Solution-Processable and Eco-Friendly Functionalization of Conductive Silver Nanoparticles Inks for Printable Electronics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Synthesis Methods
2.2. Characterization
3. Results and Discussion
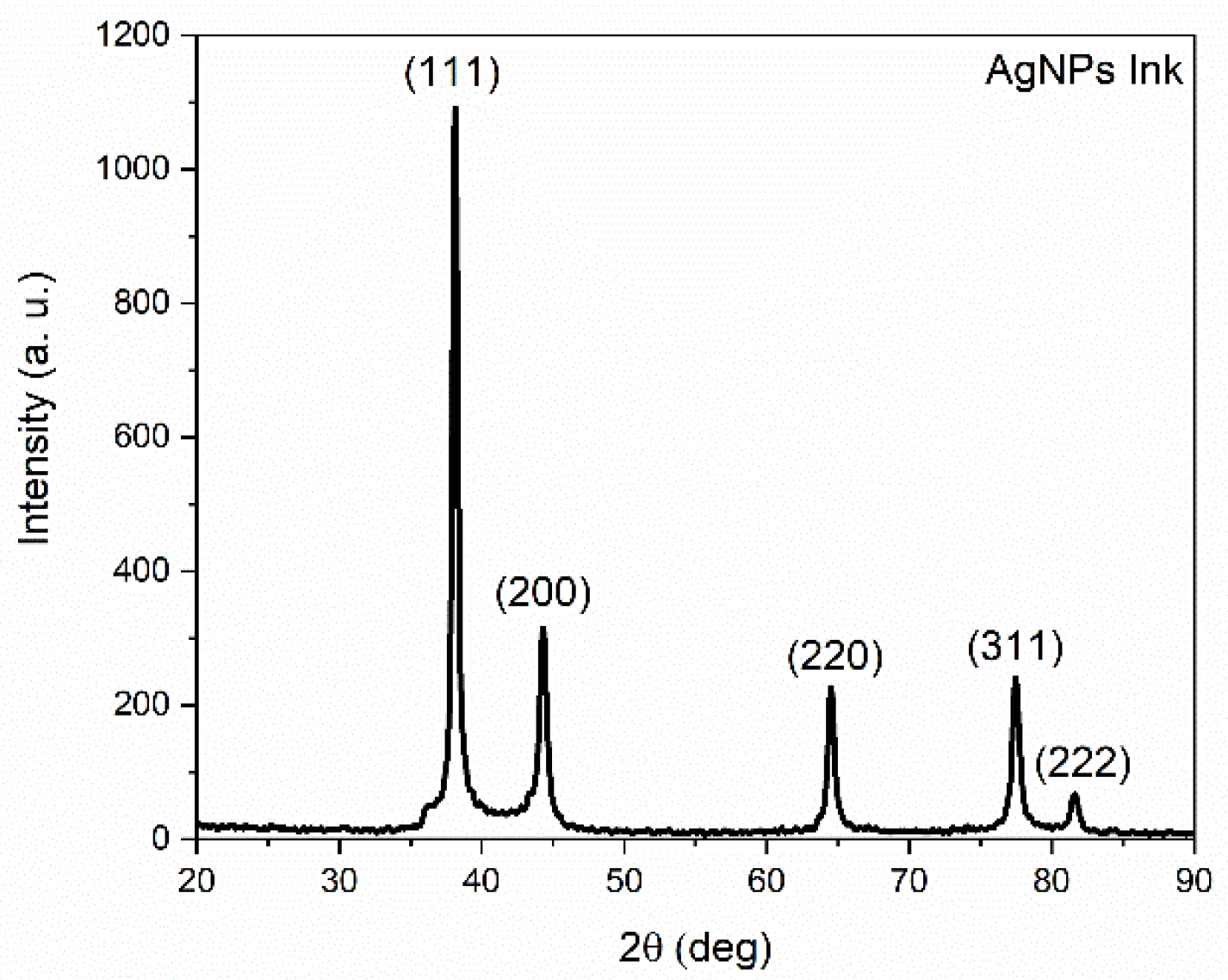
3.1. XRD Measurements
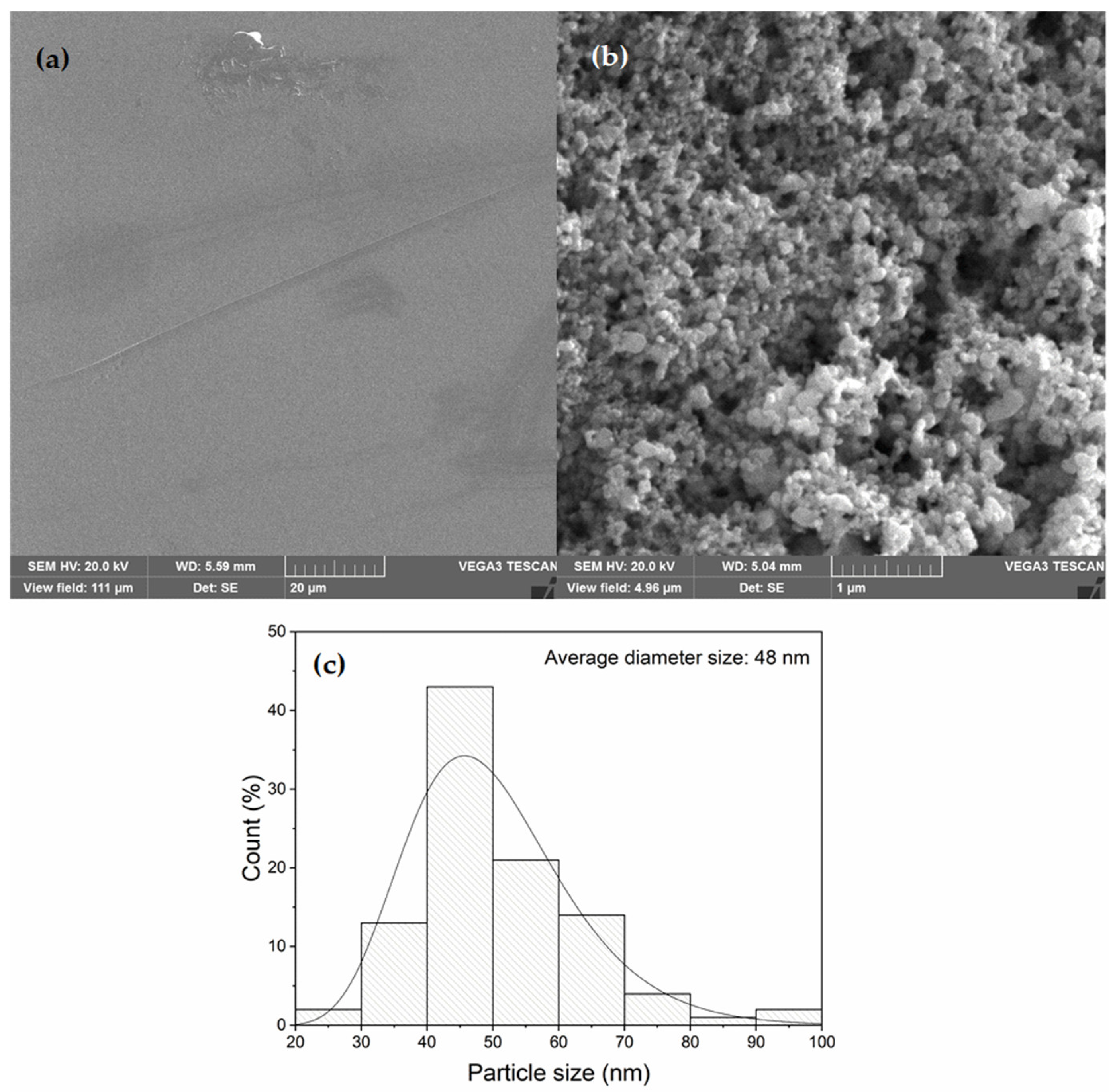
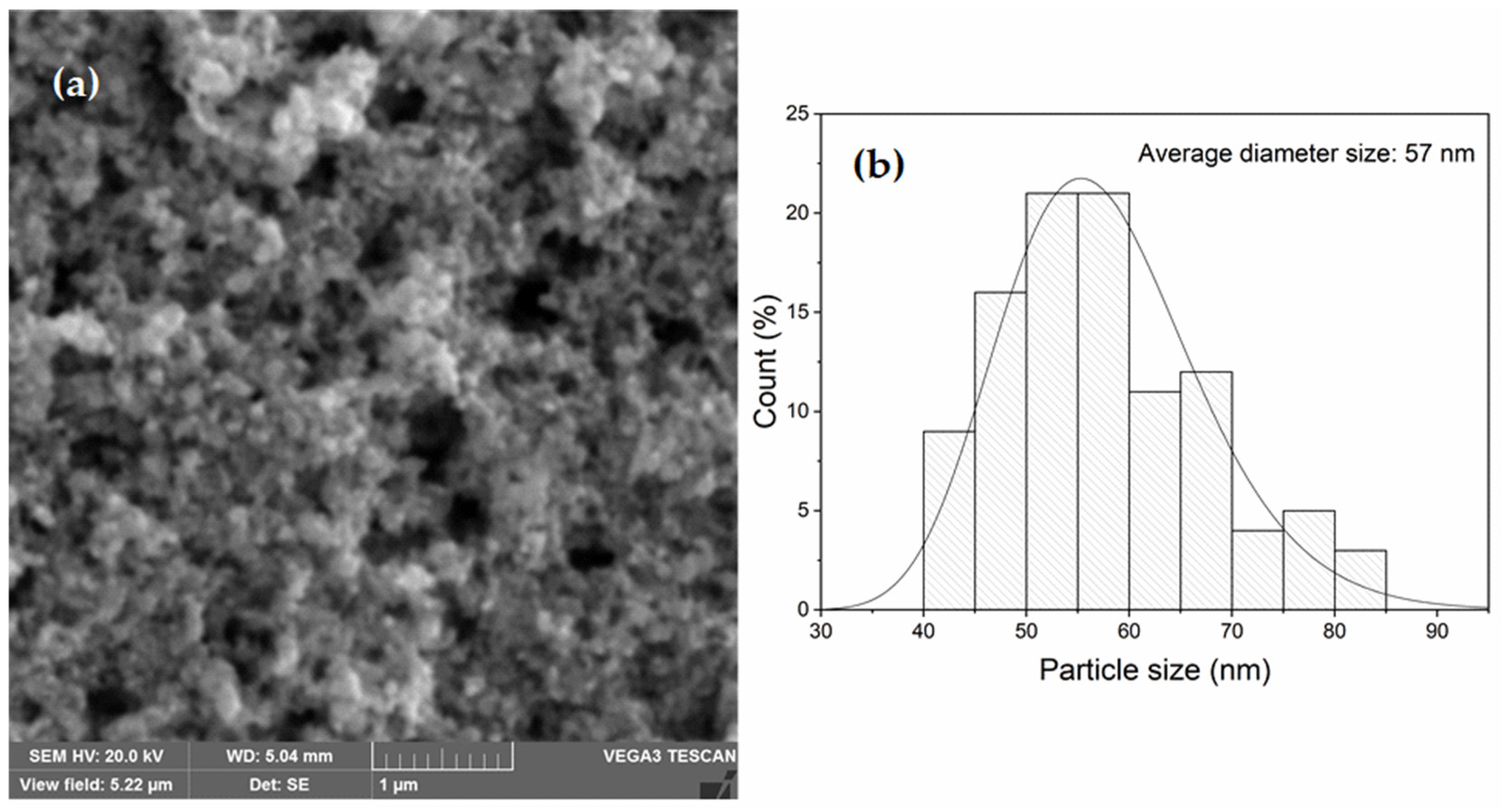
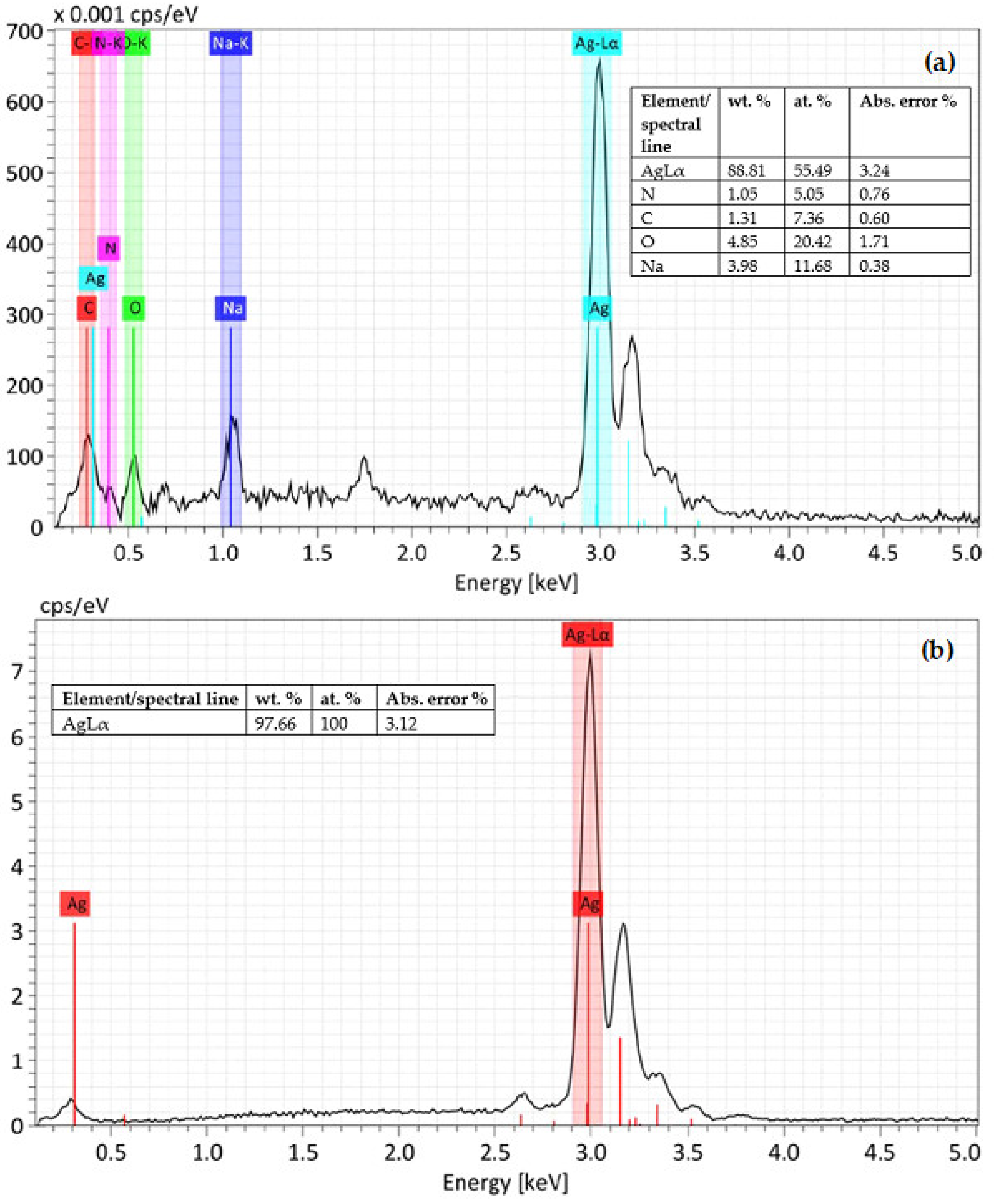
3.2. SEM/EDS Analysis
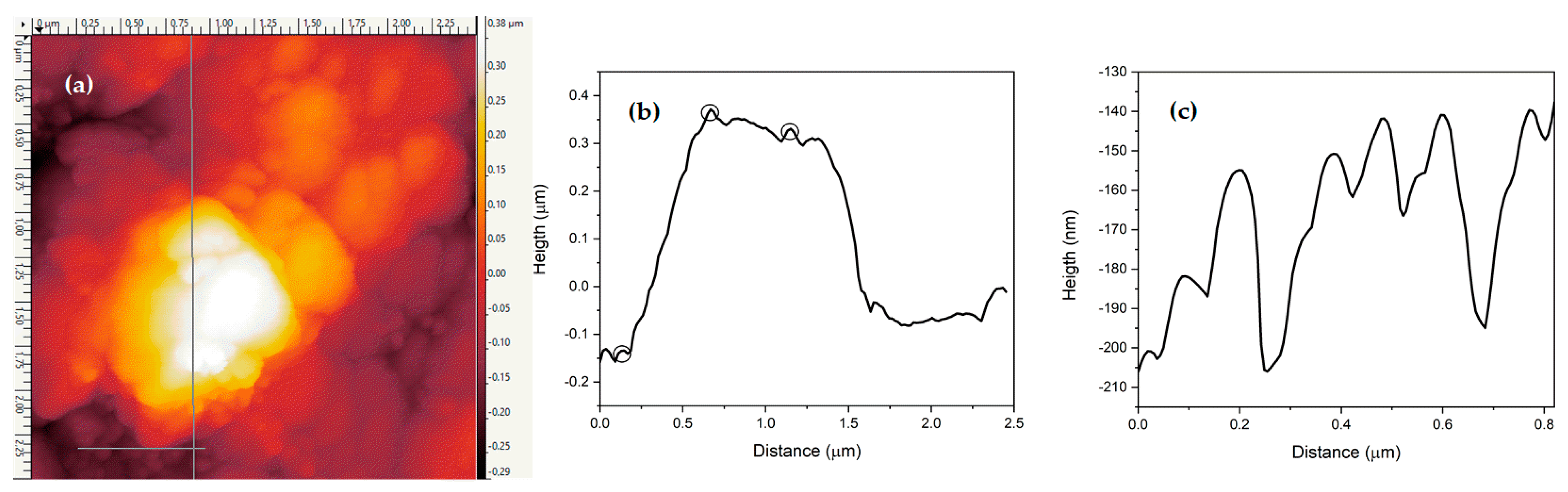
3.3. Topography Measurement by AFM
3.4. Dynamic Light Scattering (DLS) and Zeta Potential Analyses
3.5. Electrical Characterization and Testing after Bending
3.6. Application Perspective
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cano-Raya, C.; Denchev, Z.Z.; Cruz, S.F.; Viana, J.C. Chemistry of solid metal-based inks and pastes for printed electronics—A review. Appl. Mater. Today 2019, 29, 416–430. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, H.; Xing, L.; Bu, Q.; Ren, D.; Sun, B. Recent advances in inkjet-printing technologies for flexible/wearable electronics. Nanoscale 2023, 15, 6025–6051. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, Y.; Cai, Z.; Han, Z.; Liu, X.; Wang, F.; Cao, Y.; Wang, Z.; Li, H.; Chen, Y.; et al. Flexible Hybrid Electronics for Digital Healthcare. Adv. Mater. 2019, 32, 1902062. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.E. Flexible Hybrid Electronic Circuits and Systems. IEEE J. Emerg. Sel. 2017, 7, 27–37. [Google Scholar] [CrossRef]
- Li, Z.; Chang, S.; Khuje, S.; Ren, S. Recent Advancement of Emerging Nano Copper-Based Printable Flexible Hybrid Electronics. ACS Nano 2021, 15, 6211–6232. [Google Scholar] [CrossRef] [PubMed]
- Soum, V.; Lehmann, V.; Lee, H.; Khan, S.; Kwon, O.-S.; Shin, K. A Novel Polymeric Substrate with Dual-Porous Structures for High-Performance Inkjet-Printed Flexible Electronic Devices. Macromol. Mater. Eng. 2023, 308, 2300107. [Google Scholar] [CrossRef]
- Li, D.; Lai, W.Y.; Zhang, Y.-Z.; Huang, W. Printable Transparent Conductive Films for Flexible Electronics. Adv. Mater. 2018, 30, 17047738. [Google Scholar] [CrossRef]
- Zhan, N.; Huang, C.; Wan, S.; Kang, L.; Hu, M.; Zhang, Y.; Wu, X.; Zhang, J. A Novel Flexible Silver Heater Fabricated by a Solution-Based Polyimide Metalization and Inkjet-Printed Carbon Masking Technique. ACS Appl. Electron. Mater. 2019, 1, 928–935. [Google Scholar] [CrossRef]
- Mo, L.; Guo, Z.; Yang, L.; Zhang, Q.; Fang, Y.; Xin, Z.; Chen, Z.; Hu, K.; Han, L.; Li, L. Silver Nanoparticles Based Ink with Moderate Sintering in Flexible and Printed Electronics. Int. J. Mol. Sci. 2019, 20, 2124. [Google Scholar] [CrossRef]
- Arya, N.; Chandran, Y.; Singh, A.; Sharma, R.; Halder, A.; Balakrishnan, V. Substrate Versatile Roller Ball Pen Writing of Nanoporous MoS2 for Energy Storage Devices. ACS Appl. Mater. Interfaces 2023, 15, 41447–41456. [Google Scholar] [CrossRef]
- Ersu, G.; Sozen, Y.; Sánchez-Viso, E.; Kuriakose, S.; Juárez, B.H.; Mompean, F.J.; Garcia-Hernandez, M.; Visscher, L.; Magdaleno, A.J.; Prins, F.; et al. Pen Plotter as a Low-Cost Platform for Rapid Device Prototyping with Solution-Processable Nanomaterials. Adv. Eng. Mater. 2023, 25, 2300226. [Google Scholar] [CrossRef]
- Orrill, M.; Abele, D.; Wagner, M.J.; LeBlanc, S. Sterically Stabilized Multilayer Graphene Nanoshells for Inkjet Printed Resistors. Electron. Mater. 2021, 2, 394–412. [Google Scholar] [CrossRef]
- Campos-Martin, J.M.; Blanco-Brieva, G.; Fierro, J.L.G. Hydrogen Peroxide Synthesis: An Outlook beyond the Anthraquinone Process. Angew. Chem. Int. Ed. 2006, 45, 6962–6984. [Google Scholar] [CrossRef] [PubMed]
- Gatemala, H.; Ekgasit, S.; Wongravee, K. High purity silver microcrystals recovered from silver wastes by eco-friendly process using hydrogen peroxide. Chemosphere 2017, 178, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Mypati, S.; Dhanushkodi, S.R.; McLaren, M.; Docoslis, A.; Peppley, B.A.; Barz, D.P. Optimized inkjet-printed silver nanoparticle films: Theoretical and experimental investigations. RSC Adv. 2018, 8, 19679–19689. [Google Scholar] [CrossRef]
- Mahy, J.G.; Kiendrebeogo, M.; Farcy, A.; Drogui, P. Enhanced Decomposition of H2O2 Using Metallic Silver Nanoparticles under UV/Visible Light for the Removal of p-Nitrophenol from Water. Catalysts 2023, 13, 842. [Google Scholar] [CrossRef]
- Rajan, K.; Roppolo, I.; Chiappone, A.; Bocchini, S.; Perrone, D.; Chiolerio, A. Silver nanoparticle ink technology: State of the art. Nanotechnol. Sci. Appl. 2016, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nayak, L.; Mohanty, S.; Nayak, S.; Ramadoss, A. A review on inkjet printing of nanoparticles inks for flexible electronics. J. Mater. Chem. C. 2019, 7, 8771–8795. [Google Scholar] [CrossRef]
- Kabekkodu, S.N.; Faber, J.; Fawcett, T. New Powder Diffraction File (PDF-4) in relational database format: Advantages and data-mining capabilities. Acta Crystallogr. Sect. B Struct. Sci. 2002, 58, 333–337. [Google Scholar] [CrossRef]
- Nhugn, N.T.H.; Dat, N.T.; Thi, C.M.; Viet, P.V. Fast and simple synthesis of triangular silver nanoparticles under the assistance of light. Colloids Surf. A Physicochem. Eng. 2020, 594, 124659. [Google Scholar] [CrossRef]
- Adio, S.O.; Rana, A.; Chanabsha, B.; BoAli, A.A.K.; Essa, M.; Alsaadi, A. Silver Nanoparticle-Loaded Activated Carbon as an Adsorbent for the Removal of Mercury from Arabian Gas-Condensate. Arab. J. Sci. Eng. 2019, 44, 6285–6293. [Google Scholar] [CrossRef]
- Parnklang, T.; Lamlua, B.; Gatemala, H.; Thammacharoen, C.; Kuimalee, S.; Lohwongwatana, B. Shape transformation of silver nanospheres to silver nanoplates induced by redox reaction of hydrogen peroxide. Mater. Chem. Phys. 2015, 153, 127–134. [Google Scholar] [CrossRef]
- Meng, F.; Zhu, X.; Miao, P. Study of autocatalytic oxidation reaction of silver nanoparticles and the application for nonenzymatic H2O2 assay. Chem. Phys. Lett. 2015, 635, 213–216. [Google Scholar] [CrossRef]
- Wang, G.-L.; Zhu, X.-Y.; Dong, Y.-M.; Jiao, H.-J.; Wu, X.-M.; Li, Z.-J. The pH-dependent interaction of silver nanoparticles and hydrogen peroxide: A new platform for visual detection of iodide with ultra-sensitivity. Talanta 2013, 107, 146–153. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Garg, S.; Waite, D. H2O2-Mediated Oxidation of Zero-Valent Silver and Resultant Interactions among Silver Nanoparticles, Silver Ions, and Reactive Oxygen Species. Langmiur 2012, 28, 10266–10275. [Google Scholar] [CrossRef] [PubMed]
- Shigemune, H.; Maeda, S.; Cacucciolo, V.; Iwata, Y.; Iwase, E.; Hashimoto, S.; Sugano, S. Printed Paper Robot Driven by Electrostatic Actuator. IEEE Robot. Autom. Lett. 2017, 2, 1001–1007. [Google Scholar] [CrossRef]
- Paswan, S.K.; Kumari, S.; Kar, M.; Singh, A.; Pathak, H.; Borah, J.P.; Kumar, L. Optimization of structure-property relationships in nickel ferrite nanoparticles annealed at different temperature. J. Phys. Chem. Solids 2021, 151, 109928. [Google Scholar] [CrossRef]
- Arguelles, A.P.; Turner, J.A. Ultrasonic attenuation of polycrystalline materials with a distribution of grain sizes. J. Acoust. Soc. Am. 2017, 141, 4347–4353. [Google Scholar] [CrossRef]
- Gao, P.; Zhang, T.S.; Wei, J.X.; Yu, Q.J. Evaluation of RRSB distribution and lognormal distribution for describing the particle size distribution of graded cementitious materials. Powder Technol. 2018, 331, 137–145. [Google Scholar] [CrossRef]
- Riera-Franco de Sarabia, E.; Gallego-Juárez, J.A.; Rodríguez-Corral, G.; Elvira-Segura, L.; González-Gómez, I. Application of high-power ultrasound to enhance fluid/solid particle separation processes. Ultrasonics 2000, 38, 642–646. [Google Scholar] [CrossRef]
- Krause, B.; Mende, M.; Pötschke, P.; Petzold, G. Dispersability and particle size distribution of CNTs in an aqueous surfactant dispersion as a function of ultrasonic treatment time. Carbon 2010, 48, 2746–2754. [Google Scholar] [CrossRef]
- Jiang, H.; Tang, C.; Wang, Y.; Mao, L.; Sun, Q.; Zhang, L.; Song, H.; Huang, F.; Zuo, C. Low content and low-temperature cured silver nanoparticles/silver ion composite ink for flexible electronic applications with robust mechanical performance. Appl. Surf. Sci. 2021, 564, 150447. [Google Scholar] [CrossRef]
- Mou, Y.; Zhang, Y.; Cheng, H.; Peng, Y.; Chen, M. Fabrication of highly conductive and flexible printed electronics by low temperature sintering reactive silver ink. Appl. Surf. Sci. 2018, 459, 249–256. [Google Scholar] [CrossRef]
- Bhat, K.S.; Nakate, U.T.; Yoo, J.-Y.; Wang, Y.; Mahmoudi, T.; Hahn, Y.-B. Cost-effective silver ink for printable and flexible electronics with robust mechanical performance. J. Chem. Eng. 2019, 373, 355–364. [Google Scholar] [CrossRef]
- Fernandes, I.J.; Aroche, A.F.; Schuck, A.; Lamberty, P.; Peter, C.R.; Hasenkamp, W.; Rocha, T.L.A.C. Silver nanoparticle conductive inks: Synthesis, characterization, and fabrication of inkjet-printed flexible electrodes. Sci. Rep. 2020, 10, 8878. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, V.; Moneghan, J.W.; Willets, K.A. Visualizing the Effect of Partial Oxide Formation on Single Silver Nanoparticle Electrodissolution. J. Phys. Chem. C 2018, 122, 3138–3145. [Google Scholar] [CrossRef]
- Chicea, D.; Nicolae-Maranciuc, A.; Doroshkevich, A.S.; Chicea, L.M.; Ozkendir, O.M. Comparative Synthesis of Silver Nanoparticles: Evaluation of Chemical Reduction Procedures, AFM and DLS Size Analysis. Materials 2023, 16, 5244. [Google Scholar] [CrossRef]
- Viguié, J.-R.; Sukmanowski, J.; Nölting, B.; Royer, F.-X. Study of agglomeration of alumina nanoparticles by atomic force microscopy (AFM) and photon correlation spectroscopy (PCS). Colloids Surf. A Physicochem. Eng. 2007, 302, 269–275. [Google Scholar] [CrossRef]
- Worldwide, M.I. Dynamic Light Scattering, Common Terms Defined, Inform White Paper; Malvern Instruments Limited: Malvern, UK, 2011. [Google Scholar]
- Baalousha, M.; Lead, J.R. Nanoparticles dispersity in toxicology. Nat. Nanotechnol. 2013, 8, 308–309. [Google Scholar] [CrossRef]
- Gupta, V.; Trivedi, P. Chapter 15-In vitro and in vivo characterization of pharmaceutical topical nanocarriers containing anticancer drugs for skin cancer treatment. In Lipid Nanocarriers for Drug Targeting; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 563–627. [Google Scholar] [CrossRef]
- Raval, N.; Maheshwari, R.; Kalyane, D.; Youngren-Ortiz, S.R.; Chougule, M.B.; Tekade, R.K. Importance of Physicochemical Characterization of Nanoparticles in Pharmaceutical Product Development. In Basic Fundamentals of Drug Delivery; Academic Press: Cambridge, MA, USA, 2019; pp. 369–400. [Google Scholar] [CrossRef]
- Martin, D.P.; Melby, N.L.; Jordan, S.M.; Bednar, A.J.; Kennedy, A.J.; Negrete, M.E.; Chapell, M.A.; Poda, A.R. Nanosilver conductive ink: A case study for evaluating the potential risk of nanotechnology under hypothetical use scenarios. Chemosphere 2016, 162, 222–227. [Google Scholar] [CrossRef]
- Htwe, Y.Z.N.; Abdullah, M.K.; Mariatti, M. Water-based graphene/AgNPs hybrid conductive inks for flexible electronic applications. JMR&T 2022, 16, 59–73. [Google Scholar] [CrossRef]
- Htwe, Y.Z.N.; Chow, W.S.; Suriati, G.; Thant, A.A.; Mariatti, M. Properties enhancement of graphene and chemical reduction silver nanoparticles conductive inks printed on polyvinyl alcohol (PVA) substrate. Synth. Met. 2019, 256, 116120. [Google Scholar] [CrossRef]
- Jang, J.; Cho, K.; Lee, S.; Kim, S. Transparent and flexible thin-film transistors with channel layers composed of sintered HgTe nanocrystals. Nanotechnology 2008, 9, 015204. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, C.; Lan, D.; Ji, W.; Wang, Y. Solution Printing of Electronics and Sensors: Applicability and Application in Space. Adv. Eng. Mater. 2022, 24, 2200173. [Google Scholar] [CrossRef]
- Escobedo, P.; Bhattacharjee, M.; Nikbakhtnasrabadi, F.; Dahiya, R. Flexible Strain and Temperature Sensing NFC Tag for Smart Food Packaging Applications. IEEE Sens. J. 2021, 21, 26406–26414. [Google Scholar] [CrossRef]
- Liao, Y.; Zhang, R.; Qian, J. Printed electronics based on inorganic conductive nanomaterials and their applications in intelligent food packaging. RSC Adv. 2019, 9, 29154–29172. [Google Scholar] [CrossRef]
- Herbert, R.; Lim, H.-R.; Yeo, W.-H. Printed, Soft, Nanostructures Strain Sensors for Monitoring of Structural Health and Human Physiology. ACS Appl. Mater. Interfaces 2020, 12, 25020–25030. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, M.; Zhang, L.; Xu, J.; Xiao, X.; Zhang, X. Inkjet-printed flexible sensors: From function materials, manufacture process, and applications perspective. Mater. Today Commun. 2022, 31, 103263. [Google Scholar] [CrossRef]
- Rauter, L.; Zikulnig, J.; Sinani, T.; Zangl, H.; Faller, L.-M. Evaluation of Standard Electrical Bonding Strategies for the Hybrid Integration of Inkjet-Printed Electronics. Electron. Mater. 2020, 1, 2–16. [Google Scholar] [CrossRef]


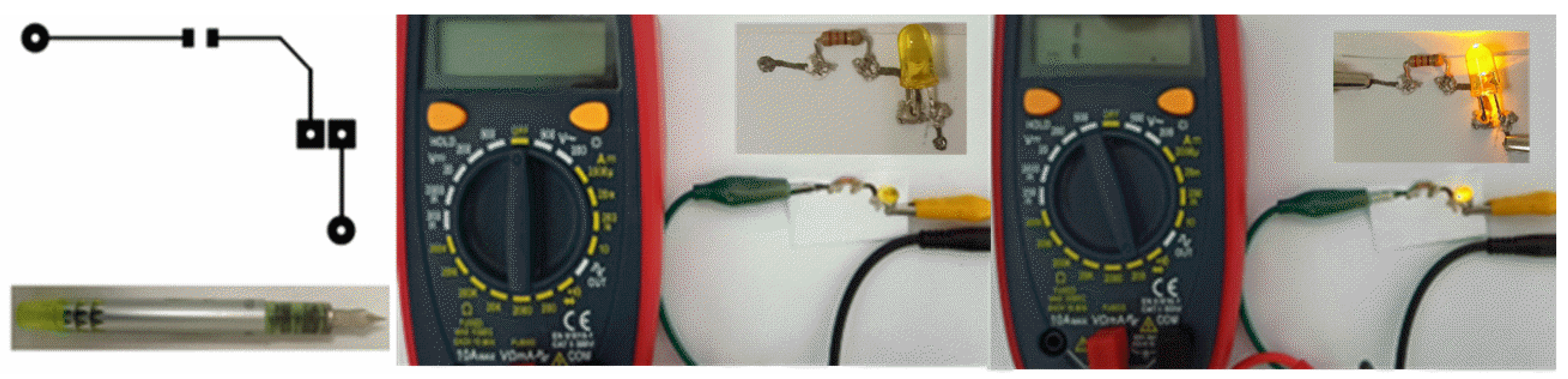
| Bending Radius | Strain (%) | Resistance (Ω) | Bending Cycles | Resistance (Ω) |
|---|---|---|---|---|
| 12.5 mm | 0.8 | 17.99 | 0 | 11.74 |
| 10 mm | 1 | 20.11 | 200 | 14.00 |
| 7.5 mm | 1.4 | 20.93 | 500 | 15.97 |
| 5 mm | 2 | 20.96 | 1000 | 17.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceron, S.; Barba, D.; Dominguez, M.A. Solution-Processable and Eco-Friendly Functionalization of Conductive Silver Nanoparticles Inks for Printable Electronics. Electron. Mater. 2024, 5, 45-55. https://doi.org/10.3390/electronicmat5020004
Ceron S, Barba D, Dominguez MA. Solution-Processable and Eco-Friendly Functionalization of Conductive Silver Nanoparticles Inks for Printable Electronics. Electronic Materials. 2024; 5(2):45-55. https://doi.org/10.3390/electronicmat5020004
Chicago/Turabian StyleCeron, Sonia, David Barba, and Miguel A. Dominguez. 2024. "Solution-Processable and Eco-Friendly Functionalization of Conductive Silver Nanoparticles Inks for Printable Electronics" Electronic Materials 5, no. 2: 45-55. https://doi.org/10.3390/electronicmat5020004
APA StyleCeron, S., Barba, D., & Dominguez, M. A. (2024). Solution-Processable and Eco-Friendly Functionalization of Conductive Silver Nanoparticles Inks for Printable Electronics. Electronic Materials, 5(2), 45-55. https://doi.org/10.3390/electronicmat5020004






