Research on the Withstand Voltage Properties of Cr/Mn-Doped Al2O3 Ceramics in Vacuum
Abstract
1. Introduction
2. Experimental Procedures
2.1. Samples Preparation
2.2. Samples Characterization
2.2.1. Characterization of Microstructure, Bulk Density and Resistivity
2.2.2. Test of Secondary Electron Emission Property
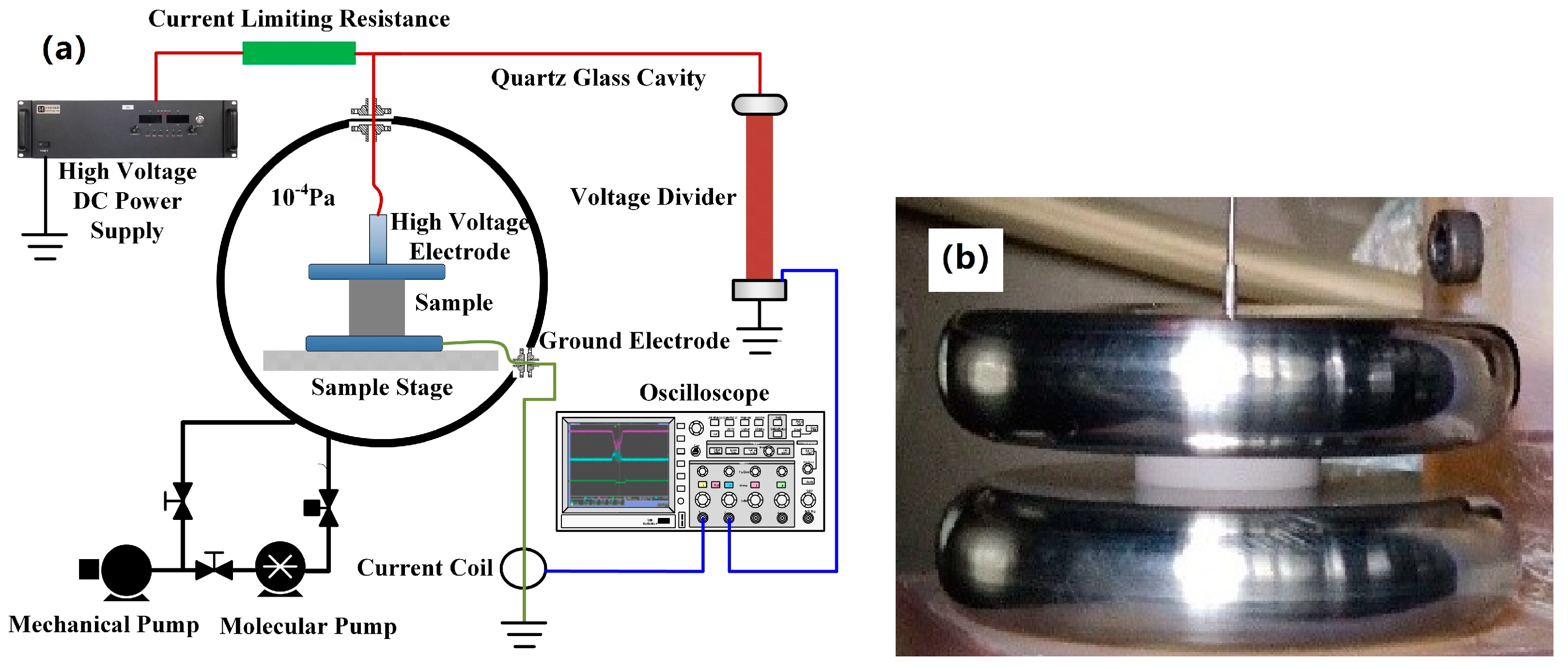
2.2.3. Test of Withstand Voltage in Vacuum
3. Results and Discussion
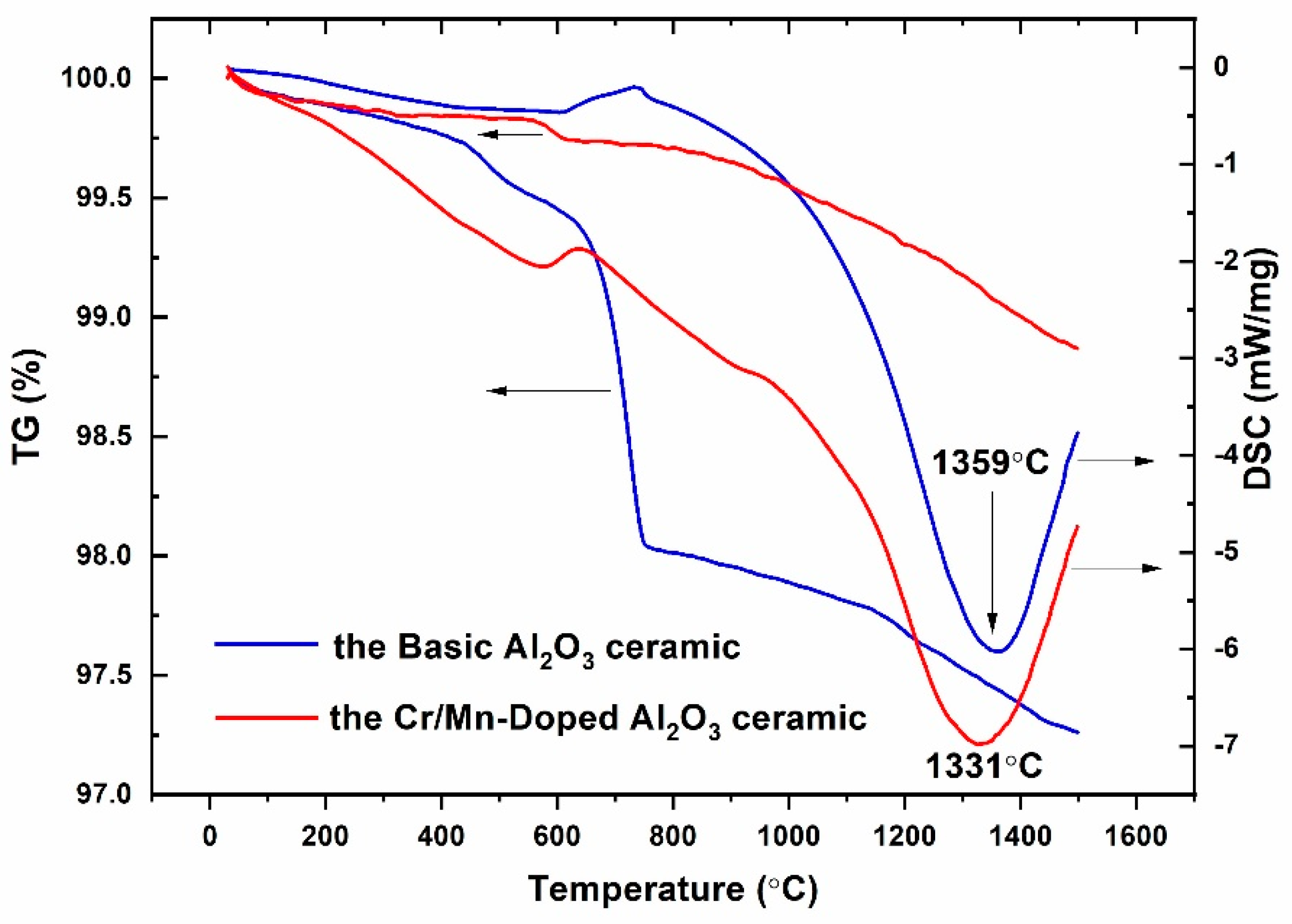
3.1. Thermal Analysis
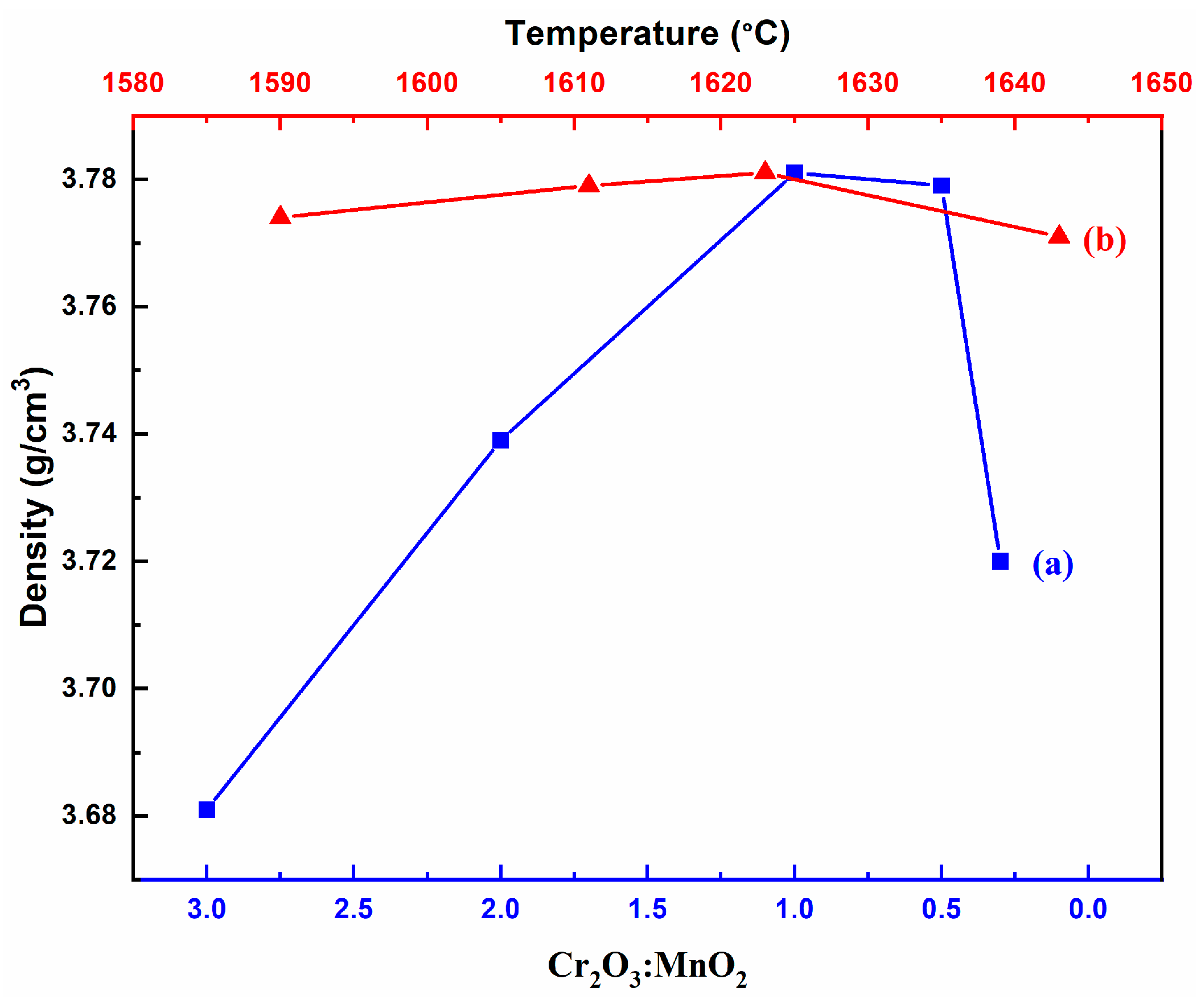
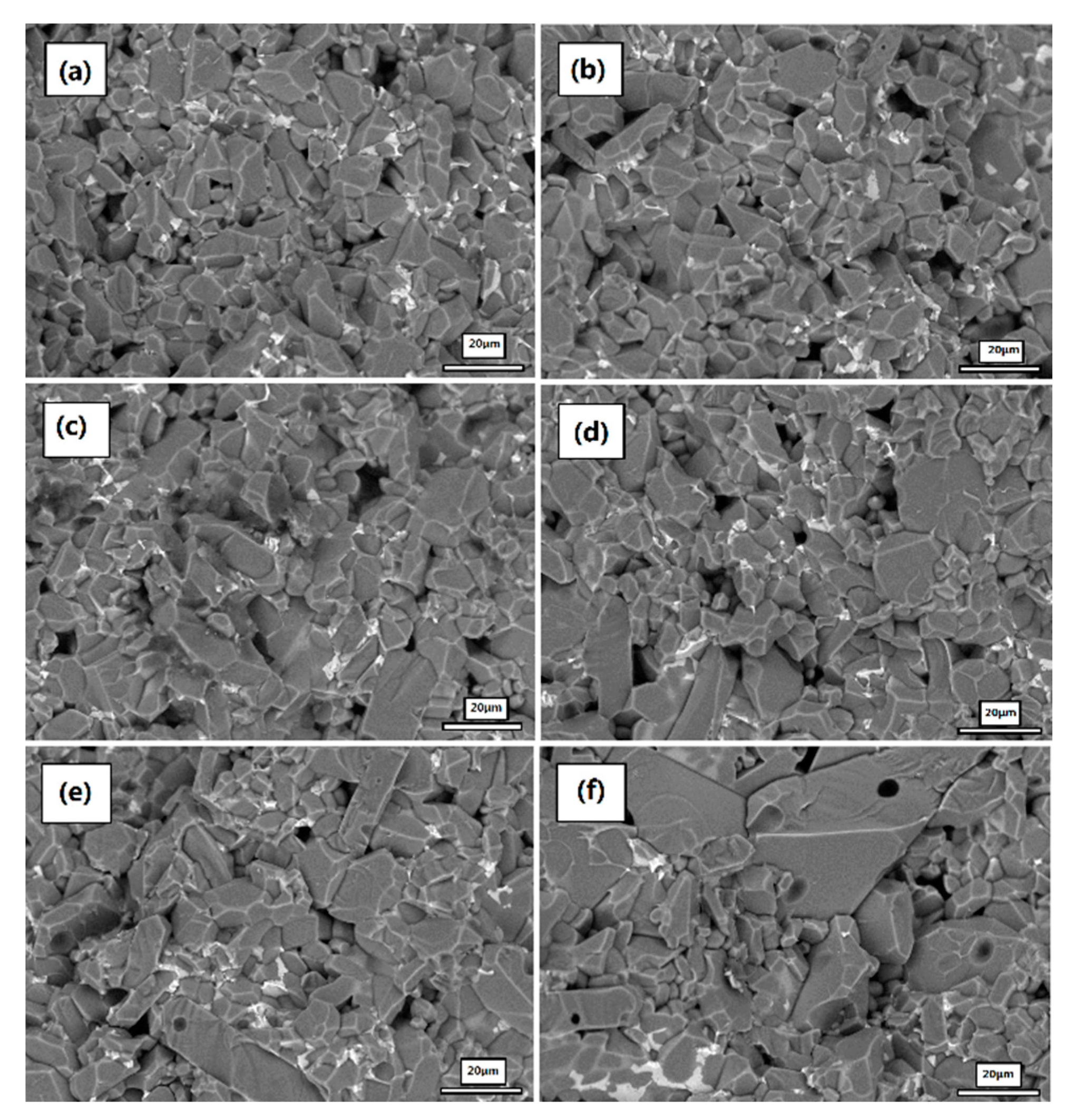
3.2. Bulk Density and Micromorphology
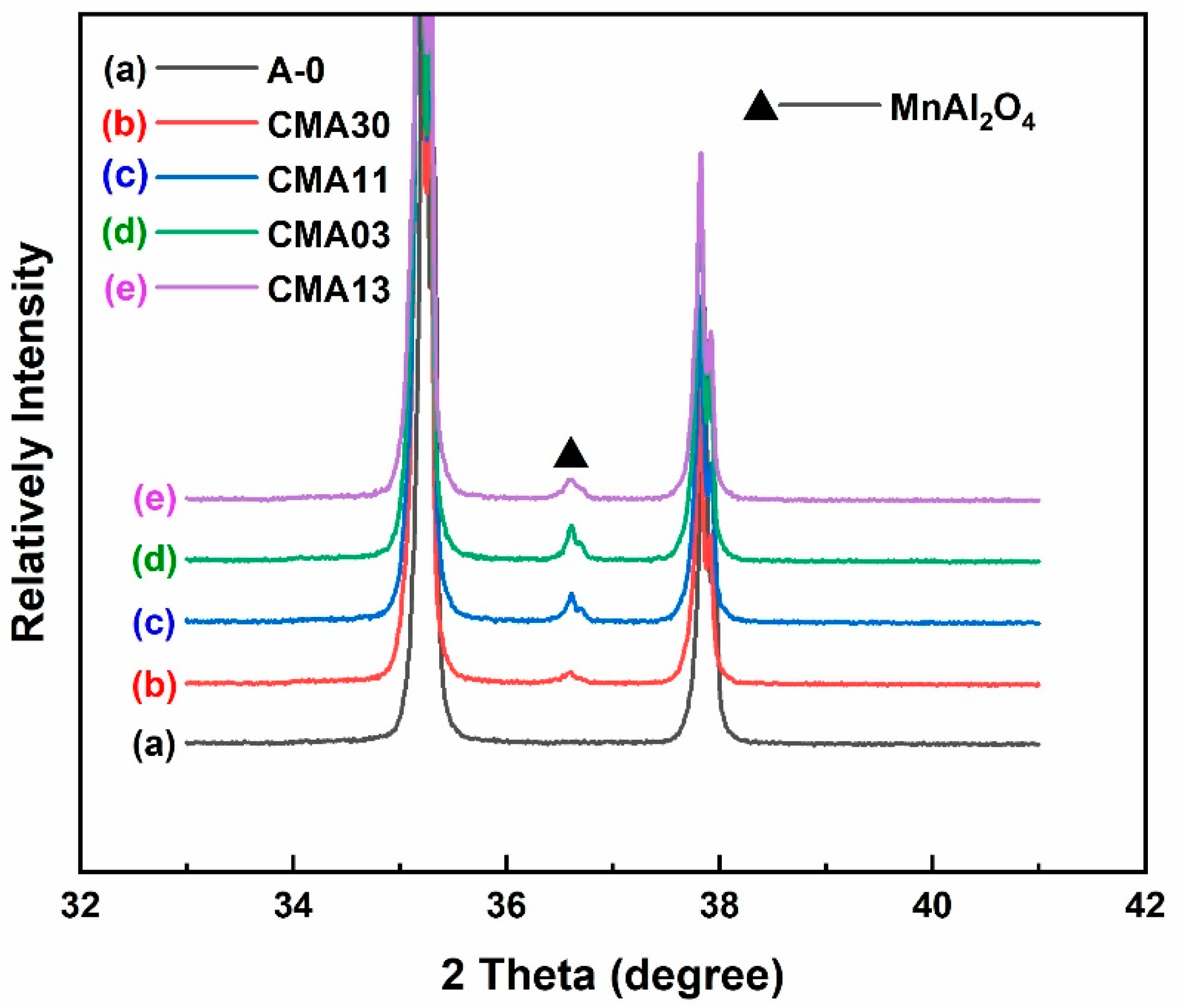
3.3. Phase Composition
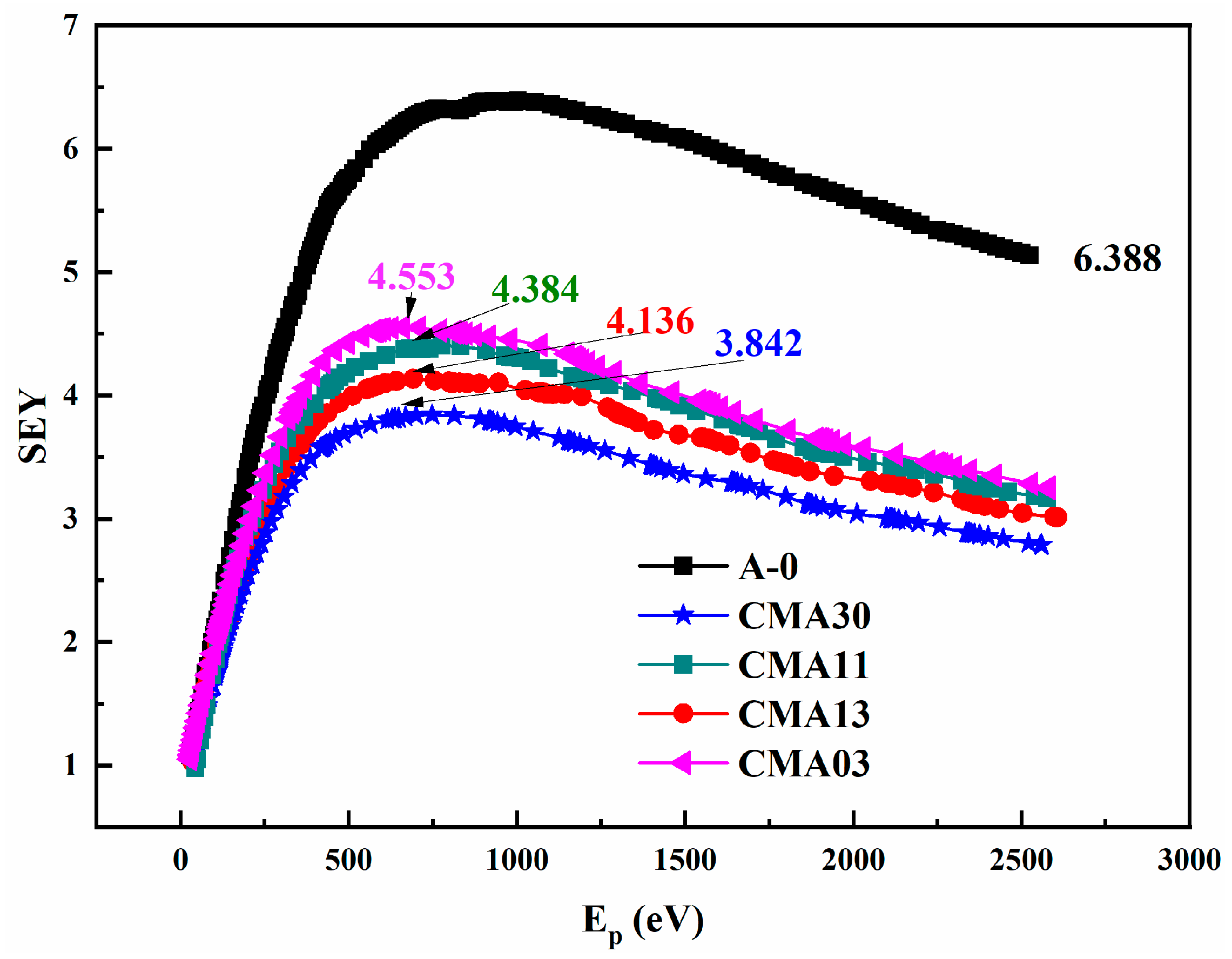
3.4. Secondary Electron Emission Property
3.5. Resistivity
3.6. Withstand Voltage in Vacuum
4. Conclusions
- (1)
- Cr2O3 and MnO2 have an influence on the microstructure and the sintering performance of Al2O3 ceramics. Al2O3 ceramics with an appropriate amount of Cr2O3 and MnO2, sintered at 1623 °C, exhibit uniform and dense microstructure and high voltage withstand stability.
- (2)
- The addition of Cr2O3 and MnO2 significantly reduced the SEY and Rs of Al2O3 ceramics. Compared to basic Al2O3 ceramics, the SEY of Cr/Mn-doped Al2O3 ceramics decreased by about 50%, while the Rs of Cr/Mn-doped Al2O3 ceramics decreased by up to three orders of magnitude.
- (3)
- The withstand voltage properties of the prepared Cr/Mn-doped Al2O3 ceramics are significantly improved. The Uho of the CMA13 sample reached 31 kV, which was 24% higher than that of the basic Al2O3 ceramics. In addition, the voltage withstand stability of the CMA13 sample was relatively high.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olsommer, Y.; Ihmig, F.R. Experimental Characterization of Ferroelectric Capacitor Circuits for the Realization of Simply Designed Electroceuticals. Electron. Mater. 2021, 2, 299–311. [Google Scholar] [CrossRef]
- Vanjaria, J.; Hariharan, V.; Arjunan, A.C.; Wu, Y.; Tompa, G.S.; Yu, H. One-Step Cost-Effective Growth of High-Quality Epitaxial Ge Films on Si (100) Using a Simplified PECVD Reactor. Electron. Mater. 2021, 2, 482–494. [Google Scholar] [CrossRef]
- Suchaneck, G.; Artiukh, E.; Kalanda, N.; Yarmolich, M.; Gerlach, G. Nanogranular Strontium Ferromolybdate/Strontium Molybdate Ceramics—A Magnetic Material Possessing a Natural Core-Shell Structure. Electron. Mater. 2024, 5, 1–16. [Google Scholar] [CrossRef]
- Goswami, A.P.; Roy, S.; Das, G.C. Effect of powder, chemistry and morphology on the dielectric properties of liquid-phase-sintered alumina. Ceram. Int. 2002, 28, 439–445. [Google Scholar] [CrossRef]
- Naseer, D.; Ha, J.H.; Lee, J.; Song, I.H. Preparation of Al2O3 Multichannel Cylindrical-Tube-Type Microfiltration Membrane with Surface Modification. Appl. Sci. 2022, 12, 7993. [Google Scholar] [CrossRef]
- Ahmad, R.; Kim, J.K.; Kim, J.H.; Kim, J. Nanostructured Ceramic Photocatalytic Membrane Modified with a Polymer Template for Textile Wastewater Treatment. Appl. Sci. 2017, 7, 1824. [Google Scholar] [CrossRef]
- Aleksandr, K.; Ilya, B.; Aleksey, Z. Electron-Beam Processing of Aluminum-Containing Ceramics in the Forevacuum Pressure Range. Ceramics 2023, 6, 2098–2116. [Google Scholar] [CrossRef]
- Sun, G.-Y.; Guo, B.-H.; Mu, H.-B.; Song, B.-P.; Zhou, R.-D.; Zhang, S.; Zhang, G.-J. Flashover strength improvement and multipactor suppression in vacuum using surface charge preconditioning on insulator. J. Appl. Phys. 2018, 124, 134102. [Google Scholar] [CrossRef]
- Pillai, A.S.; Hackam, R. Surface flashover of solid insulators in atmospheric air and in vacuum. J. Appl. Phys. 1985, 58, 146–153. [Google Scholar] [CrossRef]
- Miller, C.H. Flashover of insulators in vacuum: The last twenty years. IEEE Trans. Dielectr. Electr. Insul. 2016, 22, 3641–3657. [Google Scholar] [CrossRef]
- Yan, P.; Shao, T.; Wang, J.; Gao, W.; Yuan, W.; Pan, R.; Zhang, S.; Sun, G. Experimental investigation of surface flashover in vacuum using nanosecond pulses. IEEE Trans. Dielectr. Electr. Insul. 2007, 14, 634–642. [Google Scholar] [CrossRef]
- Liebault, J.; Vallayer, J.; Goeuriot, D.; Treheux, D.; Thevenot, F. How the trapping of charges can explain the dielectric breakdown performance of alumina ceramics. J. Eur. Ceram. Soc. 2001, 21, 389–397. [Google Scholar] [CrossRef]
- Hawley, R. Solid insulators in vacuum: A Review. Vacuum 1968, V18, 383–390. [Google Scholar] [CrossRef]
- Seif, M.N.; Balk, T.J.; Beck, M.J. Desorption from Hot Scandate Cathodes: Effects on Vacuum Device Interior Surfaces after Long-Term Operation. Materials 2020, 13, 5149. [Google Scholar] [CrossRef]
- Li, S. Improvement of surface flashover in vacuum. High Volt. 2020, 5, 122–133. [Google Scholar] [CrossRef]
- Vlieks, A.E.; Allen, M.A.; Callin, R.S.; Fowkes, W.R.; Hoyt, E.W.; Lebacqz, J.V.; Lee, T.G. Breakdown phenomena in high power klystrons. IEEE Trans. Electr. Insul. 1989, 24, 1023–1028. [Google Scholar] [CrossRef][Green Version]
- Tafazoli, M. A study of on-orbit spacecraft failures. Acta Astronaut. 2009, 64, 195–205. [Google Scholar] [CrossRef]
- Tyunkov, A.V.; Klimov, A.S.; Savkin, K.P.; Yushkov, Y.G.; Zolotukhin, D.B. Electron-Beam Deposition of Metal and Ceramic-Based Composite Coatings in the Fore-Vacuum Pressure Range. Ceramics 2022, 5, 789–797. [Google Scholar] [CrossRef]
- Jang, S.O.; Cho, C.; Kim, J.H.; Kang, I.J.; Chang, H.; Park, H.; Lee, K.; Kim, D.G.; Seok, H.W. Microwave Plasma Assisted Aerosol Deposition (µ-PAD) for Ceramic Coating Applications. Ceramics 2022, 5, 1174–1184. [Google Scholar] [CrossRef]
- Anderson, R.A.; Tucker, W.K. Vacuum surface flashover from bipolar stress. J. Appl. Phys. 1985, 58, 3346–3349. [Google Scholar] [CrossRef]
- Anderson, R.A. Mechanism of pulsed surface flashover involving electron-stimulated desorption. J. Appl. Phys. 1980, 51, 1414–1421. [Google Scholar] [CrossRef]
- Cross, J. High speed photography of surface flashover in vacuum. IEEE Trans. Electr. Insul. 1978, EI-13, 145–148. [Google Scholar] [CrossRef]
- Sudarshan, T.S.; Cross, J.D. The Effect of Chromium Oxide Coatings on Surface Flashover of Alumina Spacers in Vacuum. IEEE Trans. Electr. Insul. 1976, EI-11, 32–35. [Google Scholar] [CrossRef]
- Zheng, J.G.; Jiang, L.X.; Cai, Y.P.; Luo, S.W.; Zhou, X.M. Secondary Electron Emission Characteristic of Insulated Al2O3 Ceramics with Cr2O3 Coating. J. Inorg. Mater. 2001, 16, 497–502. [Google Scholar]
- Hosono, T.; Kato, K.; Morita, A.; Okubo, H. Charging Control on Alumina Surface in Vacuum by Surface Roughness and Electric Field Distribution. In Proceedings of the 2006 International Symposium on Discharges and Electrical Insulation in Vacuum, Matsue, Japan, 25–29 September 2006. [Google Scholar]
- Zhang, H.; Xu, Y.; Qiao, G.; Ma, D.; Jin, H. Effects of Cr2O3 Addition on Microstructure and Vacuum Flashover Performance of Glass-Ceramics. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 1882–1886. [Google Scholar] [CrossRef]
- Wang, F.; He, L.; Khan, M.Z.; Zhang, T.; Zhao, Q.; He, Y.; Huang, Z.; Zhao, H.; Li, J. Accelerated Charge Dissipation by Gas-Phase Fluorination on Nomex Paper. Appl. Sci. 2019, 9, 3879. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, D.; Qi, K.; He, Y. Measurement of total electron emission yield of insulators based on self-terminating charge neutralization. Rev. Sci. Instrum. 2022, 93, 055103-1–055103-7. [Google Scholar] [CrossRef]
- Hauet, T.; Piraux, L.; Srivastava, S.K.; Antohe, V.A.; Lacour, D.; Hehn, M.; Montaigne, F.; Schwenk, J.; Marioni, M.A.; Hug, H.J.; et al. Reversal mechanism, switching field distribution, and dipolar frustrations in Co/Pt bit pattern media based on auto-assembled anodic alumina hexagonal nanobump arrays. Phys. Rev. B 2014, 89, 174421. [Google Scholar] [CrossRef]
- Shinozaki, K.; Ishikura, Y.; Uematsu, K.; Mizutani, N.; Kato, M. The sintering behavior and mechanical properties of the MgO doped Al2O3-Cr2O3 solid solution. Nippon. Kagaku Kaishi 1981, 7, 1093–1098. [Google Scholar] [CrossRef]
- Klimov, A.; Bakeev, I.; Dolgova, A.; Oks, E.; Tran, V.T.; Zenin, A. Electron-Beam Sintering of Al2O3-Cr-Based Composites Using a Forevacuum Electron Source. Ceramics 2022, 5, 748–760. [Google Scholar] [CrossRef]
- Molak, A.; Szeremeta, A.Z.; Koperski, J. Vis and NIR Diffuse Reflectance Study in Disordered Bismuth Manganate—Lead Titanate Ceramics. Electron. Mater. 2022, 3, 101–114. [Google Scholar] [CrossRef]
- Grigoriev, S.N.; Volosova, M.A.; Peretyagin, P.Y.; Seleznev, A.E.; Okunkova, A.A.; Smirnov, A. The Effect of TiC Additive on Mechanical and Electrical Properties of Al2O3 Ceramic. Appl. Sci. 2018, 8, 2385. [Google Scholar] [CrossRef]
- Huang, Y.; Min, D.; Li, S.; Li, Z.; Xie, D.; Wang, X.; Lin, S. Surface flashover performance of epoxy resin microcomposites improved by electron beam irradiation. Appl. Surf. Sci. A J. Devoted Prop. Interfaces Relat. Synth. Behav. Mater. 2017, 406, 39–45. [Google Scholar] [CrossRef]
- Du, B.X.; Li, J.; Du, W. Surface charge accumulation and decay on directfluorinated polyimide/Al2O3 nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 1764–1771. [Google Scholar] [CrossRef]
- Sudarshan, T.S.; Li, C.R. Dielectric surface flashover in vacuum. Experimental design issues. IEEE Trans. Dielectr. Electr. Insul. 1997, 4, 657–662. [Google Scholar] [CrossRef]



| Temperature, °C | 25–520 | 520–520 | 520–1100 | 1100–1300 | 1300–* | *–* |
| Time, h | 5 | 2 | 3 | 2 | 3 | 4 |
| Sample | Cr2O3: MnO2 | Sintering Temperature, °C |
|---|---|---|
| A-0 | - | 1645 |
| CMA30 | 3:1 | 1623 |
| CMA20 | 2:1 | 1623 |
| CMA11 | 1:1 | 1590 |
| CMA12 | 1:1 | 1611 |
| CMA13 | 1:1 | 1623 |
| CMA14 | 1:1 | 1643 |
| CMA05 | 1:2 | 1623 |
| CMA03 | 1:3 | 1623 |
| Sample | Rs (Surface Resistance), Ω | Rv (Volume Resistance), Ω·cm |
|---|---|---|
| A-0 | 7.41 × 1015 | 2.35 × 1016 |
| CMA30 | 4.78 × 1014 | 1.23 × 1016 |
| CMA20 | 1.56 × 1014 | 5.23 × 1015 |
| CMA11 | 7.55 × 1014 | 6.46 × 1015 |
| CMA12 | 7.21 × 1014 | 3.81 × 1015 |
| CMA13 | 5.32 × 1014 | 4.81 × 1015 |
| CMA14 | 5.62 × 1014 | 3.48 × 1015 |
| CMA05 | 8.94 × 1013 | 8.37 × 1014 |
| CMA03 | 3.44 × 1012 | 1.64 × 1012 |
| Sample | Uho, kV | Uho, kV | Standard Deviation, kV | ||||
|---|---|---|---|---|---|---|---|
| A-0 | 18 | 23 | 26 | 31 | 26 | 25 | 4.76 |
| CMA30 | 23 | 27 | 28 | 32 | 30 | 28 | 3.39 |
| CMA20 | 22 | 30 | 28 | 27 | 30 | 29 | 3.28 |
| CMA11 | 23 | 25 | 23 | 29 | 25 | 25 | 2.45 |
| CMA12 | 28 | 25 | 30 | 31 | 28 | 28 | 2.30 |
| CMA13 | 30 | 32 | 28 | 29 | 35 | 31 | 2.77 |
| CMA14 | 35 | 27 | 26 | 36 | 37 | 32 | 5.26 |
| CMA05 | 24 | 28 | 34 | 23 | 18 | 25 | 5.98 |
| CMA03 | 19 | 33 | 23 | 24 | 30 | 26 | 5.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, D.; Wang, X.; Han, X.; Yu, Z.; Feng, J.; Wang, H. Research on the Withstand Voltage Properties of Cr/Mn-Doped Al2O3 Ceramics in Vacuum. Electron. Mater. 2025, 6, 4. https://doi.org/10.3390/electronicmat6010004
Feng D, Wang X, Han X, Yu Z, Feng J, Wang H. Research on the Withstand Voltage Properties of Cr/Mn-Doped Al2O3 Ceramics in Vacuum. Electronic Materials. 2025; 6(1):4. https://doi.org/10.3390/electronicmat6010004
Chicago/Turabian StyleFeng, Dandan, Xiaojing Wang, Xueying Han, Zhiqiang Yu, Jialun Feng, and Hefei Wang. 2025. "Research on the Withstand Voltage Properties of Cr/Mn-Doped Al2O3 Ceramics in Vacuum" Electronic Materials 6, no. 1: 4. https://doi.org/10.3390/electronicmat6010004
APA StyleFeng, D., Wang, X., Han, X., Yu, Z., Feng, J., & Wang, H. (2025). Research on the Withstand Voltage Properties of Cr/Mn-Doped Al2O3 Ceramics in Vacuum. Electronic Materials, 6(1), 4. https://doi.org/10.3390/electronicmat6010004





