Abstract
Acromegaly is a rare endocrine syndrome characterized by unrestrained growth hormone (GH) secretion from a GH-secreting pituitary neuroendocrine tumor (PitNET). Data on sleep disorders are scanty and mainly linked to Obstructive Sleep Apnea Syndrome (OSAS). This study aimed to evaluate the prevalence of insomnia and sleep quality in a cohort of patients with a low risk of OSAS before and after therapies for acromegaly. A total of 27 naïve acromegalic patients (mean age 55.15 ± 10.53 years) were submitted to a psychometric sleep evaluation and compared to a matched control group of 24 Non-Functioning Pituitary micro-Adenoma patients (mean age 51.08 ± 11.02 years). A psychometric sleep evaluation was carried out 4 years later, after achieving acromegaly control in all patients. The role of different therapies for acromegaly (somatostatin analogues, pegvisomant, or adenomectomy) was evaluated. At the initial evaluation, most untreated acromegalic patients had a higher rate of impaired sleep quality and clinical insomnia than NFPA patients (p = 0.001 ES = 1.381, p = 0.001 ES = 1.654, respectively). Patients treated with somatostatin analogues or pituitary adenomectomy had an improvement in insomnia parameters (p = 0.046 ES = 0.777, p = 0.038 ES = 0.913, respectively). Conversely, in patients treated with pegvisomant, sleep quality and insomnia worsened (p = 0.028 ES = 1.002, p = 0.009 ES = 1.398, respectively). In summary, therapies for acromegaly seem to have divergent effects on perceived sleep disorders. Concerning sleep, somatostatin analogues and adenomectomy seem to have favorable effects on the psychometric parameters of sleep.
1. Introduction
Acromegaly is a rare endocrine syndrome, with an incidence of four to six subjects per million per year, sustained by excessive GH secretion due to a PitNET. This results in increased serum Insulin-like Growth Factor 1 (IGF-1) concentrations, which, in turn, suppresses hypothalamic GHRH secretion [1]. Acromegaly can be treated with different alternative therapies: pituitary adenomectomy, radiotherapy, cabergoline, long-acting somatostatin analogues, or pegvisomant [2]. Pegvisomant is a selective antagonist of the GH receptor that reduces serum IGF-1 concentrations by inhibiting GH receptor dimerization while leaving GH pituitary secretion and serum levels unaltered [3,4].
Acromegaly is considered in remission or controlled, on a biochemical ground, when serum IGF-1 concentrations are within the normal range for the age. Importantly, pegvisomant does not cross the blood–brain barrier [5] due to its large molecular size and, consequently, it primarily targets peripheral GH receptors, leaving GH receptors in the Central Nervous System (CNS) free to respond to circulating GH.
In contrast, somatostatin analogues may cross the blood–brain barrier. This allows them to exert modulatory effects on the CNS, effectively achieving a balanced modulation of hormones participating in the regulation of GH [6]. Similarly, pituitary adenomectomy, when effective, can restore physiological Somatotropic Axis control and normalize hormone levels [7].
Although acromegalic patients have several systemic complications [1], data on sleep disorders are scanty [8,9] and mainly related to sleep apnea [10]. The relationship between sleep and the Somatotropic Axis is complex and bidirectional. Physiologically, hypothalamic neuropeptide Growth Hormone Releasing Hormone (GHRH) induces Slow Wave Sleep (SWS) and pituitary GH secretion [11]. Indeed, GH pulses are higher during SWS sleep and the removal of GHRH function alters the sleep cycle as well as GH release [12]. GH and its peripheral effector Insulin-like Growth Factor 1 (IGF-1) exert a negative feedback on hypothalamic GHRH-secreting neurons [13]. On this basis, it is expected that acromegalic patients have sleep disruption, owing to low GHRH and increased serum GH and IGF-1 concentrations, even in the absence of Obstructive Sleep Apnea Syndrome (OSAS). Moreover, the chronic excess of GH and IGF-1 is responsible for physical changes such as facial features, the enlargement of extremities, and soft tissue swelling. These abnormalities can contribute to breathing-related sleep disorders through high-frequency OSAS [14], a leading cause of sleep disorders and a risk factor for cardiometabolic disease in the general population. The tight link between sleep and the Somatotropic Axis is further supported by the inhibitory effect of OSAS on GH secretion [15].
Therefore, this study aimed to evaluate the extent of insomnia and sleep quality in a cohort of untreated acromegalic patients. To minimize the impact of sleep apnea on sleep, only patients with a low risk of OSAS were enrolled in the present study. The study followed patients for years after diagnosis to investigate patterns of sleep as a function of treatments.
2. Methods
2.1. Study Design and Population
This is a historical prospective study conducted from 2017 to 2022, in which sleep quality and insomnia were evaluated in a cohort of 55 consecutive naive acromegalic patients referred to the Endocrinology Unit of the University of Pisa. Also, a control group of 25 subjects with Non-Functioning Pituitary micro-Adenoma (NFPA) was enrolled.
Criteria that precluded study participation were psychiatric diseases, medical history of brain injuries, cerebrovascular or neurological disorders, high risk of OSAS, and history of drugs or alcohol abuse. OSAS risk was assessed for both groups using the Berlin questionnaire [16,17].
The study adhered to the guidelines of Helsinki Declaration, and it received approval from the internal board of the Department of Clinical and Experimental Medicine at Pisa University. Informed written consent was obtained from all participants prior to their enrollment, ensuring their voluntary participation and understanding of the study’s procedures and potential risks.
2.2. Diagnosis of Acromegaly
All patients who participated in the study underwent a detailed baseline evaluation at the Endocrinology Unit of the University of Pisa. The evaluation included clinical, biochemical, and radiological assessments, involving a pituitary MRI. To assess pituitary function in both acromegalic patients and controls, baseline, fasting 8 AM serum levels of gonadotropins, estradiol (in females) or testosterone (in males), prolactin, free Thyroxine (fT4), free Triiodothyronine (fT3), Thyrotropin (TSH), and plasma ACTH were measured. In all subjects, serum levels of GH and IGF-1 were measured twice in the morning in fasting conditions. Also, a standard ACTH (cosyntropin 250 mcg iv) stimulation test with cortisol sampling was performed in both acromegalic and NFPA patients. This test was conducted on all patients as a routine clinical practice to thoroughly evaluate pituitary function at diagnosis and after surgery. A standard oral glucose tolerance test (OGTT) was performed in acromegalic patients with serum GH samples.
The diagnostic criteria for acromegaly were based on clinical symptoms, consistently elevated serum IGF-1 levels that exceeded age-specific range, and the inability of an OGTT to suppress serum GH levels below 0.4 mcg/dL [18].
The attainment of disease control in acromegalic treated patients was defined by maintaining serum IGF-1 concentrations within the age-appropriate normal range. This criterion was applied following pituitary adenomectomy or during the course of treatment with long-acting somatostatin analogues or pegvisomant therapy, with a minimum treatment duration of 6 months.
The diagnosis of pituitary defects followed international guidelines. Central hypothyroidism was diagnosed by low Thyroxine levels, and inappropriately low or normal TSH levels. In pre-menopausal women, hypogonadotropic hypogonadism was diagnosed by low FSH and estradiol levels in the presence of amenorrhea and normal serum prolactin levels. In post-menopausal women, the diagnosis relied solely on FSH levels. For males, hypogonadotropic hypogonadism was diagnosed when gonadotropin and testosterone levels were below the normal range. Total testosterone levels were measured, and free testosterone was subsequently calculated using the ISSAM calculator. Central hypoadrenalism was identified by a serum cortisol response to the cosyntropin stimulation test lower than 18.0 mcg/dL, along with low-to-normal plasma ACTH levels.
In controls, confirmation of non-functioning pituitary adenomas was established through basal pituitary profiles, as described.
Hormonal Assays
Serum GH concentrations were assessed using the Access Ultrasensitive chemiluminescent human growth hormone (hGH) assay (Beckman Coulter, Fullerton, CA, USA), calibrated against IS 98/574 standards [19]. The assay’s lower limit of quantification (LOQ) was established at 0.05 µg/L. Intra-assay coefficients of variation ranged from 1.48% to 11.26%, while inter-assay coefficients of variation spanned from 1.96% to 14.4%. Our laboratory’s normative values for GH concentrations are as follows: males 0.01–0.97 µg/L, females 0.01–3.61 µg/L.
Serum IGF1 levels were determined through the IDS-iSYS IGF1 assay, an automated chemiluminescence immunoassay calibrated against IS 02/254 standards [20]. The LOQ for this IGF1 assay was set at 8.8 µg/L. Intra-assay coefficients of variation ranged from 1.9% to 4.2%, and inter-assay coefficients of variation ranged from 3.9% to 7.2%. Specific cut-off values for adults were derived from previous normative studies and were age dependent. The normal range for adults in our laboratory can be provided upon request. All other hormonal assays were performed using standard methods at the Endocrinology laboratory of Azienda-Ospedaliero Universitaria Pisana.
2.3. Sleep Assessment
Validated questionnaires were used to perform comprehensive assessments of sleep parameters. These included the following: (a) The Pittsburgh Sleep Quality Index (PSQI) which evaluates sleep quality based on subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disorders, use of sleep medications, and daytime dysfunction. Scores above 5 indicate poor sleep quality [21,22]. (b) The Insomnia Severity Index (ISI) which assesses the severity and impact of insomnia symptoms through seven items that measure the perceived severity of difficulties falling asleep, staying asleep, and the overall satisfaction with sleep. Scores above 7 indicate the presence of insomnia [23,24]. (c) The Ford Insomnia Response to Stress Test (FIRST) which investigates the relationship between stress and insomnia symptoms through 10 items that capture the individual’s perception of how stress impacts their ability to fall asleep, stay asleep, and their overall sleep quality. Scores above 18 indicate “stress-related sleep reactivity” [25,26]. (d) The Epworth Sleepiness Scale (ESS) which assesses the daytime sleepiness and propensity to doze off in various situations. This consists of eight items that measure the likelihood of falling asleep or feeling drowsy during activities such as sitting and reading, watching TV, or in social situations. Scores above 10 indicate increased daytime sleepiness compared to physiological levels [27,28].
2.4. Study Procedure
All patients underwent evaluation at the time of the acromegaly diagnosis (first visit) prior to any specific medical or surgical intervention. The acromegalic group was then compared to a control group of NFPA patients to estimate the extent of sleep impairment.
Following the initial evaluation and comparison with the control group, it was questioned whether achieving acromegaly control, via different therapies, could improve sleep quality. Therefore, the patients underwent a second sleep evaluation after at least 12 months of biochemical monitoring following the achievement of acromegaly control (last visit). Therapies for acromegaly were based on clinical aspects, independently of sleep disorders. Therapies were the following: (a) pituitary adenomectomy; (b) long-acting somatostatin analogues (Lanreotide autogel or Octreotide); (c) pegvisomant. Therefore, to explore the role of different therapies on sleep disorders, acromegalic patients were stratified into groups based on the specific therapeutic interventions they received: pegvisomant (PEG), long-acting somatostatin analogues (SSAs), or pituitary adenomectomy (Hx) (see Supplementary Material for the study flow chart).
2.5. Statistical Analysis
Data management and analysis were performed using Matlab (R2017b, Mathworks Inc., Natick, Massachusetts, US).
Group comparisons were conducted based on appropriate statistical tests: we used χ2 test, Independent Sample t-test, and one-way Analysis Of Variance (ANOVA), while follow-up comparisons were tested through Paired Samples t-test. The normality of continuous data and the homogeneity of the variance were tested by the Shapiro–Wilk test and Levene’s test, respectively.
Qualitative values were reported using numbers and percentages, while mean and standard deviation were used for quantitative values.
For each variable, we derived the effect size (ES) [29] and its magnitude was interpreted according to Cohen classification: 0.0–0.1 (no effect), 0.2–0.4 (small effect), 0.5–0.7 (intermediate effect), and 0.8–1 (large effect) [30].
Statistical significance was set at p < 0.05, each post hoc p-value was adjusted for multiple comparisons (HDS of Turkey), and all tests were 2-tailed.
3. Results
3.1. Clinical and Biochemical Features
Out of the initial cohort of fifty-five acromegalic patients, twenty-eight (50.9%) were excluded from the study due to the high risk of OSAS based on the Berlin questionnaire. Likewise, a patient of the NFPA group was excluded for a positive Berlin index.
The remaining patients included twenty-seven naïve acromegalic patients (12 men and 15 women) with a mean age of 55.2 ± 10.6, and twenty-four NFPA patients (9 men and 15 women) with a mean age of 51.1 ± 11.0 completed the initial evaluation. None of the patients in either group were taking medication for the treatment of insomnia or other sleep disorders. The acromegalic and NFPA patients did not differ in age, sex, or educational level. As expected, the two groups differed in terms of biochemical parameters related to acromegaly, specifically GH and IGF-1 concentrations. Of the 27 patients with acromegaly, 8 were treated exclusively with somatostatin analogues, achieving excellent biochemical control of the disease. The remaining 19 patients underwent surgery. Of these, 12 had persistent disease and undertook therapy with pegvisomant, and 2 undertook therapy with somatostatin analogues. Pegvisomant therapy was always administered as monotherapy. No patient had optic chiasm compression. The mean follow-up time was 44 months (±11 months), while the mean duration to achieve biochemical control was 12 months (±1 month). Demographic and clinical data are reported in Table 1.

Table 1.
Demographic and clinical characteristics of experimental and control groups.
3.2. Sleep Evaluation: Baseline Sleep Assessment and Group Comparison
At the first evaluation, the response rate for acromegalic patients was 49.1% (27 out of 55), while the response rate for NFPA patients was 96% (24 out of 25). Acromegalic patients had significantly higher mean scores on three out of four sleep questionnaires compared to NFPA patients (PSQI p-value < 0.001, ES = 1.381; ISI p-value < 0.001, ES = 1.654; ESS p-value < 0.013, ES = 0.719) (Table 2).

Table 2.
Sleep comparison at first clinical visit between acromegaly and NFPA.
The majority of naïve acromegalic patients experienced disrupted sleep patterns: 74.07% had alterations in sleep quality, 70.37% had clinical insomnia, 48.14% heightened sleep reactivity to stress, and 25.29% reported daytime drowsiness. Furthermore, the percentage of sleep quality alteration and clinical insomnia was significantly higher in acromegalic patients than in NFPA patients (PSQI p-value < 0.001, ISI p-value < 0.001) (Table 3).

Table 3.
Comparison of the percentage of sleep alteration at the first clinical visit between acromegaly and NFPA.
3.3. Sleep Evaluation: Treatment-Based Comparison at Last Evaluation
At the last clinic visit, all patients had controlled acromegaly (IGF-1 index = 0.85 ± 0.15), and the acromegalic response rate on sleep evaluation was 96.29% (26 out of 27 patients).
Post hoc comparisons revealed that patients treated with pegvisomant had worse sleep quality (PSQI, mean difference = 5.669, p-value < 0.001) and higher insomnia severity (ISI, mean difference = 9.913, p-value < 0.0001) compared to patients treated with somatostatin analogues.
Similarly, patients undergoing pegvisomant therapy had worse sleep quality (PSQI, mean difference = 4.735, p-value < 0.016) and higher insomnia severity (ISI, mean difference = 11.463 p-value < 0.001) compared to patients who underwent surgery. No statistically significant difference was observed between the patients treated with somatostatin analogues and the patients undergoing surgery, either in terms of sleep quality or severity of insomnia (Table 4).

Table 4.
ANOVA post hoc comparison between treatment-based groups at last clinical visit.
3.4. Sleep Evaluation: Treatment-Based Sleep Characterization
To investigate the progression of sleep disorders during the treatment of acromegaly, a comparison was conducted for each group of patients between the period before they underwent treatment and the period after achieving disease control.
As shown in Table 5, a statistically significant reduction in insomnia severity was found both in the SSA (ISI, p-value < 0.046, ES = 0.77) and in Hx (ISI, p-value < 0.038, ES = 0.91). No other significant differences were found for the other sleep parameters, indicating their stability between the two visits.

Table 5.
Sleep evaluation of acromegalic patients based on the type of treatment for acromegaly: comparison between first evaluation and the last clinical visit.
In contrast, sleep in the group of patients receiving pegvisomant showed a different trend (Table 5). During the interval between the initial clinical evaluation and the concluding visit, there was a statistically significant increase in the alteration in sleep quality (PSQI, p-value < 0.028; ES = 1.00) and in the severity of insomnia (ISI, p-value < 0.009; ES = 1.39).
4. Discussion
The present study retrospectively investigated sleep disorders in patients with acromegaly and their longitudinal changes after achieving disease control with different therapies.
Naïve acromegalic patients frequently had insomnia and a notable alteration in sleep quality, which, conversely, was absent in the control group. It is noteworthy that all patients included in the present study had a low risk of OSAS based on the Berlin questionnaire.
Data on the role of the Somatotropic Axis in the regulation of sleep suggest that GHRH, the hormone responsible for stimulating the pituitary gland to release GH, represents the endocrine sleep-promoting marker. As a matter of fact, GHRH promotes Non-Rapid Eye Movement (NREM) sleep through a hypothalamic mechanism involving GABAergic neurons situated in the medial preoptic area [31]. It has been shown that micro-injections of GHRH in the anterior hypothalamus and the medial preoptic region (AH/MPO) result in a dose-dependent increase in the duration and intensity of NREM sleep [32]. Actually, in normal physiology, the maximal GH secretory burst occurs within minutes after the first period of SWS [33].
In addition, administering GHRH receptor antagonists locally in the same sites reduces spontaneous NREM sleep and delta wave power [11].
Moreover, GH has been associated with the Rapid Eye Movement (REM) phase of sleep, whereas IGF-1 appears to have a complex influence on sleep architecture by affecting orexin neurons, which play a crucial role in facilitating and sustaining wakefulness [34]. Here, it is hypothesized that disruption of neuroregulation of the Somatotropic Axis, resulting in reduced pulsatile GH secretion and persistently elevated serum levels throughout the day [20,21,22], could lead the high levels of GH/IGF-1 to exert constant negative feedback on GHRH secretion, thus potentially explaining the observed sleep alteration. Indeed, our findings revealed a significant difference in sleep dynamics between patients receiving pegvisomant therapy compared to those receiving somatostatin analogues or undergoing pituitary surgery (Figure 1). It should be pointed out that, during the last 6 months leading up to their most recent visit, all patients consistently maintained their serum IGF-1 levels within the normal range for the age. Specifically, patients undergoing pegvisomant treatment not only experienced more pronounced sleep impairment compared to those undergoing other therapies, but this impairment worsened over time. In contrast, sleep disorders in patients receiving somatostatin analogues or undergoing pituitary surgery typically exhibit an improvement trend, falling below the clinical cut-off for the ISI questionnaire. This raises an interesting question regarding the underlying mechanisms that may contribute to sleep alterations in acromegaly, particularly in patients treated with pegvisomant since this drug does not cross the blood–brain barrier.
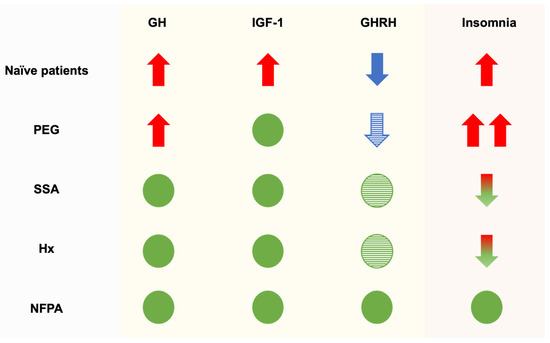
Figure 1.
Proposed mechanisms of sleep alteration in acromegalic patients. Naïve patients: acromegalic patients at the first diagnosis; PEG: acromegalic patients undergoing pegvisomant therapy; SSA: acromegalic patients undergoing long-acting somatostatin analogue therapy; Hx: acromegalic patients undergoing pituitary surgery; NFPA: Non-Functioning Pituitary micro-Adenoma patients. Red arrow: high levels; blue arrow: low levels; green circle: normal level (absence of insomnia); red and green arrows represent elevated values that are gradually reverting to their baseline levels; striped blue arrow: likely low levels; striped green circle: likely normal levels. The proposed model considers hormonal dynamics at the brain level.
Patients treated with pegvisomant may have the worst combination of GH and IGF-1 levels in the brain for sleep rhythms. Considering naïve acromegalic patients, they had high GH and IGF-1 concentrations in peripheral tissues and in the brain. Those cured with surgery or under somatostatin analogue therapy similarly had normal GH and IGF-1 concentrations in peripheral tissues and in the brain. Conversely, patients treated with pegvisomant achieved normal IGF-1 concentrations in peripheral tissue, where GH action is inhibited. However, the brain was exposed to normal circulating IGF-1 levels and unrestricted action of GH levels. Thus, it looks like a combination of normal IGF-1 and high GH concentrations could be a worse association than high GH and IGF-1 levels, the last observed in naïve patients (Figure 1). This hypothesis should be further confirmed in a large sample of acromegalic patients.
5. Limitation
The main limitations of the study are the lack of polysomnography for the exclusion of OSAS and the small sample size. We could assume that some patients with OSAS were not correctly identified by the Berlin questionnaire. However, the effect of different therapies for acromegaly on sleep was observed longitudinally and was reinforced by achieving control of acromegaly, which may improve sleep disorders attributed to the potential presence of OSAS. A further limitation could be attributed to the presence of hypopituitarism (25.92%), which could impact sleep dynamics. However, during the initial visit, a baseline assessment of pituitary function was performed and any hormone deficiencies were corrected with hormone replacement therapy.
6. Conclusions
In summary, the results of our study indicate that untreated acromegalic patients with a low risk of OSAS consistently exhibited sleep disorders. Significantly, the choice of therapy for acromegaly exerted a different impact on sleep despite normal serum IGF-1 levels. Our efforts to enroll patients with a low risk of OSAS were meticulous. Importantly, the longitudinal evaluation of patients ensured that our findings regarding the impact of therapies remained sound, unaffected by the initial selection criteria. It is worth noting that, despite achieving disease control and thereby reducing the potential presence of OSAS (if the Berlin questionnaire yielded some false negatives), patients treated with pegvisomant still reported worsening sleep indices.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/endocrines5030030/s1, Supplementary File “study flow chart”.
Author Contributions
G.A., F.B. and A.G. conceived and planned the experiment. G.A. performed sleep assessment and together with D.M. performed statistical analyses. G.M., C.U. and F.B. took care of the diagnosis and management of acromegaly, and patient enrollment. D.A.C. and V.D.G. contributed to data collection. F.B. and A.G. served as the supervisors for the entire project, and together with D.M. reviewed the manuscript in its entirety. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was approved by the internal board of the Department of Clinical and Experimental Medicine at Pisa University N19/2013.
Informed Consent Statement
Informed consent was obtained from all participants involved in the study.
Data Availability Statement
The data of this study are available from the corresponding author, upon reasonable request.
Conflicts of Interest
The authors report no competing interests.
References
- Dineen, R.; Stewart, P.M.; Sherlock, M. Acromegaly—Diagnosis and Clinical Management. QJM Int. J. Med. 2016, 110, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Barkhoudarian, G.; Beckers, A.; Ben-Shlomo, A.; Biermasz, N.; Biller, B.; Boguszewski, C.; Bolanowski, M.; Bollerslev, J.; Bonert, V.; et al. Multidisciplinary management of acromegaly: A consensus. Rev. Endocr. Metab. Disord. 2020, 21, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Kopchick, J.J.; Parkinson, C.; Stevens, E.C.; Trainer, P.J. Growth Hormone Receptor Antagonists: Discovery, Development, and Use in Patients with Acromegaly. Endocr. Rev. 2002, 23, 623–646. [Google Scholar] [CrossRef]
- Tritos, N.A.; Chanson, P.; Jimenez, C.; King, D.; Jönsson, P.J.; Klibanski, A.; Biller, B.M.K. Effectiveness of first-line pegvisomant monotherapy in acromegaly: An ACROSTUDY analysis. Eur. J. Endocrinol. 2017, 176, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Veldhuis, J.D.; Bidlingmaier, M.; Bailey, J.; Erickson, D.; Sandroni, P. A Pegylated Growth Hormone Receptor Antagonist, Pegvisomant, Does Not Enter the Brain in Humans. J. Clin. Endocrinol. Metab. 2010, 95, 3844–3847. [Google Scholar] [CrossRef]
- Song, Y.H.; Yoon, J.; Lee, S.H. The role of neuropeptide somatostatin in the brain and its application in treating neurological disorders. Exp. Mol. Med. 2021, 53, 328–338. [Google Scholar] [CrossRef]
- Bray, D.P.; Mannam, S.; Rindler, R.S.; Quillin, J.W.; Oyesiku, N.M. Surgery for acromegaly: Indications and goals. Front. Endocrinol. 2022, 13, 924589. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, N.; Min, K.T.; Kim, E.H.; Oh, H.; Choi, S.H. Sleep disturbance and delirium in patients with acromegaly in the early postoperative period after transsphenoidal pituitary surgery. Medicine 2020, 99, e23157. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, X.; Guo, J.; Wang, L.; Zhao, H.; Wang, Y.; Wang, J.; Sun, X.; Jiang, W.; Liu, G.; et al. Sleep quality in acromegaly and changes after transsphenoidal surgery: A prospective longitudinal study. Sleep Med. 2020, 67, 164–170. [Google Scholar] [CrossRef]
- Cho, J.; Kim, J.H.; Kim, Y.H.; Lee, J. Obstructive Sleep Apnea Screening and Effects of Surgery in Acromegaly: A Prospective Study. Endocrinol. Metab. 2024, 39, 641–652. [Google Scholar] [CrossRef]
- Peterfi, Z.; McGinty, D.; Sarai, E.; Szymusiak, R. Growth hormone-releasing hormone activates sleep regulatory neurons of the rat preoptic hypothalamus. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2010, 298, R147–R156. [Google Scholar] [CrossRef] [PubMed]
- Van Cauter, E.; Plat, L. Physiology of growth hormone secretion during sleep. J. Pediatr. 1996, 128, S32–S37. [Google Scholar] [CrossRef] [PubMed]
- Müller, E.E.; Locatelli, V.; Cocchi, D. Neuroendocrine Control of Growth Hormone Secretion. Physiol. Rev. 1999, 79, 511–607. [Google Scholar] [CrossRef] [PubMed]
- Parolin, M.; Dassie, F.; Alessio, L.; Wennberg, A.; Rossato, M.; Vettor, R.; Maffei, P.; Pagano, C. Obstructive Sleep Apnea in Acromegaly and the Effect of Treatment: A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2020, 105, e23–e31. [Google Scholar] [CrossRef] [PubMed]
- Hoyos, C.M.; Killick, R.; Keenan, D.M.; Baxter, R.C.; Veldhuis, J.D.; Liu, P.Y. Continuous Positive Airway Pressure Increases Pulsatile Growth Hormone Secretion and Circulating Insulin-like Growth Factor-1 in a Time-Dependent Manner in Men With Obstructive Sleep Apnea: A Randomized Sham-Controlled Study. Sleep 2014, 37, 733–741. [Google Scholar] [CrossRef]
- Netzer, N.C.; Stoohs, R.A.; Netzer, C.M.; Clark, K.; Strohl, K.P. Using the Berlin Questionnaire to Identify Patients at Risk for the Sleep Apnea Syndrome. Ann. Intern. Med. 1999, 131, 485. [Google Scholar] [CrossRef]
- Gassino, G.; Cicolin, A.; Erovigni, F.; Carossa, S.; Preti, G. Obstructive sleep apnea, depression, and oral status in elderly occupants of residential homes. Int. J. Prosthodont. 2005, 18, 316–322. [Google Scholar]
- Giustina, A.; Barkan, A.; Beckers, A.; Biermasz, N.; Biller, B.M.K.; Boguszewski, C.; Bolanowski, M.; Bonert, V.; Bronstein, M.D.; Casanueva, F.F.; et al. A Consensus on the Diagnosis and Treatment of Acromegaly Comorbidities: An Update. J. Clin. Endocrinol. Metab. 2020, 105, e937–e946. [Google Scholar] [CrossRef]
- Somatropin WHO International Standard 98/574. Available online: https://nibsc.org/products/brm_product_catalogue/detail_page.aspx?catid=98/574 (accessed on 4 September 2024).
- WHO International Standard Insulin-Like Growth Factor-I, Recombinant, Human, for Immunoassay NIBSC Code: 02/254. Available online: https://nibsc.org/documents/ifu/02-254.pdf (accessed on 4 September 2024).
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Curcio, G.; Tempesta, D.; Scarlata, S.; Marzano, C.; Moroni, F.; Rossini, P.M.; Ferrara, M.; De Gennaro, L. Validity of the Italian Version of the Pittsburgh Sleep Quality Index (PSQI). Neurol. Sci. 2013, 34, 511–519. [Google Scholar] [CrossRef]
- Morin, C.M. Insomnia: Psychological Assessment and Management; Guilford: New York, NY, USA, 1993. [Google Scholar]
- Castronovo, V.; Galbiati, A.; Marelli, S.; Brombin, C.; Cugnata, F.; Giarolli, L.; Anelli, M.M.; Rinaldi, F.; Ferini-Strambi, L. Validation study of the Italian version of the Insomnia Severity Index (ISI). Neurol. Sci. 2016, 37, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Drake, C.; Richardson, G.; Roehrs, T.; Scofield, H.; Roth, T. Ford Insomnia Response to Stress Test; American Psychological Association: Washington, DC, USA, 2012. [Google Scholar] [CrossRef]
- Palagini, L.; Bruno, R.M.; Paolo, T.; Caccavale, L.; Gronchi, A.; Mauri, M.; Riemann, D.; Drake, C.L. Association between Stress-Related Sleep Reactivity and Metacognitive Beliefs about Sleep in Insomnia Disorder: Preliminary Results. Behav. Sleep Med. 2016, 14, 636–649. [Google Scholar] [CrossRef] [PubMed]
- Johns, M.W. A New Method for Measuring Daytime Sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Vignatelli, L.; Plazzi, G.; Barbato, A.; Ferini-Strambi, L.; Manni, R.; Pompei, F.; D’Alessandro, R. Italian version of the Epworth sleepiness scale: External validity. Neurol. Sci. 2003, 23, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect size estimates: Current use, calculations, and interpretation. J. Exp. Psychol. Gen. 2012, 141, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: London, UK, 2013. [Google Scholar] [CrossRef]
- Obal, F.; Krueger, J.M. GHRH and sleep. Sleep Med. Rev. 2004, 8, 367–377. [Google Scholar] [CrossRef]
- Zhang, J.; Obál, F.; Zheng, T.; Fang, J.; Taishi, P.; Krueger, J.M. Intrapreoptic Microinjection of GHRH or Its Antagonist Alters Sleep in Rats. J. Neurosci. 1999, 19, 2187–2194. [Google Scholar] [CrossRef]
- Chennaoui, M.; Léger, D.; Gomez-Merino, D. Sleep and the GH/IGF-1 axis: Consequences and countermeasures of sleep loss/disorders. Sleep Med. Rev. 2020, 49, 101223. [Google Scholar] [CrossRef]
- Zegarra-Valdivia, J.A.; Pignatelli, J.; Fernandez de Sevilla, M.E.; Fernandez, A.M.; Munive, V.; Martinez-Rachadell, L.; Nuñez, A.; Aleman, I.T. Insulin-like growth factor I modulates sleep through hypothalamic orexin neurons. FASEB J. 2020, 34, 15975–15990. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).