevent the Formation of Intrauterine Adhesions Following Endometrial Cavity Surgery. J. Med. Econ. 2024, 27, 170–183. [Google Scholar] [CrossRef]
- Mouhayar, Y.; Yin, O.; Mumford, S.L.; Segars, J.H. Hysteroscopic Polypectomy Prior to Infertility Treatment: A Cost Analysis and Systematic Review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 213, 107–115. [Google Scholar] [CrossRef]
- AAGL. Practice Report: Practice Guidelines on Intrauterine Adhesions Developed in Collaboration with the European Society of Gynaecological Endoscopy (ESGE). Gynecol. Surg. 2017, 14, 6. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Tammy, C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brenna, S.E. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Br. Med. J. 2021, 372, n71. [Google Scholar] [CrossRef]
- De Silva, P.M.; Smith, P.P.; Cooper, N.A.M.; Clark, T.J.; the Royal College of Obstetricians and Gynaecologists. Outpatient Hysteroscopy. Int. J. Obstet. Gynaecol. 2024, 131, 669–678. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Tao, Y.; Cai, R.; Zhang, J.; Gao, H.; Chen, Q.; Kuang, Y.; Zhang, S. Cross-Linked Hyaluronan Gel to Improve Pregnancy Rate of Women Patients with Moderate to Severe Intrauterine Adhesion Treated with IVF: A Randomized Controlled Trial. Arch. Gynecol. Obstet. 2020, 301, 199–205. [Google Scholar] [CrossRef]
- Esteban Manchado, B.; Lopez-Yarto, M.; Fernandez-Parra, J.; Rodriguez-Oliver, A.; Gonzalez-Paredes, A.; Laganà, A.S.; Garzon, S.; Haimovich, S. Office Hysteroscopic Metroplasty with Diode Laser for Septate Uterus: A Multicenter Cohort Study. Minim. Invasive Ther. Allied Technol. 2022, 31, 441–447. [Google Scholar] [CrossRef]
- Di Spiezio Sardo, A.; Campo, R.; Zizolfi, B.; Santangelo, F.; Meier Furst, R.; Di Cesare, C.; Bettocchi, S.; Vitagliano, A.; Om-belet, W. Long-Term Reproductive Outcomes after Hysteroscopic Treatment of Dysmorphic Uteri in Women with Reproductive Failure: An European Multicenter Study. J. Minim. Invasive Gynecol. 2020, 27, 755–762. [Google Scholar] [CrossRef]
- Dan, J.; Cao, Y. Yangmo Decoction versus Hyaluronic Acid Gel in Women with Intrauterine Re-Adhesion after Hysteroscopic Adhesiolysis: A Retrospective Efficacy and Safety Analysis. BMC Womens Health 2023, 23, 25. [Google Scholar] [CrossRef]
- Trinh, T.T.; Nguyen, K.D.; Pham, H.V.; Ho, T.V.; Nguyen, H.T.; O’Leary, S.; Le, H.T.T.; Pham, H.M. Effectiveness of Hyaluronic Acid Gel and Intrauterine Devices in Prevention of Intrauterine Adhesions after Hysteroscopic Adhesiolysis in Infertile Women. J. Minim. Invasive Gynecol. 2022, 29, 284–290. [Google Scholar] [CrossRef]
- Luerti, M.; Vitagliano, A.; Di Spiezio Sardo, A.; Angioni, S.; Garuti, G.; De Angelis, C.; Del Zoppo, S.; Dealberti, D.; Nappi, L.; Perrini, G. Effectiveness of Hysteroscopic Techniques for Endometrial Polyp Removal: The Italian Multicenter Trial. J. Minim. Invasive Gynecol. 2019, 26, 1169–1176. [Google Scholar] [CrossRef]
- Litta, P.; Cosmi, E.; Saccardi, C.; Esposito, C.; Rui, R.; Ambrosini, G. Outpatient Operative Polypectomy Using a 5 mm-Hysteroscope without Anaesthesia and/or Analgesia: Advantages and Limits. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 139, 210–214. [Google Scholar] [CrossRef]
- Walker, S.H.; Gokhale, L. Safety Aspects of Hysteroscopy, Specifically in Relation to Entry and Specimen Retrieval: A UK Survey of Practice. Gynecol. Surg. 2018, 15, 2. [Google Scholar] [CrossRef]
- Vitale, S.G.; Riemma, G.; Carugno, J.; Perez-Medina, T.; Alonso Pacheco, L.; Haimovich, S.; Parry, J.P.; Di Spiezio Sardo, A.; De Franciscis, P. Postsurgical Barrier Strategies to Avoid the Recurrence of Intrauterine Adhesion Formation after Hysteroscopic Adhesiolysis: A Network Meta-Analysis of Randomized Controlled Trials. Am. J. Obstet. Gynecol. 2022, 226, 487–498.e8. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Fang, T.; Dong, Y.; Mao, J.; Wang, J.; Zhao, M.; Wu, R. Comparison of Secondary Prevention Following Hysteroscopic Adhesiolysis in the Improvement of Reproductive Outcomes: A Retrospective Cohort Study. J. Clin. Med. 2024, 13, 73. [Google Scholar] [CrossRef]
- Tang, R.; Zhang, W.; Xiao, X.; Li, W.; Chen, X.; Wang, X. Intrauterine Interventions Options for Preventing Recurrence after Hysteroscopic Adhesiolysis: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Arch. Gynecol. Obstet. 2024, 309, 1847–1861. [Google Scholar] [CrossRef]
- Pan, L.-Z.; Wang, Y.; Chen, X. A Randomized Controlled Study on an Integrated Approach to Prevent and Treat Re-Adhesion after Transcervical Resection of Moderate-to-Severe Intrauterine Adhesions. Clinics 2021, 76, e1987. [Google Scholar] [CrossRef] [PubMed]
- Winata, I.G.S.; Pradnyana, I.W.A.S.; Yusrika, M.U.; Pradnyaan, I.G.B.M.A.; Hartano, E. The Role of Hysteroscopy in Patients with Recurrent Implantation Failure before Starting in Vitro Fertilization: A Systematic Review and Meta-Analysis. Trocar 2023, 4, 10–25. [Google Scholar] [CrossRef]
- Sroussi, J.; Bourret, A.; Pourcelot, A.-G.; Thubert, T.; Lesavre, M.; Legendre, G.; Tuffet, S.; Rousseau, A.; Benifla, J.-L.; HYFACO Group. Does Hyaluronic Acid Gel Reduce Intrauterine Adhesions after Dilation and Curettage in Women with Miscarriage? A Multicentric Randomized Controlled Trial (HYFACO Study). Am. J. Obstet. Gynecol. 2022, 227, 597.e1–597.e8. [Google Scholar] [CrossRef]
- George, J.S.; Naert, M.N.; Lanes, A.; Yin, S.; Bharadwa, S.; Ginsburg, E.S.; Srouji, S.S. Utility of Office Hysteroscopy in Diagnosing Retained Products of Conception in Women with Normal or Inconclusive Ultrasound Scans. Obstet. Gynecol. 2023, 142, 100–110. [Google Scholar] [CrossRef]
- Bailey, A.P.; Jaslow, C.R.; Kutteh, W.H. Minimally Invasive Surgical Options for Congenital and Acquired Uterine Factors Associated with Recurrent Pregnancy Loss. Womens Health 2015, 11, 239–249. [Google Scholar] [CrossRef]
- Lessey, B.A.; Young, S.L. Physiological and Molecular Determinants of Embryo Implantation. Mol. Hum. Reprod. 2014, 20, 12–22. [Google Scholar] [CrossRef]
- Chung, J.P.W.; Chau, O.S.Y.; Law, T.S.M.; Ng, K.; Ip, P.N.P.; Ng, E.Y.L.; Tso, T.K.Y.; Sahota, D.S.; Li, T.C. Incidence of Intrauterine Adhesion after Ultrasound-Guided Manual Vacuum Aspiration for First-Trimester Miscarriages: A Prospective Cohort Study. Arch. Gynecol. Obstet. 2024, 309, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Shi, X.; Song, D.; Liu, Y.; Huang, X.; Xiao, Y.; Yang, L.; Xia, E.; Li, T.-C. The Efficacy of Auto-Cross-Linked Hyaluronic Acid Gel in Addition to Oestradiol and Intrauterine Balloon Insertion in the Prevention of Adhesion Reformation after Hysteroscopic Adhesiolysis. Reprod. Biomed. Online 2022, 45, 501–507. [Google Scholar] [CrossRef]
- Torres-de la Roche, L.A.; Bérard, V.; de Wilde, M.S.; Devassy, R.; Wallwiener, M.; De Wilde, R.L. Chemically Modified Hyaluronic Acid for Prevention of Post-Surgical Adhesions: New Aspects of Gel Barriers Physical Profiles. J. Clin. Med. 2022, 11, 931. [Google Scholar] [CrossRef]
- Dou, Y.; Yu, T.; Li, Z.; Wang, J.; Jiang, Y.; Liu, Y. Short- and Long-Term Outcomes of Postoperative Intrauterine Application of Hyaluronic Acid Gel: A Meta-Analysis of Randomized Controlled Trials. J. Minim. Invasive Gynecol. 2022, 29, 934–942. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).