Abstract
Adrenocortical carcinoma (ACC) and pheochromocytoma (PCC) are malignancies originating from distinct layers of the adrenal gland. ACCs arise from the adrenal cortex, are often detected at advanced stages and are associated with poor prognosis. PCCs are mostly benign, arise from the adrenal medulla and have a variable prognosis, with 10% of PCCs resulting in metastasis. Genetic background strongly influences metastasis of PCCs, and no reliable biomarkers that predict metastatic behavior exist to date. Current therapeutic strategies for both ACCs and PCCs are overall limited. Thus, novel preclinical models and drug screening approaches need to be established to aid in the identification of more promising drugs and treatment schemes. In this review, we summarize the currently available human and murine cell lines for both tumor entities.
Keywords:
adrenocortical carcinoma; pheochromocytoma; cell lines; human; murine; NCI-H295; MUC-1; TVBF-7; C CU-ACC1; CU-ACC2; Jil-2266 1. Introduction
The adrenal gland is composed of the steroid hormone-secreting cortex and the catecholamine-secreting medulla (Figure 1). Adrenal-derived hormones are essential to regulate vital functions of the organism, including stress response, blood pressure and metabolism. Various benign and malignant tumors can originate from the adrenals, among them the highly malignant adrenocortical carcinoma (ACC) arising from the secretory cells of the adrenal cortex with an incidence rate of 0.5–2 cases per million and pheochromocytomas (PCCS) originating from the chromaffin cells of the adrenal medulla with an incidence rate of 0.8 cases per million yearly [].
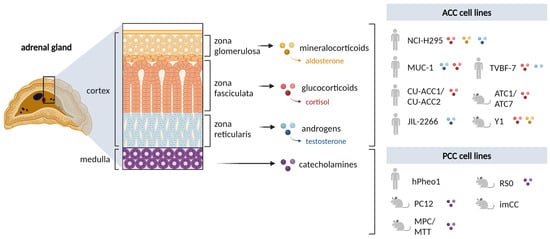
Figure 1.
Overview of the adrenal gland and the human and rodent in vitro cell lines available. Profiles for mineralocorticoids, glucocorticoids and androgen secretion by each cell line as reported to date. NCI-H295 includes all sub-strains. Created with BioRender.com.
ACC is often diagnosed at advanced and metastatic stages. While surgical resection is the treatment of choice, the high number of recurrences and advanced staging often require systemic treatment strategies. Unfortunately, these remain inefficient in many cases, resulting in a 5-year survival rate of less than 44% []. For a long time, preclinical research was hampered due to the lack of appropriate in vitro models. However, currently, the field is rapidly progressing with six cell lines derived from human ACC which better reflect the complexities of this aggressive disease.
PCCs are mostly benign, with a malignancy rate of around 10%, strongly dependent on the genetic background/molecular cluster. Left untreated, these tumors can lead to life-threatening emergencies due to high catecholamine release and cardiovascular comorbidities []. Metastasis occurs in less than 15% of patients, whereas the rate of tumor recurrence after resection is 6–16% and correlates with primary tumor size and genetic background [,]. Nevertheless, all PCCs are considered to have the ability to spread to distant sites since no reliable predictors for metastatic behavior exist to date []. Surgical resection is the first-line therapy against PCCs, while chemotherapies, radionuclide therapies, and tyrosine kinase inhibitors are used to treat metastatic cases with reasonable but limited efficiency []. The availability of a greater variety of preclinical cell line models, especially from human tumors, would significantly support the discovery of new biomarkers and novel therapeutic strategies. However, human cell lines for PCCs remain unavailable.
2. Adrenal Cortex
2.1. Rodent Cell Lines
In 1966, the first rodent model of ACC was the Y1 mouse cell line established from a mouse exposed to radiation []. These cells have an epithelial-like morphology, are unresponsive to ACTH stimulation, have a partially functional steroid biosynthesis pathway and produce corticosterone and aldosterone [,]. For many years, Y1 cells were the only available in vitro adrenal model [,,,,]. Later, ATC1 [] and ATC7 [] were generated from adrenocortical tumors of transgenic mice expressing SV40-Tag under the akd1b7 (aldo-keto reductase 1B7) promoter. Both cell lines synthesize and secrete corticosterone in response to ACTH and prolactin (ATC1 only). Additionally, both cell lines exhibit the phenotype of zona fasciculate cells and, therefore, they are mostly used in mechanistic and functional studies of the adrenal [,,,]. For example, Fudulu et al. investigated how immune cells influence steroidogenic gene expression [], while Hazel et al. explored pulsatile ACTH stimulation of steroidogenic gene expression []. Most recently, ATC7 was used in a study on the role of HOX genes in the formation of ACC []. Thus, these recent cell lines have been primarily used to understand the regulation of the steroidogenic pathway.
Mouse cell line models of ACC have been crucial to adrenal cancer research, particularly since Y1 was introduced some five decades ago when human primary cells were not available. Nonetheless, differences exist between rodent and human adrenal cells. For example, cytochrome P450 17A1 (CYP17A1), a crucial enzyme in the production of glucocorticoids and sex steroids, is differentially expressed between humans and rodents [,,,,]. Due to variations in the expression of CYP17A1 in rodents (ranging from completely absent to lowly expressed), cortisol and androgens are hardly produced in the adrenals of mice and rats. Therefore, cortisol is the most abundant glucocorticoid in humans, while corticosterone is predominant in rodents []. As for androgens, unlike human adrenals, which produce and secrete sex steroids and vast amounts of androgen precursors (DHEA, DHEAS) [], adrenal androgen production in adult mice and rats is rather low/non-existent and highly strain-dependent [,,]. This variation has led to the debatable view that adult mice do not have a comparatively functional zona reticularis to adult humans []. Although CYP17A1 can be expressed in Y1 cells treated with compounds that target DNA methylation, androgen precursors could not be detected []. Consistently, in vitro rodent cell line models of ACC are devoid of the capability to secrete androgens, as mentioned above. In contrast, ACCs synthesize and secrete various steroid hormones and precursor metabolites from the steroidogenic pathway, alone or in combination [,,,]. In fact, steroid profiling by LC-MS/MS (liquid chromatography tandem mass spectrometry) or GC-MS (gas chromatography–mass spectrometry) in either serum or urine, respectively, can be a tool to discriminate between benign or malignant disease as well as detect recurrent or progressive disease [,,,]. As will be discussed next (also depicted in Figure 1), human cell lines of ACC reflect many of these characteristics by secreting various combinations of glucocorticoids, mineralocorticoids or androgens and numerous steroid precursors.
2.2. Human Cell Lines
NCI-H295. NCI-H295 was the first human adult ACC cell line established from a primary ACC surgically removed from a female patient in 1980 and subsequently reported in 1990 by Gazdar and colleagues (Figure 2) []. Over the years, different sub-strains from the original NCI-H295 cells were generated by modulating growth conditions, the most commonly used being H295R and H295A, H295RA, HA13, HAC15, and HAC50. Growth condition and passage number are highly relevant for the identity of NCI-H295 since the ability of drugs to affect cell survival varies according to these two parameters []. Of note, unlike the parental clone, the resulting sub-strains grow in adhesion []. Interestingly, HAC13, HAC-15, and HAC50 display high responsiveness to Angiotensin II and K+ stimulation as well as a modest ACTH response with an increase in steroidogenic enzyme expression []. Over the years, NCI-H295 became the gold-standard model in preclinical ACC research, as extensively reviewed in [,,]. As demonstrated already in the original publication, this cell line produces all major adrenal hormones []. Most recent studies elucidated the transcriptome profile of this cell line when exposed to different cortisol biosynthesis inhibitors and identified new compounds as Cyp11B1- and 2-inhibitors [,]. Additionally, NCI-H295R cells were used to understand the role of the Cn/NFATC4 pathway in CYP11B2 expression and aldosterone synthesis []. Another highly interesting in vitro and in vivo study uncovered how oncogenic drivers use tissue-specific partners to regulate ACC subtype differentiation and that these can be reversed by pharmacologic inhibition of EZH2 [].
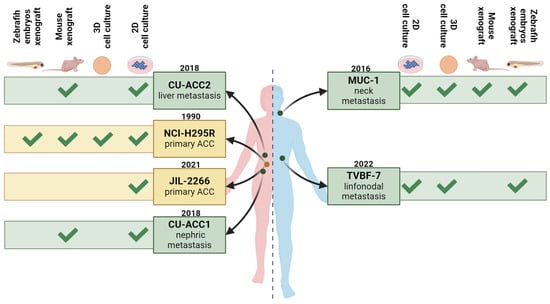
Figure 2.
Overview of the currently available human adrenocortical carcinoma cell lines. The pink semi-silhouette represents the female sex, while the blue semi-silhouette represents the male sex. Cell lines deriving from primary ACC are indicated in dark yellow, while those from metastatic disease are indicated in green. Figure created with BioRender.
The tumorigenic activity of NCI-H295 cells was demonstrated for the first time in the original publication by the injection of NCI-H295 cells and subsequent successful engraftment of the xenografts in athymic nude mice []. The ability of these cells to grow in murine xenograft models has since been widely used, as exemplified here [,,,,,]. However, consistent with its origin, the NCI-H295 cell line does not form spontaneous metastases in murine models. Although not a true model of spontaneous metastatic ACC, nonetheless, the study of Morin and colleagues is of special interest, as they created a murine xenograft model of metastatic adrenocortical carcinoma via intrasplenic grafting of NCI-H295R cells in nude mice []. The lack of spontaneous metastatic activity of NCI-H295R was also recently confirmed in a xenograft model in Danio rerio embryos [,]. However, Ruggiero and colleagues demonstrated that overexpression of Steroidogenic Factor-1 (SF-1) in NCI-H295R increases their invasive capacity in vitro and in the Danio rerio xenograft model. Invasiveness is reduced following the inactivation of fascin (FASCN1), identifying this actin-binding protein as a promising molecular target in ACC []. The establishment of new ACC cell lines and patient-derived xenografts (PDXs) originating from metastatic diseases (see below) has allowed us to overcome model limitations regarding metastatic properties and disease, as will be outlined in more detail below.
NCI-H295 cells are characterized by a large deletion in the TP53 gene [,,,] and by an activating CTNNB1 mutation [,,] (Table 1). Nicolson et al. published more recently a comprehensive analysis of the mutational landscape of NCI-H295 based on whole-exome sequencing []. From a pharmacological point of view, NCI-H295 cells are sensitive to mitotane [,,]. However, as recently demonstrated by Dedhia et al. [], NCI-H295R spheroid constructs encapsulated in a 3D hydrogel scaffold exhibit reduced sensitivity to mitotane and the first-line chemotherapy agents etoposide, doxorubicin and cisplatin (EDP). Furthermore, these NCI-H295R constructs had increased cortisol synthesis []. Therefore, the NCI-H295 cell line is extremely useful in such studies aimed at investigating the mechanism of mitotane [] or to evaluate newer formulations of this adrenolytic drug [,]. For over 25 years, NCI-H295 and its derivatives have been and still remain the gold standard human ACC cell model. However, nowadays, NCI-H295 cells are rarely used as the sole preclinical model to identify new potential drug targets of ACC [,,,,], and indeed, they are more and more implemented together with the next-generation models, outlined in the following sections.

Table 1.
Currently identified mutation of ACC cell lines (WT; wild type).
MUC-1. The first adult human metastatic ACC model was developed by Hantel and colleagues and reported in 2016 as MUC-1 (Figure 2) []. The tumor sample originated from a neck metastasis of a male patient in progression following treatment with the clinical gold standard for advanced and metastatic disease EDP plus mitotane (EDP-M). The model was originally established as both patient-derived tissue xenograft-(PDTX) and as a cell line. Consistent with the characteristics of the original patient tumor, the model was reported from the beginning to be extremely drug-resistant against EDP-M in vitro [] as well as against IGF1-receptor inhibition and liposomal doxorubicin in vivo []. Further pharmacological analysis revealed broad drug resistance against various chemotherapeutic drugs commonly applied in clinical studies but also phytochemicals, targeted therapeutic drugs and combinatorial regimens, outlining for this cell line high drug resistance against pharmacotherapy [,,,,,,,,,,,]. This feature renders MUC-1 very useful in pharmacological investigations since the identification of effective therapies in ACC in the context of advanced and multidrug-resistant disease remains challenging. Interestingly, MUC-1 cells, together with NCI-H295R cells, were recently used in the development and validation of a high-throughput multi-well plate-based format for multidimensional cell modeling for different endocrine cells and tissue sources []. In another recent study, Fei et al. co-cultured MUC-1 and NCI-H295R cells with adipose stem cells (ASCs) []. By comparing the results from these two co-cultures, one can observe quite different interplays between tumor cells and adipose tissue resulting from primary and metastatic ACC []. Additionally, at a metabolic level, MUC-1 cells display different mechanisms for cholesterol and lipid droplet handling than NCI-H295R cells. In particular, in MUC-1 cells, the lipid droplets contain triacylglycerol with low amounts of cholesteryl ester. Since the effectiveness of mitotane has been attributed to the toxic intracellular accumulation of free cholesterol, this difference was proposed as part of the mechanism of resistance to mitotane in MUC-1 cells []. Furthermore, it has been demonstrated that pharmacological modulation of the estrogen-related alpha receptor-in variance to NCI-H295R does not cause a reduction in ATP consumption in MUC-1 but increases glycolysis activity, indicating increased cellular plasticity at the metabolic level [].
From a genetic point of view, a somatic deletion/frameshift mutation was found in the TP53 gene, while the gene encoding for β-catenin, CTNNB1, reflects wildtype [,,] (Table 1). This feature made this cell line suitable for testing the activity of Polo/Like kinase 1 inhibitors (PLK1i), which have been shown to be more effective in tumors with TP53 mutation []. Indeed, Warmington and colleagues tested two PLK1i (rigosertib, a multi-target inhibitor, and poloxin, a new specific PLK1i) in a panel of human ACC cell lines comprised of cells with mutated or wild-type TP53 []. The response of this cell line to both compounds was poor, but it should be underlined that although MUC-1 cells have a mutation in the TP53 gene, the expression of the target (PLK1) was demonstrated to be poor. It can be furthermore cautiously speculated, upon evaluation of the activity of caspases 3/7 together with the proliferation and cell viability data, that upon treatment, the remaining MUC-1 cells might switch to a state of quiescence instead of undergoing cell death []. The same study also reported for MUC-1 a nonsense mutation in the ATRX gene, which is a central player in the regulating of DNA damage response and chromosomal stability []. In another recent study, Kerdivel et al. evaluated the molecular driver of DNA hypermethylation in ACC and the usefulness of DNA demethylating agents []. The results of this study revealed a low CpG island methylator phenotype for MUC-1 cells and a weak response to the demethylating agent.
In line with the patient’s clinical report, the MUC-1 cell line presents a comparably low but significant and inducible steroidogenic activity; however, high cellular plasticity maintains dynamic modulability. Thus, it is interesting to note an increased androgenic activity following forskolin exposure, with an abundant production of androgens, accompanied by an augmented gene expression of essential steroidogenic enzymes, androgen receptors and gonadotropin-releasing hormone receptors []. Moreover, a significant cortisol secretion was reported in vivo []. Recently, MUC-1 cells were reported to spontaneously form distant metastases in Danio rerio embryos three days after xenotransplantation into the zebrafish yolk sac [,]. In contrast, metastases were not observed in embryos xenografted with NCI-H295R. Interestingly, the number of metastasis-positive MUC-1 embryos was reduced following progesterone or trabectedin treatments [,], two drugs whose in vitro effects on ACC have already been demonstrated [,,]. The results of the divergent metastatic capacities are in line with other recent findings reported on the activity and modulability of the CXCL12/CXCR4 axis in these two cell lines [].
CU-ACC1 and CU-ACC2. Two cell models were established in 2018 from metastatic ACC in female patients, reported as CU-ACC1 and CU-ACC2 (Figure 2). The CU-ACC1 cells originate from a perinephric ACC metastasis, while CU-ACC2 cells were derived from a liver recurrence in a patient with Lynch syndrome with non-secreting primary ACC treated with mitotane []. Both models were established as PDTX in athymic nude mice as well as cell lines using ROCK inhibitor and feeder cells. Moreover, the CU-ACC2 PDX was used to set up the first humanized ACC PDTX mouse, aiming to elucidate the effects of immunotherapy in an ACC tumor microenvironment [].
Secretome analysis revealed that CU-ACC1 produces higher levels of cortisol compared to NCI-H295R cells. In contrast, CU-ACC2 cells secrete only small amounts of cortisol, in line with the marginal gene expression of CYP11B1. Following stimulation with forskolin, CU-ACC1 increased cortisol secretion, while CU-ACC2 is refractory to cAMP-dependent stimuli []. Although CU-ACC2 is, thereby, defined as a rather low steroidogenic cell line, Weigand and colleagues detected elevated CYP11A1 expression and response to ketoconazole, a drug used in this study to inhibit steroidogenesis and reverse the effects of a ferroptosis inducer []. As reported by the authors, these data support the idea that this cell line has the characteristics of steroidogenic cells despite very low steroid production.
From a genetic point of view, a G34R mutation within the ubiquitination recognition motif of the protein predicts a gain-of-function mutation in the CTNNB1 gene for CU-ACC1 (Table 1) []. In CU-ACC2, a predicted loss-of-function mutation for the TP53 gene is reported, as well as with a heterozygous deletion of exons 1–6 of the MSH2 gene, in line with the clinically reported Lynch syndrome (Table 1) []. CU-ACC1, representing a wild type model for TP53, was also implemented in the study relating to PLK1i already mentioned, proving to be poorly/not sensitive to the inhibition of this target []. Finally, these models have been implemented in several studies with the aim of finding potential pharmacological targets for ACC, in particular, Maternal Embryonic Leucine Zipper Kinase (MELK) [], mitotic PDZ-binding kinase (PBK) [] and Aurora kinase inhibition with blockade of the Wnt/β-catenin pathway [].
JIL-2266. JIL-2266 cells were derived from a primary ACC in a female patient with severe Cushing’s syndrome and androgen excess who had undergone treatment with mitotane []. This cell line was first reported in 2021 and represents the second adult human primary ACC-derived cell line available today (Figure 2). The cell line is characterized by a high mutational burden, consistent with the reported germline mutation in the MUTYH gene in the donor patient. Moreover, a hemizygous stop-gain mutation in the TP53 gene and a somatic nucleotide variant in the ZNRF3 gene were reported (Table 1). In line with the clinical history, JIL-2266 cells were reported to be insensitive to mitotane. SF-1 expression analysis demonstrated an intermediate-to-low positivity for these cells, subsequently confirmed by Ruggiero and colleagues []. Endocrine characterization revealed medium- and passage-dependent adrenal hormone production. Cortisol production was not detected under any conditions [].
TVBF-7. TVBF-7 cells, derived from a male ACC patient in progression after EDP-M therapy, are the most recently introduced ACC cell line (Figure 2) []. Endocrine characterization in direct comparison to NCI-H295R and MUC-1 showed that the glucocorticoid axis is highly active in TVBF-7 with abundant cortisol secretion. Moreover, TVBF-7 cells are unresponsive to ACTH and FSK stimulation, indicative of an autonomous glucocorticoid secretion profile. []. Mutational analysis revealed a non-sense APC mutation, suggestive of potentially altered Wnt/β-Catenin signaling in TVBF-7 cells, a pathway often affected in ACC. As mentioned above, other ACC cell models harbor CTNNB1 mutations; however, an APC mutation has only been identified in TVBF-7 cells so far (Table 1) [].
This cell line was established from a primary culture initially referred to as ACC115m []. In this study by Rossini et al., TVBF-7 cells were found to express low levels of both the nuclear progesterone receptor (PgR) and the nuclear estrogen receptors ERα and Erβ. In line with decreased PgR expression, TVBF-7 are moderately sensitive to progesterone, which has cytotoxic activity in other ACC cell models. Interestingly, exposure of TVBF-7 to progesterone does not induce apoptosis as observed in NCI-H295R and MUC-1 but activates autophagic cell death []. Abate et al. later demonstrated that this cell model maintains the expression of the pocket proteins, a group of tumor-suppressing proteins composed of retinoblastoma proteins and P107 and P130 []. From a pharmacological point of view, the authors also demonstrated low sensitivity to mitotane, in line with the patient’s clinical history. As already mentioned, xenografts of different ACC cell lines were recently achieved in Danio rerio embryos in order to assess the effect of trabectedin and progesterone on the formation of metastasis. While NCI-H295R could not metastasize, besides MUC-1, TVBF-7 also had metastatic ability in this in vivo preclinical model. Interestingly, the two cell lines formed metastases in different anatomical regions of the embryo, particularly the pericardial zone for TVBF-7 and the tail for MUC-1 cells, reflecting again important characteristics of the original patient material obtained from local and distant metastases, respectively. Additionally, both cell lines secrete metalloproteinase-2 (MMP-2) in vitro, whose expression is known to correlate with ACC aggressiveness [,,]. In direct comparison with NCI-H295R and MUC-1, TVBF-7 was also shown to form tumor spheroids in a high-throughput multi-well format, thereby also providing interesting features for 3D modeling in vitro [].
At the transcriptional level, ACC can be divided into two molecular subgroups, C1A and C1B, depending on the molecular signature [,]. For example, the C1A group is enriched in transcriptional and mitotic cell cycle genes, while C1B is enriched in cell metabolism genes. Moreover, most tumors within the C1A subgroup exhibit a steroid phenotype []. Interestingly, in a recent study, TVBF-7, NCI-H295R and MUC-1 were classified in direct comparison to patient samples according to this molecular subgrouping. Based on their specific molecular signature and in line with their steroidogenic profiles, TVBF-7 and NCI-H295R cells were allocated to the C1A subgroup, while MUC-1 cells were classified to the C1B subgroup. Interestingly, exposure to forskolin or mitotane led to dynamic shifts on the C1B to C1A axis []. In sum, over the last years, the subsequently established panel of human ACC cell lines with their different characteristics (from primary tumor to metastatic origin), varying mutational status in essential genes and therapeutic sensitivities improved the preclinical landscape and allowed for an increasingly complex preclinical modeling of this disease.
3. Adrenal Medulla
3.1. Rodent Cell Lines
PC12. Although PCCs are very rare in rodents, they do occur spontaneously in response to chemical or radiation stimuli []. PC12 was the first rodent cell line derived in 1976 from adrenal PCC in an irradiated rat []. The line was difficult to establish since the cells did not readily adhere to tissue culture dishes and, therefore, were passaged multiple times in collagen-coated plates. PC12 cells synthesize and store the catecholamines norepinephrine and dopamine but not epinephrine. Additionally, these cells have the unique ability to develop neurites upon nerve growth factor (NGF) stimulation and upon withdrawal of the growth factor, the neurites degenerate and cell division continues. Therefore, PC12 cells are used in both adrenal functional studies as well as in developmental neurobiology [,,,,,]. Their phenotype and morphology resembles adrenal chromaffin cells and can be maintained over multiple generations. In preclinical studies, PC12 cells were used in mouse xenografts to determine the efficacy of radiotherapy [] and receptor tyrosine kinase inhibitors sunitinib and sorafenib [].
MPC/MTT. Mouse pheochromocytoma (MPC) refers to six cell lines derived from adrenal PCCs from different heterozygous NF1 knockout mice, with and without irradiation []. The cell lines exhibit various morphologies, from primitive progenitors to more differentiated chromaffin cells, and express cellular markers accordingly. Additionally, five out of six lines show basal and dexamethasome-stimulated expression of phenylethanolamine N-methyltransferase (PNMT), the enzyme responsible for converting norepinephrine to epinephrine. The cell lines have chromosomal abnormalities and express developmental genes from the nervous system []. Transcriptome analysis shows that the cell lines have distinct gene expression signatures between each other and normal adrenal chromaffin cells []. Following the xenograft of MPC cell lines, one MPC-derived liver tumor in mice was cultured in vitro and gave rise to the more aggressive MTT (mouse tumor tissue-derived) cell line []. MTT cells grow as clusters in 3D structures and are often cultured as spheroids. In mechanistic studies, migration of SDHB-deficient MTT cells grown as monolayers [] or spheroids [] was significantly enhanced in co-cultures with lactate-secreting cancer-activated fibroblasts. Both MPC and MTT have been used extensively to investigate signaling pathways and the action of therapeutic agents [,,,,,,,,,,]. Additionally, the cell lines were used in studies in vitro and in vivo (mouse) to establish immunotherapy markers [] and immunotherapeutic approaches against PCCs [].
RS0. RS0 cells originated from an irradiated rat heterozygous for SDH []. These cells also grow as spheroids and require low to no serum or the addition of stem cell growth factors. Similar to human SDH-deficient PCCs, this cell line produces dopamine and low levels of norepinephrine. The cell line is well characterized at the genomic, transcriptomic and metabolomic levels [].
imCC. imCC (immortalized mouse chromaffin cells) originate from a conditional SDHBlox/lox knockout mouse []. This cell line was developed by maintaining the adrenal medulla cells long-term in culture until proliferation was observed. The proliferating cells were then transduced with adenovirus-Cre, which rendered these cells homozygous null for SDHB. They are characterized by fast migratory capabilities, mesenchymal appearance and a phenotype/genotype similar to an epithelial-to-mesenchymal transition (ETM), which is in contrast to mature chromaffin cells []. imCC, along with MPC/MTT (monolayer and spheroid cultures) and fresh human patient-derived tumor primary cultures, were used together to assess the synergistic potential of tumor therapies [].
3.2. Human Cell Lines
Human chromaffin cells show little/no proliferation, rendering in vitro models for chromaffin cells as well as for PCCs difficult to establish. Initially, two cell lines were created from benign human PCCs, KNA and KAT45 [,]. Both cell lines expressed markers of chromaffin cells and could release catecholamines. However, these lines have not been used for tumor research in recent years []. The only available human PCC to date, known as hPheo1, was derived from an adrenal PCC and immortalized using human telomerase reverse transcriptase (hTERT) []. Culturing these cells in the presence of growth factors stimulates the expression of chromaffin cellular markers such as chromogranin-A together with PNMT, the only enzyme expressed from the catecholamine pathway. Moreover, the cells resemble mesenchymal, but not chromaffin, cells and differ at the genomic level from the original tumor. However, in transcriptomic comparisons with the original tumor and normal adrenal, hPheo1 does cluster with the original tumor, suggesting that it is more tumor-like than wild-type adrenal cells. hPheo1 cells have been used to understand the mechanisms involved in tumor development. For example, succinate dehydrogenase subunit B (SDHB) is an enzyme involved in mitochondrial bioenergetics and frequently mutated in metastatic PCC [,]. Knockdown of SDHB caused the cells to metabolize glutamine as a primary fuel source, decreased extracellular adhesion of the cells and significantly increased their proliferation []. Additionally, in hPeo1-SDHB-knockdown cells, membrane potential contributes to the aggressive migratory behavior of the cells, which can be normalized by treatment with glibenclamide, a specific potassium channel inhibitor []. Therefore, as the only human cell line available for PCC, hPeo1 cells play a role in understanding tumor progression. It is worth mentioning that, due to the lack of reliable human cell line models, fresh patient-derived PCC and paraganglioma primary cultures have been established in order to perform multiple drug testing and correlate drug responsiveness of the individual patient-derived tumor primary cultures with the genetic background [,,].
4. Conclusions
Research on ACC and PCC are hot topics, particularly for the identification of biomarkers of tumor progression and effective therapies. Over the past decade, the development of new human ACC cell lines derived from both primary and metastatic diseases have prompted remarkable progress over the past in basic and translational research. Moreover, the recently established Danio rerio xenograft model has become a useful new tool for rapid drug screening. This model allows not only for the evaluation of drug effect on tumor growth but also on the mechanisms underlying the metastatic process, as the two metastasis-derived cell models retain the ability to form distal (MUC-1) and local (TVBF-7) metastasis in the embryo in contrast to the primary-derived cells (NCI-H295R), which remain confined to the injection site. If vast progress has been made in ACC; unfortunately, the same thing cannot be said for PCC to date. Indeed, the unmet need for the development of cellular models of human PCC remains open. However, although there is a lack of human PCC cell lines, several studies have been conducted using large panels of patient-derived primary cultures for extended culture periods [,]. Finally, a 3D in vitro model termed adrenoid, consisting of human ACC cells (NCI-H295R cells) and murine PCC cells (MTT cells), was recently generated []. This adrenoid, together with the other co-culturing models [,,], represents a valuable tool and gives a view into the future of next-generation modeling within adrenal research.
Author Contributions
Conceptualization, C.H.; writing—original draft preparation, E.L., A.A., K.W. and C.H.; writing—review and editing, E.L., A.A., K.W., F.B., S.S., S.B., S.N. and C.H.; project administration, C.H. and S.N.; funding acquisition, C.H., E.L., S.N., K.W., F.B. and S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was kindly supported by the “Swiss 3R Competence Centre” (to C.H. and E.L.), by the Deutsche Forschungsgemeinschaft (DFG) within the CRC/Transregio 205/1 to S.B., K.W., F.B., and S.N. and by the University Medicine Zurich through the Immuno-TargET project.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fassnacht, M.; Assie, G.; Baudin, E.; Eisenhofer, G.; De La Fouchardiere, C.; Haak, H.R.; de Krijger, R.; Porpiglia, F.; Terzolo, M.; Berruti, A.; et al. Adrenocortical carcinomas and malignant phaeochromocytomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1476–1490. [Google Scholar] [CrossRef] [PubMed]
- Sedlack, A.J.H.; Hatfield, S.J.; Kumar, S.; Arakawa, Y.; Roper, N.; Sun, N.Y.; Nilubol, N.; Kiseljak-Vassiliades, K.; Hoang, C.D.; Bergsland, E.K.; et al. Preclinical Models of Adrenocortical Cancer. Cancers 2023, 15, 2873. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, C.; Fazeli, S.; Roman-Gonzalez, A. Antiangiogenic therapies for pheochromocytoma and paraganglioma. Endocr. Relat. Cancer 2020, 27, R239–R254. [Google Scholar] [CrossRef] [PubMed]
- Nolting, S.; Bechmann, N.; Taieb, D.; Beuschlein, F.; Fassnacht, M.; Kroiss, M.; Eisenhofer, G.; Grossman, A.; Pacak, K. Personalized Management of Pheochromocytoma and Paraganglioma. Endocr. Rev. 2022, 43, 199–239. [Google Scholar] [CrossRef] [PubMed]
- Press, D.; Akyuz, M.; Dural, C.; Aliyev, S.; Monteiro, R.; Mino, J.; Mitchell, J.; Hamrahian, A.; Siperstein, A.; Berber, E. Predictors of recurrence in pheochromocytoma. Surgery 2014, 156, 1523–1527; discussion 1527–1528. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.K. Update on Adrenal Tumours in 2017 World Health Organization (WHO) of Endocrine Tumours. Endocr. Pathol. 2017, 28, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Kloos, S.; Remde, H.; Dischinger, U.; Pamporaki, C.; Timmers, H.; Robledo, M.; Fliedner, S.M.J.; Wang, K.; Maurer, J.; et al. Responses to systemic therapy in metastatic pheochromocytoma/paraganglioma: A retrospective multicenter cohort study. Eur. J. Endocrinol. 2023, 189, 546–565. [Google Scholar] [CrossRef] [PubMed]
- Yasumura, Y.; Buonassisi, V.; Sato, G. Clonal analysis of differentiated function in animal cell cultures. I. Possible correlated maintenance of differentiated function and the diploid karyotype. Cancer Res. 1966, 26, 529–535. [Google Scholar]
- Rainey, W.E.; Saner, K.; Schimmer, B.P. Adrenocortical cell lines. Mol. Cell Endocrinol. 2004, 228, 23–38. [Google Scholar] [CrossRef]
- Schimmer, B.P. Adrenocortical Y1 cells. Methods Enzymol. 1979, 58, 570–574. [Google Scholar] [CrossRef]
- Weber, M.M.; Fottner, C.; Wolf, E. The role of the insulin-like growth factor system in adrenocortical tumourigenesis. Eur. J. Clin. Investig. 2000, 30 (Suppl. S3), 69–75. [Google Scholar] [CrossRef] [PubMed]
- Ramchandani, S.; MacLeod, A.R.; Pinard, M.; von Hofe, E.; Szyf, M. Inhibition of tumorigenesis by a cytosine-DNA, methyltransferase, antisense oligodeoxynucleotide. Proc. Natl. Acad. Sci. USA 1997, 94, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Mellon, S.H.; Miller, W.L.; Bair, S.R.; Moore, C.C.; Vigne, J.L.; Weiner, R.I. Steroidogenic adrenocortical cell lines produced by genetically targeted tumorigenesis in transgenic mice. Mol. Endocrinol. 1994, 8, 97–108. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lozano, R.C.; Maloberti, P.; Mendez, C.F.; Paz, C.; Podesta, E.J. ACTH regulation of mitochondrial acyl-CoA thioesterase activity in Y1 adrenocortical tumour cells. Endocr. Res. 2002, 28, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Le, T.; Schimmer, B.P. The regulation of MAPKs in Y1 mouse adrenocortical tumor cells. Endocrinology 2001, 142, 4282–4287. [Google Scholar] [CrossRef] [PubMed]
- Ragazzon, B.; Lefrancois-Martinez, A.M.; Val, P.; Tournaire, C.; Berger, M.; Gachancard-Bouya, J.L.; Begue, R.J.; Veyssiere, G.; Martinez, A. ACTH and PRL sensitivity of highly differentiated cell lines obtained by adrenocortical targeted oncogenesis. Endocr. Res. 2004, 30, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Ragazzon, B.; Lefrancois-Martinez, A.M.; Val, P.; Sahut-Barnola, I.; Tournaire, C.; Chambon, C.; Gachancard-Bouya, J.L.; Begue, R.J.; Veyssiere, G.; Martinez, A. Adrenocorticotropin-dependent changes in SF-1/DAX-1 ratio influence steroidogenic genes expression in a novel model of glucocorticoid-producing adrenocortical cell lines derived from targeted tumorigenesis. Endocrinology 2006, 147, 1805–1818. [Google Scholar] [CrossRef] [PubMed][Green Version]
- de Joussineau, C.; Sahut-Barnola, I.; Tissier, F.; Dumontet, T.; Drelon, C.; Batisse-Lignier, M.; Tauveron, I.; Pointud, J.C.; Lefrancois-Martinez, A.M.; Stratakis, C.A.; et al. mTOR pathway is activated by PKA in adrenocortical cells and participates in vivo to apoptosis resistance in primary pigmented nodular adrenocortical disease (PPNAD). Hum. Mol. Genet. 2014, 23, 5418–5428. [Google Scholar] [CrossRef]
- Dufour, D.; Dumontet, T.; Sahut-Barnola, I.; Carusi, A.; Onzon, M.; Pussard, E.; Wilmouth, J.J.; Olabe, J.; Lucas, C.; Levasseur, A.; et al. Loss of SUMO-specific protease 2 causes isolated glucocorticoid deficiency by blocking adrenal cortex zonal transdifferentiation in mice. Nat. Commun. 2022, 13, 7858. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.I.F.; Huang, V.; Olah, M.; Trinh, L.; Liu, Y.; Hazell, G.; Conway-Campbell, B.; Zhao, Z.; Martinez, A.; Lefrancois-Martinez, A.M.; et al. Involvement of CREB-regulated transcription coactivators (CRTC) in transcriptional activation of steroidogenic acute regulatory protein (Star) by ACTH. Mol. Cell Endocrinol. 2020, 499, 110612. [Google Scholar] [CrossRef] [PubMed]
- Walczak, E.M.; Kuick, R.; Finco, I.; Bohin, N.; Hrycaj, S.M.; Wellik, D.M.; Hammer, G.D. Wnt signaling inhibits adrenal steroidogenesis by cell-autonomous and non-cell-autonomous mechanisms. Mol. Endocrinol. 2014, 28, 1471–1486. [Google Scholar] [CrossRef] [PubMed]
- Fudulu, D.P.; Horn, G.; Hazell, G.; Lefrancois-Martinez, A.M.; Martinez, A.; Angelini, G.D.; Lightman, S.L.; Spiga, F. Co-culture of monocytes and zona fasciculata adrenal cells: An in vitro model to study the immune-adrenal cross-talk. Mol. Cell Endocrinol. 2021, 526, 111195. [Google Scholar] [CrossRef] [PubMed]
- Hazell, G.; Horn, G.; Lightman, S.L.; Spiga, F. Dynamics of ACTH-Mediated Regulation of Gene Transcription in ATC1 and ATC7 Adrenal Zona Fasciculata Cell Lines. Endocrinology 2019, 160, 587–604. [Google Scholar] [CrossRef] [PubMed]
- Francis, J.C.; Gardiner, J.R.; Renaud, Y.; Chauhan, R.; Weinstein, Y.; Gomez-Sanchez, C.; Lefrancois-Martinez, A.M.; Bertherat, J.; Val, P.; Swain, A. HOX genes promote cell proliferation and are potential therapeutic targets in adrenocortical tumours. Br. J. Cancer 2021, 124, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Raff, H. CORT, Cort, B, Corticosterone, and now Cortistatin: Enough Already! Endocrinology 2016, 157, 3307–3308. [Google Scholar] [CrossRef]
- Burris-Hiday, S.D.; Scott, E.E. Steroidogenic cytochrome P450 17A1 structure and function. Mol. Cell Endocrinol. 2021, 528, 111261. [Google Scholar] [CrossRef] [PubMed]
- Basham, K.J.; Hung, H.A.; Lerario, A.M.; Hammer, G.D. Mouse models of adrenocortical tumors. Mol. Cell Endocrinol. 2016, 421, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Mostaghel, E.A.; Zhang, A.; Hernandez, S.; Marck, B.T.; Zhang, X.; Tamae, D.; Biehl, H.E.; Tretiakova, M.; Bartlett, J.; Burns, J.; et al. Contribution of Adrenal Glands to Intratumor Androgens and Growth of Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2019, 25, 426–439. [Google Scholar] [CrossRef] [PubMed]
- van Weerden, W.M.; Bierings, H.G.; van Steenbrugge, G.J.; de Jong, F.H.; Schroder, F.H. Adrenal glands of mouse and rat do not synthesize androgens. Life Sci. 1992, 50, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Poutanen, M.; Hagberg Thulin, M.; Harkonen, P. Targeting sex steroid biosynthesis for breast and prostate cancer therapy. Nat. Rev. Cancer 2023, 23, 686–709. [Google Scholar] [CrossRef] [PubMed]
- Dumontet, T.; Martinez, A. Adrenal androgens, adrenarche, and zona reticularis: A human affair? Mol. Cell Endocrinol. 2021, 528, 111239. [Google Scholar] [CrossRef] [PubMed]
- Missaghian, E.; Kempna, P.; Dick, B.; Hirsch, A.; Alikhani-Koupaei, R.; Jegou, B.; Mullis, P.E.; Frey, B.M.; Fluck, C.E. Role of DNA methylation in the tissue-specific expression of the CYP17A1 gene for steroidogenesis in rodents. J. Endocrinol. 2009, 202, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, S.; Kunz, M.; Kurlbaum, M.; Vey, J.; Kendl, S.; Deutschbein, T.; Hahner, S.; Fassnacht, M.; Dandekar, T.; Kroiss, M. Plasma steroid metabolome profiling for the diagnosis of adrenocortical carcinoma. Eur. J. Endocrinol. 2019, 180, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.R.; Ghataore, L.; Couchman, L.; Vincent, R.P.; Whitelaw, B.; Lewis, D.; Diaz-Cano, S.; Galata, G.; Schulte, K.M.; Aylwin, S.; et al. A 13-Steroid Serum Panel Based on LC-MS/MS: Use in Detection of Adrenocortical Carcinoma. Clin. Chem. 2017, 63, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Chortis, V.; Bancos, I.; Nijman, T.; Gilligan, L.C.; Taylor, A.E.; Ronchi, C.L.; O’Reilly, M.W.; Schreiner, J.; Asia, M.; Riester, A.; et al. Urine Steroid Metabolomics as a Novel Tool for Detection of Recurrent Adrenocortical Carcinoma. J. Clin. Endocrinol. Metab. 2020, 105, e307–e318. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Minamidate, T.; Shiga, A.; Ruike, Y.; Ishiwata, K.; Naito, K.; Ishida, A.; Deguchi, H.; Fujimoto, M.; Koide, H.; et al. Steroid metabolites for diagnosing and predicting clinicopathological features in cortisol-producing adrenocortical carcinoma. BMC Endocr. Disord. 2020, 20, 173. [Google Scholar] [CrossRef] [PubMed]
- Berke, K.; Constantinescu, G.; Masjkur, J.; Kimpel, O.; Dischinger, U.; Peitzsch, M.; Kwapiszewska, A.; Dobrowolski, P.; Nolting, S.; Reincke, M.; et al. Plasma Steroid Profiling in Patients With Adrenal Incidentaloma. J. Clin. Endocrinol. Metab. 2022, 107, e1181–e1192. [Google Scholar] [CrossRef] [PubMed]
- Bancos, I.; Taylor, A.E.; Chortis, V.; Sitch, A.J.; Jenkinson, C.; Davidge-Pitts, C.J.; Lang, K.; Tsagarakis, S.; Macech, M.; Riester, A.; et al. Urine steroid metabolomics for the differential diagnosis of adrenal incidentalomas in the EURINE-ACT study: A prospective test validation study. Lancet Diabetes Endocrinol. 2020, 8, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Kimpel, O.; Altieri, B.; Dischinger, U.; Fuss, C.T.; Kurlbaum, M.; Fassnacht, M. Early Detection of Recurrence and Progress Using Serum Steroid Profiling by LC-MS/MS in Patients with Adrenocortical Carcinoma. Metabolites 2023, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Gazdar, A.F.; Oie, H.K.; Shackleton, C.H.; Chen, T.R.; Triche, T.J.; Myers, C.E.; Chrousos, G.P.; Brennan, M.F.; Stein, C.A.; La Rocca, R.V. Establishment and characterization of a human adrenocortical carcinoma cell line that expresses multiple pathways of steroid biosynthesis. Cancer Res. 1990, 50, 5488–5496. [Google Scholar] [PubMed]
- Kurlbaum, M.; Sbiera, S.; Kendl, S.; Martin Fassnacht, M.; Kroiss, M. Steroidogenesis in the NCI-H295 Cell Line Model is Strongly Affected By Culture Conditions and Substrain. Exp. Clin. Endocrinol. Diabetes 2020, 128, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Nanba, K.; Blinder, A.R.; Rainey, W.E. Primary Cultures and Cell Lines for In Vitro Modeling of the Human Adrenal Cortex. Tohoku J. Exp. Med. 2021, 253, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Parmar, J.; Key, R.E.; Rainey, W.E. Development of an adrenocorticotropin-responsive human adrenocortical carcinoma cell line. J. Clin. Endocrinol. Metab. 2008, 93, 4542–4546. [Google Scholar] [CrossRef] [PubMed]
- Sigala, S.; Rossini, E.; Abate, A.; Tamburello, M.; Bornstein, S.R.; Hantel, C. An update on adrenocortical cell lines of human origin. Endocrine 2022, 77, 432–437. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, H.J.; Jung, J.W.; Chung, S.; Son, G.H. Transcriptomic data of human adrenocortical NCI-H295R cells treated with cortisol biosynthesis inhibitors. Data Brief. 2024, 52, 109948. [Google Scholar] [CrossRef] [PubMed]
- Maier, P.; Heinze, B.; Gabor, S.; Reese, S.; Hahner, S.; Schirbel, A. Fluorinated aldosterone synthase (CYP11B2)-inhibitors for differential diagnosis between bilateral and unilateral conditions of primary aldosteronism. Bioorg. Med. Chem. Lett. 2023, 96, 129501. [Google Scholar] [CrossRef] [PubMed]
- Berber, M.; Leng, S.; Wengi, A.; Winter, D.V.; Odermatt, A.; Beuschlein, F.; Loffing, J.; Breault, D.T.; Penton, D. Calcineurin regulates aldosterone production via dephosphorylation of NFATC4. JCI Insight 2023, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.R.; Borges, K.S.; Finco, I.; LaPensee, C.R.; Rege, J.; Solon, A.L.; Little, D.W.; Else, T.; Almeida, M.Q.; Dang, D.; et al. beta-Catenin-Driven Differentiation Is a Tissue-Specific Epigenetic Vulnerability in Adrenal Cancer. Cancer Res. 2023, 83, 2123–2141. [Google Scholar] [CrossRef] [PubMed]
- Hantel, C.; Beuschlein, F. Xenograft models for adrenocortical carcinoma. Mol. Cell Endocrinol. 2016, 421, 28–33. [Google Scholar] [CrossRef]
- Logie, A.; Boudou, P.; Boccon-Gibod, L.; Baudin, E.; Vassal, G.; Schlumberger, M.; Le Bouc, Y.; Gicquel, C. Establishment and characterization of a human adrenocortical carcinoma xenograft model. Endocrinology 2000, 141, 3165–3171. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lindhe, O.; Skogseid, B. Mitotane effects in a H295R xenograft model of adjuvant treatment of adrenocortical cancer. Horm. Metab. Res. 2010, 42, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Gaujoux, S.; Hantel, C.; Launay, P.; Bonnet, S.; Perlemoine, K.; Lefevre, L.; Guillaud-Bataille, M.; Beuschlein, F.; Tissier, F.; Bertherat, J.; et al. Silencing mutated beta-catenin inhibits cell proliferation and stimulates apoptosis in the adrenocortical cancer cell line H295R. PLoS ONE 2013, 8, e55743. [Google Scholar] [CrossRef] [PubMed]
- Nagy, Z.; Baghy, K.; Hunyadi-Gulyas, E.; Micsik, T.; Nyiro, G.; Racz, G.; Butz, H.; Perge, P.; Kovalszky, I.; Medzihradszky, K.F.; et al. Evaluation of 9-cis retinoic acid and mitotane as antitumoral agents in an adrenocortical xenograft model. Am. J. Cancer Res. 2015, 5, 3645–3658. [Google Scholar] [PubMed]
- Cerquetti, L.; Bucci, B.; Carpinelli, G.; Lardo, P.; Proietti, A.; Saporito, R.; Rindi, G.; Petrangeli, E.; Toscano, V.; Stigliano, A. Antineoplastic Effect of a Combined Mitotane Treatment/Ionizing Radiation in Adrenocortical Carcinoma: A Preclinical Study. Cancers 2019, 11, 1768. [Google Scholar] [CrossRef]
- Morin, A.; Ruggiero, C.; Robidel, E.; Doghman-Bouguerra, M.; Das, A.T.; Castellano, R.; Josselin, E.; Favier, J.; Lalli, E. Establishment of a mouse xenograft model of metastatic adrenocortical carcinoma. Oncotarget 2017, 8, 51050–51057. [Google Scholar] [CrossRef] [PubMed]
- Abate, A.; Rossini, E.; Tamburello, M.; Lagana, M.; Cosentini, D.; Grisanti, S.; Fiorentini, C.; Tiberio, G.A.M.; Scatolini, M.; Grosso, E.; et al. Ribociclib Cytotoxicity Alone or Combined With Progesterone and/or Mitotane in in Vitro Adrenocortical Carcinoma Cells. Endocrinology 2022, 163, bqab248. [Google Scholar] [CrossRef] [PubMed]
- Tamburello, M.; Abate, A.; Rossini, E.; Basnet, R.M.; Zizioli, D.; Cosentini, D.; Hantel, C.; Lagana, M.; Tiberio, G.A.M.; Grisanti, S.; et al. Preclinical Evidence of Progesterone as a New Pharmacological Strategy in Human Adrenocortical Carcinoma Cell Lines. Int. J. Mol. Sci. 2023, 24, 6829. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, C.; Tamburello, M.; Rossini, E.; Zini, S.; Durand, N.; Cantini, G.; Cioppi, F.; Hantel, C.; Kiseljak-Vassiliades, K.; Wierman, M.E.; et al. FSCN1 as a new druggable target in adrenocortical carcinoma. Int. J. Cancer 2023, 153, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Cerquetti, L.; Bucci, B.; Marchese, R.; Misiti, S.; De Paula, U.; Miceli, R.; Muleti, A.; Amendola, D.; Piergrossi, P.; Brunetti, E.; et al. Mitotane increases the radiotherapy inhibitory effect and induces G2-arrest in combined treatment on both H295R and SW13 adrenocortical cell lines. Endocr. Relat. Cancer 2008, 15, 623–634. [Google Scholar] [CrossRef]
- Pinto, E.M.; Kiseljak-Vassiliades, K.; Hantel, C. Contemporary preclinical human models of adrenocortical carcinoma. Curr. Opin. Endocr. Metab. Res. 2019, 8, 139–144. [Google Scholar] [CrossRef]
- Reincke, M.; Karl, M.; Travis, W.H.; Mastorakos, G.; Allolio, B.; Linehan, H.M.; Chrousos, G.P. p53 mutations in human adrenocortical neoplasms: Immunohistochemical and molecular studies. J. Clin. Endocrinol. Metab. 1994, 78, 790–794. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sigala, S.; Bothou, C.; Penton, D.; Abate, A.; Peitzsch, M.; Cosentini, D.; Tiberio, G.A.M.; Bornstein, S.R.; Berruti, A.; Hantel, C. A Comprehensive Investigation of Steroidogenic Signaling in Classical and New Experimental Cell Models of Adrenocortical Carcinoma. Cells 2022, 11, 1439. [Google Scholar] [CrossRef] [PubMed]
- Tissier, F.; Cavard, C.; Groussin, L.; Perlemoine, K.; Fumey, G.; Hagnere, A.M.; Rene-Corail, F.; Jullian, E.; Gicquel, C.; Bertagna, X.; et al. Mutations of beta-catenin in adrenocortical tumors: Activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res. 2005, 65, 7622–7627. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, N.G.; Korah, R.; Carling, T. Adrenocortical cancer cell line mutational profile reveals aggressive genetic background. J. Mol. Endocrinol. 2019, 62, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Hantel, C.; Jung, S.; Mussack, T.; Reincke, M.; Beuschlein, F. Liposomal polychemotherapy improves adrenocortical carcinoma treatment in a preclinical rodent model. Endocr. Relat. Cancer 2014, 21, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, T.P.; Wrzesinski, T.; Jagodzinski, P.P. The effect of mitotane on viability, steroidogenesis and gene expression in NCI-H295R adrenocortical cells. Mol. Med. Rep. 2013, 7, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Germano, A.; Rapa, I.; Volante, M.; Lo Buono, N.; Carturan, S.; Berruti, A.; Terzolo, M.; Papotti, M. Cytotoxic activity of gemcitabine, alone or in combination with mitotane, in adrenocortical carcinoma cell lines. Mol. Cell Endocrinol. 2014, 382, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dedhia, P.H.; Sivakumar, H.; Rodriguez, M.A.; Nairon, K.G.; Zent, J.M.; Zheng, X.; Jones, K.; Popova, L.V.; Leight, J.L.; Skardal, A. A 3D adrenocortical carcinoma tumor platform for preclinical modeling of drug response and matrix metalloproteinase activity. Sci. Rep. 2023, 13, 15508. [Google Scholar] [CrossRef] [PubMed]
- Rossini, E.; Tamburello, M.; Abate, A.; Beretta, S.; Fragni, M.; Cominelli, M.; Cosentini, D.; Hantel, C.; Bono, F.; Grisanti, S.; et al. Cytotoxic Effect of Progesterone, Tamoxifen and Their Combination in Experimental Cell Models of Human Adrenocortical Cancer. Front. Endocrinol. 2021, 12, 669426. [Google Scholar] [CrossRef] [PubMed]
- Langer, C.; Koll-Weber, M.; Holzer, M.; Hantel, C.; Suss, R. Mitotane Nanocarriers for the Treatment of Adrenocortical Carcinoma: Evaluation of Albumin-Stabilized Nanoparticles and Liposomes in a Preclinical In Vitro Study with 3D Spheroids. Pharmaceutics 2022, 14, 1891. [Google Scholar] [CrossRef]
- Haider, M.S.; Schreiner, J.; Kendl, S.; Kroiss, M.; Luxenhofer, R. A Micellar Mitotane Formulation with High Drug-Loading and Solubility: Physico-Chemical Characterization and Cytotoxicity Studies in 2D and 3D In Vitro Tumor Models. Macromol. Biosci. 2020, 20, e1900178. [Google Scholar] [CrossRef] [PubMed]
- Laha, D.; Grant, R.R.C.; Mishra, P.; Boufraqech, M.; Shen, M.; Zhang, Y.Q.; Hall, M.D.; Quezado, M.; De Melo, M.S.; Del Rivero, J.; et al. Preclinical assessment of synergistic efficacy of MELK and CDK inhibitors in adrenocortical cancer. J. Exp. Clin. Cancer Res. 2022, 41, 282. [Google Scholar] [CrossRef] [PubMed]
- Penny, M.K.; Lerario, A.M.; Basham, K.J.; Chukkapalli, S.; Mohan, D.R.; LaPensee, C.; Converso-Baran, K.; Hoenerhoff, M.J.; Suarez-Fernandez, L.; Rey, C.G.D.; et al. Targeting Oncogenic Wnt/beta-Catenin Signaling in Adrenocortical Carcinoma Disrupts ECM Expression and Impairs Tumor Growth. Cancers 2023, 15, 3559. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhu, D.; Luo, B.; Kou, W.; Cheng, Y.; Zhu, Y. IFNgamma enhances ferroptosis by increasing JAK-STAT pathway activation to suppress SLCA711 expression in adrenocortical carcinoma. Oncol. Rep. 2022, 47, 8308. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, X.; Li, B.; Li, Y.; Zhang, B. KIF11 is a potential prognostic biomarker and therapeutic target for adrenocortical carcinoma. Transl. Androl. Urol. 2023, 12, 594–611. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, S.; Shapiro, I.; Mazumdar, A.; Zitzmann, K.; Nolting, S.; Luca, E.; Beuschlein, F.; Sharma, A.; Hantel, C. The Vault Complex Is Significantly Involved in Therapeutic Responsiveness of Endocrine Tumors and Linked to Autophagy under Chemotherapeutic Conditions. Cancers 2023, 15, 1783. [Google Scholar] [CrossRef]
- Hantel, C.; Shapiro, I.; Poli, G.; Chiapponi, C.; Bidlingmaier, M.; Reincke, M.; Luconi, M.; Jung, S.; Beuschlein, F. Targeting heterogeneity of adrenocortical carcinoma: Evaluation and extension of preclinical tumor models to improve clinical translation. Oncotarget 2016, 7, 79292–79304. [Google Scholar] [CrossRef] [PubMed]
- Beuschlein, F.; Jakoby, J.; Mentz, S.; Zambetti, G.; Jung, S.; Reincke, M.; Suss, R.; Hantel, C. IGF1-R inhibition and liposomal doxorubicin: Progress in preclinical evaluation for the treatment of adrenocortical carcinoma. Mol. Cell Endocrinol. 2016, 428, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Abate, A.; Rossini, E.; Bonini, S.A.; Fragni, M.; Cosentini, D.; Tiberio, G.A.M.; Benetti, D.; Hantel, C.; Lagana, M.; Grisanti, S.; et al. Cytotoxic Effect of Trabectedin In Human Adrenocortical Carcinoma Cell Lines and Primary Cells. Cancers 2020, 12, 928. [Google Scholar] [CrossRef] [PubMed]
- Bothou, C.; Sharma, A.; Oo, A.; Kim, B.; Perge, P.; Igaz, P.; Ronchi, C.L.; Shapiro, I.; Hantel, C. Novel Insights into the Molecular Regulation of Ribonucleotide Reductase in Adrenocortical Carcinoma Treatment. Cancers 2021, 13, 4200. [Google Scholar] [CrossRef] [PubMed]
- Cantini, G.; Fei, L.; Canu, L.; Lazzeri, E.; Sottili, M.; Francalanci, M.; Angelotti, M.L.; De Filpo, G.; Ercolino, T.; Gelmini, S.; et al. Stimulated Expression of CXCL12 in Adrenocortical Carcinoma by the PPARgamma Ligand Rosiglitazone Impairs Cancer Progression. J. Pers. Med. 2021, 11, 1097. [Google Scholar] [CrossRef] [PubMed]
- Fragni, M.; Palma Lopez, L.P.; Rossini, E.; Abate, A.; Cosentini, D.; Salvi, V.; Vezzoli, S.; Poliani, P.L.; Bosisio, D.; Hantel, C.; et al. In vitro cytotoxicity of cabazitaxel in adrenocortical carcinoma cell lines and human adrenocortical carcinoma primary cell cultures(☆). Mol. Cell Endocrinol. 2019, 498, 110585. [Google Scholar] [CrossRef] [PubMed]
- Hasanovic, A.; Ruggiero, C.; Jung, S.; Rapa, I.; Signetti, L.; Ben Hadj, M.; Terzolo, M.; Beuschlein, F.; Volante, M.; Hantel, C.; et al. Targeting the multidrug transporter Patched potentiates chemotherapy efficiency on adrenocortical carcinoma in vitro and in vivo. Int. J. Cancer 2018, 143, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Weigand, I.; Lippert, J.; Kircher, S.; Altieri, B.; Steinhauer, S.; Hantel, C.; Rost, S.; Rosenwald, A.; Kroiss, M.; et al. Targeted Gene Expression Profile Reveals CDK4 as Therapeutic Target for Selected Patients With Adrenocortical Carcinoma. Front. Endocrinol. 2020, 11, 219. [Google Scholar] [CrossRef] [PubMed]
- Nocito, M.C.; Avena, P.; Zavaglia, L.; De Luca, A.; Chimento, A.; Hamad, T.; La Padula, D.; Stancati, D.; Hantel, C.; Sirianni, R.; et al. Adrenocortical Carcinoma (ACC) Cells Rewire Their Metabolism to Overcome Curcumin Antitumoral Effects Opening a Window of Opportunity to Improve Treatment. Cancers 2023, 15, 1050. [Google Scholar] [CrossRef] [PubMed]
- Rossini, E.; Giacopuzzi, E.; Gangemi, F.; Tamburello, M.; Cosentini, D.; Abate, A.; Lagana, M.; Berruti, A.; Grisanti, S.; Sigala, S. Estrogen-Like Effect of Mitotane Explained by Its Agonist Activity on Estrogen Receptor-alpha. Biomedicines 2021, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Siebert, C.; Ciato, D.; Murakami, M.; Frei-Stuber, L.; Perez-Rivas, L.G.; Monteserin-Garcia, J.L.; Nolting, S.; Maurer, J.; Feuchtinger, A.; Walch, A.K.; et al. Heat Shock Protein 90 as a Prognostic Marker and Therapeutic Target for Adrenocortical Carcinoma. Front. Endocrinol. 2019, 10, 487. [Google Scholar] [CrossRef]
- Warde, K.M.; Lim, Y.J.; Ribes Martinez, E.; Beuschlein, F.; O’Shea, P.; Hantel, C.; Dennedy, M.C. Mitotane Targets Lipid Droplets to Induce Lipolysis in Adrenocortical Carcinoma. Endocrinology 2022, 163, bqac102. [Google Scholar] [CrossRef]
- Warmington, E.; Smith, G.; Chortis, V.; Liang, R.; Lippert, J.; Steinhauer, S.; Landwehr, L.S.; Hantel, C.; Kiseljak-Vassiliades, K.; Wierman, M.E.; et al. PLK1 inhibitors as a new targeted treatment for adrenocortical carcinoma. Endocr. Connect. 2024, 13, 1. [Google Scholar] [CrossRef]
- Bornstein, S.; Shapiro, I.; Malyukov, M.; Zullig, R.; Luca, E.; Gelfgat, E.; Beuschlein, F.; Nolting, S.; Berruti, A.; Sigala, S.; et al. Innovative multidimensional models in a high-throughput-format for different cell types of endocrine origin. Cell Death Dis. 2022, 13, 648. [Google Scholar] [CrossRef] [PubMed]
- Fei, L.; Cantini, G.; Nocentini, A.; Nardini, P.; Catarinicchia, S.; Canu, L.; Ercolino, T.; Quartararo, G.; Nesi, G.; Gacci, M.; et al. Carbonic anhydrases III and IX are new players in the crosstalk between adrenocortical carcinoma and its altered adipose microenvironment. J. Endocrinol. Investig. 2023, 46, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Avena, P.; De Luca, A.; Chimento, A.; Nocito, M.C.; Sculco, S.; La Padula, D.; Zavaglia, L.; Giulietti, M.; Hantel, C.; Sirianni, R.; et al. Estrogen Related Receptor Alpha (ERRalpha) a Bridge between Metabolism and Adrenocortical Cancer Progression. Cancers 2022, 14, 3885. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Kraikivski, P.; Shafiekhani, S.; Terhune, S.S.; Dash, R.K. Crosstalk between Plk1, p53, cell cycle, and G2/M DNA damage checkpoint regulation in cancer: Computational modeling and analysis. NPJ Syst. Biol. Appl. 2021, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Kerdivel, G.; Amrouche, F.; Calmejane, M.A.; Carallis, F.; Hamroune, J.; Hantel, C.; Bertherat, J.; Assie, G.; Boeva, V. DNA hypermethylation driven by DNMT1 and DNMT3A favors tumor immune escape contributing to the aggressiveness of adrenocortical carcinoma. Clin. Epigenetics 2023, 15, 121. [Google Scholar] [CrossRef]
- Kiseljak-Vassiliades, K.; Zhang, Y.; Bagby, S.M.; Kar, A.; Pozdeyev, N.; Xu, M.; Gowan, K.; Sharma, V.; Raeburn, C.D.; Albuja-Cruz, M.; et al. Development of new preclinical models to advance adrenocortical carcinoma research. Endocr. Relat. Cancer 2018, 25, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.; Capasso, A.; Jordan, K.R.; French, J.D.; Kar, A.; Bagby, S.M.; Barbee, J.; Yacob, B.W.; Head, L.S.; Tompkins, K.D.; et al. Development of an Adrenocortical Cancer Humanized Mouse Model to Characterize Anti-PD1 Effects on Tumor Microenvironment. J. Clin. Endocrinol. Metab. 2020, 105, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Weigand, I.; Schreiner, J.; Rohrig, F.; Sun, N.; Landwehr, L.S.; Urlaub, H.; Kendl, S.; Kiseljak-Vassiliades, K.; Wierman, M.E.; Angeli, J.P.F.; et al. Active steroid hormone synthesis renders adrenocortical cells highly susceptible to type II ferroptosis induction. Cell Death Dis. 2020, 11, 192. [Google Scholar] [CrossRef] [PubMed]
- Kiseljak-Vassiliades, K.; Zhang, Y.; Kar, A.; Razzaghi, R.; Xu, M.; Gowan, K.; Raeburn, C.D.; Albuja-Cruz, M.; Jones, K.L.; Somerset, H.; et al. Elucidating the Role of the Maternal Embryonic Leucine Zipper Kinase in Adrenocortical Carcinoma. Endocrinology 2018, 159, 2532–2544. [Google Scholar] [CrossRef] [PubMed]
- Kar, A.; Zhang, Y.; Yacob, B.W.; Saeed, J.; Tompkins, K.D.; Bagby, S.M.; Pitts, T.M.; Somerset, H.; Leong, S.; Wierman, M.E.; et al. Targeting PDZ-binding kinase is anti-tumorigenic in novel preclinical models of ACC. Endocr. Relat. Cancer 2019, 26, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Maria, A.G.; Silva Borges, K.; Lira, R.C.P.; Hassib Thome, C.; Berthon, A.; Drougat, L.; Kiseljak-Vassiliades, K.; Wierman, M.E.; Faucz, F.R.; Faca, V.M.; et al. Inhibition of Aurora kinase A activity enhances the antitumor response of beta-catenin blockade in human adrenocortical cancer cells. Mol. Cell Endocrinol. 2021, 528, 111243. [Google Scholar] [CrossRef] [PubMed]
- Landwehr, L.S.; Schreiner, J.; Appenzeller, S.; Kircher, S.; Herterich, S.; Sbiera, S.; Fassnacht, M.; Kroiss, M.; Weigand, I. A novel patient-derived cell line of adrenocortical carcinoma shows a pathogenic role of germline MUTYH mutation and high tumour mutational burden. Eur. J. Endocrinol. 2021, 184, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Volante, M.; Sperone, P.; Bollito, E.; Frangipane, E.; Rosas, R.; Daffara, F.; Terzolo, M.; Berruti, A.; Papotti, M. Matrix metalloproteinase type 2 expression in malignant adrenocortical tumors: Diagnostic and prognostic significance in a series of 50 adrenocortical carcinomas. Mod. Pathol. 2006, 19, 1563–1569. [Google Scholar] [CrossRef] [PubMed]
- Abate, A.; Tamburello, M.; Rossini, E.; Basnet, R.M.; Ribaudo, G.; Gianoncelli, A.; Hantel, C.; Cosentini, D.; Lagana, M.; Grisanti, S.; et al. Trabectedin impairs invasiveness and metastasis in adrenocortical carcinoma preclinical models. Endocr. Relat. Cancer 2023, 30, 2. [Google Scholar] [CrossRef] [PubMed]
- de Reynies, A.; Assie, G.; Rickman, D.S.; Tissier, F.; Groussin, L.; Rene-Corail, F.; Dousset, B.; Bertagna, X.; Clauser, E.; Bertherat, J. Gene expression profiling reveals a new classification of adrenocortical tumors and identifies molecular predictors of malignancy and survival. J. Clin. Oncol. 2009, 27, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Giordano, T.J.; Kuick, R.; Else, T.; Gauger, P.G.; Vinco, M.; Bauersfeld, J.; Sanders, D.; Thomas, D.G.; Doherty, G.; Hammer, G. Molecular classification and prognostication of adrenocortical tumors by transcriptome profiling. Clin. Cancer Res. 2009, 15, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Cherniack, A.D.; Dewal, N.; Moffitt, R.A.; Danilova, L.; Murray, B.A.; Lerario, A.M.; Else, T.; Knijnenburg, T.A.; Ciriello, G.; et al. Comprehensive Pan-Genomic Characterization of Adrenocortical Carcinoma. Cancer Cell 2016, 29, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Gunz, S.; Kerdivel, G.; Meirer, J.; Shapiro, I.; Ragazzon, B.; Amrouche, F.; Calmejane, M.-A.; Hamroune, J.; Sigala, S.; Berruti, A.; et al. The super-enhancer landscape reflects molecular subgroups of adrenocortical carcinoma. bioRxiv 2023, bioRxiv:2023.2004.2005.535576. [Google Scholar] [CrossRef]
- Bayley, J.P.; Devilee, P. Advances in paraganglioma-pheochromocytoma cell lines and xenografts. Endocr. Relat. Cancer 2020, 27, R433–R450. [Google Scholar] [CrossRef] [PubMed]
- Greene, L.A.; Tischler, A.S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc. Natl. Acad. Sci. USA 1976, 73, 2424–2428. [Google Scholar] [CrossRef]
- Wiatrak, B.; Kubis-Kubiak, A.; Piwowar, A.; Barg, E. PC12 Cell Line: Cell Types, Coating of Culture Vessels, Differentiation and Other Culture Conditions. Cells 2020, 9, 958. [Google Scholar] [CrossRef] [PubMed]
- Westerink, R.H.; Ewing, A.G. The PC12 cell as model for neurosecretion. Acta Physiol. 2008, 192, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Yano, N.; Kora, K.; Yokoyama, A.; Maizuru, K.; Kayaki, T.; Nishikawa, K.; Osawa, M.; Niwa, A.; Takenouchi, T.; et al. Involvement of mTOR pathway in neurodegeneration in NSF-related developmental and epileptic encephalopathy. Hum. Mol. Genet. 2023, 32, 1683–1697. [Google Scholar] [CrossRef] [PubMed]
- Oprea, D.; Sanz, C.G.; Barsan, M.M.; Enache, T.A. PC-12 Cell Line as a Neuronal Cell Model for Biosensing Applications. Biosensors 2022, 12, 500. [Google Scholar] [CrossRef] [PubMed]
- Delenclos, M.; Burgess, J.D.; Lamprokostopoulou, A.; Outeiro, T.F.; Vekrellis, K.; McLean, P.J. Cellular models of alpha-synuclein toxicity and aggregation. J. Neurochem. 2019, 150, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.M.; Oliveira, A.; Oliveira, J.M.A.; Pinho, B.R. Stress response mechanisms in protein misfolding diseases: Profiling a cellular model of Huntington’s disease. Arch. Biochem. Biophys. 2023, 745, 109711. [Google Scholar] [CrossRef] [PubMed]
- Rutgers, M.; Buitenhuis, C.K.; van der Valk, M.A.; Hoefnagel, C.A.; Voute, P.A.; Smets, L.A. [131I] and [125I] metaiodobenzylguanidine therapy in macroscopic and microscopic tumors: A comparative study in SK-N-SH human neuroblastoma and PC12 rat pheochromocytoma xenografts. Int. J. Cancer 2000, 90, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Denorme, M.; Yon, L.; Roux, C.; Gonzalez, B.J.; Baudin, E.; Anouar, Y.; Dubessy, C. Both sunitinib and sorafenib are effective treatments for pheochromocytoma in a xenograft model. Cancer Lett. 2014, 352, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.F.; Evinger, M.J.; Tsokas, P.; Bedri, S.; Alroy, J.; Shahsavari, M.; Tischler, A.S. Pheochromocytoma cell lines from heterozygous neurofibromatosis knockout mice. Cell Tissue Res. 2000, 302, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.F.; Tischler, A.S.; Mohammed, M.; Naeem, R. Microarray-based comparative genomic hybridization of pheochromocytoma cell lines from neurofibromatosis knockout mice reveals genetic alterations similar to those in human pheochromocytomas. Cancer Genet. Cytogenet. 2005, 159, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.F.; Evinger, M.J.; Zhi, J.; Picard, K.L.; Tischler, A.S. Pheochromocytomas in Nf1 knockout mice express a neural progenitor gene expression profile. Neuroscience 2007, 147, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Martiniova, L.; Lai, E.W.; Elkahloun, A.G.; Abu-Asab, M.; Wickremasinghe, A.; Solis, D.C.; Perera, S.M.; Huynh, T.T.; Lubensky, I.A.; Tischler, A.S.; et al. Characterization of an animal model of aggressive metastatic pheochromocytoma linked to a specific gene signature. Clin. Exp. Metastasis 2009, 26, 239–250. [Google Scholar] [CrossRef] [PubMed]
- D’Antongiovanni, V.; Martinelli, S.; Richter, S.; Canu, L.; Guasti, D.; Mello, T.; Romagnoli, P.; Pacak, K.; Eisenhofer, G.; Mannelli, M.; et al. The microenvironment induces collective migration in silenced mouse pheochromocytoma spheroids. Endocr. Relat. Cancer 2017, 24, 555–564. [Google Scholar] [CrossRef]
- Martinelli, S.; Riverso, M.; Mello, T.; Amore, F.; Parri, M.; Simeone, I.; Mannelli, M.; Maggi, M.; Rapizzi, E. SDHB and SDHD silenced pheochromocytoma spheroids respond differently to tumour microenvironment and their aggressiveness is inhibited by impairing stroma metabolism. Mol. Cell Endocrinol. 2022, 547, 111594. [Google Scholar] [CrossRef] [PubMed]
- Bechmann, N.; Ehrlich, H.; Eisenhofer, G.; Ehrlich, A.; Meschke, S.; Ziegler, C.G.; Bornstein, S.R. Anti-Tumorigenic and Anti-Metastatic Activity of the Sponge-Derived Marine Drugs Aeroplysinin-1 and Isofistularin-3 against Pheochromocytoma In Vitro. Mar. Drugs 2018, 16, 172. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.C.; Venara, M.; Nowicki, S.; Chemes, H.E.; Barontini, M.; Pennisi, P.A. Igf-I regulates pheochromocytoma cell proliferation and survival in vitro and in vivo. Endocrinology 2012, 153, 3724–3734. [Google Scholar] [CrossRef] [PubMed]
- Nolting, S.; Garcia, E.; Alusi, G.; Giubellino, A.; Pacak, K.; Korbonits, M.; Grossman, A.B. Combined blockade of signalling pathways shows marked anti-tumour potential in phaeochromocytoma cell lines. J. Mol. Endocrinol. 2012, 49, 79–96. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nolting, S.; Giubellino, A.; Tayem, Y.; Young, K.; Lauseker, M.; Bullova, P.; Schovanek, J.; Anver, M.; Fliedner, S.; Korbonits, M.; et al. Combination of 13-Cis retinoic acid and lovastatin: Marked antitumor potential in vivo in a pheochromocytoma allograft model in female athymic nude mice. Endocrinology 2014, 155, 2377–2390. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.F.; Korgaonkar, P.G.; Fliedner, S.; Giubellino, A.; Pacak, K.; Sahagian, G.G.; Tischler, A.S. Cytocidal activities of topoisomerase 1 inhibitors and 5-azacytidine against pheochromocytoma/paraganglioma cells in primary human tumor cultures and mouse cell lines. PLoS ONE 2014, 9, e87807. [Google Scholar] [CrossRef] [PubMed]
- Schovanek, J.; Bullova, P.; Tayem, Y.; Giubellino, A.; Wesley, R.; Lendvai, N.; Nolting, S.; Kopacek, J.; Frysak, Z.; Pommier, Y.; et al. Inhibitory Effect of the Noncamptothecin Topoisomerase I Inhibitor LMP-400 on Female Mice Models and Human Pheochromocytoma Cells. Endocrinology 2015, 156, 4094–4104. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, M.; Liers, J.; Peitzsch, M.; Feldmann, A.; Bergmann, R.; Sommer, U.; Richter, S.; Bornstein, S.R.; Bachmann, M.; Eisenhofer, G.; et al. Strain-specific metastatic phenotypes in pheochromocytoma allograft mice. Endocr. Relat. Cancer 2018, 25, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Fankhauser, M.; Bechmann, N.; Lauseker, M.; Goncalves, J.; Favier, J.; Klink, B.; William, D.; Gieldon, L.; Maurer, J.; Spottl, G.; et al. Synergistic Highly Potent Targeted Drug Combinations in Different Pheochromocytoma Models Including Human Tumor Cultures. Endocrinology 2019, 160, 2600–2617. [Google Scholar] [CrossRef] [PubMed]
- Nolting, S.; Maurer, J.; Spottl, G.; Aristizabal Prada, E.T.; Reuther, C.; Young, K.; Korbonits, M.; Goke, B.; Grossman, A.; Auernhammer, C.J. Additive Anti-Tumor Effects of Lovastatin and Everolimus In Vitro through Simultaneous Inhibition of Signaling Pathways. PLoS ONE 2015, 10, e0143830. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Schober, L.; Fischer, A.; Bechmann, N.; Maurer, J.; Peischer, L.; Reul, A.; Hantel, C.; Reincke, M.; Beuschlein, F.; et al. Opposing effects of cannabidiol in patient-derived neuroendocrine tumor, pheochromocytoma/paraganglioma primary cultures. J. Clin. Endocrinol. Metab. 2024, dgae241. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Schutze, I.; Gulde, S.; Bechmann, N.; Richter, S.; Helm, J.; Lauseker, M.; Maurer, J.; Reul, A.; Spoettl, G.; et al. Personalized drug testing in human pheochromocytoma/paraganglioma primary cultures. Endocr. Relat. Cancer 2022, 29, 285–306. [Google Scholar] [CrossRef] [PubMed]
- Papewalis, C.; Kouatchoua, C.; Ehlers, M.; Jacobs, B.; Porwol, D.; Schinner, S.; Willenberg, H.S.; Anlauf, M.; Raffel, A.; Eisenhofer, G.; et al. Chromogranin A as potential target for immunotherapy of malignant pheochromocytoma. Mol. Cell Endocrinol. 2011, 335, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Caisova, V.; Li, L.; Gupta, G.; Jochmanova, I.; Jha, A.; Uher, O.; Huynh, T.T.; Miettinen, M.; Pang, Y.; Abunimer, L.; et al. The Significant Reduction or Complete Eradication of Subcutaneous and Metastatic Lesions in a Pheochromocytoma Mouse Model after Immunotherapy Using Mannan-BAM, TLR Ligands, and Anti-CD40. Cancers 2019, 11, 654. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.F.; Cochran, B.; Baleja, J.D.; Sikes, H.D.; Pattison, A.D.; Zhang, X.; Lomakin, I.; Shepard-Barry, A.; Pacak, K.; Moon, S.J.; et al. A xenograft and cell line model of SDH-deficient pheochromocytoma derived from Sdhb+/− rats. Endocr. Relat. Cancer 2020, 27, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Letouze, E.; Martinelli, C.; Loriot, C.; Burnichon, N.; Abermil, N.; Ottolenghi, C.; Janin, M.; Menara, M.; Nguyen, A.T.; Benit, P.; et al. SDH mutations establish a hypermethylator phenotype in paraganglioma. Cancer Cell 2013, 23, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Loriot, C.; Domingues, M.; Berger, A.; Menara, M.; Ruel, M.; Morin, A.; Castro-Vega, L.J.; Letouze, E.; Martinelli, C.; Bemelmans, A.P.; et al. Deciphering the molecular basis of invasiveness in Sdhb-deficient cells. Oncotarget 2015, 6, 32955–32965. [Google Scholar] [CrossRef] [PubMed]
- Pfragner, R.; Behmel, A.; Smith, D.P.; Ponder, B.A.J.; Wirnsberger, G.; Rinner, I.; Porta, S.; Henn, T.; Niederle, B. First continuous human pheochromocytoma cell line: KNA—Biological, cytogenetic and molecular characterization of KNA cells. J. Neurocytol. 1998, 27, 175–186. [Google Scholar] [CrossRef]
- Venihaki, M.; Ain, K.; Dermitzaki, E.; Gravanis, A.; Margioris, A.N. KAT45, a noradrenergic human pheochromocytoma cell line producing corticotropin-releasing hormone. Endocrinology 1998, 139, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Ghayee, H.K.; Bhagwandin, V.J.; Stastny, V.; Click, A.; Ding, L.H.; Mizrachi, D.; Zou, Y.S.; Chari, R.; Lam, W.L.; Bachoo, R.M.; et al. Progenitor cell line (hPheo1) derived from a human pheochromocytoma tumor. PLoS ONE 2013, 8, e65624. [Google Scholar] [CrossRef] [PubMed]
- Amar, L.; Baudin, E.; Burnichon, N.; Peyrard, S.; Silvera, S.; Bertherat, J.; Bertagna, X.; Schlumberger, M.; Jeunemaitre, X.; Gimenez-Roqueplo, A.P.; et al. Succinate dehydrogenase B gene mutations predict survival in patients with malignant pheochromocytomas or paragangliomas. J. Clin. Endocrinol. Metab. 2007, 92, 3822–3828. [Google Scholar] [CrossRef] [PubMed]
- Buffet, A.; Burnichon, N.; Favier, J.; Gimenez-Roqueplo, A.P. An overview of 20 years of genetic studies in pheochromocytoma and paraganglioma. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101416. [Google Scholar] [CrossRef] [PubMed]
- Tabebi, M.; Kumar Dutta, R.; Skoglund, C.; Soderkvist, P.; Gimm, O. Loss of SDHB Induces a Metabolic Switch in the hPheo1 Cell Line toward Enhanced OXPHOS. Int. J. Mol. Sci. 2022, 23, 560. [Google Scholar] [CrossRef] [PubMed]
- Amore, F.; Garella, R.; Santi, A.; Guasti, D.; Martinelli, S.; Canu, L.; Bani, D.; Neuzil, J.; Maggi, M.; Squecco, R.; et al. The aggressiveness of succinate dehydrogenase subunit B-deficient chromaffin cells is reduced when their bioelectrical properties are restored by glibenclamide. Endocr. Relat. Cancer 2023, 30, 10. [Google Scholar] [CrossRef] [PubMed]
- Bayley, J.P.; Rebel, H.G.; Scheurwater, K.; Duesman, D.; Zhang, J.; Schiavi, F.; Korpershoek, E.; Jansen, J.C.; Schepers, A.; Devilee, P. Long-term in vitro 2D-culture of SDHB and SDHD-related human paragangliomas and pheochromocytomas. PLoS ONE 2022, 17, e0274478. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, S.; Cantini, G.; Propato, A.P.; Bani, D.; Guasti, D.; Nardini, P.; Calosi, L.; Mello, T.; Bechmann, N.; Danza, G.; et al. The 3D in vitro Adrenoid cell model recapitulates the complexity of the adrenal gland. Sci. Rep. 2024, 14, 8044. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).