Differences in Exercise-Linked Biomarkers between Premenopausal and Postmenopausal Middle-Aged Females
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Familiarization
2.3. Experimental Session
2.4. Blood Collection and Analysis
2.5. Statistical Analysis
3. Results
3.1. Group Characteristics
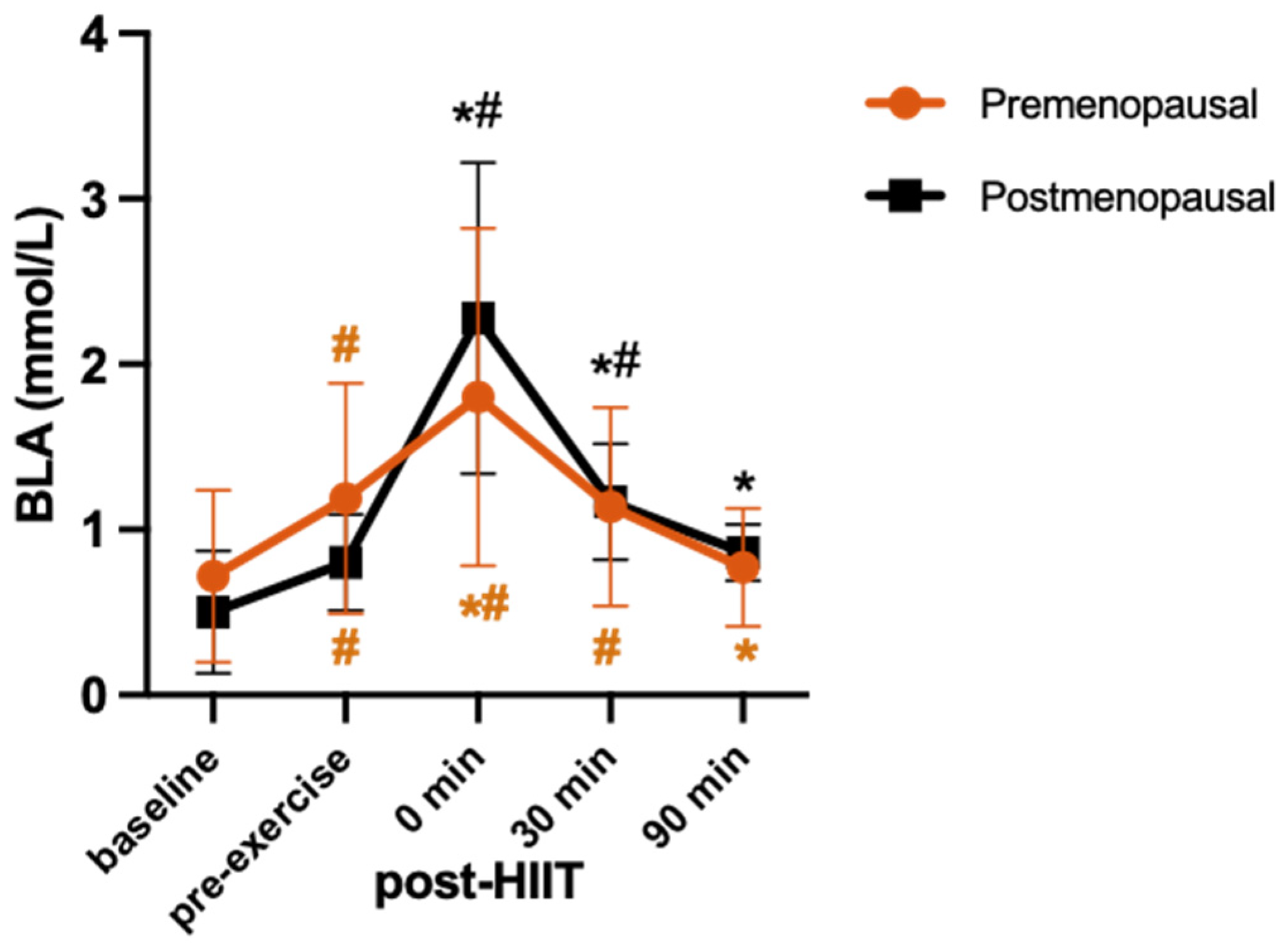
3.2. HIIT Session
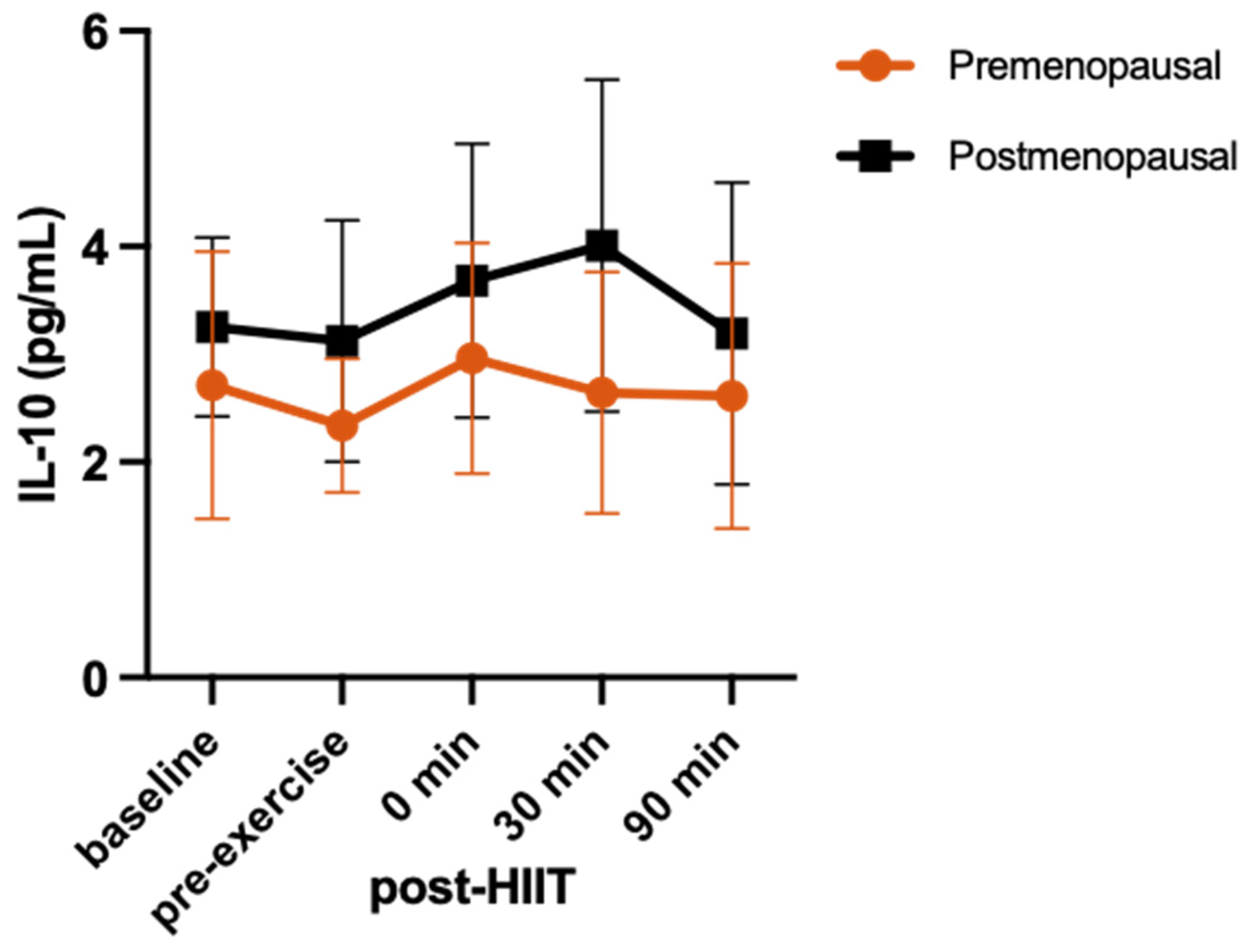
3.3. Cytokine Response
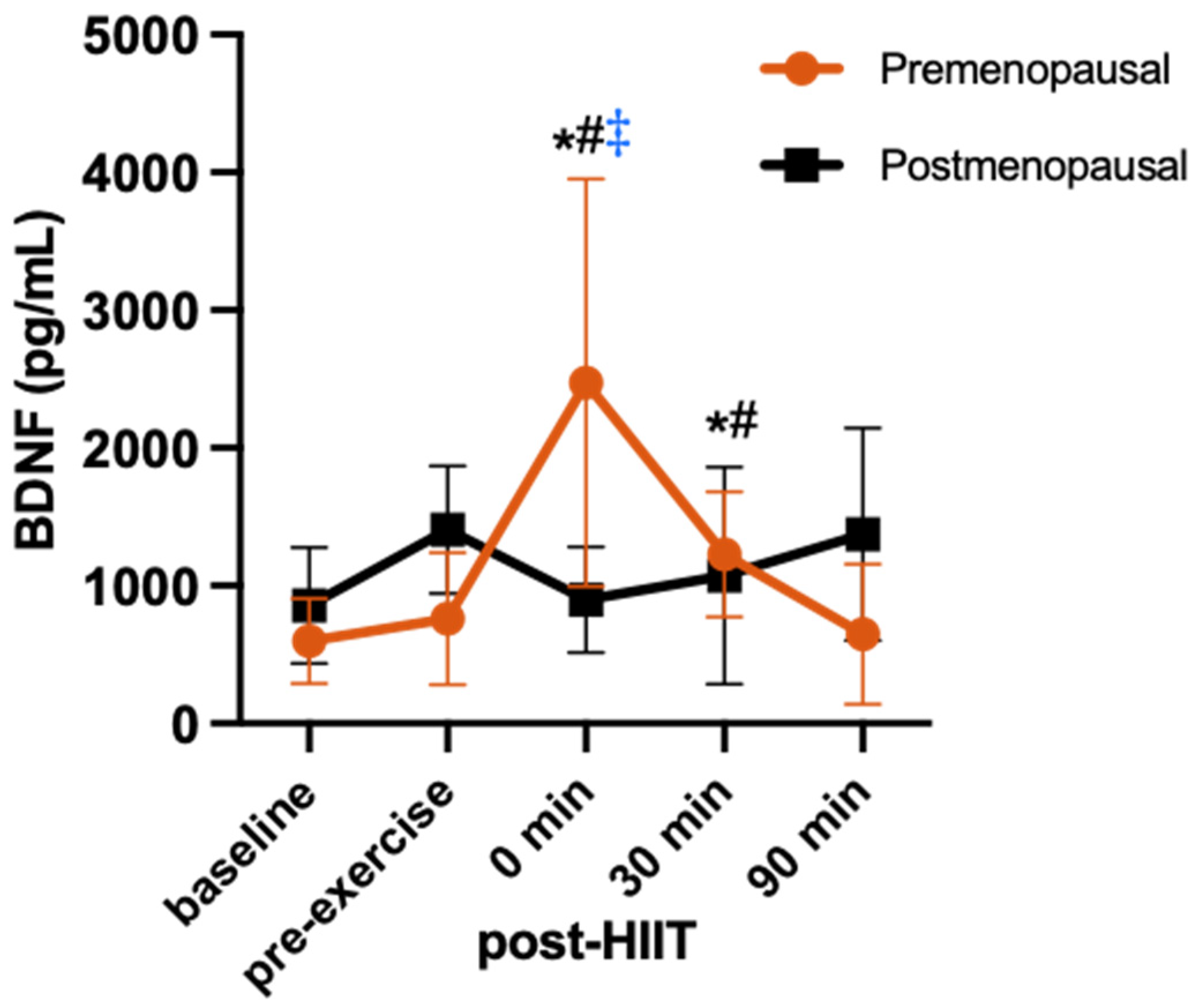
3.4. BDNF Response
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nelson, H.D. Menopause. Lancet 2008, 371, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Malutan, A.M.; Dan, M.; Nicolae, C.; Carmen, M. Proinflammatory and anti-inflammatory cytokine changes related to menopause. Menopause Rev. 2014, 13, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Pfeilschifter, J.; Köditz, R.; Pfohl, M.; Schatz, H. Changes in Proinflammatory Cytokine Activity after Menopause. Endocr. Rev. 2002, 23, 90–119. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, R.; Brown, C.; Puscheck, E.; Friedrich, E.; Slatopolsky, E.; Maggio, D.; McCracken, R.; Avioli, L.V. Effect of surgical menopause and estrogen replacement on cytokine release from human blood mononuclear cells. Proc. Natl. Acad. Sci. USA 1991, 88, 5134. [Google Scholar] [CrossRef] [PubMed]
- Sinatora, R.; Chagas, E.; Mattera, F.; Mellem, L.; de Oliveira dos Santos, A.; Pereira, L.; Aranão, A.; Landgraf Guiguer, E.; Cressoni Araujo, A.; Haber, J.; et al. Relationship of Inflammatory Markers and Metabolic Syndrome in Postmenopausal Women. Metabolites 2022, 12, 73. [Google Scholar] [CrossRef] [PubMed]
- Caiazzo, E.; Sharma, M.; Rezig, A.O.; Morsy, M.; Ialenti, A.; Pellicori, P.; Bruzzese, D.; Maffia, P.; Guzik, T.J. Inflammatory Cytokines and Risk of Developing Hypertension: A Systematic Review and Meta-Analysis. In Proceedings of the 19th World Congress of Basic & Clinical Pharmacology (WCP2023), Glasgow, Scotland, 2–7 July 2023. [Google Scholar] [CrossRef]
- Su, J.-H.; Luo, M.-Y.; Liang, N.; Gong, S.-X.; Chen, W.; Huang, W.-Q.; Tian, Y.; Wang, A.-P. Interleukin-6: A Novel Target for Cardio-Cerebrovascular Diseases. Front. Pharmacol. 2021, 12, 745061. [Google Scholar] [CrossRef] [PubMed]
- Feghali, C.A.; Wright, T.M. Cytokines in acute and chronic inflammation. Front. Biosci. 1997, 2, d12–d26. [Google Scholar] [CrossRef] [PubMed]
- Docherty, S.; Harley, R.; McAuley, J.J.; Crowe, L.A.N.; Pedret, C.; Kirwan, P.D.; Siebert, S.; Millar, N.L. The effect of exercise on cytokines: Implications for musculoskeletal health: A narrative review. BMC Sports Sci. Med. Rehabil. 2022, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.; Sun, Y.; Woods, J. Exercise and the Regulation of Inflammatory Responses. Prog. Mol. Biol. Transl. Sci. 2015, 135, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Azizbeigi, K.; Azarbayjani, M.A.; Atashak, S.; Stannard, S. Effect of Moderate and High Resistance Training Intensity on Indices of Inflammatory and Oxidative Stress. Res. Sports Med. 2015, 23, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, É.; Marinho, D.A.; Neiva, H.P.; Lourenço, O. Inflammatory Effects of High and Moderate Intensity Exercise—A Systematic Review. Front. Physiol. 2020, 10, 1550. [Google Scholar] [CrossRef] [PubMed]
- Zwetsloot, K.A.; John, C.S.; Lawrence, M.M.; Battista, R.A.; Shanely, R.A. High-intensity interval training induces a modest systemic inflammatory response in active, young men. J. Inflamm. Res. 2014, 7, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Kapilevich, L.V.; Zakharova, A.N.; Kabachkova, A.V.; Kironenko, T.A.; Orlov, S.N. Dynamic and Static Exercises Differentially Affect Plasma Cytokine Content in Elite Endurance- and Strength-Trained Athletes and Untrained Volunteers. Front. Physiol. 2017, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, W.J.; Ratamess, N.A. Hormonal Responses and Adaptations to Resistance Exercise and Training. Sports Med. 2005, 35, 339–361. [Google Scholar] [CrossRef] [PubMed]
- Fragala, M.S.; Kraemer, W.J.; Denegar, C.R.; Maresh, C.M.; Mastro, A.M.; Volek, J.S. Neuroendocrine-Immune Interactions and Responses to Exercise. Sports Med. 2011, 41, 621–639. [Google Scholar] [CrossRef] [PubMed]
- Bogdanis, G.C. Effects of Physical Activity and Inactivity on Muscle Fatigue. Front. Physiol. 2012, 3, 142. [Google Scholar] [CrossRef] [PubMed]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed]
- Pluchino, N.; Russo, M.; Santoro, A.N.; Litta, P.; Cela, V.; Genazzani, A.R. Steroid hormones and BDNF. Neuroscience 2013, 239, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Gyorkos, A.; Baker, M.H.; Miutz, L.N.; Lown, D.A.; Jones, M.A.; Houghton-Rahrig, L.D. Carbohydrate-restricted Diet and Exercise Increase Brain-derived Neurotrophic Factor and Cognitive Function: A Randomized Crossover Trial. Cureus 2019, 11, e5604. [Google Scholar] [CrossRef] [PubMed]
- Marston, K.J.; Newton, M.J.; Brown, B.M.; Rainey-Smith, S.R.; Bird, S.; Martins, R.N.; Peiffer, J.J. Intense resistance exercise increases peripheral brain-derived neurotrophic factor. J. Sci. Med. Sport 2017, 20, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, P.; Brassard, P.; Adser, H.; Pedersen, M.V.; Leick, L.; Hart, E.; Secher, N.H.; Pedersen, B.K.; Pilegaard, H. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp. Physiol. 2009, 94, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Reycraft, J.T.; Islam, H.; Townsend, L.K.; Hayward, G.C.; Hazell, T.J.; Macpherson, R.E.K. Exercise Intensity and Recovery on Circulating Brain-derived Neurotrophic Factor. Med. Sci. Sports Exerc. 2020, 52, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Barha, C.K.; Falck, R.S.; Davis, J.C.; Nagamatsu, L.S.; Liu-Ambrose, T. Sex differences in aerobic exercise efficacy to improve cognition: A systematic review and meta-analysis of studies in older rodents. Front. Neuroendocrinol. 2017, 46, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Sohrabji, F.; Miranda, R.C.; Toran-Allerand, C.D. Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc. Natl. Acad. Sci. USA 1995, 92, 11110–11114. [Google Scholar] [CrossRef] [PubMed]
- Solum, D.T.; Handa, R.J. Estrogen Regulates the Development of Brain-Derived Neurotrophic Factor mRNA and Protein in the Rat Hippocampus. J. Neurosci. 2002, 22, 2650–2659. [Google Scholar] [CrossRef]
- Begliuomini, S.; Casarosa, E.; Pluchino, N.; Lenzi, E.; Centofanti, M.; Freschi, L.; Pieri, M.; Genazzani, A.D.; Luisi, S.; Genazzani, A.R. Influence of endogenous and exogenous sex hormones on plasma brain-derived neurotrophic factor. Hum. Reprod. 2007, 22, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- El-Sayes, J.; Turco, C.V.; Skelly, L.E.; Nicolini, C.; Fahnestock, M.; Gibala, M.J.; Nelson, A.J. The Effects of Biological Sex and Ovarian Hormones on Exercise-Induced Neuroplasticity. Neuroscience 2019, 410, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Fowles, J.R.; O’Brien, M.W.; Wojcik, W.R.; d’Entremont, L.; Shields, C.A. A pilot study: Validity and reliability of the CSEP-PATH PASB-Q and a new leisure time physical activity questionnaire to assess physical activity and sedentary behaviours. Appl. Physiol. Nutr. Metab. 2017, 42, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Hagobian, T.A.; Braun, B. Physical activity and hormonal regulation of appetite: Sex differences and weight control. Exerc. Sport Sci. Rev. 2010, 38, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Elliott-Sale, K.J.; Minahan, C.L.; de Jonge, X.A.K.J.; Ackerman, K.E.; Sipilä, S.; Constantini, N.W.; Lebrun, C.M.; Hackney, A.C. Methodological Considerations for Studies in Sport and Exercise Science with Women as Participants: A Working Guide for Standards of Practice for Research on Women. Sports Med. 2021, 51, 843–861. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, S.F.; Leung, J.M.P.; Hazell, T.J. Is a verification phase needed to determine VO2max across fitness levels? Eur. J. Appl. Physiol. 2021, 121, 861–870. [Google Scholar] [CrossRef] [PubMed]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise TESTING and Prescription, 9th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Islam, H.; Townsend, L.K.; Hazell, T.J. Modified sprint interval training protocols. Part I. Physiological responses. Appl. Physiol. Nutr. Metab. 2017, 42, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: London, UK, 1988. [Google Scholar] [CrossRef]
- Cumming, G. Cohen’s d needs to be readily interpretable: Comment on Shieh (2013). Behav. Res. Methods 2013, 45, 968–971. [Google Scholar] [CrossRef] [PubMed]
- Kouvelioti, R.; Kurgan, N.; Falk, B.; Ward, W.E.; Josse, A.R.; Klentrou, P. Cytokine and Sclerostin Response to High-Intensity Interval Running versus Cycling. Med. Sci. Sports Exerc. 2019, 51, 2458–2464. [Google Scholar] [CrossRef]
- Karim, R.; Stanczyk, F.Z.; Hodis, H.N.; Cushman, M.; Lobo, R.A.; Hwang, J.; Mack, W.J. Associations between markers of inflammation and physiological and pharmacological levels of circulating sex hormones in postmenopausal women. Menopause 2010, 17, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Arent, S.M.; Senso, M.; Golem, D.L.; McKeever, K.H. The effects of theaflavin-enriched black tea extract on muscle soreness, oxidative stress, inflammation, and endocrine responses to acute anaerobic interval training: A randomized, double-blind, crossover study. J. Int. Soc. Sports Nutr. 2010, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Mezil, Y.A.; Allison, D.; Kish, K.; Ditor, D.; Ward, W.E.; Tsiani, E.; Klentrou, P. Response of Bone Turnover Markers and Cytokines to High-Intensity Low-Impact Exercise. Med. Sci. Sports Exerc. 2015, 47, 1495–1502. [Google Scholar] [CrossRef]
- Febbraio, M.A.; Pedersen, B.K. Muscle-derived interleukin-6: Mechanisms for activation and possible biological roles. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2002, 16, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Hackney, A.C.; Kallman, A.L.; Ağgön, E. Female sex hormones and the recovery from exercise: Menstrual cycle phase affects responses. Biomed. Hum. Kinet. 2019, 11, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Hojman, P.; Brolin, C.; Nørgaard-Christensen, N.; Dethlefsen, C.; Lauenborg, B.; Olsen, C.K.; Åbom, M.M.; Krag, T.; Gehl, J.; Pedersen, B.K. IL-6 release from muscles during exercise is stimulated by lactate-dependent protease activity. American Journal of Physiology. Endocrinol. Metab. 2019, 316, E940–E947. [Google Scholar] [CrossRef]
- Zaldivar, F.; Wang-Rodriguez, J.; Nemet, D.; Schwindt, C.; Galassetti, P.; Mills, P.J.; Wilson, L.D.; Cooper, D.M. Constitutive pro- and anti-inflammatory cytokine and growth factor response to exercise in leukocytes. J. Appl. Physiol. 2006, 100, 1124–1133. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, C.A.Z.; Sierra, A.P.R.; Martínez Galán, B.S.; Maciel, J.F.d.S.; Manoel, R.; Barbeiro, H.V.; de Souza, H.P.; Cury-Boaventura, M.F. Time Course and Role of Exercise-Induced Cytokines in Muscle Damage and Repair After a Marathon Race. Front. Physiol. 2021, 12, 752144. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.M.W.; Pedersen, B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Islam, H.; Neudorf, H.; Mui, A.L.; Little, J.P. Interpreting “anti-inflammatory” cytokine responses to exercise: Focus on interleukin-10. J. Physiol. 2021, 599, 5163–5177. [Google Scholar] [CrossRef] [PubMed]
- Kasapis, C.; Thompson, P.D. The effects of physical activity on serum C-reactive protein and inflammatory markers: A systematic review. J. Am. Coll. Cardiol. 2005, 45, 1563–1569. [Google Scholar] [CrossRef] [PubMed]
- Townsend, J.R.; Hoffman, J.R.; Fragala, M.S.; Jajtner, A.R.; Gonzalez, A.M.; Wells, A.J.; Mangine, G.T.; Fukuda, D.H.; Stout, J.R. TNF-α and TNFR1 responses to recovery therapies following acute resistance exercise. Front. Physiol. 2015, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Knaepen, K.; Goekint, M.; Heyman, E.M.; Meeusen, R. Neuroplasticity—Exercise-induced response of peripheral brain-derived neurotrophic factor: A systematic review of experimental studies in human subjects. Sports Med. 2010, 40, 765–801. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Abou Haidar, E.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R.; et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. eLife 2016, 5, e15092. [Google Scholar] [CrossRef] [PubMed]
- Fulgenzi, G.; Hong, Z.; Tomassoni-Ardori, F.; Barella, L.F.; Becker, J.; Barrick, C.; Swing, D.; Yanpallewar, S.; Croix, B.S.; Wess, J.; et al. Novel metabolic role for BDNF in pancreatic β-cell insulin secretion. Nat. Commun. 2020, 11, 1950. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.-H.; Chua, S.C., Jr. The Brain–Liver Connection Between BDNF and Glucose Control. Diabetes 2013, 62, 1367–1368. [Google Scholar] [CrossRef] [PubMed]
- Podyma, B.; Parekh, K.; Güler, A.D.; Deppmann, C.D. Metabolic Homeostasis via BDNF and its Receptors. Trends Endocrinol. Metab. 2021, 32, 488–499. [Google Scholar] [CrossRef]
- Rentería, I.; García-Suárez, P.C.; Fry, A.C.; Moncada-Jiménez, J.; Machado-Parra, J.P.; Antunes, B.M.; Jiménez-Maldonado, A. The Molecular Effects of BDNF Synthesis on Skeletal Muscle: A Mini-Review. Front. Physiol. 2022, 13, 934714. [Google Scholar] [CrossRef]
- Matthews, V.B.; Aström, M.-B.; Chan, M.H.S.; Bruce, C.R.; Krabbe, K.S.; Prelovsek, O.; Akerström, T.; Yfanti, C.; Broholm, C.; Mortensen, O.H.; et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia 2009, 52, 1409–1418. [Google Scholar] [CrossRef]
- Vaynman, S.; Ying, Z.; Gomez-Pinilla, F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur. J. Neurosci. 2004, 20, 2580–2590. [Google Scholar] [CrossRef]
- Mazo, C.E.; Miranda, E.R.; Shadiow, J.; Vesia, M.; Haus, J.M. High Intensity Acute Aerobic Exercise Elicits Alterations in Circulating and Skeletal Muscle Tissue Expression of Neuroprotective Exerkines. Brain Plast. 2022, 8, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Wiet, R. The Effects of Acute Aerobic Exercise on BDNF Levels and Cognition in Postmenopausal Women; Cleveland State University: Cleveland, OH, USA, 2018; Available online: https://etd.ohiolink.edu/acprod/odb_etd/etd/r/1501/10?clear=10&p10_accession_num=csu1527873335485353 (accessed on 17 June 2024).
- Berchtold, N.C.; Kesslak, J.P.; Pike, C.J.; Adlard, P.A.; Cotman, C.W. Estrogen and exercise interact to regulate brain-derived neurotrophic factor mRNA and protein expression in the hippocampus. Eur. J. Neurosci. 2001, 14, 1992–2002. [Google Scholar] [CrossRef] [PubMed]
- Vanderheyden, L.W.; McKie, G.L.; Howe, G.J.; Hazell, T.J. Greater lactate accumulation following an acute bout of high-intensity exercise in males suppresses acylated ghrelin and appetite postexercise. J. Appl. Physiol. 2020, 128, 1321–1328. [Google Scholar] [CrossRef] [PubMed]


| Premenopausal Females (n = 8) | Postmenopausal Females (n = 7) | |
|---|---|---|
| Age (years) | 44 ± 3 ‡ | 57 ± 2 ‡ |
| BMI (kg/m2) | 28 ± 5 | 28 ± 2 |
| VO2max (mL/kg/min) | 29.2 ± 7.4 | 30.2 ± 6.5 |
| Estradiol (pmol/L) | 137.3 ± 31.9 ‡ | 56.7 ± 19.2 ‡ |
| Progesterone (nmol/L) | 19.7 ± 4.8 ‡ | 0.60 ± 0.27 ‡ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannopoulos, A.J.; Mohammad, A.; Retsidou, M.I.; Tucker, J.A.L.; Bornath, D.P.D.; McCarthy, S.F.; MacPherson, R.E.K.; Hazell, T.J.; Klentrou, P. Differences in Exercise-Linked Biomarkers between Premenopausal and Postmenopausal Middle-Aged Females. Endocrines 2024, 5, 290-303. https://doi.org/10.3390/endocrines5030021
Giannopoulos AJ, Mohammad A, Retsidou MI, Tucker JAL, Bornath DPD, McCarthy SF, MacPherson REK, Hazell TJ, Klentrou P. Differences in Exercise-Linked Biomarkers between Premenopausal and Postmenopausal Middle-Aged Females. Endocrines. 2024; 5(3):290-303. https://doi.org/10.3390/endocrines5030021
Chicago/Turabian StyleGiannopoulos, Anthony J., Ahmad Mohammad, Maria I. Retsidou, Jessica A. L. Tucker, Derek P. D. Bornath, Seth F. McCarthy, Rebecca E. K. MacPherson, Tom J. Hazell, and Panagiota Klentrou. 2024. "Differences in Exercise-Linked Biomarkers between Premenopausal and Postmenopausal Middle-Aged Females" Endocrines 5, no. 3: 290-303. https://doi.org/10.3390/endocrines5030021






