Mechanisms of Insulin Resistance in Patients with Obesity
Abstract
:1. Introduction
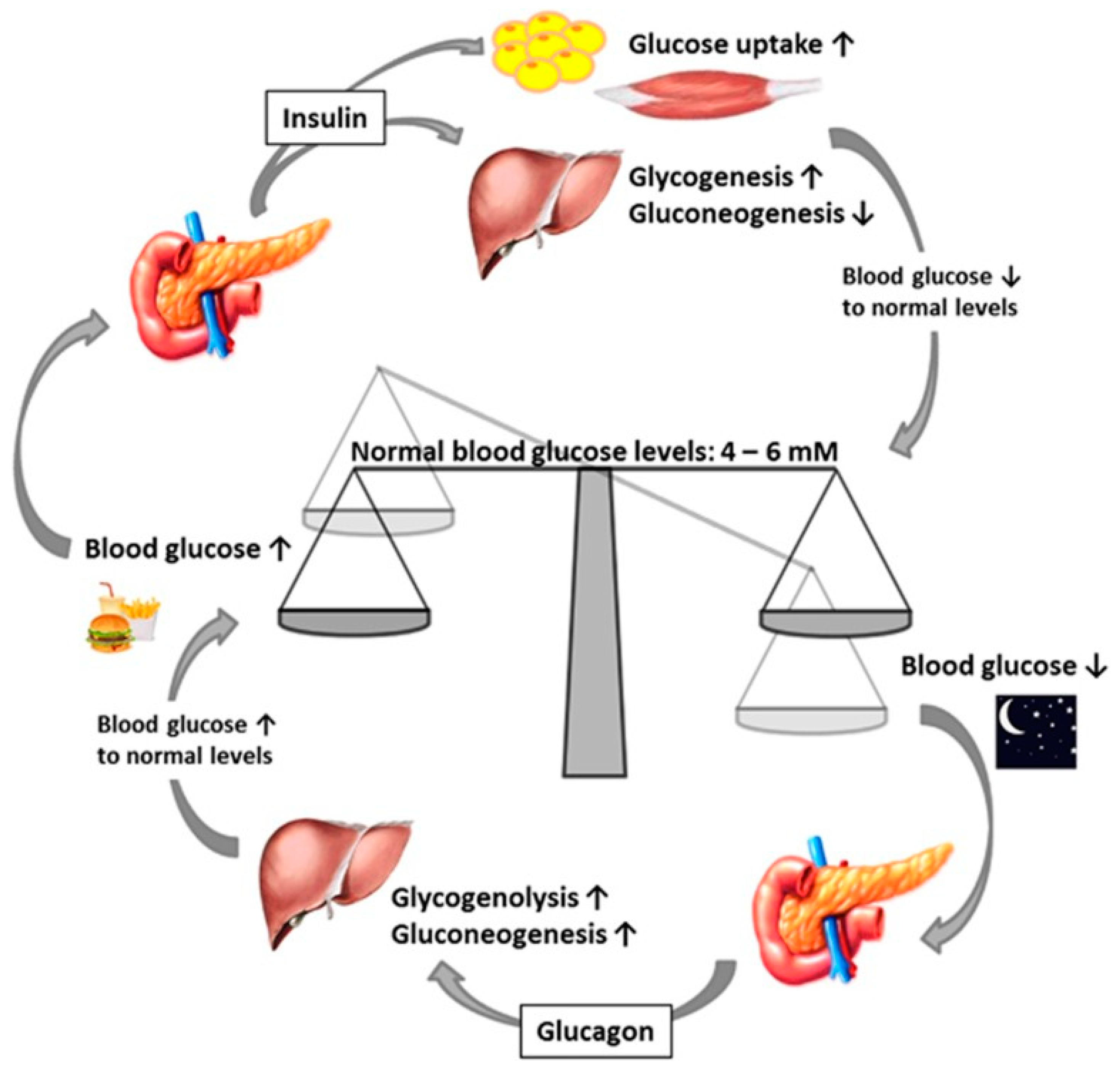
Normal Insulin Function
2. Methodology
2.1. Arteriosclerosis in Obesity and Diabetes
2.2. Inflammatory Mechanisms of Insulin Resistance
2.3. Neural Mechanisms of Insulin Resistance
2.4. Biochemical Mechanisms: Ectopic Fat, Oxidative Stress, and Mitochondrial Dysfunction
3. Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Akt | Agder Kollektivtrafikk/Protein kinase B |
| ATF-6 | Transcription factor 6 |
| CaV1.2 | Cardiac L-type Ca2+ channel |
| CRP | C-reactive protein |
| CPT-1 | Carnitine palmitoyltransferase-1 |
| HbA1c | Glycosylated hemoglobin |
| IRS-1 | Inhibiting insulin receptor substrate 1 |
| FFA | Free fatty acid |
| TNFα | Tumor necrosis factor-α |
| IL-6 | Interleukin 6 |
| ER | Endoplasmic reticulum |
| FOXO1 | Forkhead box protein O1 |
| GSK3 | Glycogen synthase kinase-3 |
| IκB | Inhibitor of nuclear factor kappa light chain enhancer of activated B cells |
| IKBKB | Inhibitor of kappa light polypeptide gene enhancer in B-cells, kinase beta |
| IKKβ | IκB kinase β |
| IL-1β | Interleukin 1 beta |
| IRE-1 | Inositol-requiring kinase/endoribonuclease 1 |
| IRS | Insulin receptor substrate |
| IRS-1 | Insulin receptor substrate 1 |
| IRS-2 | Insulin receptor substrate 2 |
| JNK | Jun N-terminal kinases |
| JNK1 | JUN N-terminal kinase1 |
| NF-Κb | Nuclear factor kappa light chain enhancer of activated B cells |
| p38 MAPK | Mitogen-activated protein kinase p38 |
| PERK | Protein kinase RNA-like endoplasmic reticulum kinase |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1α |
| PGC-1β | Peroxisome proliferator-activated receptor gamma coactivator 1β |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| ROS | Reactive oxygen species |
| SOCS | Suppressor of cytokine signaling proteins |
| SOCS-1 | Suppressor of cytokine signaling proteins 1 |
| SOCS-3 | Suppressor of cytokine signaling proteins 3 |
| SOCS-6 | Suppressor of cytokine signaling proteins 6 |
| TNF-a | Tumor necrosis factor-α |
| TZD | Thiazolidinedione |
| UPR | Unfolded protein response |
| β cells | Beta cells |
References
- He, Q.; Gao, Z.; Yin, J.; Zhang, J.; Yun, Z.; Ye, J. Regulation of HIF-1α activity in adipose tissue by obesity-associated factors: Adipogenesis, insulin, and hypoxia. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E877–E885. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; McGuinness, O.P. Inflammation during obesity is not all bad: Evidence from animal and human studies. Am. J. Physiol. Endocrinol. Metab. 2012, 304, E466–E477. [Google Scholar] [CrossRef] [PubMed]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Investig. 2016, 116, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Chan, Z.; Chooi, Y.C.; Choo, J.; Sadananthan, S.A.; Chang, A.; Sasikala, S.; Michael, N.; Velan, S.S.; Magkos, F. Regulation of glucose metabolism in nondiabetic, metabolically obese normal-weight Asians. Am. J. Physiol. Endocrinol. Metab. 2018, 314, E494–E502. [Google Scholar] [CrossRef] [PubMed]
- Röder, P.V.; Wu, B.; Liu, Y.; Han, W. Pancreatic regulation of glucose homeostasis. Exp. Mol. Med. 2016, 48, e219. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, A.; Gaz, C.; Palumbo, P.; Panunzi, S. A unifying organ model of pancreatic insulin secretion. PLoS ONE 2015, 10, e0142344. [Google Scholar] [CrossRef] [PubMed]
- Beckwith, S. The relationship between diabetes and atherosclerosis. Br. J. Card. Nurs. 2014, 9, 237–242. [Google Scholar] [CrossRef]
- Katakami, N. Mechanism of Development of Atherosclerosis and Cardiovascular Disease in Diabetes Mellitus. J. Atheroscler. Thromb. 2018, 25, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, R.; Wang, H.; Liang, F. Mechanisms linking inflammation to insulin resistance. Int. J. Endocrinol. 2015, 2015, 508409. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Akash, M.S.H. Nutrition and Diabetes Mellitus: How are They Interlinked? Crit. Rev. Eukaryot. Gene Expr. 2016, 26, 317–332. [Google Scholar] [CrossRef]
- Feve, B.; Bastard, J.P. The role of interleukins in insulin resistance and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2009, 5, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Akash, M.S.H.; Rehman, K.; Chen, S. Role of inflammatory mechanisms in the pathogenesis of type 2 diabetes mellitus. J. Cell. Biochem. 2013, 114, 525–531. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K.; Chen, S. IL-1Ra and its delivery strategies: Inserting the association in perspective. Pharm. Res. 2013, 30, 2951–2966. [Google Scholar] [CrossRef]
- Swaroop, J.J.; Rajarajeswari, D.; Naidu, J.N. Association of TNF-alpha with insulin resistance in type 2 diabetes mellitus. Indian J. Med. Res. 2012, 135, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Kitade, H.; Ni, Y.; Ota, T. Roles of chem Okines and chemokine receptors in obesity-associated insulin resistance and nonalcoholic fatty liver disease. Biomolecules 2015, 5, 1563–1579. [Google Scholar] [CrossRef]
- Kitade, H.; Sawamoto, K.; Nagashimada, M.; Inoue, H.; Yamamoto, Y.; Sai, Y.; Takamura, T.; Yamamoto, H.; Miyamoto, K.; Ginsberg, H.N.; et al. CCR5 plays a critical role in obesity-induced adipose tissue inflammation and insulin resistance by regulating both macrophage recruitment and M1/M2 status. Diabetes 2012, 61, 1680–1690. [Google Scholar] [CrossRef]
- Lee, C.T.; Harris, S.B.; Retnakaran, R.; Gerstein, H.C.; Perkins, B.A.; Zinman, B.; Hanley, A.J. White blood cell subtypes, insulin resistance and beta-cell dysfunction in high-risk individuals—The PROMISE cohort. Clin. Endocrinol. 2014, 81, 536–541. [Google Scholar] [CrossRef]
- Vella, C.A.; Burgos, X.; Ellis, C.J.; Zubia, R.Y.; Ontiveros, D.; Reyes, H.; Lozano, C. Associations of insulin resistance with cardiovascular risk factors and inflammatory cytokines in normal-weight Hispanic women. Diabetes Care 2013, 36, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- de Vries, M.A.; Alipour, A.; Klop, B.; van de Geijn, G.J.; Janssen, H.W.; Njo, T.L.; van der Meulen, N.; Rietveld, A.P.; Liem, A.H.; Westerman, E.M.; et al. Glucose-dependent leukocyte activation in patients with type 2 diabetes mellitus, familial combined hyperlipidemia, and healthy controls. Metabolism 2015, 64, 213–217. [Google Scholar] [CrossRef]
- Abu-Farha, M.; Behbehani, K.; Elkum, N. Comprehensive analysis of circulating adipokines and hsCRP association with cardiovascular disease risk factors and metabolic syndrome in Arabs. Cardiovasc. Diabetol. 2014, 13, 76. [Google Scholar] [CrossRef]
- Doyle, S.L.; Donohoe, C.L.; Lysaght, J.; Reynolds, J.V. Visceral obesity, metabolic syndrome, insulin resistance, and cancer. Proc. Nutr. Soc. 2012, 71, 181–189. [Google Scholar] [CrossRef]
- Al-Hamodi, Z.; Al-Habori, M.; Al-Meeri, A.; Saif-Ali, R. Association of adipokines, leptin/adiponectin ratio and C-reactive protein with obesity and type 2 diabetes mellitus. Diabetol. Metab. Syndr. 2014, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Farooq, W.; Farwa, U.; Khan, F.R. The metabolic syndrome and inflammation: The role of insulin resistance and increased adiposity. Oman Med. J. 2015, 30, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Ye, J. Mechanisms of insulin resistance in obesity. Front. Med. 2013, 7, 14–24. [Google Scholar] [CrossRef]
- Choi, K.; Kim, Y.B. Molecular mechanism of insulin resistance in obesity and type 2 diabetes. Korean J. Intern. Med. 2010, 25, 119–129. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. Mechanisms for insulin resistance: Common threads and missing links. Cell 2012, 148, 852–871. [Google Scholar] [CrossRef]
- Matveyenko, A.V.; Liuwantara, D.; Gurlo, T.; Kirakossian, D.; Dalla Man, C.; Cobelli, C.; White, M.F.; Copps, K.D.; Volpi, E.; Fujita, S.; et al. Pulsatile portal vein insulin delivery enhances hepatic insulin action and signaling. Diabetes 2012, 61, 2269–2279. [Google Scholar] [CrossRef]
- Wan, M.; Leavens, K.F.; Hunter, R.W.; Koren, S.; von Wilamowitz-Moellendorff, A.; Lu, M.; Satapati, S.; Chu, Q.; Sakamoto, K.; Burgess, S.C.; et al. A noncanonical, GSK3-independent pathway controls postprandial hepatic glycogen deposition. Cell Metab. 2013, 18, 99–105. [Google Scholar] [CrossRef]
- Lu, M.; Wan, M.; Leavens, K.F.; Chu, Q.; Monks, B.R.; Fernandez, S.; Ahima, R.S.; Ueki, K.; Kahn, C.R.; Birnbaum, M.J. Insulin regulates liver metabolism in vivo in the absence of hepatic Akt and Foxo1. Nat. Med. 2012, 18, 388–395. [Google Scholar] [CrossRef]
- Perry, R.J.; Camporez, J.P.G.; Kursawe, R.; Titchenell, P.M.; Zhang, D.; Perry, C.J.; Jurczak, M.J.; Abudukadier, A.; Han, M.S.; Zhang, X.M.; et al. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell 2015, 160, 745–758. [Google Scholar] [CrossRef]
- Bogan, J.S.; Rubin, B.R.; Yu, C.; Löffler, M.G.; Orme, C.M.; Belman, J.P.; McNally, L.J.; Hao, M.; Cresswell, J.A. Endoproteolytic cleavage of TUG protein regulates GLUT4 glucose transporter translocation. J. Biol. Chem. 2012, 287, 23932–23947. [Google Scholar] [CrossRef]
- Kersten, S. Physiological regulation of lipoprotein lipase. Biochim. Biophys. Acta 2014, 1841, 919–933. [Google Scholar] [CrossRef]
- Ruud, J.; Steculorum, S.M.; Brüning, J.C. Neuronal control of peripheral insulin sensitivity and glucose metabolism. Nat. Commun. 2017, 8, 15259. [Google Scholar] [CrossRef] [PubMed]
- Berglund, E.D.; Liu, T.; Kong, X.; Sohn, J.W.; Vong, L.; Deng, Z.; Lee, C.E.; Lee, S.; Williams, K.W.; Olson, D.P.; et al. Melanocortin 4 receptors in autonomic neurons regulate thermogenesis and glycemia. Nat. Neurosci. 2014, 17, 911–913. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.W.; Elias, C.F.; Fukuda, M.; Williams, K.W.; Berglund, E.D.; Holland, W.L.; Cho, Y.R.; Chuang, J.C.; Xu, Y.; Choi, M.; et al. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab. 2010, 11, 286–297. [Google Scholar] [CrossRef]
- Barazzoni, R.; Gortan Cappellari, G.; Ragni, M.; Nisoli, E. Insulin resistance in obesity: An overview of fundamental alterations. Eat. Weight Disord. 2018, 23, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.P.; David Cheng, T.Y.; Tsai, S.P.; Chan, H.T.; Hsu, H.L.; Hsu, C.C.; Hsu, C.C.; Eriksen, M.P. Are Asians at greater mortality risks for being overweight than Caucasians? Redefining obesity for Asians. Public Health Nutr. 2010, 12, 497–506. [Google Scholar] [CrossRef]
- de Groot, P.C.; Dekkers, O.M.; Romijn, J.A.; Dieben, S.W.; Helmerhorst, F.M. PCOS, coronary heart disease, stroke and the influence of obesity: A systematic review and meta-analysis. Hum. Reprod. Update 2011, 17, 495–500. [Google Scholar] [CrossRef]
- Sironi, A.M.; Sicari, R.; Folli, F.; Gastaldelli, A. Ectopic fat storage, insulin resistance, and hypertension. Curr. Pharm. Des. 2011, 17, 3074–3080. [Google Scholar] [CrossRef]
- Stinkens, R.; Goossens, G.H.; Jocken, J.W.; Blaak, E.E. Targeting fatty acid metabolism to improve glucose metabolism. Obes. Rev. 2015, 16, 715–757. [Google Scholar] [CrossRef]
- Hocking, S.; Samocha-Bonet, D.; Milner, K.L.; Greenfield, J.R.; Chisholm, D.J. Adiposity and insulin resistance in humans: The role of the different tissue and cellular lipid depots. Endocr. Rev. 2013, 34, 463–500. [Google Scholar] [CrossRef] [PubMed]
- Jans, A.; Konings, E.; Goossens, G.H.; Bouwman, F.G.; Moors, C.C.; Boekschoten, M.V.; Afman, L.A.; Müller, M.; Mariman, E.C.; Blaak, E.E. PUFAs acutely affect triacylglycerol-derived skeletal muscle fatty acid uptake and increase postprandial insulin sensitivity. Am. J. Clin. Nutr. 2012, 95, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Gross, M.; Lee, D.H.; Holvoet, P.; Himes, J.H.; Shikany, J.M.; Jacobs, D.R., Jr. Oxidative stress and insulin resistance: The coronary artery risk development in young adults’ study. Diabetes Care 2009, 32, 1302–1307. [Google Scholar] [CrossRef]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia, and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456–480. [Google Scholar] [CrossRef] [PubMed]
- Cossarizza, A.; Ferraresi, R.; Troiano, L.; Roat, E.; Gibellini, L.; Bertoncelli, L.; Nasi, M.; Pinti, M. Simultaneous analysis of reactive oxygen species and reduced glutathione content in living cells by polychromatic flow cytometry. Nat. Protoc. 2010, 4, 1790–1797. [Google Scholar] [CrossRef]
- Choi, C.S.; Zhou, L.; Park, S.Y.; Xu, L.; Xia, X.; Ye, J.; Su, L.; Jeong, K.H.; Hur, J.H.; Oh, H.; et al. Insulin resistance and white adipose tissue inflammation are uncoupled in energetically challenged Fsp27-deficient mice. Nat. Commun. 2015, 6, 5949. [Google Scholar] [CrossRef]
- Narayanan, D.; Xi, Q.; Pfeffer, L.M.; Jaggar, J.H. Mitochondria control functional CaV1.2 expression in smooth muscle cells of cerebral arteries. Circ. Res. 2010, 107, 631–641. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Manna, P.; Gachhui, R.; Sil, P.C. D-saccharic acid 1,4-lactone protects diabetic rat kidney by ameliorating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via NF-κB and PKC signaling. Toxicol. Appl. Pharmacol. 2013, 267, 16–29. [Google Scholar] [CrossRef]
- Loh, K.; Deng, H.; Fukushima, A.; Cai, X.; Boivin, B.; Galic, S.; Bruce, C.; Shields, B.; Skiba, B. Reactive oxygen species enhance insulin sensitivity. Cell Metab. 2009, 10, 260–272. [Google Scholar] [CrossRef]
- Leloup, C.; Tourrel-Cuzin, C.; Magnan, C.; Karaca, M.; Castel, J.; Carneiro, L.; Colombani, A.L.; Ktorza, A.; Casteilla, L.; Pénicaud, L. Mitochondrial reactive oxygen species are obligatory signals for glucose-induced insulin secretion. Diabetes 2009, 58, 673–681. [Google Scholar] [CrossRef]
- Tangvarasittichai, S.; Poonsub, P.; Tangvarasittichai, O. Association of serum lipoprotein ratios with insulin resistance in type 2 diabetes mellitus. Indian J. Med. Res. 2010, 131, 641–648. [Google Scholar]
- Otero, Y.F.; Stafford, J.M.; McGuinness, O.P. Pathway-selective insulin resistance and metabolic disease: The importance of nutrient flux. J. Biol. Chem. 2014, 289, 20462–20469. [Google Scholar] [CrossRef]
- Guerrero-Hernández, A.; Leon-Aparicio, D.; Chavez-Reyes, J.; Olivares-Reyes, J.A.; DeJesus, S. Endoplasmic reticulum stress in insulin resistance and diabetes. Cell Calcium 2014, 56, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Flamment, M.; Hajduch, E.; Ferré, P.; Foufelle, F. New insights into ER stress-induced insulin resistance. Trends Endocrinol. Metab. 2012, 23, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Sha, H.; He, Y.; Yang, L.; Qi, L. Stressed out about obesity: IRE1a-XBP1 in metabolic disorders. Trends Endocrinol. Metab. 2011, 22, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Boden, G. Endoplasmic reticulum stress: Another link between obesity and insulin resistance/inflammation? Diabetes 2009, 58, 518–519. [Google Scholar] [CrossRef]
- Khan, S.; Wang, C.H. ER stress in adipocytes and insulin resistance: Mechanisms and significance (Review). Mol. Med. Rep. 2014, 10, 2234–2240. [Google Scholar] [CrossRef]
- Kawasaki, N.; Asada, R.; Saito, A.; Kanemoto, S.; Imaizumi, K. Obesity-induced endoplasmic reticulum stress causes chronic inflammation in adipose tissue. Sci. Rep. 2012, 2, 799. [Google Scholar] [CrossRef]
- Lee, J.; Ozcan, U. Unfolded protein response signaling and metabolic diseases. J. Biol. Chem. 2014, 289, 1203–1211. [Google Scholar] [CrossRef]

| Studies Related to Inflammatory Mechanisms | ||
| Study | Design | Key Findings |
| Chen et al. (2015) [9] | Systematic review | A wide range of inflammatory processes and molecules, such as cytokines and macrophages, increase the risk of insulin resistance |
| Rehman and Akash (2016) [10] | Systematic review | Inflammation is a critical physiological process associated with increased levels of pro-inflammatory cytokines and white blood cells in the body |
| Xu et al. (2015) [15] | Systematic review | Inflammatory markers, such as chemokines and chemokine receptors, play a vital role in the development of insulin resistance and the progression of type 2 diabetes mellitus |
| Vella et al. (2013) [18] | Experimental study | Surrogate markers of inflammation are associated with insulin resistance and the risk of cardiovascular disease among Hispanic women |
| de Vries et al. (2015) [19] | Randomized controlled trial | Insulin resistance is associated with acute and chronic hyperglycemia and postprandial leukocyte activation |
| Al-Hamodi et al. (2014) [22] | Randomized controlled trial | A significant association exists among insulin resistance, adiposity, adipokines, C-reactive protein, and the leptin/adiponectin ratio |
| Studies Related to Neural mechanisms | ||
| Study | Design | Key Findings |
| Samuel and Shulman (2016) [26] | Systematic review | Insulin resistance is a complex disorder caused by inflammatory and neural signaling processes and substrate flux |
| Samuel and Shulman (2012) [26] | Systematic review | Insulin resistance is caused by unfolded protein response (UPR) activation, ectopic lipid metabolite accumulation, and innate immune system responses |
| Wan M et al. (2013) [29] | Systematic review | The GSK3-independent pathway and postprandial hepatic glycogen deposition contribute to the development of insulin resistance |
| Lu M et al. (2012) [30] | Experimental study (gene expression analysis) | Deletion of Akt results in the activation of FoX01–dependent gene expression and eventually insensitivity to insulin level changes |
| Kersten (2012) [33] | Systematic review | Liver-derived apolipoproteins influence the risk of insulin resistance |
| Hill et al. (2010) [36] | Systematic review | Insulin action and sensitivity are regulated by pro-opiomelanocortin neurons. These neurons also control glucose homeostasis |
| Studies Related to Cellular Mechanisms | ||
| Study | Design | Key Findings |
| Sironi et al. (2011) [40] | Systematic review | Increased uptake of fatty acids and lipids can result in obesity and the subsequent ectopic storage of fats |
| Stinkens et al. (2015) [41] | Systematic review | Fatty acid metabolism and accumulation may result in the emergence and progression of complications, such as insulin resistance |
| Tangvarasittichai (2015) [45] | Systematic review | Oxidative stress leads to dyslipidemia, β-cell dysfunction, loss of glucose tolerance, and insulin resistance |
| Cossarizza et al. (2010) [46] | Experimental study (polychromatic flow cytometry) | A significant association exists among reactive oxygen species, oxidative stress, and cell death |
| Narayanan et al. (2010) [48] | Systematic review | Mitochondria dysfunction affects the expression of CaV1.2 in muscles and contributes to the development of insulin resistance |
| Tangvarasittichai et al. (2010) [52] | Systematic review and meta-analysis | A significant association exists between the serum lipoprotein ratios and insulin resistance among patients with type 2 diabetes mellitus |
| Khan and Wang (2014) [58] | Systematic review | ER stress leads to the development of insulin resistance through neural and inflammatory mechanisms |
| Kawasaki et al. (2012) [59] | Systematic review | Obesity-induced ER leads to chronic inflammation in adipose tissues and increases the risk of insulin resistance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arneth, B. Mechanisms of Insulin Resistance in Patients with Obesity. Endocrines 2024, 5, 153-165. https://doi.org/10.3390/endocrines5020011
Arneth B. Mechanisms of Insulin Resistance in Patients with Obesity. Endocrines. 2024; 5(2):153-165. https://doi.org/10.3390/endocrines5020011
Chicago/Turabian StyleArneth, Borros. 2024. "Mechanisms of Insulin Resistance in Patients with Obesity" Endocrines 5, no. 2: 153-165. https://doi.org/10.3390/endocrines5020011
APA StyleArneth, B. (2024). Mechanisms of Insulin Resistance in Patients with Obesity. Endocrines, 5(2), 153-165. https://doi.org/10.3390/endocrines5020011





