The Old and the New in Subacute Thyroiditis: An Integrative Review
Abstract
1. Introduction
2. Materials and Methods
3. Integrative Review
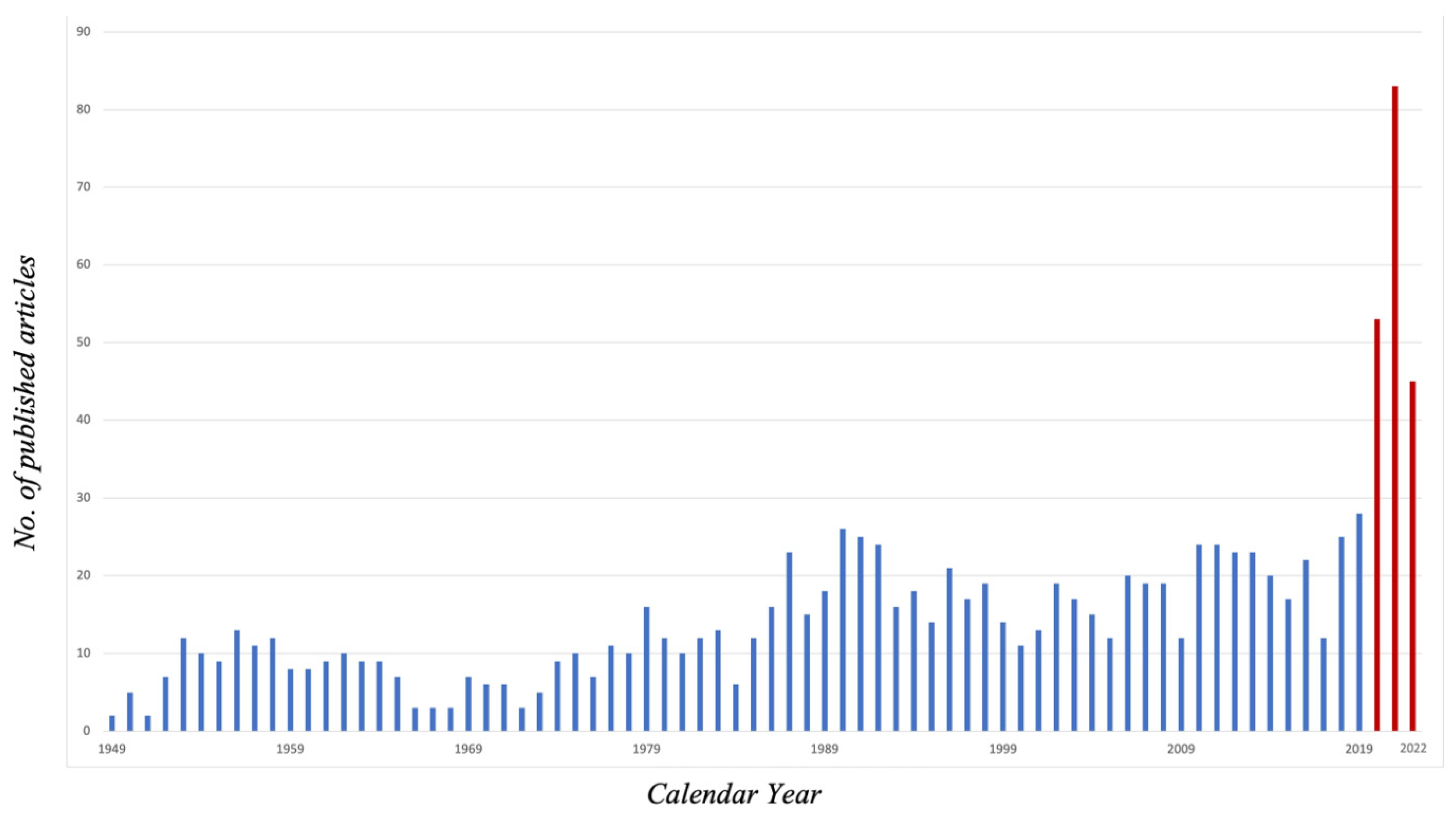
3.1. Epidemiology
3.2. Pathophysiology
3.3. Natural History
3.4. Clinical Manifestations
3.5. Laboratory Findings
3.6. Imaging
3.7. Cyto-Histopathology
3.8. Diagnosis
3.9. Treatment
3.10. Follow-Up
3.11. SAT and COVID-19
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Fatourechi, V.; Aniszewski, J.P.; Fatourechi, G.Z.E.; Atkinson, E.J.; Jacobsen, S. Clinical Features and Outcome of Subacute Thyroiditis in an Incidence Cohort: Olmsted County, Minnesota, Study. J. Clin. Endocrinol. Metab. 2003, 88, 2100–2105. [Google Scholar] [CrossRef] [PubMed]
- Samuels, M.H. Subacute, silent, and postpartum thyroiditis. Med. Clin. N. Am. 2012, 96, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Hiraiwa, T.; Kubota, S.; Imagawa, A.; Sasaki, I.; Ito, M.; Miyauchi, A.; Hanafusa, T. Two cases of subacute thyroiditis presenting in pregnancy. J. Endocrinol. Investig. 2006, 29, 924–927. [Google Scholar] [CrossRef]
- Bai, C.-F.; Shen, G.-H.; Yang, Y.; Yang, K.; Hayden, M.R.; Zhou, Y.-Y.; Geng, X.-Q. Subacute thyroiditis during early pregnancy: A case report and literature review. BMC Pregnancy Childbirth 2022, 22, 19. [Google Scholar] [CrossRef]
- Bilbao, N.A.; Kaulfers, A.D.; Bhowmick, S.K. Subacute thyroiditis in a child. AACE Clin. Case Rep. 2019, 5, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Ramineni, P.; Kamath, S.P.; Joshi, J.; Rao, S. Subacute thyroiditis with airway compromise in a 5-year-old boy. BMJ Case Rep. 2020, 13, e236909. [Google Scholar] [CrossRef] [PubMed]
- Tabassom, A.; Chippa, V.; Edens, M.A. De Quervain Thyroiditis. [Updated 1 May 2022]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK526066/ (accessed on 15 April 2022).
- Martino, E.; Buratti, L.; Bartalena, L.; Mariotti, S.; Cupini, C.; Aghini-Lombardi, F.; Pinchera, A. High prevalence of subacute thyroiditis during summer season in Italy. J. Endocrinol. Investig. 1987, 10, 321–323. [Google Scholar] [CrossRef]
- Iitaka, M.; Momotani, N.; Ishii, J.; Ito, K. Incidence of subacute thyroiditis recurrences after a prolonged latency: 24-year survey. J. Clin. Endocrinol. Metab. 1996, 81, 466–469. [Google Scholar] [CrossRef][Green Version]
- Oksa, H.; Järvenpää, P.; Metsähonkala, L.; Pasternack, A.; Leinikki, P. No seasonal distribution in subacute de Quervain’s thyroiditis in Finland. J. Endocrinol. Investig. 1989, 12, 495. [Google Scholar] [CrossRef]
- Greer, M.A. Subacute thyroiditis. West J. Med. 1991, 155, 83. [Google Scholar]
- Nishihara, E.; Ohye, H.; Amino, N.; Takata, K.; Arishima, T.; Kudo, T.; Ito, M.; Kubota, S.; Fukata, S.; Miyauchi, A. Clinical Characteristics of 852 Patients with Subacute Thyroiditis before Treatment. Intern. Med. 2008, 47, 725–729. [Google Scholar] [CrossRef]
- Calapkulu, M.; Sencar, M.E.; Sakiz, D.; Unsal, I.O.; Ozbek, M.; Cakal, E. The Importance of Vitamin D Level in Subacute Thyroiditis Disease and the Effect of Vitamin D on Disease Prognosis. Endocr. Pract. 2020, 26, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Stasiak, M.; Lewiński, A. New aspects in the pathogenesis and management of subacute thyroiditis. Rev. Endocr. Metab. Disord. 2021, 22, 1027–1039. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Artola, Y.; Poncino, D.; García, M.L.; Munné, M.S.; González, J.; García, D.S. Acute hepatitis E virus infection and association with a subacute thyroiditis. Ann. Hepatol. 2015, 14, 141–142. [Google Scholar] [CrossRef]
- Assir, M.Z.K.; Jawa, A.; Ahmed, H.I. Expanded dengue syndrome: Subacute thyroiditis and intracerebral hemorrhage. BMC Infect. Dis. 2012, 12, 240. [Google Scholar] [CrossRef]
- Mo, Z.; Dong, Y.; Chen, X.; Yao, H.; Zhang, B. Acute transverse myelitis and subacute thyroiditis associated with dengue viral infection: A case report and literature review. Exp. Ther. Med. 2016, 12, 2331–2335. [Google Scholar] [CrossRef]
- Ippolito, S.; Gallo, D.; Rossini, A.; Patera, B.; Lanzo, N.; Fazzino, G.F.M.; Piantanida, E.; Tanda, M.L. SARS-CoV-2 vaccine-associated subacute thyroiditis: Insights from a systematic review. J. Endocrinol. Investig. 2022, 45, 1189–1200. [Google Scholar] [CrossRef]
- Ippolito, S.; Dentali, F.; Tanda, M.L. SARS-CoV-2: A potential trigger for subacute thyroiditis? Insights from a case report. J. Endocrinol. Investig. 2020, 43, 1171–1172. [Google Scholar] [CrossRef]
- Stasiak, M.; Tymoniuk, B.; Michalak, R.; Stasiak, B.; Kowalski, M.L.; Lewiński, A. Subacute Thyroiditis is Associated with HLA-B*18:01, -DRB1*01 and -C*04:01—The Significance of the New Molecular Background. J. Clin. Med. 2020, 9, 534. [Google Scholar] [CrossRef]
- Kramer, A.B.; Roozendaal, C.; Dullaart, R.P. Familial Occurrence of Subacute Thyroiditis Associated with Human Leukocyte Antigen-B35. Thyroid 2004, 14, 544–547. [Google Scholar] [CrossRef]
- Ohsako, N.; Tamai, H.; Sudo, T.; Mukuta, T.; Tanaka, H.; Kuma, K.; Kimura, A.; Sasazuki, T. Clinical characteristics of subacute thyroiditis classified according to human leukocyte antigen typing. J. Clin. Endocrinol. Metab. 1995, 80, 3653–3656. [Google Scholar] [CrossRef] [PubMed]
- Collazos, J.; Gener, B.; de Miguel, J. Bacterial sinusitis associated with subacute, granulomatous thyroiditis. J. Infect. 1997, 34, 158–159. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, T.S.; Baek, H.S.; Jin, H.Y. Subacute painful thyroiditis accompanied by scrub typhus infection. Endocrine 2013, 44, 546–548. [Google Scholar] [CrossRef] [PubMed]
- Abad, S.; Blanche, P.; Moachon, L.; Brunet, A.; Héripret-Fredouille, L.; Sicard, D.; Salmon-Céron, D. Unusual thyroiditis after recombinant interleukin-2 therapy. Int. J. STD AIDS 2002, 13, 790–791. [Google Scholar] [CrossRef] [PubMed]
- Cañas, C.A.; Tobón, G.J.; Arango, L.G.; Guarin, N. Developing of granulomatous thyroiditis during etanercept therapy. Clin. Rheumatol. 2009, 28 (Suppl. 1), 17–19. [Google Scholar] [CrossRef]
- Paraná, R.; Cruz, M.; Lyra, L.; Cruz, T. Subacute thyroiditis during treatment with combination therapy (interferon plus ribavirin) for hepatitis C virus. J. Viral Hepat. 2000, 7, 393–395. [Google Scholar] [CrossRef]
- Sinnott, M.J.; McIntyre, H.D.; Pond, S.M. Granulomatous thyroiditis and lithium therapy. Aust. N. Z. J. Med. 1992, 22, 84. [Google Scholar] [CrossRef]
- Girgis, C.M.; Russo, R.R.; Benson, K. Subacute thyroiditis following the H1N1 vaccine. J. Endocrinol. Investig. 2010, 33, 506. [Google Scholar] [CrossRef]
- Altay, F.A.; Güz, G.; Altay, M. Subacute thyroiditis following seasonal influenza vaccination. Hum. Vaccines Immunother. 2016, 12, 1033–1034. [Google Scholar] [CrossRef]
- Hsiao, J.-Y.; Hsin, S.-C.; Hsieh, M.-C.; Hsia, P.-J.; Shin, S.-J. Subacute Thyroiditis Following Influenza Vaccine (Vaxigrip®) in A Young Female. Kaohsiung J. Med Sci. 2006, 22, 297–300. [Google Scholar] [CrossRef]
- Xie, Q.; Mu, X.Y.; Li, S.Q. Subacute Thyroiditis Following HPV Vaccination: A Case Report. Sichuan Da Xue Xue Bao Yi Xue Ban. 2021, 52, 1047–1048. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, P.; Perrone, V.; Pozzi, M.; Carnovale, C.; Perrotta, C.; Clementi, E.; Radice, S. The epidemiological profile of ASIA syndrome after HPV vaccination: An evaluation based on the Vaccine Adverse Event Reporting Systems. Immunol. Res. 2014, 61, 90–96. [Google Scholar] [CrossRef]
- Toft, J.; Larsen, S.; Toft, H. Subacute thyroiditis after hepatitis B vaccination. Endocr. J. 1998, 45, 135. [Google Scholar] [PubMed]
- Alkis, N.; Baysal, M. Subacute thyroiditis after SARS-CoV-2 BNT162b2 vaccine in a multiple myeloma patient. SAGE Open Med Case Rep. 2022, 10, 2050313X221091392. [Google Scholar] [CrossRef]
- Oyibo, S.O. Subacute Thyroiditis After Receiving the Adenovirus-Vectored Vaccine for Coronavirus Disease (COVID-19). Cureus 2021, 13, e16045. [Google Scholar] [CrossRef] [PubMed]
- Iremli, B.G.; Şendur, S.N.; Ünlütürk, U. Three Cases of Subacute Thyroiditis Following SARS-CoV-2 Vaccine: Postvaccination ASIA Syndrome. J. Clin. Endocrinol. Metab. 2021, 106, 2600–2605. [Google Scholar] [CrossRef]
- Yorulmaz, G.; Tekin, M.S. SARS-CoV-2 vaccine-associated subacute thyroiditis. J. Endocrinol. Investig. 2022, 45, 1341–1347. [Google Scholar] [CrossRef]
- Das, L.; Bhadada, S.K.; Sood, A. Post-COVID-vaccine autoimmune/inflammatory syndrome in response to adjuvants (ASIA syndrome) manifesting as subacute thyroiditis. J. Endocrinol. Investig. 2021, 45, 465–467. [Google Scholar] [CrossRef]
- Shoenfeld, Y.; Agmon-Levin, N. ‘ASIA’—Autoimmune/inflammatory syndrome induced by adjuvants. J. Autoimmun. 2011, 36, 4–8. [Google Scholar] [CrossRef]
- Vojdani, A.; Kharrazian, D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin. Immunol. 2020, 217, 108480. [Google Scholar] [CrossRef]
- Dotan, A.; Muller, S.; Kanduc, D.; David, P.; Halpert, G.; Shoenfeld, Y. The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun. Rev. 2021, 20, 102792. [Google Scholar] [CrossRef]
- Şendur, S.N.; Özmen, F.; Oğuz, S.H.; Iremli, B.G.; Malkan, Y.; Gürlek, A.; Erbas, T.; Ünlütürk, U. Association of Human Leukocyte Antigen Genotypes with Severe Acute Respiratory Syndrome Coronavirus 2 Vaccine-Induced Subacute Thyroiditis. Thyroid 2022, 32, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Stasiak, M.; Zawadzka-Starczewska, K.; Lewiński, A. Clinical Manifestation of Subacute Thyroiditis Triggered by SARS-CoV-2 Infection Can Be HLA-Dependent. Viruses 2021, 13, 2447. [Google Scholar] [CrossRef] [PubMed]
- Ginde, A.A.; Mansbach, J.M.; Camargo, C.A., Jr. Association Between Serum 25-Hydroxyvitamin D Level and Upper Respiratory Tract Infection in the Third National Health and Nutrition Examination Survey. Arch. Intern. Med. 2009, 169, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Bostan, H.; Sencar, M.E.; Calapkulu, M.; Hepsen, S.; Duger, H.; Unsal, I.O.; Ozbek, M.; Cakal, E. Two Important Issues in Subacute Thyroiditis Management: Delayed Diagnosis and Inappropriate Use of Antibiotics. Eur. Thyroid J. 2021, 10, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Sari, O.; Erbaş, B.; Erbaş, T. Subacute thyroiditis in a single lobe. Clin. Nucl. Med. 2001, 26, 400–401. [Google Scholar] [CrossRef] [PubMed]
- Zacharia, T.T.; Perumpallichira, J.J.; Sindhwani, V.; Chavhan, G. Gray-scale and color Doppler sonographic findings in a case of subacute granulomatous thyroiditis mimicking thyroid carcinoma. J. Clin. Ultrasound 2002, 30, 442–444. [Google Scholar] [CrossRef]
- Candrina, R.; Giustina, G. Case Report: Iodine-Induced Subacute Thyroiditis with Thyrotoxicosis Presenting as Fever of Unknown Origin. Am. J. Med Sci. 1990, 300, 37–40. [Google Scholar] [CrossRef]
- Raj, R.; Yada, S.; Jacob, A.; Unnikrishnan, D.; Ghali, W. Fever of Unknown Origin as a Sole Presentation of Subacute Thyroiditis in an Elderly Patient: A Case Report with Literature Review. Case Rep. Endocrinol. 2018, 2018, 5041724. [Google Scholar] [CrossRef]
- Bahowairath, F.A.; Woodhouse, N.; Hussain, S.; Al Busaidi, M. Lesson of the month 1: Subacute thyroiditis: A rare cause of fever of unknown origin. Clin. Med. 2017, 17, 86–87. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Lee, J.-J.; Liu, C.-L.; Tzen, C.-Y.; Cheng, S.-P. Atypical subacute thyroiditis. Surgery 2010, 147, 461–462. [Google Scholar] [CrossRef] [PubMed]
- Duhig, T.J.; McKeag, D. Thyroid Disorders in Athletes. Curr. Sports Med. Rep. 2009, 8, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Obuobie, K.; Al-Sabah, A.; Lazarus, J. Subacute thyroiditis in an immunosuppressed patient. J. Endocrinol. Investig. 2002, 25, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Sakane, S.; Murakami, Y.; Sasaki, M.; Yamano, Y.; Takamatsu, J.; Kuma, K.; Ohsawa, N. Serum Concentrations of Granulocyte Colony-Stimulating Factor (G-CSF) Determined by A Highly-Sensitive Chemiluminescent Immunoassay during the Clinical Course of Subacute Thyroiditis. Endocr. J. 1995, 42, 391–396. [Google Scholar] [CrossRef][Green Version]
- Calapkulu, M.; Sencar, M.E.; Sakiz, D.; Duger, H.; Unsal, I.O.; Ozbek, M.; Cakal, E. The prognostic and diagnostic use of hematological parameters in subacute thyroiditis patients. Endocrine 2019, 68, 138–143. [Google Scholar] [CrossRef]
- Taşkaldiran, I.; Omma, T.; Önder, E.; Firat, S.N.; Koç, G.; Kiliç, M.K.; Kuşkonmaz, M.; Çulha, C. Neutrophil to lymphocyte ratio, Monocyte to lymphocyte ratio, platelet to lymphocyte ratio in different etiological causes of thyrotoxicosis. Turk. J. Med. Sci. 2019, 49, 1687–1692. [Google Scholar] [CrossRef]
- Cengiz, H.; Varım, C.; Demirci, T.; Cetin, S. Hemogram parameters in the patients with subacute thyroiditis. Pak. J. Med. Sci. 2019, 36, 240–245. [Google Scholar] [CrossRef]
- Bartalena, L.; Brogioni, S.; Grasso, L.; Martino, E. Increased serum interleukin-6 concentration in patients with subacute thyroiditis: Relationship with concomitant changes in serum T4-binding globulin concentration. J. Endocrinol. Investig. 1993, 16, 213–218. [Google Scholar] [CrossRef]
- Amino, N.; Yabu, Y.; Miki, T.; Morimoto, S.; Kumahara, Y.; Mori, H.; Iwatani, Y.; Nishi, K.; Nakatani, K.; Miyai, K. Serum Ratio of Triiodothyronine to Thyroxine, and Thyroxine-Binding Globulin and Calcitonin Concentrations in Graves’ Disease and Destruction- Induced Thyrotoxicosis*. J. Clin. Endocrinol. Metab. 1981, 53, 113–116. [Google Scholar] [CrossRef]
- Hennessey, J. Subacute Thyroiditis, Acute and Subacute, and Riedel’s Thyroiditis (Endotext [Internet] 2018; MDText.com, Inc.: South Dartmouth, MA, USA, 2018; Available online: https://www.endotext.org/chapter/subacute-thyroiditis/ (accessed on 15 April 2022).
- Tachibana, T.; Orita, Y.; Ogawara, Y.; Matsuyama, Y.; Abe, I.; Nakada, M.; Sato, Y.; Nishizaki, K. Time-lag between symptom onset and laboratory findings in patients with subacute thyroiditis. Auris Nasus Larynx 2014, 41, 369–372. [Google Scholar] [CrossRef]
- Ito, M.; Takamatsu, J.; Yoshida, S.; Murakami, Y.; Sakane, S.; Kuma, K.; Ohsawa, N. Incomplete Thyrotroph Suppression Determined by Third Generation Thyrotropin Assay in Subacute Thyroiditis Compared to Silent Thyroiditis or Hyperthyroid Graves’ Disease. J. Clin. Endocrinol. Metab. 1997, 82, 616–619. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lio, S.; Pontecorvi, A.; Caruso, M.; Monaco, F.; D’Armiento, M. Transitory subclinical and permanent hypothyroidism in the course of subacute thyroiditis (de Quervain). Eur. J. Endocrinol. 1984, 106, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Engkakul, P.; Mahachoklertwattana, P.; Poomthavorn, P. Eponym. Eur. J. Pediatr. 2010, 170, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Stasiak, M.; Michalak, R.; Stasiak, B.; Lewinski, A. Clinical characteristics of subacute thyroiditis is different than it used to be-current state based on 15 years own material. Neuro Endocrinol. Lett. 2019, 39, 489–495. [Google Scholar]
- Erdem, N.; Erdogan, M.; Ozbek, M.; Karadeniz, M.; Cetinkalp, S.; Ozgen, A.G.; Saygılı, F.; Yilmaz, C.; Tuzun, M.; Kabalak, T. Demographic and clinical features of patients with subacute thyroiditis: Results of 169 patients from a single University Center in Turkey. J. Endocrinol. Investig. 2007, 30, 546–550. [Google Scholar] [CrossRef]
- Nishihara, E.; Amino, N.; Kudo, T.; Kohsaka, K.; Ito, M.; Fukata, S.; Nishikawa, M.; Nakamura, H.; Miyauchi, A. Moderate Frequency of Anti-Thyroglobulin Antibodies in the Early Phase of Subacute Thyroiditis. Eur. Thyroid J. 2019, 8, 268–272. [Google Scholar] [CrossRef]
- Prajapati, S.; Hernandez-Prera, J.C. Putting All the Pieces Together: Clinical, Macroscopic and Microscopic Characteristics of Subacute Thyroiditis. Head Neck Pathol. 2018, 13, 231–234. [Google Scholar] [CrossRef]
- Zhuo, L.; Nie, Y.; Ma, L.; Shen, L.; Zhou, X.; Li, F. Diagnostic value of nuclear medicine imaging and ultrasonography in subacute thyroiditis. Am. J. Transl. Res. 2021, 13, 5629–5634. [Google Scholar]
- Mundy-Baird, G.; Kyriacou, A.; Syed, A.A. De Quervain subacute thyroiditis. Can. Med. Assoc. J. 2021, 193, E1007. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, E.-K.; Kim, M.J.; Kim, B.M.; Oh, K.K.; Hong, S.W.; Park, C.S. Ultrasonographic Characteristics of Subacute Granulomatous Thyroiditis. Korean J. Radiol. 2006, 7, 229–234. [Google Scholar] [CrossRef]
- Kunz, A.; Blank, W.; Braun, B. De Quervain’s Subacute Thyroiditis-Colour Doppler Sonography Findings. Ultraschall der Med.-Eur. J. Ultrasound 2005, 26, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Omori, N.; Omori, K.; Takano, K. Association of the Ultrasonographic Findings of Subacute Thyroiditis with Thyroid Pain and Laboratory Findings. Endocr. J. 2008, 55, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Daniels, G.H.; Barbesino, G. Painful Subacute Thyroiditis is Commonly Misdiagnosed as Suspicious Thyroid Nodular Disease. Mayo Clin. Proc. Innov. Qual. Outcomes 2021, 5, 330–337. [Google Scholar] [CrossRef]
- Park, H.K.; Kim, D.W.; Lee, Y.J.; Ha, T.K.; Kim, D.H.; Bae, S.K.; Jung, S.J. Suspicious sonographic and cytological findings in patients with subacute thyroiditis: Two case reports. Diagn. Cytopathol. 2014, 43, 399–402. [Google Scholar] [CrossRef]
- Ucan, B.; Delibasi, T.; Cakal, E.; Arslan, M.S.; Bozkurt, N.C.; Demirci, T.; Ozbek, M.; Sahin, M. Papillary thyroid cancer case masked by subacute thyroiditis. Arq. Bras. Endocrinol. Metabol. 2014, 58, 851–854. [Google Scholar] [CrossRef]
- Valentini, R.B.; De Macedo, B.M.; Izquierdo, R.F.; Meyer, E.L.S. Painless thyroiditis associated to thyroid carcinoma: Role of initial ultrasonography evaluation. Arch. Endocrinol. Metab. 2016, 60, 178–182. [Google Scholar] [CrossRef]
- Leboulleux, S.; Girard, E.; Rose, M.; Travagli, J.P.; Sabbah, N.; Caillou, B.; Hartl, D.M.; Lassau, N.; Baudin, E.; Schlumberger, M. Ultrasound Criteria of Malignancy for Cervical Lymph Nodes in Patients Followed Up for Differentiated Thyroid Cancer. J. Clin. Endocrinol. Metab. 2007, 92, 3590–3594. [Google Scholar] [CrossRef]
- Xie, P.; Xiao, Y.; Liu, F. Real-time ultrasound elastography in the diagnosis and differential diagnosis of subacute thyroiditis. J. Clin. Ultrasound 2011, 39, 435–440. [Google Scholar] [CrossRef]
- Ruchała, M.; Szczepanek-Parulska, E.; Zybek, A.; Moczko, J.; Czarnywojtek, A.; Kaminski, G.; Sowinski, J. The role of sonoelastography in acute, subacute and chronic thyroiditis: A novel application of the method. Eur. J. Endocrinol. 2012, 166, 425–432. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, D.W. Sonographic Characteristics and Interval Changes of Subacute Thyroiditis. J. Ultrasound Med. 2016, 35, 1653–1659. [Google Scholar] [CrossRef]
- Solbiati, L.; Osti, V.; Cova, L.; Tonolini, M. Ultrasound of thyroid, parathyroid glands and neck lymph nodes. Eur. Radiol. 2001, 11, 2411–2424. [Google Scholar] [CrossRef] [PubMed]
- De Leo, S.; Lee, S.Y.; Braverman, L.E. Hyperthyroidism. Lancet 2016, 388, 906–918. [Google Scholar] [CrossRef]
- Peter, S.A. Painful subacute thyroiditis (de Quervain’s thyroiditis). J. Natl. Med. Assoc. 1992, 84, 877. [Google Scholar] [PubMed]
- Mariani, G.; Tonaccehra, M.; Grosso, M.; Fiore, E.; Falcetta, P.; Montanelli, L.; Bagattini, B.; Vitti, P.; Strauss, H.W. The Role of Nuclear Medicine in the Clinical Management of Benign Thyroid Disorders. Part 2. Nodular Goiter, Hypothyroidism, and Subacute Thyroiditis. J. Nucl. Med. 2021, 62, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Hiromatsu, Y.; Ishibashi, M.; Miyake, I.; Nonaka, K. Technetium-99m tetrofosmin imaging in patients with subacute thyroiditis. Eur. J. Pediatr. 1998, 25, 1448–1452. [Google Scholar] [CrossRef]
- Hiromatsu, Y.; Ishibashi, M.; Nishida, H.; Kawamura, S.; Kaku, H.; Baba, K.; Kaida, H.; Miyake, I. Technetium-99m Sestamibi Imaging in Patients with Subacute Thyroiditis. Endocr. J. 2003, 50, 239–244. [Google Scholar] [CrossRef][Green Version]
- Loevner, L.A. Imaging of the thyroid gland. Semin. Ultrasound CT MRI 1996, 17, 539–562. [Google Scholar] [CrossRef]
- Jhaveri, K.; Shroff, M.M.; Fatterpekar, G.M.; Som, P.M. CT and MR Imaging Findings Associated with Subacute Thyroiditis. Am. J. Neuroradiol. 2003, 24, 143–146. [Google Scholar]
- Otsuka, N.; Nagai, K.; Morita, K.; Fukunaga, M.; Okazaki, S.; Onishi, M.; Nanba, K.; Sato, M.; Taketa, K. Magnetic resonance imaging of subacute thyroiditis. Radiat. Med. 1994, 12, 273–276. [Google Scholar]
- Yeo, S.; Lee, S.; Hwang, I.; Ahn, E. Subacute Thyroiditis Presenting as a Focal Lesion on [18F] Fluorodeoxyglucose Whole-Body Positron-Emission Tomography/CT. Am. J. Neuroradiol. 2010, 32, E58–E60. [Google Scholar] [CrossRef]
- Sahin, D.; Akpolat, I. Diagnostic cytological features and differential diagnosis of subacute granulomatous (De Quervain’s) thyroiditis. Diagn. Cytopathol. 2019, 47, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Vural, C.; Paksoy, N.; Gök, N.D.; Yazal, K. Subacute granulomatous (De Quervain’s) thyroiditis: Fine-needle aspiration cytology and ultrasonographic characteristics of 21 cases. Cytojournal 2015, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Öfner, C.; Hittmair, A.; Kröll, I.; Bangerl, I.; Zechmann, W.; Totsch, M.; Ladurner, D.; Bocker, W.; Schmid, K.W. Fine Needle Aspiration Cytodiagnosis of Subacute (De Quervain’s) Thyroiditis In an Endemic Goitre Area. Cytopathology 1994, 5, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, R.S.; Brennan, M.D.; McConahey, W.M.; Goellner, J.R.; Gharib, H. Hashimoto’s Thyroiditis: An uncommon cause of painful thyroid unresponsive to corticosteroid therap. Ann. Intern. Med. 1986, 104, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Houghton, D.J.; Gray, H.W.; MacKenzie, K. The tender neck: Thyroiditis or thyroid abscess? Clin. Endocrinol. 1998, 48, 521–524. [Google Scholar] [CrossRef]
- Jin, M.; Kim, T.Y. Anaplastic Thyroid Carcinoma with Initial Ultrasonography Features Mimicking Subacute Thyroiditis. Endocrinol. Metab. 2021, 36, 201–202. [Google Scholar] [CrossRef]
- García Solano, J.; Giménez Bascuñana, A.; Sola Pérez, J.; Campos Fernández, J.; Martínez Parra, D.; Sánchez Sánchez, C.; Montalbán Romero, S.; Pérez-Guillermo, M. Fine-needle aspiration of subacute granulomatous thyroiditis (De Quervain’s thyroiditis): A clinico-cytologic review of 36 cases. Diagn. Cytopathol. 1997, 16, 214–220. [Google Scholar] [CrossRef]
- Toda, S.; Tokuda, Y.; Koike, N.; Yonemitsu, N.; Watanabe, K.; Koike, K.; Fujitani, N.; Hiromatsu, Y.; Sugihara, H. Growth Factor-Expressing Mast Cells Accumulate at the Thyroid Tissue-Regenerative Site of Subacute Thyroiditis. Thyroid 2000, 10, 381–386. [Google Scholar] [CrossRef]
- Shabb, N.S.; Salti, I. Subacute thyroiditis: Fine-needle aspiration cytology of 14 cases presenting with thyroid nodules. Diagn. Cytopathol. 2005, 34, 18–23. [Google Scholar] [CrossRef]
- Nishihara, E.; Hirokawa, M.; Ohye, H.; Ito, M.; Kubota, S.; Fukata, S.; Amino, N.; Miyauchi, A. Papillary Carcinoma Obscured by Complication with Subacute Thyroiditis: Sequential Ultrasonographic and Histopathological Findings in Five Cases. Thyroid 2008, 18, 1221–1225. [Google Scholar] [CrossRef]
- Lu, C.-P.; Chang, T.-C.; Wang, C.-Y.; Hsiao, Y.-L. Serial Changes in Ultrasound-Guided Fine Needle Aspiration Cytology in Subacute Thyroiditis. Acta Cytol. 1997, 41, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.; O’Callaghan, K.; Sinclair, H.; Hawke, K.; Love, A.; Hajkowicz, K.; Stewart, A.G. Risk factors, treatment and outcomes of subacute thyroiditis secondary to COVID-19: A systematic review. Intern. Med. J. 2021, 52, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Stasiak, M.; Michalak, R.; Stasiak, B.; Lewiński, A. Time-Lag Between Symptom Onset and Diagnosis of Subacute Thyroiditis–How to Avoid the Delay of Diagnosis and Unnecessary Overuse of Antibiotics. Horm. Metab. Res. 2019, 52, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-S.; Lin, S.-Y.; Sheu, W.H.-H. Graves’ Disease Presented as Painful Goiter. Horm. Res. Paediatr. 2002, 57, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.S. Syndromes of thyrotoxicosis with low radioactive iodine uptake. Endocrinol. Metab. Clin. N. Am. 1998, 27, 169–185. [Google Scholar] [CrossRef]
- Guarda, M.L.A.; Baskin, M.H.J. Inflammatory and Lymphoid Lesions of the Thyroid Gland: Cytopathology by Fine-Needle Aspiration. Am. J. Clin. Pathol. 1987, 87, 14–22. [Google Scholar] [CrossRef]
- Gupta, N.; Sharma, K.; Barwad, A.; Sharma, M.; Rajwanshi, A.; Dutta, P.; Sharma, A. Thyroid tuberculosis-role of PCR in diagnosis of a rare entity. Cytopathology 2010, 22, 392–396. [Google Scholar] [CrossRef]
- Kasagi, K. Painful Hashimoto’s Thyroiditis. Intern. Med. 2006, 45, 351–352. [Google Scholar] [CrossRef][Green Version]
- Meier, D.A.; Nagle, C.E. Differential diagnosis of a tender goiter. J. Nucl. Med. 1996, 37, 1745. [Google Scholar]
- Stasiak, M.; Michalak, R.; Lewinski, A. Thyroid primary and metastatic malignant tumours of poor prognosis may mimic subacute thyroiditis-time to change the diagnostic criteria: Case reports and a review of the literature. BMC Endocr. Disord. 2019, 19, 86. [Google Scholar] [CrossRef]
- Byrd, J.C.; Dow, N.S.; Gaertner, E.; Hargis, J.B.; Raber, T.R.; Burrell, L.; Weiss, R.B. Leukemic thyroiditis as the initial relapsing sign in a patient with acute lymphocytic leukemia and blast expression of the neural cell adhesion molecule. Am. J. Hematol. 1997, 55, 212–215. [Google Scholar] [CrossRef]
- Ranganath, R.; Shaha, M.A.; Xu, B.; Migliacci, J.; Ghossein, R.; Shaha, A.R. de Quervain’s thyroiditis: A review of experience with surgery. Am. J. Otolaryngol. 2016, 37, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Sato, J.; Uchida, T.; Komiya, K.; Goto, H.; Takeno, K.; Suzuki, R.; Honda, A.; Himuro, M.; Watada, H. Comparison of the therapeutic effects of prednisolone and nonsteroidal anti-inflammatory drugs in patients with subacute thyroiditis. Endocrine 2016, 55, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Szabo, S.M.; Allen, D.B. Thyroiditis. Differentiation of acute suppurative and subacute. Case report and review of the literature. Clin. Pediatr. 1989, 28, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Masuoka, H.; Miyauchi, A.; Tomoda, C.; Inoue, H.; Takamura, Y.; Ito, Y.; Kobayashi, K.; Miya, A. Imaging Studies in Sixty Patients with Acute Suppurative Thyroiditis. Thyroid 2011, 21, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Anastasilakis, A.D.; Karanicola, V.; Kourtis, A.; Makras, P.; Kampas, L.; Gerou, S.; Giomisi, A. A case report of subacute thyroiditis during pregnancy: Difficulties in differential diagnosis and changes in cytokine levels. Gynecol. Endocrinol. 2010, 27, 384–390. [Google Scholar] [CrossRef]
- Doniach, D.; Hudson, R.V.; Roitt, I.M. Human Auto-immune Thyroiditis: Clinical Studies. BMJ 1960, 1, 365–373. [Google Scholar] [CrossRef]
- Seo, H.M.; Kim, M.; Bae, J.; Kim, J.-H.; Lee, J.W.; Lee, S.A.; Koh, G.; Lee, D.H. A Case of Painful Hashimoto Thyroiditis that Mimicked Subacute Thyroiditis. Chonnam Med. J. 2012, 48, 69–72. [Google Scholar] [CrossRef]
- Kon, Y.C.; DeGroot, L.J. Painful Hashimoto’s Thyroiditis as an Indication for Thyroidectomy: Clinical Characteristics and Outcome in Seven Patients. J. Clin. Endocrinol. Metab. 2003, 88, 2667–2672. [Google Scholar] [CrossRef]
- Ross, D.S.; Burch, H.B.; Cooper, D.S.; Greenlee, M.C.; Laurberg, P.; Maia, A.L.; Rivkees, S.A.; Samuels, M.; Sosa, J.A.; Stan, M.N.; et al. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid 2016, 26, 1343–1421. [Google Scholar] [CrossRef]
- Sencar, M.E.; Calapkulu, M.; Sakiz, D.; Hepsen, S.; Kus, A.; Akhanli, P.; Unsal, I.O.; Kizilgul, M.; Ucan, B.; Ozbek, M.; et al. An Evaluation of the Results of the Steroid and Non-steroidal Anti-inflammatory Drug Treatments in Subacute Thyroiditis in relation to Persistent Hypothyroidism and Recurrence. Sci. Rep. 2019, 9, 16899. [Google Scholar] [CrossRef] [PubMed]
- Volpé, R. The Management of Subacute (DeQuervain’s) Thyroiditis. Thyroid 1993, 3, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Topuzovic, N.; Smoje, J.; Karner, I. The therapeutic approach in subacute (de Quervain’s) thyroiditis. J. Nucl. Med. 1997, 38, 1665. [Google Scholar] [PubMed]
- Duan, L.; Feng, X.; Zhang, R.; Tan, X.; Xiang, X.; Shen, R.; Zheng, H. Short-Term Versus 6-Week Prednisone In The Treatment Of Subacute Thyroiditis: A Randomized Controlled Trial. Endocr. Pract. 2020, 26, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Su, Y.; Zhang, M.; Zhang, X.; Guan, Q. Successful Management of Recurrent Subacute Thyroiditis by Adding Colchicine to Glucocorticoid Treatment: A Case Series Study. Horm. Metab. Res. 2020, 52, 712–717. [Google Scholar] [CrossRef]
- Koirala, K.P. Treatment of Acute Painful Thyroiditis with Low Dose Prednisolone: A Study on Patients from Western Nepal. J. Clin. Diagn. Res. 2015, 9, MC01. [Google Scholar] [CrossRef]
- Kubota, S.; Nishihara, E.; Kudo, T.; Ito, M.; Amino, N.; Miyauchi, A. Initial Treatment with 15 mg of Prednisolone Daily Is Sufficient for Most Patients with Subacute Thyroiditis in Japan. Thyroid 2013, 23, 269–272. [Google Scholar] [CrossRef]
- Hepsen, S.; Akhanli, P.; Sencar, M.E.; Duger, H.; Sakiz, D.; Kizilgul, M.; Unsal, I.O.; Ucan, B.; Ozbek, M.; Cakal, E. The Evaluation of Low- and High-Dose Steroid Treatments in Subacute Thyroiditis: A Retrospective Observational Study. Endocr. Pract. 2020, 27, 594–600. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Jiang, L.; Li, Z.; Li, F.; Chen, H.; Feng, L. Efficacy and safety of ultrasound-guided intrathyroidal injection of glucocorticoids versus routine oral administration of glucocorticoids for subacute thyroiditis: Protocol of systematic review and meta-analysis. Medicine 2019, 98, e18564. [Google Scholar] [CrossRef]
- Forkert, I.O.; Melekhovets, O.K.; Kalynychenko, D.O.; Melekhovets, Y.V.; Kovalenko, E.L. Painful subacute thyroiditis treatment approach. Wiad. Lek. 2021, 74, 1921–1924. [Google Scholar] [CrossRef]
- Mazza, E.; Quaglino, F.; Suriani, A.; Palestini, N.; Gottero, C.; Leli, R.; Taraglio, S. Thyroidectomy for Painful Thyroiditis Resistant to Steroid Treatment: Three New Cases with Review of the Literature. Case Rep. Endocrinol. 2015, 2015, 138327. [Google Scholar] [CrossRef]
- Duininck, T.M.; van Heerden, J.A.; Fatourechi, V.; Curlee, K.J.; Farley, D.R.; Thompson, G.B.; Grant, C.S.; Lloyd, R.V. De Quervain’s Thyroiditis: Surgical Experience. Endocr. Pract. 2002, 8, 255–258. [Google Scholar] [CrossRef]
- Dumitriu, L.; Gudovan, E.; Ursu, H. Radioiodine treatment in recurrences of subacute thyroiditis. Endocrinologie 1990, 28, 21–23. [Google Scholar] [PubMed]
- Shen, X.; Yang, R.; An, J.; Zhong, X. Analysis of the Molecular Mechanisms of the Effects of Prunella vulgaris against Subacute Thyroiditis Based on Network Pharmacology. Evid.-Based Complement. Altern. Med. 2020, 2020, 9810709. [Google Scholar] [CrossRef] [PubMed]
- Saklamaz, A. Is There a Drug Effect on the Development of Permanent Hypothyroidism in Subacute Thyroiditis? Acta Endocrinol. 2017, 13, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ding, G.; Li, J.; Li, X.; Ding, L.; Li, X.; Yang, S.; Tang, F. Risk Factors for Subacute Thyroiditis Recurrence: A Systematic Review and Meta-Analysis of Cohort Studies. Front. Endocrinol. 2021, 12, 783439. [Google Scholar] [CrossRef]
- Arao, T.; Okada, Y.; Torimoto, K.; Kurozumi, A.; Narisawa, M.; Yamamoto, S.; Tanaka, Y. Prednisolone Dosing Regimen for Treatment of Subacute Thyroiditis. J. UOEH 2015, 37, 103–110. [Google Scholar] [CrossRef]
- Benbassat, C.A.; Olchovsky, D.; Tsvetov, G.; Shimon, I. Subacute thyroiditis: Clinical characteristics and treatment outcome in fifty-six consecutive patients diagnosed between 1999 and 2005. J. Endocrinol. Investig. 2007, 30, 631–635. [Google Scholar] [CrossRef]
- Nishihara, E.; Amino, N.; Ohye, H.; Ota, H.; Ito, M.; Kubota, S.; Fukata, S.; Miyauchi, A. Extent of hypoechogenic area in the thyroid is related with thyroid dysfunction after subacute thyroiditis. J. Endocrinol. Investig. 2009, 32, 33–36. [Google Scholar] [CrossRef]
- Teixeira, V.L.; Romaldini, J.; Rodrigues, H.F.; Tanaka, L.M.; Farah, C.S. Thyroid function during the spontaneous course of subacute thyroiditis. J. Nucl. Med. 1985, 26, 457–460. [Google Scholar]
- Görges, J.; Ulrich, J.; Keck, C.; Müller-Wieland, D.; Diederich, S.; Janssen, O.E. Long-term Outcome of Subacute Thyroiditis. Exp. Clin. Endocrinol. Diabetes 2019, 128, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Dong, Y.; Lu, L.; Zhang, N. C-reactive protein and thyroid-stimulating hormone levels as risk factors for hypothyroidism in patients with subacute thyroiditis. Endocr. Connect. 2021, 10, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Tamai, H.; Nozaki, T.; Mukuta, T.; Morita, T.; Matsubayashi, S.; Kuma, K.; Kumagai, L.F.; Nagataki, S. The Incidence of Thyroid Stimulating Blocking Antibodies during the Hypothyroid Phase in Patients with Subacute Thyroiditis. J. Clin. Endocrinol. Metab. 1991, 73, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.A.; Papaly, R.; Maliakal, A.; Chandra, L.; Antony, M.A. Elevated Graves’ Disease-Specific Thyroid-Stimulating Immunoglobulin and Thyroid Stimulating Hormone Receptor Antibody in a Patient with Subacute Thyroiditis. Cureus 2021, 13, e19448. [Google Scholar] [CrossRef]
- Minciullo, P.L.; Ruggeri, R.M.; Vita, G.; Benvenga, S.; Gangemi, S. Development of Hashimoto’s Thyroiditis After Subacute Thyroiditis: An Unusual Patient. Thyroid 2009, 19, 73–74. [Google Scholar] [CrossRef]
- Iitaka, M.; Kakinuma, S.; Yamanaka, K.; Fujimaki, S.; Oosuga, I.; Wada, S.; Katayama, S. Induction of Autoimmune Hypothyroidism and Subsequent Hyperthyroidism by TSH Receptor Antibodies following Subacute Thyroiditis: A Case Report. Endocr. J. 2001, 48, 139–142. [Google Scholar] [CrossRef][Green Version]
- Hallengren, B.; Planck, T.; Åsman, P.; Lantz, M. Presence of Thyroid-Stimulating Hormone Receptor Antibodies in a Patient with Subacute Thyroiditis followed by Hypothyroidism and Later Graves’ Disease with Ophthalmopathy: A Case Report. Eur. Thyroid J. 2015, 4, 197–200. [Google Scholar] [CrossRef][Green Version]
- Fukata, S.; Matsuzuka, F.; Kobayashi, A.; Hirai, K.; Kuma, K.; Sugawara, M. Development of Graves’ disease after subacute thyroiditis: Two unusual cases. Eur. J. Endocrinol. 1992, 126, 495–496. [Google Scholar] [CrossRef]
- Yamamoto, M.; Saito, S.; Sakurada, T.; Tamura, M.; Kudo, Y.; Yoshida, K.; Kaise, K.; Kaise, N.; Fukazawa, H.; Itagaki, Y.; et al. Recurrence of Subacute Thyroiditis Over 10 Years after the First Attack in Three Cases. Endocrinol. Jpn. 1988, 35, 833–839. [Google Scholar] [CrossRef]
- Stasiak, M.; Tymoniuk, B.; Stasiak, B.; Lewiński, A. The Risk of Recurrence of Subacute Thyroiditis Is HLA-Dependent. Int. J. Mol. Sci. 2019, 20, 1089. [Google Scholar] [CrossRef]
- Brancatella, A.; Ricci, D.; Viola, N.; Sgrò, D.; Santini, F.; Latrofa, F. Subacute Thyroiditis After Sars-COV-2 Infection. J. Clin. Endocrinol. Metab. 2020, 105, 2367–2370. [Google Scholar] [CrossRef] [PubMed]
- Tanda, M.L.; Ippolito, S.; Gallo, D.; Baj, A.; Novazzi, F.; Genoni, A.; Annoni, M.; Mancini, N.; Clementi, N.; Finzi, G.; et al. SARS-CoV-2 detection in primary thyroid sarcoma: Coincidence or interaction? J. Endocrinol. Investig. 2022, 45, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Poma, A.M.; Bonuccelli, D.; Giannini, R.; Macerola, E.; Vignali, P.; Ugolini, C.; Torregrossa, L.; Proietti, A.; Pistello, M.; Basolo, A.; et al. COVID-19 autopsy cases: Detection of virus in endocrine tissues. J. Endocrinol. Investig. 2021, 45, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Rotondi, M.; Coperchini, F.; Ricci, G.; Denegri, M.; Croce, L.; Ngnitejeu, S.T.; Villani, L.; Magri, F.; Latrofa, F.; Chiovato, L. Detection of SARS-COV-2 receptor ACE-2 mRNA in thyroid cells: A clue for COVID-19-related subacute thyroiditis. J. Endocrinol. Investig. 2020, 44, 1085–1090. [Google Scholar] [CrossRef]
- Lania, A.; Sandri, M.T.; Cellini, M.; Mirani, M.; Lavezzi, E.; Mazziotti, G. Thyrotoxicosis in patients with COVID-19: The thyrcov study. Eur. J. Endocrinol. 2020, 183, 381–387. [Google Scholar] [CrossRef]
- Bartalena, L.; Grasso, L.; Brogioni, S.; Aghini-Lombardi, F.; Braverman, L.E.; Martino, E. Serum interleukin-6 in amiodarone-induced thyrotoxicosis. J. Clin. Endocrinol. Metab. 1994, 78, 423–427. [Google Scholar] [CrossRef]
- Ippolito, S.; Di Dalmazi, G.; Pani, F.; Sabini, E.; Caturegli, P. Distinct Cytokine Signatures in Thyroiditis Induced by PD-1 or CTLA-4 Blockade: Insights from a New Mouse Model. Thyroid 2021, 31, 1839–1849. [Google Scholar] [CrossRef]
- Muller, I.; Cannavaro, D.; Dazzi, D.; Covelli, D.; Mantovani, G.; Muscatello, A.; Ferrante, E.; Orsi, E.; Resi, V.; Longari, V.; et al. SARS-CoV-2-related atypical thyroiditis. Lancet Diabetes Endocrinol. 2020, 8, 739–741. [Google Scholar] [CrossRef]
- Lui, D.T.W.; Lee, C.H.; Chow, W.S.; Lee, A.C.H.; Tam, A.R.; Fong, C.H.Y.; Law, C.Y.; Leung, E.K.H.; To, K.K.W.; Tan, K.C.B.; et al. Thyroid Dysfunction in Relation to Immune Profile, Disease Status, and Outcome in 191 Patients with COVID-19. J. Clin. Endocrinol. Metab. 2020, 106, e926–e935. [Google Scholar] [CrossRef]
- Trimboli, P.; Cappelli, C.; Croce, L.; Scappaticcio, L.; Chiovato, L.; Rotondi, M. COVID-19-Associated Subacute Thyroiditis: Evidence-Based Data From a Systematic Review. Front. Endocrinol. 2021, 12, 707726. [Google Scholar] [CrossRef]
- Khoo, B.; Tan, T.; Clarke, S.A.; Mills, E.G.; Patel, B.; Modi, M.; Phylactou, M.; Eng, P.C.; Thurston, L.; Alexander, E.C.; et al. Thyroid Function Before, During, and After COVID-19. J. Clin. Endocrinol. Metab. 2020, 106, e803–e811. [Google Scholar] [CrossRef] [PubMed]
- Campi, I.; Bulgarelli, I.; Dubini, A.; Perego, G.B.; Tortorici, E.; Torlasco, C.; Torresani, E.; Rocco, L.; Persani, L.; Fugazzola, L. The spectrum of thyroid function tests during hospitalization for SARS COV-2 infection. Eur. J. Endocrinol. 2021, 184, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Pirola, I.; Gandossi, E.; Rotondi, M.; Marini, F.; Cristiano, A.; Chiovato, L.; Castellano, M.; Ferlin, A.; Cappelli, C. Incidence of De Quervain’s thyroiditis during the COVID-19 pandemic in an area heavily affected by Sars-CoV-2 infection. Endocrine 2021, 74, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Brancatella, A.; Viola, N.; Rutigliano, G.; Sgrò, D.; Santini, F.; Latrofa, F. Subacute thyroiditis at the time of SARS-CoV-2 pandemic. J. Endocr. Soc. 2021, 5, bvab130. [Google Scholar] [CrossRef]
- Bragazzi, N.L.; Hejly, A.; Watad, A.; Adawi, M.; Amital, H.; Shoenfeld, Y. ASIA syndrome and endocrine autoimmune disorders. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101412. [Google Scholar] [CrossRef]
- Ruggeri, R.M.; Campennì, A.; Deandreis, D.; Siracusa, M.; Tozzoli, R.; Petranović Ovčariček, P.; Giovanella, L. SARS-CoV-2-related immune-inflammatory thyroid disorders: Facts and perspectives. Expert Rev. Clin. Immunol. 2021, 17, 737–759. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanzo, N.; Patera, B.; Fazzino, G.F.M.; Gallo, D.; Lai, A.; Piantanida, E.; Ippolito, S.; Tanda, M.L. The Old and the New in Subacute Thyroiditis: An Integrative Review. Endocrines 2022, 3, 391-410. https://doi.org/10.3390/endocrines3030031
Lanzo N, Patera B, Fazzino GFM, Gallo D, Lai A, Piantanida E, Ippolito S, Tanda ML. The Old and the New in Subacute Thyroiditis: An Integrative Review. Endocrines. 2022; 3(3):391-410. https://doi.org/10.3390/endocrines3030031
Chicago/Turabian StyleLanzo, Nicola, Bohdan Patera, Gaia Francesca Maria Fazzino, Daniela Gallo, Adriana Lai, Eliana Piantanida, Silvia Ippolito, and Maria Laura Tanda. 2022. "The Old and the New in Subacute Thyroiditis: An Integrative Review" Endocrines 3, no. 3: 391-410. https://doi.org/10.3390/endocrines3030031
APA StyleLanzo, N., Patera, B., Fazzino, G. F. M., Gallo, D., Lai, A., Piantanida, E., Ippolito, S., & Tanda, M. L. (2022). The Old and the New in Subacute Thyroiditis: An Integrative Review. Endocrines, 3(3), 391-410. https://doi.org/10.3390/endocrines3030031






