The Predictive Value of the Triglycerides/HDL-Cholesterol Ratio for Diabetes Incidence
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Clinical and Laboratory
2.3. Definitions
2.4. Statistical Analysis
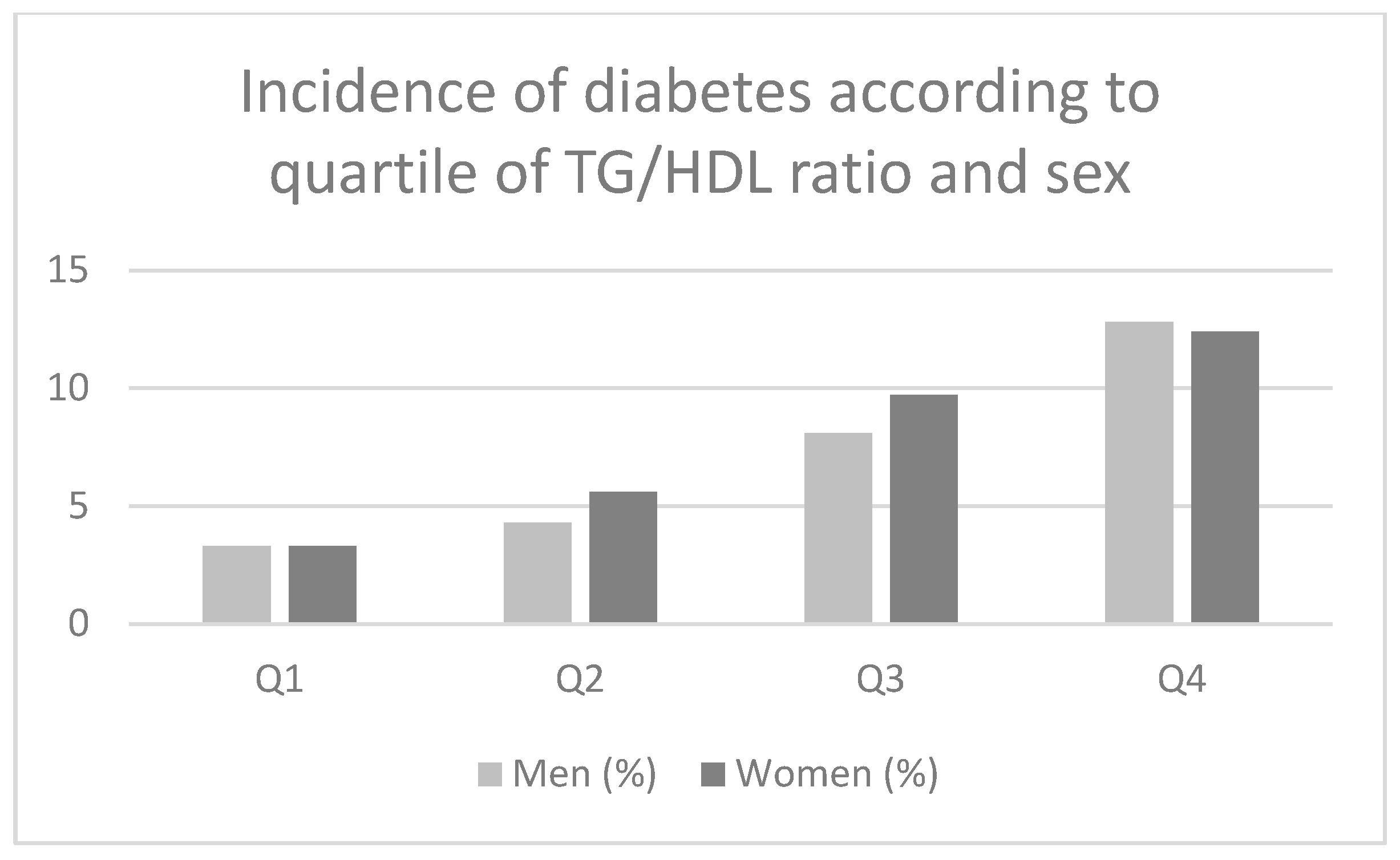
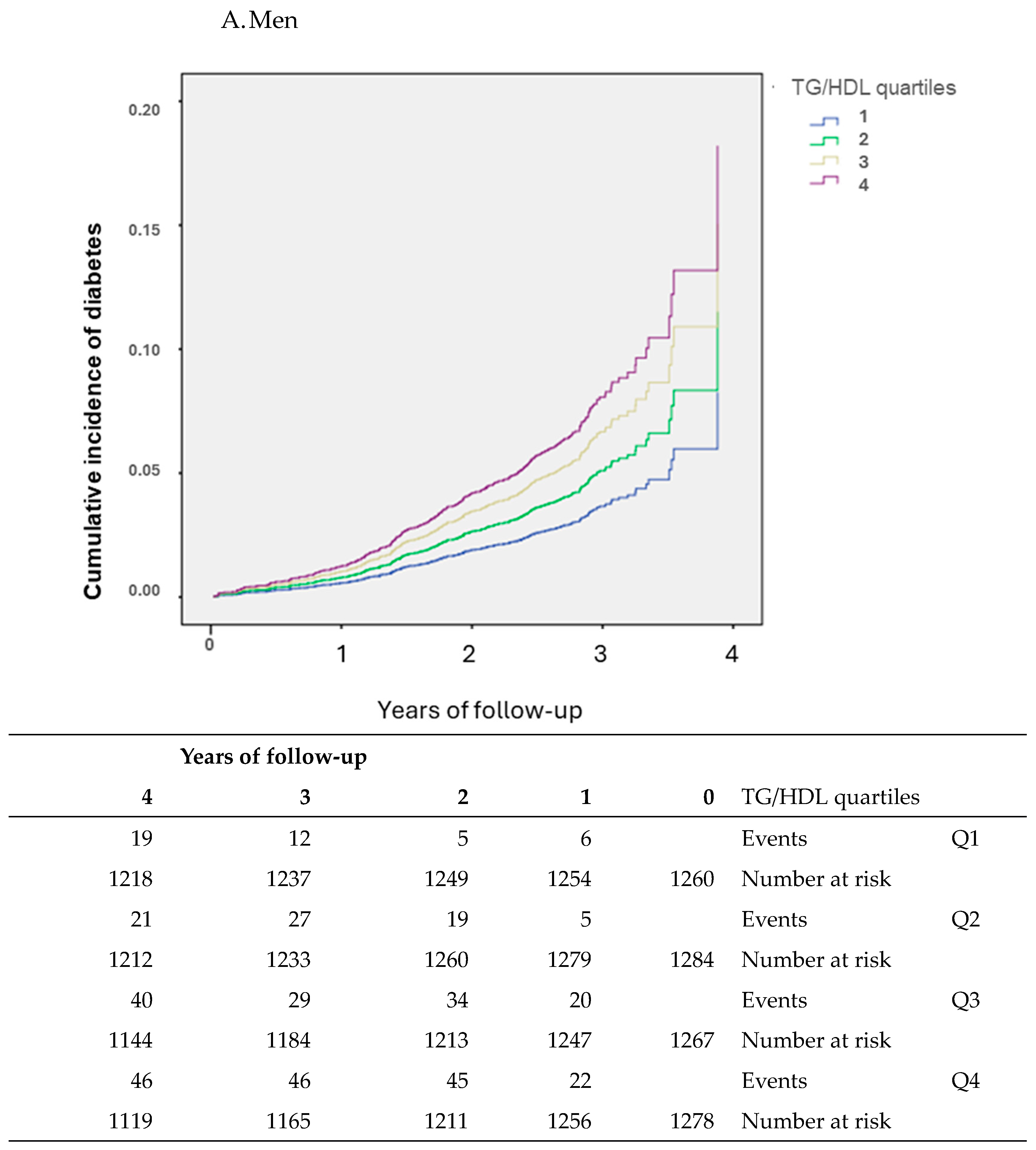
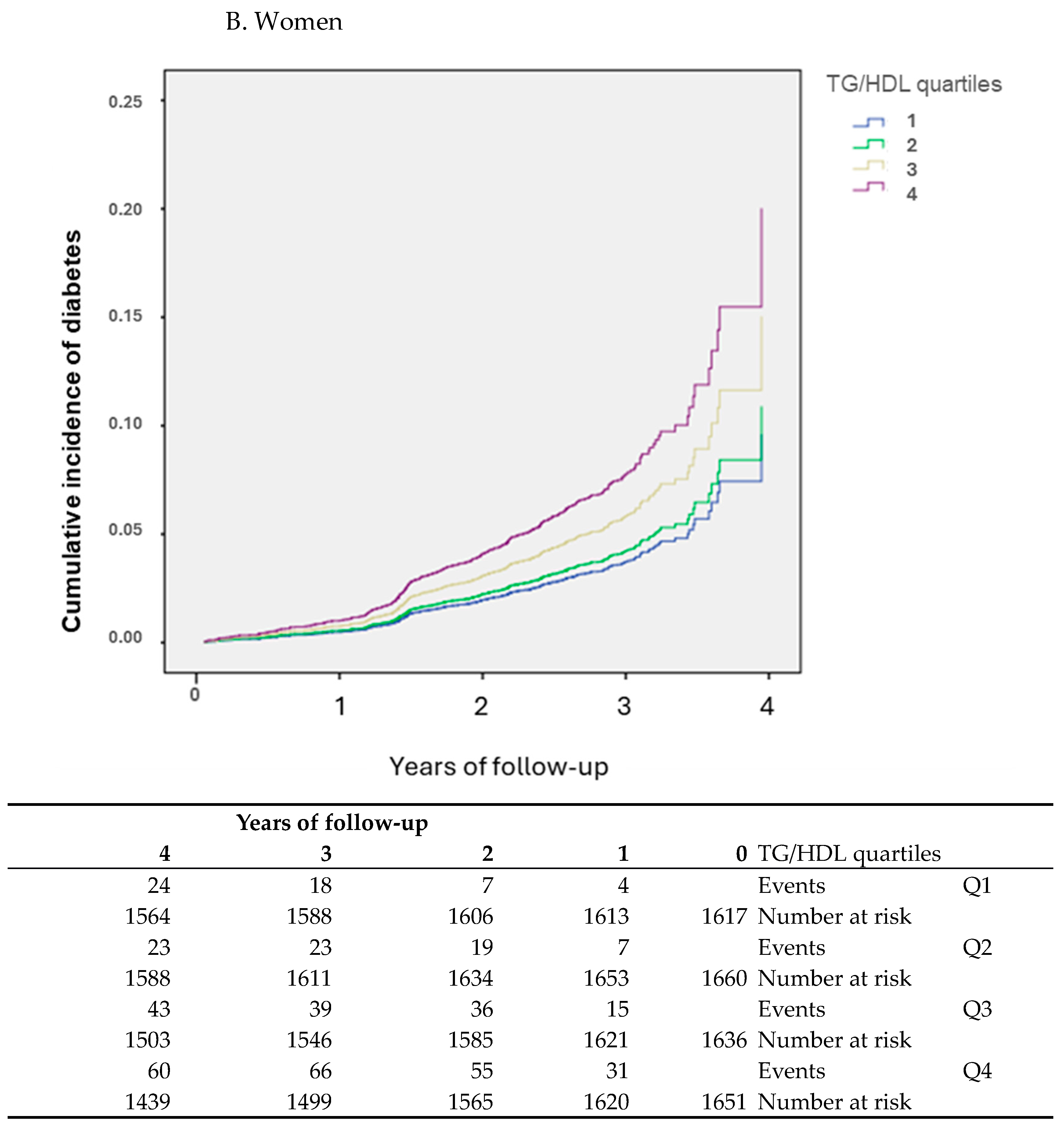
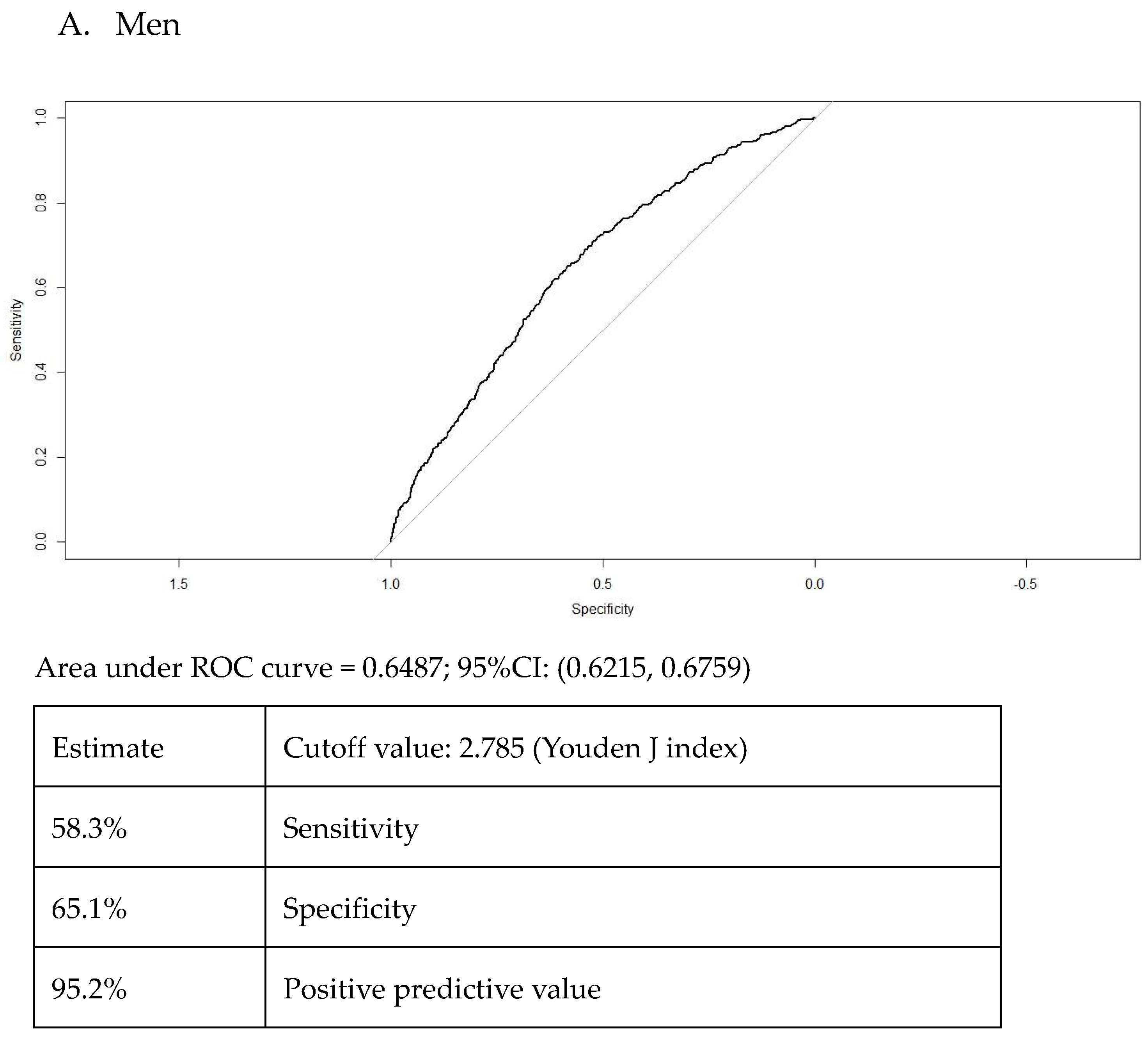
3. Results
Participants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chi, X.; Wang, Y.; Setrerrahmane, S.; Xie, W.; Xu, H. Trends in Insulin Resistance: Insights into Mechanisms and Therapeutic Strategy. Signal Transduct. Target. Ther. 2022, 7, 216. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.J. Insulin Resistance as the Core Defect in Type 2 Diabetes Mellitus. Am. J. Cardiol. 2002, 90, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Park, S.Y.; Choi, C.S. Insulin Resistance: From Mechanisms to Therapeutic Strategies. Diabetes Metab. J. 2022, 46, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Singh, B. Surrogate Markers of Insulin Resistance: A Review. World J. Diabetes 2010, 1, 36–47. [Google Scholar] [CrossRef]
- Biernacka-Bartnik, A.; Kocełak, P.; Owczarek, A.J.; Choręza, P.S.; Markuszewski, L.; Madej, P.; Puzianowska-Kuźnicka, M.; Chudek, J.; Olszanecka-Glinianowicz, M. The Cut-off Value for HOMA-IR Discriminating the Insulin Resistance Based on the SHBG Level in Women with Polycystic Ovary Syndrome. Front. Med. 2023, 10, 1100547. [Google Scholar] [CrossRef]
- Vergès, B. Pathophysiology of Diabetic Dyslipidaemia: Where Are We? Diabetologia 2015, 58, 886–899. [Google Scholar] [CrossRef]
- Liu, H.; Liu, J.; Liu, J.; Xin, S.; Lyu, Z.; Fu, X. Triglyceride to High-Density Lipoprotein Cholesterol (TG/HDL-C) Ratio, a Simple but Effective Indicator in Predicting Type 2 Diabetes Mellitus in Older Adults. Front. Endocrinol. 2022, 13, 828581. [Google Scholar] [CrossRef]
- Bhowmik, B.; Siddiquee, T.; Mujumder, A.; Afsana, F.; Ahmed, T.; Mdala, I.A.; Nayla, N.C.; Khan, A.K.A.; Hussain, A.; Holmboe-Ottesen, G.; et al. Serum Lipid Profile and Its Association with Diabetes and Prediabetes in a Rural Bangladeshi Population. Int. J. Environ. Res. Public Health 2018, 15, 1944. [Google Scholar] [CrossRef]
- Vega, G.L.; Barlow, C.E.; Grundy, S.M.; Leonard, D.; DeFina, L.F. Triglyceride-to-High-Density-Lipoprotein-Cholesterol Ratio Is an Index of Heart Disease Mortality and of Incidence of Type 2 Diabetes Mellitus in Men. J. Investig. Med. 2014, 62, 345–349. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.; Xuan, X.; Xie, Z.; Qiu, Y.; Qin, H.; Xiaoning, Z. Association between Triglyceride to High-Density Lipoprotein Cholesterol Ratio and Type 2 Diabetes Risk in Japanese. Sci. Rep. 2023, 13, 3719. [Google Scholar] [CrossRef] [PubMed]
- Yuge, H.; Okada, H.; Hamaguchi, M.; Kurogi, K.; Murata, H.; Ito, M.; Fukui, M. Triglycerides/HDL Cholesterol Ratio and Type 2 Diabetes Incidence: Panasonic Cohort Study 10. Cardiovasc. Diabetol. 2023, 22, 308. [Google Scholar] [CrossRef]
- Da Luz, P.L.; Favarato, D.; Faria-Neto, J.R.; Lemos, P.; Chagas, A.C.P. High Ratio of Triglycerides to HDL-Cholesterol Predicts Extensive Coronary Disease. Clinics 2008, 63, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.I.; Duncan, B.B.; Mill, J.G.; Lotufo, P.A.; Chor, D.; Barreto, S.M.; Aquino, E.M.L.; Passos, V.M.A.; Matos, S.M.A.; Molina, M.d.C.B.; et al. Cohort Profile: Longitudinal Study of Adult Health (ELSA-Brasil). Int. J. Epidemiol. 2015, 44, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Aquino, E.M.L.; Barreto, S.M.; Bensenor, I.M.; Carvalho, M.S.; Chor, D.; Duncan, B.B.; Lotufo, P.A.; Mill, J.G.; Molina, M.D.C.; Mota, E.L.A.; et al. Brazilian Longitudinal Study of Adult Health (ELSA-Brasil): Objectives and Design. Am. J. Epidemiol. 2012, 175, 315–324. [Google Scholar] [CrossRef]
- Bensenor, I.M.; Griep, R.H.; Pinto, K.A.; De Faria, C.P.; Felisbino-Mendes, M.; Caetano, E.I.; da Silva Albuquerque, L.; Schmidt, M.I. Routines of Organization of Clinical Tests and Interviews in the ELSA-Brasil Investigation Center. Rev. Saude Publica 2013, 47, 37–47. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44 (Suppl. S1), S15–S33. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, D.; Maiorino, M.I.; Bellastella, G.; Chiodini, P.; Esposito, K. Glycemic Control, Preexisting Cardiovascular Disease, and Risk of Major Cardiovascular Events in Patients with Type 2 Diabetes Mellitus: Systematic Review With Meta-Analysis of Cardiovascular Outcome Trials and Intensive Glucose Control Trials. J. Am. Heart Assoc. 2019, 8, e012356. [Google Scholar] [CrossRef]
- WHO World Health Organisation. 2021. Available online: https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 4 September 2024).
- Sampson, U.K.; Fazio, S.; Linton, M.F. Residual Cardiovascular Risk despite Optimal LDL Cholesterol Reduction with Statins: The Evidence, Etiology, and Therapeutic Challenges. Curr. Atheroscler. Rep. 2012, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.K.; Lee, H.S.; Lee, Y.J. Triglyceride to HDL-Cholesterol Ratio and the Incidence Risk of Type 2 Diabetes in Community Dwelling Adults: A Longitudinal 12-Year Analysis of the Korean Genome and Epidemiology Study. Diabetes Res. Clin. Pract. 2020, 163, 108150. [Google Scholar] [CrossRef]
- Che, B.; Zhong, C.; Zhang, R.; Pu, L.; Zhao, T.; Zhang, Y.; Han, L. Triglyceride-Glucose Index and Triglyceride to High-Density Lipoprotein Cholesterol Ratio as Potential Cardiovascular Disease Risk Factors: An Analysis of UK Biobank Data. Cardiovasc. Diabetol. 2023, 22, 34. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Liu, Y.; Li, L.; Zheng, Y.; Wang, Y.; Su, J.; Yang, R.; Luo, M.; Yu, C. Correlation between the triglyceride –to-high-density lipoprotein cholesterol ratio and other unconvencional lipid parameters with the risk of prediabetes and Type 2 diabetes in pactients with coronary heart disease: A RCSCD-TCM study in China. Cardiovasc. Diabetol. 2022, 21, 93. [Google Scholar] [CrossRef] [PubMed]
- Baez-Duarte, B.G.; Zamora-Gínez, I.; González-Duarte, R.; Torres-Rasgado, E.; Ruiz-Vivanco, G.; Pérez-Fuentes, R.; Guerrero-Romero, F.; Rodriguez-Moran, M.; De La Pena, J.E.; Wacher, N.; et al. Triglyceride/High-Density Lipoprotein Cholesterol (TG/HDL-C) Index as a Reference Criterion of Risk for Metabolic Syndrome (MetS) and Low Insulin Sensitivity in Apparently Healthy Subjects. Gac. Med. Mex. 2017, 153, 152–158. [Google Scholar]
- Salazar, M.R.; Carbajal, H.A.; Espeche, W.G.; Aizpúrua, M.; Sisnieguez, C.E.; March, C.E.; Balbín, E.; Stavile, R.N.; Reaven, G.M. Identifying cardiovascular disease risk and outcome: Use of the plasma triglyceride/high-density lipoprotein cholesterol concentration ratio versus metabolic syndrome criteria. J. Intern. Med. 2013, 273, 595–601. [Google Scholar] [CrossRef]
- George MM, A.K.; Zimmet, P.; George Alberti, K.M.; Kaufman, F.; Tajima, N.; Silink, M.; Arslanian, S.; Wong, G.; Bennett, P.; Shaw, J.; et al. The Metabolic Syndrome in Children and Adolescents—An IDF Consensus Report. Pediatr. Diabetes 2007, 8, 299–306. [Google Scholar]
- Radetti, G.; Grugni, G.; Lupi, F.; Fanolla, A.; Caroli, D.; Bondesan, A.; Sartorio, A. High Tg/HDL-Cholesterol Ratio Highlights a Higher Risk of Metabolic Syndrome in Children and Adolescents with Severe Obesity. J. Clin. Med. 2022, 11, 4488. [Google Scholar] [CrossRef]
- Nie, G.; Hou, S.; Zhang, M.; Peng, W. High TG/HDL Ratio Suggests a Higher Risk of Metabolic Syndrome among an Elderly Chinese Population: A Cross-Sectional Study. BMJ Open 2021, 11, e041519. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, J.; Hein, H.O.; Suadicani, P.; Gyntalberg, F. Relation of high TG-Low HDL cholesterol and LDL cholesterol to the incidence of ischemic heart disease. Arter. Thromb. Vasc. Biol. 1997, 17, 1114–1120. [Google Scholar] [CrossRef]
- Du, T.; Yuan, G.; Zhang, M.; Zhou, X.; Sun, X.; Yu, X. Clinical Usefulness of Lipid Ratios, Visceral Adiposity Indicators, and the Triglycerides and Glucose Index as Risk Markers of Insulin Resistance. Cardiovasc. Diabetol. 2014, 13, 146. [Google Scholar] [CrossRef]
- Aquino, E.M.L.; Vasconcellos-Silva, P.R.; Coeli, C.M.; Araujo, M.J.; Santos, S.M.; Figueiredo, R.C.d.; Duncan, B.B. Aspectos eticos em estudos longitudinais: O caso do ELSA-Brasil. Rev. Saúde Pública 2013, 47 (Suppl. S2), 19–26. [Google Scholar] [CrossRef]

| A. Men | ||||||
| TG_HDL Quartiles | ||||||
| Q1 <1.69 | Q2 1.69–<2.54 | Q3 2.54–<3.85 | Q4 ≥3.85 | p-value | p-for-trend | |
| N | 1260 | 1284 | 1267 | 1278 | ||
| Age (years) | 51 (9.5) | 51 (9.5) | 51 (8.7) | 50 (8.5) | 0.286 | 0.053 |
| Race/Ethnicity, n (%) | 0.127 | 0.021 | ||||
| 667 (53.6) | 669 (53.1) | 724 (57.7) | 695 (55.0) | ||
| 355 (28.5) | 381 (30.2) | 353 (28.1) | 393 (31.1) | ||
| 178 (14.3) | 176 (14.0) | 144 (11.5) | 140 (11.1) | ||
| 27 (2.2) | 22 (1.7) | 17 (1.4) | 18 (1.4) | ||
| 17 (1.4) | 13 (1.0) | 16 (1.3) | 18 (1.4) | ||
| Higher educational level, n (%) | 1071 (85.0) | 1122 (87.4) | 1093 (86.3) | 1106 (86.5) | 0.008 | 0.280 |
| Pre-diabetes, n (%) | 558 (44.3) | 671 (52.3) | 756 (59.7) | 825 (64.6) | <0.001 | <0.001 |
| Family history of DM, n (%) | 378 (30.6) | 400 (31.7) | 433 (34.7) | 447 (35.5) | 0.024 | 0.003 |
| Physically active, n (%) | 452 (36.3) | 372 (29.3) | 306 (24.6) | 270 (21.5) | <0.001 | <0.001 |
| Smoking, n (%) | 114 (23.2) | 132 (23.2) | 132 (23.2) | 181 (26.5) | 0.187 | 0.078 |
| Body mass index (kg/m2) | 24.6 (3.6) | 27.2 (3.8) a | 27.3 (4.1) a,b | 28.0 (3.9) a,b,c | <0.001 | <0.001 |
| Waist circumference (cm) | 87.8 (10.5) | 93.0 (10.5) a | 96.3 (10.9) a,b | 98.2 (10.1) a,b,c | <0.001 | <0.001 |
| Systolic BP (mmHg) | 122 (16) | 123 (15) | 124 (16) a | 126 (16) a,b | <0.001 | <0.001 |
| Diastolic BP (mmHg) | 76 (11) | 77 (10) a | 79 (10) a,b | 81 (10) a,b,c | <0.001 | <0.001 |
| Total cholesterol (mg/dL) | 187.8 (35.8) | 194.4 (37.7) a | 202.4 (39.6) a,b | 209.1 (40.6) a,b,c | <0.001 | <0.001 |
| HDL-cholesterol (mg/dL) | 58.0 (11.3) | 49.0 (8.2) a | 45.3 (7.4) a,b | 41.7 (6.8) a,b,c | <0.001 | <0.001 |
| LDL-cholesterol (mg/dL) | 113.0 (31.7) | 122.8 (33.4) a | 126.8 (34.8) a,b | 119.2 (38.1) a,b,c | <0.001 | <0.001 |
| Triglycerides (mg/dL) # | 69.6 (16.1) | 101.9 (18.8) a | 140.4 (26.9) a,b | 233.8 (70.4) a,b,c | <0.001 | <0.001 |
| Fasting glucose (mg/dL) | 99.0 (8.3) | 101.0 (8.0) a | 102.2 (8.3) a,b | 103.4 (8.4) a,b,c | <0.001 | <0.001 |
| 2 h glucose (mg/dL) | 111.1 (26.8) | 115.9 (27.0) a | 124.5 (27.7) a,b | 129.5 (27.9) | <0.001 | <0.001 |
| HbA1c (%) | 5.1 (0.5) | 5.2 (0.5) | 5.2 (0.5) a | 5.2 (0.5) a | <0.001 | <0.001 |
| HOMA-IR # | 2.0 (1.2) | 2.6 (1.8) a | 3.2 (2.0) a,b | 3.8 (2,2) a,b,c | <0.001 | <0.001 |
| TG/HDL ratio | 1.2 (0.3) | 2.1 (0.2) a | 3.1 (0.4) a,b | 5.7 (1.7) a,b,c | <0.001 | <0.001 |
| B. Women | ||||||
| TG_HDL Quartiles | ||||||
| Q1 <1.13 | Q2 1.13–<1.63 | Q3 1.63–<2.39 | Q4 ≥2.39 | p-value | p-for-trend | |
| N | 1617 | 1660 | 1636 | 1651 | ||
| Age (years) | 50 (8.6) | 50 (8.5) | 52 (8.8) a,b | 52 (8.3) a,b | <0.001 | <0.001 |
| Race/Ethnicity, n (%) | <0.001 | 0.434 | ||||
| 852 (53.3) | 881 (53.8) | 869 (53.5) | 878 (53.4) | ||
| 381 (23.8) | 418 (25.5) | 441 (28.8) | 474 (28.8) | ||
| 322 (20.1) | 286 (17.5) | 249 (15.3) | 214 (13.0) | ||
| 37 (2.3) | 46 (2.8) | 52 (3.2) | 56 (3.4) | ||
| 8 (0.5) | 7 (0.4) | 12 (0.7) | 22 (1.3) | ||
| Higher educational level, n (%) | 1534 (94.9) | 1558 (93.9) | 1487 (90.9) | 1498 (90.7) | <0.001 | <0.001 |
| Pre-diabetes, n (%) | 359 (22.2) | 498 (30.0) | 596 (36.4) | 811 (49.1) | <0.001 | <0.001 |
| Family history of DM, n(%) | 571 (35.7) | 610 (37.1) | 653 (40.3) | 656 (40.1) | 0.015 | 0.002 |
| Physically active, n(%) | 393 (24.7) | 342 (21.0) | 330 (20.4) | 266 (16.4) | <0.001 | <0.001 |
| Smoking, n (%) | 114 (23.0) | 181 (31.9) | 173 (29.2) | 202 (30.3) | 0.009 | 0.038 |
| Body mass index (kg/m2) | 24.7 (4.1) | 25.8 (4.5) a | 27.3 (4.9) a,b | 28.4 (4.9) a,b,c | <0.001 | <0.001 |
| Waist circumference (cm) | 80.5 (10.2) | 84.4 (11.3) a | 88.5 (11.5) a,b | 91.9 (11.4) a,b,c | <0.001 | <0.001 |
| Systolic BP (mmHg) | 113 (14.9) | 114 (15.4) a | 117 (16.1) a,b | 119 (15.7) a,b,c | <0.001 | <0.001 |
| Diastolic BP (mmHg) | 71 (9.6) | 72 (9.7) a | 74 (10.0) a,b | 76 (9.8) a,b,c | <0.001 | <0.001 |
| Total cholesterol (mg/dL) | 192.3 (33.9) | 197.2 (36.5) a | 204.2 (38.6) a,b | 212.7 (40.8) a,b,c | <0.001 | <0.001 |
| HDL-cholesterol (mg/dL) | 69.0 (11.7) | 60.0 (103) a | 55.3 (9.7) a,b | 48.8 (8.4) a,b,c | <0.001 | <0.001 |
| LDL-cholesterol (mg/dL) | 108.0 (29.0) | 118.0 (29.0) a | 124.6 (34.5) a,b | 127.3 (36.3) a,b,c | <0.001 | <0.001 |
| Triglycerides (mg/dL) # | 59.0 (12.8) | 81.8 (15.3) a | 108.4(21.2) a,b | 170.9 (54.5) | <0.001 | <0.001 |
| Fasting glucose (mg/dL) | 94.2 (7.6) | 96.0 (7.9) a | 97.6 (8.4) a,b | 99.9 (8.6) a,b,c | <0.001 | <0.001 |
| 2 h glucose (mg/dL) | 105.6 (23.7) | 113.0 (24.9) a | 120.6 (26.8) a,b | 128.8(27.1) a,b,c | <0.001 | <0.001 |
| HbA1c (%) | 5.1 (0.5) | 5.1 (0.5) | 5.2 (0.5) a | 5.2 (0.5) a,b | <0.001 | <0.001 |
| HOMA-IR # | 1.8 (1.0) | 2.3 (1.6) a | 2.8 (1.7) a,b | 3.4 (2.1) a,b,c | <0.001 | <0.001 |
| TG/HDL ratio | 0.9 (0.2) | 1.4 (0.1) a | 2.0 (0.2) a,b | 3.6 (1.3) a,b,c | <0.001 | <0.001 |
| Crude | Model 1 | Model 2 | |
|---|---|---|---|
| Men | HR (95%CI) | HR (95%CI) | HR (95%CI) |
| Q1 | 1 | 1 | 1 |
| Q2 | 1.75 (1.19–2.56) | 1.78 (1.21–2.60) | 1.39 (0.92–2.12) |
| Q3 | 3.07 (2.16–4.36) | 3.15 (2.22–4.48) | 1.82 (1.23–2.71) |
| Q4 | 3.95 (2.81–5.55) | 4.16(2.96–5.85) | 2.20 (1.49–3.26) |
| Women | |||
| Q1 | 1 | 1 | 1 |
| Q2 | 1.32 (0.93–1.89) | 1.30 (0,91–1,86) | 1.13 (0.78–1.65) |
| Q3 | 2.51 (1.83–3.46) | 2.38 (1.73–3.27) | 1.57 (1.11–2.22) |
| Q4 | 4.11 (3.04–5.55) | 3.87 (2.86–5.23) | 2.08 (1.48–2.93) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Almeida-Pititto, B.; Branda, J.I.; de Oliveira, J.M.; Dualib, P.M.; de Aquino Fernandes Dias, L.B.; Bensenor, I.M.; Lotufo, P.A.; Ferreira, S.R.G. The Predictive Value of the Triglycerides/HDL-Cholesterol Ratio for Diabetes Incidence. Endocrines 2024, 5, 418-429. https://doi.org/10.3390/endocrines5030031
de Almeida-Pititto B, Branda JI, de Oliveira JM, Dualib PM, de Aquino Fernandes Dias LB, Bensenor IM, Lotufo PA, Ferreira SRG. The Predictive Value of the Triglycerides/HDL-Cholesterol Ratio for Diabetes Incidence. Endocrines. 2024; 5(3):418-429. https://doi.org/10.3390/endocrines5030031
Chicago/Turabian Stylede Almeida-Pititto, Bianca, Julia Ines Branda, Julia M. de Oliveira, Patrícia M. Dualib, Luisa Bittencourt de Aquino Fernandes Dias, Isabela M. Bensenor, Paulo A. Lotufo, and Sandra Roberta G. Ferreira. 2024. "The Predictive Value of the Triglycerides/HDL-Cholesterol Ratio for Diabetes Incidence" Endocrines 5, no. 3: 418-429. https://doi.org/10.3390/endocrines5030031
APA Stylede Almeida-Pititto, B., Branda, J. I., de Oliveira, J. M., Dualib, P. M., de Aquino Fernandes Dias, L. B., Bensenor, I. M., Lotufo, P. A., & Ferreira, S. R. G. (2024). The Predictive Value of the Triglycerides/HDL-Cholesterol Ratio for Diabetes Incidence. Endocrines, 5(3), 418-429. https://doi.org/10.3390/endocrines5030031









