Update on Sodium Glucose Cotransporter Type 2 Inhibitors Use in Kidney Transplant Patients
Abstract
:1. Introduction
2. Mechanism of Action
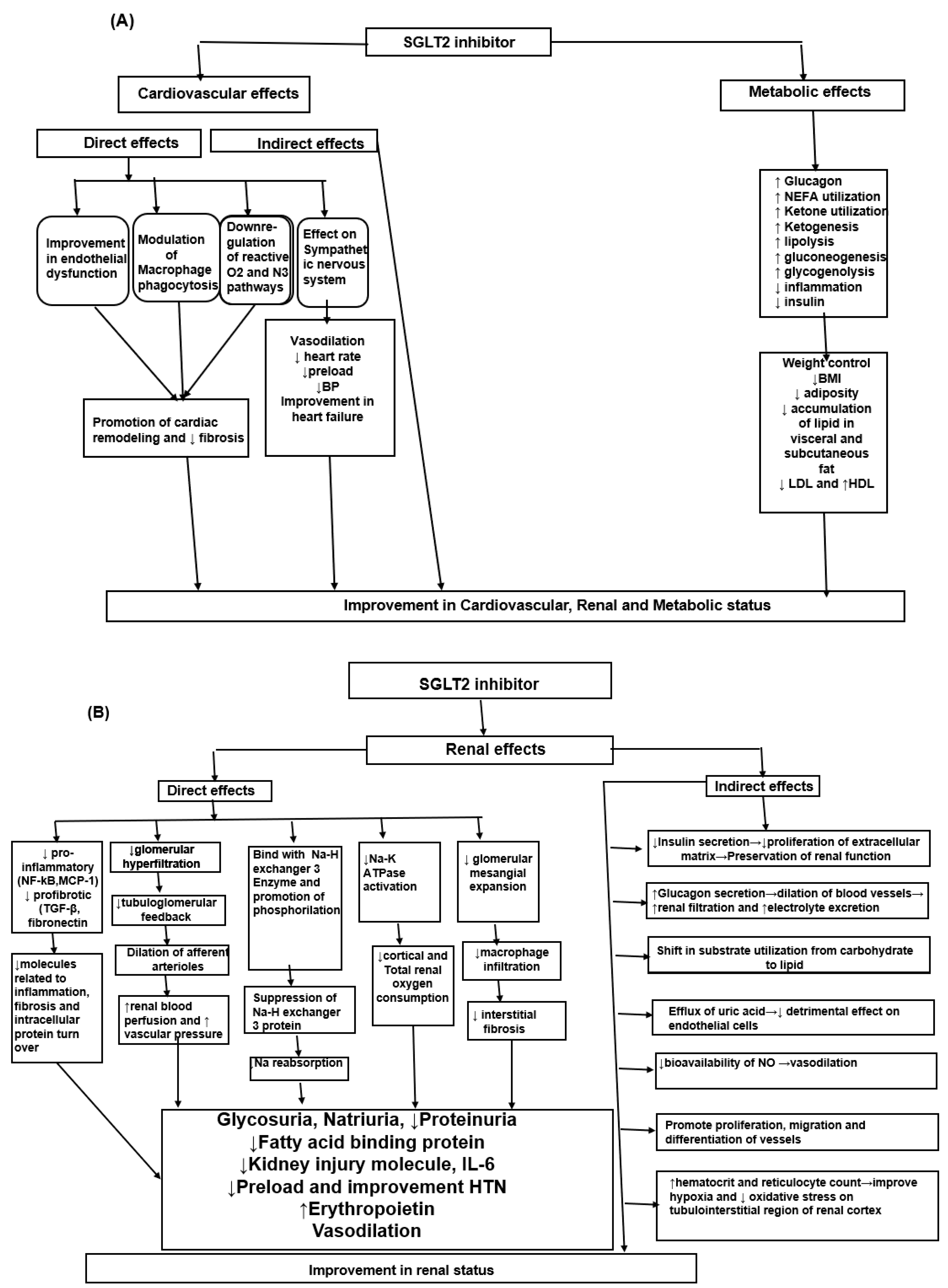
3. SGLT2i Effects on the Heart, Metabolism and Kidneys in Patients without Diabetes
4. SGLT2 Inhibitors in Kidney Transplant Patients
Side Effects Related to the Use of SGLT2
- (a)
- Start treatment with SGLT2 inhibitors at least 6 months after transplantation;
- (b)
- Start treatment if no previous rejection occurred;
- (c)
- Start treatment in patients with no history of UTI 6 months before starting therapy;
- (d)
- Start treatment only in patients without a history of vascular disease.
5. Conclusions
Funding
Conflicts of Interest
References
- McGuire, D.K.; Shih, W.J.; Cosentino, F.; Charbonnel, B.; Cherney, D.Z.I.; Dagogo-Jack, S.; Pratley, R.; Greenberg, M.; Wang, S.; Huyck, S.; et al. Association of SGLT2 Inhibitors with Cardiovascular and Kidney Outcomes in Patients with Type 2 Diabetes: A Meta-analysis. JAMA Cardiol. 2021, 6, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Hurren, K.M.; Pinelli, N.R. Drug-drug interactions with glucagon-like peptide-1 receptor agonists. Ann. Pharmacother. 2012, 46, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Lachin, J.M.; Inzucchi, S.E. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2016, 374, 1094. [Google Scholar] [CrossRef] [PubMed]
- Neal, B.; Perkovic, V.; Matthews, D.R. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 2099. [Google Scholar] [CrossRef]
- Lee, T.M.; Chang, N.C.; Lin, S.Z. Dapagliflozin, a selective SGLT2 Inhibitor, attenuated cardiac fibrosis by regulating the macrophage polarization via STAT3 signaling in infarcted rat hearts. Free Radic. Biol. Med. 2017, 104, 298–310. [Google Scholar] [CrossRef]
- Hasan, I.; Rashid, T.; Jaikaransingh, V.; Heilig, C.; Abdel-Rahman, E.M.; Awad, A.S. SGLT2 inhibitors: Beyond glycemic control. J. Clin. Transl. Endocrinol. 2024, 35, 100335. [Google Scholar] [CrossRef]
- List, J.F.; Woo, V.; Morales, E.; Tang, W.; Fiedorek, F.T. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care 2009, 32, 650–657. [Google Scholar] [CrossRef]
- Dekkers, C.C.J.; Sjöström, C.D.; Greasley, P.J.; Cain, V.; Boulton, D.W.; Heerspink, H.J.L. Effects of the sodium-glucose co-transporter-2 inhibitor dapagliflozin on estimated plasma volume in patients with type 2 diabetes. Diabetes Obes. Metab. 2019, 21, 2667–2673. [Google Scholar] [CrossRef] [PubMed]
- Crawford, P.A. Refueling the Failing Heart: A Case for Sodium-Glucose Cotransporter 2 Inhibition in Cardiac Energy Homeostasis. JACC Basic. Transl. Sci. 2018, 3, 588–590. [Google Scholar] [CrossRef]
- Packer, M. SGLT2 Inhibitors Produce Cardiorenal Benefits by Promoting Adaptive Cellular Reprogramming to Induce a State of Fasting Mimicry: A Paradigm Shift in Understanding Their Mechanism of Action. Diabetes Care 2020, 43, 508–511. [Google Scholar] [CrossRef]
- Inoue, M.K.; Matsunaga, Y.; Nakatsu, Y.; Yamamotoya, T.; Ueda, K.; Kushiyama, A.; Sakoda, H.; Fujishiro, M.; Ono, H.; Iwashita, M.; et al. Possible involvement of normalized Pin1 expression level and AMPK activation in the molecular mechanisms underlying renal protective effects of SGLT2 inhibitors in mice. Diabetol. Metab. Syndr. 2019, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.K.; Choi, H.; Jeong, J.Y.; Na, K.R.; Lee, K.W.; Lim, B.J.; Choi, D.E. Dapagliflozin, SGLT2 Inhibitor, Attenuates Renal Ischemia-Reperfusion Injury. PLoS ONE 2016, 11, e0158810. [Google Scholar]
- Aragón-Herrera, A.; Feijóo-Bandín, S.; Otero Santiago, M.; Barral, L.; Campos-Toimil, M.; Gil-Longo, J.; Costa Pereira, T.M.; García-Caballero, T.; Rodríguez-Segade, S.; Rodríguez, J.; et al. Empagliflozin reduces the levels of CD36 and cardiotoxic lipids while improving autophagy in the hearts of Zucker diabetic fatty rats. Biochem. Pharmacol. 2019, 170, 113677. [Google Scholar] [CrossRef]
- Mizuno, M.; Kuno, A.; Yano, T.; Miki, T.; Oshima, H.; Sato, T.; Nakata, K.; Kimura, Y.; Tanno, M.; Miura, T. Empagliflozin normalizes the size and number of mitochondria and prevents reduction in mitochondrial size after myocardial infarction in diabetic hearts. Physiol. Rep. 2018, 6, e13741. [Google Scholar] [CrossRef]
- Ren, F.F.; Xie, Z.Y.; Jiang, Y.N.; Guan, X.; Chen, Q.Y.; Lai, T.F.; Li, L. Dapagliflozin attenuates pressure overload-induced myocardial remodeling in mice via activating SIRT1 and inhibiting endoplasmic reticulum stress. Acta Pharmacol. Sin. 2022, 43, 1721–1732. [Google Scholar] [CrossRef]
- Wang, C.C.; Li, Y.; Qian, X.Q.; Zhao, H.; Wang, D.; Zuo, G.X.; Wang, K. Empagliflozin alleviates myocardial I/R injury and cardiomyocyte apoptosis via inhibiting ER stress-induced autophagy and the PERK/ATF4/Beclin1 pathway. J. Drug Target. 2022, 30, 858–872. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Perco, P.; Mulder, S.; Leierer, J.; Hansen, M.K.; Heinzel, A.; Mayer, G. Canagliflozin reduces inflammation and fibrosis biomarkers: A potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia 2019, 62, 1154–1166. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Scheen, A.J. Drug-drug interactions with sodium-glucose cotransporters type 2 (SGLT2) inhibitors, new oral glucose-lowering agents for the management of type 2 diabetes mellitus. Clin. Pharmacokinet. 2014, 53, 295–304. [Google Scholar] [CrossRef]
- Kruger, D.F.; Bode, B.; Spollett, G.R. Understanding GLP-1 analogs and enhancing patients success. Diabetes Educ. 2010, 36 (Suppl. S3), 44S–72S. [Google Scholar] [CrossRef]
- Whaley, J.M.; Tirmenstein, M.; Reilly, T.P.; Poucher, S.M.; Saye, J.; Parikh, S.; List, J.F. Targeting the kidney and glucose excretion with dapagliflozin: Preclinical and clinical evidence for SGLT2 inhibition as a new option for treatment of type 2 diabetes mellitus. Diabetes Metab. Syndr. Obes. 2012, 5, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Hindi, J.; Farouk, S.S. Sodium-Glucose Cotransporter 2 Inhibitors and Kidney Transplantation: What Are We Waiting For? Kidney360 2021, 2, 1174–1178. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Khan, M.S.; Marx, N.; Lam, C.S.P.; Schnaidt, S.; Ofstad, A.P.; Brueckmann, M.; Jamal, W.; et al. Effect of Empagliflozin on Cardiovascular and Renal Outcomes in Patients with Heart Failure by Baseline Diabetes Status: Results From the EMPEROR-Reduced Trial. Circulation 2021, 143, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Cruz, C.; González-Ortiz, M.; Rosales-Rivera, L.Y.; Patiño-Laguna, A.J.; Ramírez-Rodríguez, Z.G.; Díaz-Cruz, K.; Martínez-Abundis, E. Effects of dapagliflozin on blood pressure variability in patients with prediabetes and prehypertension without pharmacological treatment: A randomized trial. Blood Press. Monit. 2020, 25, 346–350. [Google Scholar] [CrossRef]
- Petrie, M.C.; Verma, S.; Docherty, K.F.; Inzucchi, S.E.; Anand, I.; Belohlávek, J.; Böhm, M.; Chiang, C.E.; Chopra, V.K.; de Boer, R.A.; et al. Effect of Dapagliflozin on Worsening Heart Failure and Cardiovascular Death in Patients with Heart Failure with and Without Diabetes. JAMA 2020, 323, 1353–1368. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Bays, H.E.; Weinstein, R.; Law, G.; Canovatchel, W. Canagliflozin: Effects in overweight and obese subjects without diabetes mellitus. Obesity 2014, 22, 1042–1049. [Google Scholar] [CrossRef]
- Neeland, I.J.; de Albuquerque Rocha, N.; Hughes, C.; Ayers, C.R.; Malloy, C.R.; Jin, E.S. Effects of Empagliflozin Treatment on Glycerol-Derived Hepatic Gluconeogenesis in Adults with Obesity: A Randomized Clinical Trial. Obesity 2020, 28, 1254–1262. [Google Scholar] [CrossRef]
- Færch, K.; Blond, M.B.; Bruhn, L.; Amadid, H.; Vistisen, D.; Clemmensen, K.K.B.; Vainø, C.T.R.; Pedersen, C.; Tvermosegaard, M.; Dejgaard, T.F.; et al. The effects of dapagliflozin, metformin or exercise on glycaemic variability in overweight or obese individuals with prediabetes (the PRE-D Trial): A multi-arm, randomised, controlled trial. Diabetologia 2021, 64, 42–55. [Google Scholar] [CrossRef]
- Veelen, A.; Andriessen, C.; Op den Kamp, Y.; Erazo-Tapia, E.; de Ligt, M.; Mevenkamp, J.; Jörgensen, J.A.; Moonen-Kornips, E.; Schaart, G.; Esterline, R.; et al. Effects of the sodium-glucose cotransporter 2 inhibitor dapagliflozin on substrate metabolism in prediabetic insulin resistant individuals: A randomized, double-blind crossover trial. Metabolism 2023, 140, 155396. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- The EMPA-KIDNEY Collaborative Group; Herrington, W.G.; Staplin, N.; Wanner, C.; Green, J.B.; Hauske, S.J.; Emberson, J.R.; Preiss, D.; Judge, P.; Mayne, K.J.; et al. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar]
- Podestà, M.A.; Sabiu, G.; Galassi, A.; Ciceri, P.; Cozzolino, M. SGLT2 Inhibitors in Diabetic and Non-Diabetic Chronic Kidney Disease. Biomedicines 2023, 11, 279. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Kwon, S.; Jeon, Y.; Kim, Y.H.; Kwon, H.; Kim, Y.S.; Lee, H.; Kim, Y.L.; Kim, C.D.; Park, S.H.; et al. The Efficacy and Safety of SGLT2 Inhibitor in Diabetic Kidney Transplant Recipients. Transplantation 2022, 106, e404–e412. [Google Scholar] [CrossRef]
- Sánchez Fructuoso, A.I.; Bedia Raba, A.; Banegas Deras, E.; Vigara Sánchez, L.A.; Valero San Cecilio, R.; Franco Esteve, A.; Cruzado Vega, L.; Gavela Martínez, E.; González Garcia, M.E.; Saurdy Coronado, P.; et al. Sodium-glucose cotransporter-2 inhibitor therapy in kidney transplant patients with type 2 or post-transplant diabetes: An observational multicentre study. Clin. Kidney J. 2023, 16, 1022–1034. [Google Scholar] [CrossRef]
- Rajasekeran, H.; Kim, S.J.; Cardella, C.J.; Schiff, J.; Cattral, M.; Cherney, D.Z.I.; Singh, S.K.S. Use of Canagliflozin in Kidney Transplant Recipients for the Treatment of Type 2 Diabetes: A Case Series. Diabetes Care 2017, 40, e75–e76. [Google Scholar] [CrossRef]
- Shah, M.; Virani, Z.; Rajput, P.; Shah, B. Efficacy and Safety of Canagliflozin in Kidney Transplant Patients. Indian J. Nephrol. 2019, 29, 278–281. [Google Scholar] [CrossRef]
- Schwaiger, E.; Burghart, L.; Signorini, L.; Ristl, R.; Kopecky, C.; Tura, A.; Pacini, G.; Wrba, T.; Antlanger, M.; Schmaldienst, S.; et al. Empagliflozin in posttransplantation diabetes mellitus: A prospective, interventional pilot study on glucose metabolism, fluid volume, and patient safety. Am. J. Transplant. 2019, 19, 907–919. [Google Scholar] [CrossRef]
- Halden, T.A.S.; Kvitne, K.E.; Midtvedt, K.; Rajakumar, L.; Robertsen, I.; Brox, J.; Bollerslev, J.; Hartmann, A.; Åsberg, A.; Jenssen, T. Efficacy and Safety of Empagliflozin in Renal Transplant Recipients with Posttransplant Diabetes Mellitus. Diabetes Care 2019, 42, 1067–1074. [Google Scholar] [CrossRef]
- Mahling, M.; Schork, A.; Nadalin, S.; Fritsche, A.; Heyne, N.; Guthoff, M. Sodium-Glucose Cotransporter 2 (SGLT2) Inhibition in Kidney Transplant Recipients with Diabetes Mellitus. Kidney Blood Press. Res. 2019, 44, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Attallah, N.; Yassine, L. Use of Empagliflozin in Recipients of Kidney Transplant: A Report of 8 Cases. Transplant. Proc. 2019, 51, 3275–3280. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Joon, J.; Chul, Y.; Eun, W.; Hyuk, K.; Hyun, S.S. Sodium/glucose cotransporter 2 inhibitor for the treatment of diabetes in kidney transplant patients. Nephrol. Dial. Transplant. 2019, 34, gfz103.SP770. [Google Scholar] [CrossRef]
- AlKindi, F.; Al-Omary, H.L.; Hussain, Q.; Al Hakim, M.; Chaaban, A.; Boobes, Y. Outcomes of SGLT2 Inhibitors Use in Diabetic Renal Transplant Patients. Transplant. Proc. 2020, 52, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Song, C.C.; Brown, A.; Winstead, R.; Yakubu, I.; Demehin, M.; Kumar, D.; Gupta, G. Early initiation of sodium-glucose linked transporter inhibitors (SGLT-2i) and associated metabolic and electrolyte outcomes in diabetic kidney transplant recipients. Endocrinol. Diabetes Metab. 2020, 4, e00185. [Google Scholar] [CrossRef]
- Lemke, A.; Brokmeier, H.M.; Leung, S.B.; Mara, K.C.; Mour, G.K.; Wadei, H.M.; Hill, J.M.; Stegall, M.; Kudva, Y.C.; Shah, P.; et al. Sodium-glucose cotransporter 2 inhibitors for treatment of diabetes mellitus after kidney transplantation. Clin. Transplant. 2022, 36, e14718. [Google Scholar] [CrossRef]
- Oikonomaki, D.; Dounousi, E.; Duni, A.; Roumeliotis, S.; Liakopoulos, V. Incretin based therapies and SGLT-2 inhibitors in kidney transplant recipients with diabetes: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2021, 172, 108604. [Google Scholar] [CrossRef]
- Nissaisorakarn, P.; Pavlakis, M.; Aala, A. Novel Glucose-Lowering Therapies in the Setting of Solid Organ Transplantation. Adv. Chronic Kidney Dis. 2021, 28, 361–370. [Google Scholar] [CrossRef]
- Kanbay, M.; Demiray, A.; Afsar, B.; Karakus, K.E.; Ortiz, A.; Hornum, M.; Covic, A.; Sarafidis, P.; Rossing, P. Sodium-glucose cotransporter 2 inhibitors for diabetes mellitus control after kidney transplantation: Review of the current evidence. Nephrology 2021, 26, 1007–1017. [Google Scholar] [CrossRef]
- Chewcharat, A.; Prasitlumkum, N.; Thongprayoon, C.; Bathini, T.; Medaura, J.; Vallabhajosyula, S.; Cheungpasitporn, W. Efficacy and Safety of SGLT-2 Inhibitors for Treatment of Diabetes Mellitus among Kidney Transplant Patients: A Systematic Review and Meta-Analysis. Med. Sci. 2020, 8, 47. [Google Scholar] [CrossRef]
- Schwarzenbach, M.; Bernhard, F.E.; Czerlau, C.; Sidler, D. Chances and risks of sodium-glucose cotransporter 2 inhibitors in solid organ transplantation: A review of literatures. World J. Transplant. 2021, 11, 254–262. [Google Scholar] [CrossRef]
- Kwon, H.; Son, S.H.; Kim, K. Sodium-Glucose Cotransprter 2 inhibitors reduce microalbuminuria in diabetic renal transplant patients. Transplantation 2020, 104 (Suppl. S3), S430. [Google Scholar] [CrossRef]
- Perrin, P.; Muller, C.; Dimitrov, I.; Chantrel, F.; Heitz, M.; Woerly, A. Assessment of SGLT2 inhibitors’ safety and discontinuation causes in patients with advanced chronic kidney disease. Clin. Kidney J. 2024, 17, sfae169. [Google Scholar] [CrossRef] [PubMed]
- Menne, J.; Dumann, E.; Haller, H.; Schmidt, B.M.W. Acute kidney injury and adverse renal events in patients receiving SGLT2-inhibitors: A systematic review and meta-analysis. PLoS Med. 2019, 16, e1002983. [Google Scholar] [CrossRef] [PubMed]
- Copur, S.; Yildiz, A.; Basile, C.; Tuttle, K.R.; Kanbay, M. Is there any robust evidence showing that SGLT2 inhibitor use predisposes to acute kidney injury? Nephrol. 2023, 36, 31–43. [Google Scholar] [CrossRef]
- Demir, M.E.; Özler, T.E.; Merhametsiz, Ö.; Sözener, U.; Uyar, M.; Ercan, Z.; Bardak Demir, S.; Sezer, S.; Türkmen Sarıyıldız, G. The results of SGLT-2 inhibitors use in kidney transplantation: 1-year experiences from two centers. Int. Urol. Nephrol. 2023, 55, 2989–2999. [Google Scholar] [CrossRef]
- Sweiss, H.; Selznick, L.; Contreras, J.; Long, C.; Hall, R.; Bhayana, S.; Patel, R.; Klein, K. Safety and Efficacy of Sodium-Glucose Cotransporter-2 Inhibitors in Solid Organ Transplant Recipients. Prog. Transplant. 2023, 33, 261–265. [Google Scholar] [CrossRef]
- Juric, I.; Puljiz, D.Z.; Jelakovic, B.; Basic-Jukic, N. Combination of SGLT2 Inhibitors and GLP-1 Receptor Agonists in PTDM Treatment in Kidney Transplant Recipients: Synergistic Effect with Added Value in Terms of Nephroprotection. Transplant. Proc. 2024, 56, 1264–1265. [Google Scholar] [CrossRef]
- Oslo University Hospital. Can Dapaglifozin Preserve Structure and Function in Transplanted Kidney? Available online: https://clinicaltrials.gov/study/NCT05788276?tab=results (accessed on 30 July 2024).
- Neto, E.D. Effect of Adding Dapaglifozin to Allograft Dysfunction of Renal Transplanted Patients. Available online: https://clinicaltrials.gov/study/NCT04743453?tab=results (accessed on 30 July 2024).
- Lai, V. Efficacy, Mechanisms and Safety of SGLT2 Inhibitors in Kidney Transplant Recipients. Available online: https://clinicaltrials.gov/study/NCT04965935?tab=results (accessed on 30 July 2024).

| Author, Year, Study Type, Follow-Up | Basal eGFR (mL/min) | Effect on Renal Function (eGFR mL/min/1.73) | Proteinuria (uPCR) g/d/(uACR) mg/g | Adverse Events |
|---|---|---|---|---|
| Rajasekeran et al., 2017 [37], CS, n = 6, 8 mo | 78.6 ± 18.2 | No differences | NA | Cellulitis |
| Shah et al., 2019 [38], PS, n = 25, 8 mo | 86 ± 20 | No differences | NA | None |
| Schwaiger et al., 2019 [39], PS, n = 14, 12 mo | 55.6 ± 20.3 | Decrease and then stabilize | ΔuACR: −25 ΔuACR: −73 | UTI 5 |
| Halden et al., 2019 [40], RCT, n = 44, 6 mo | 66 ± 10.5 | No differences | NA | UTI 3 |
| Mahling et al., 2019 [41], PS, n = 10, 6 mo | 57 ± 19.3 | No differences | NA | UTI 2 |
| Attallah et al., 2019 [42], CS, n = 25, 12 mo | NA | Decrease and then stabilize | ΔuPCR −0.6 g/d | UTI 2 |
| Kong et al., 2019 [43], PS, n = 42, 12 mo | 60.36 ± 17 | No differences | ΔuACR No significant change | Acute cystitis 3 |
| Alkindi et al., 2020 [44], CS, n = 8, 12 mo | 75.8 ± 13.4 | No differences | NA | UTI 1 |
| Song et al., 2021 [45], RS, n = 50, 6 mo | 66.7 | No differences | NA | UTI 7 |
| Lemke et al., 2021 [46], RS, n = 39, 12 mo | NA | No differences | NA | UTI 6, Ketoacidosis 1 |
| Sánchez Fructuoso et al., 2022 [36], MCO, n = 339, 12 mo | 58.4 (56.2–60.6) | No differences | ΔuPCR: −230 at 6 mo | UTI 14%, AKI 1.8% |
| Lim et al., 2022 [35], OR, PSM, n = 2083, 63 mo | S: 66.9 ± 17.7 C: 68.4 ± 20.1 | Decrease, stabilization and amelioration | ΔuPCR: urine PCR significantly decreased after SGTLi, p = 0.005 | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvadori, M.; Rosati, A.; Rosso, G. Update on Sodium Glucose Cotransporter Type 2 Inhibitors Use in Kidney Transplant Patients. Transplantology 2024, 5, 224-233. https://doi.org/10.3390/transplantology5030022
Salvadori M, Rosati A, Rosso G. Update on Sodium Glucose Cotransporter Type 2 Inhibitors Use in Kidney Transplant Patients. Transplantology. 2024; 5(3):224-233. https://doi.org/10.3390/transplantology5030022
Chicago/Turabian StyleSalvadori, Maurizio, Alberto Rosati, and Giuseppina Rosso. 2024. "Update on Sodium Glucose Cotransporter Type 2 Inhibitors Use in Kidney Transplant Patients" Transplantology 5, no. 3: 224-233. https://doi.org/10.3390/transplantology5030022
APA StyleSalvadori, M., Rosati, A., & Rosso, G. (2024). Update on Sodium Glucose Cotransporter Type 2 Inhibitors Use in Kidney Transplant Patients. Transplantology, 5(3), 224-233. https://doi.org/10.3390/transplantology5030022







