Synthetic Pathways to Pyrido[3,4-c]pyridazines and Their Polycyclic Derivatives
Abstract
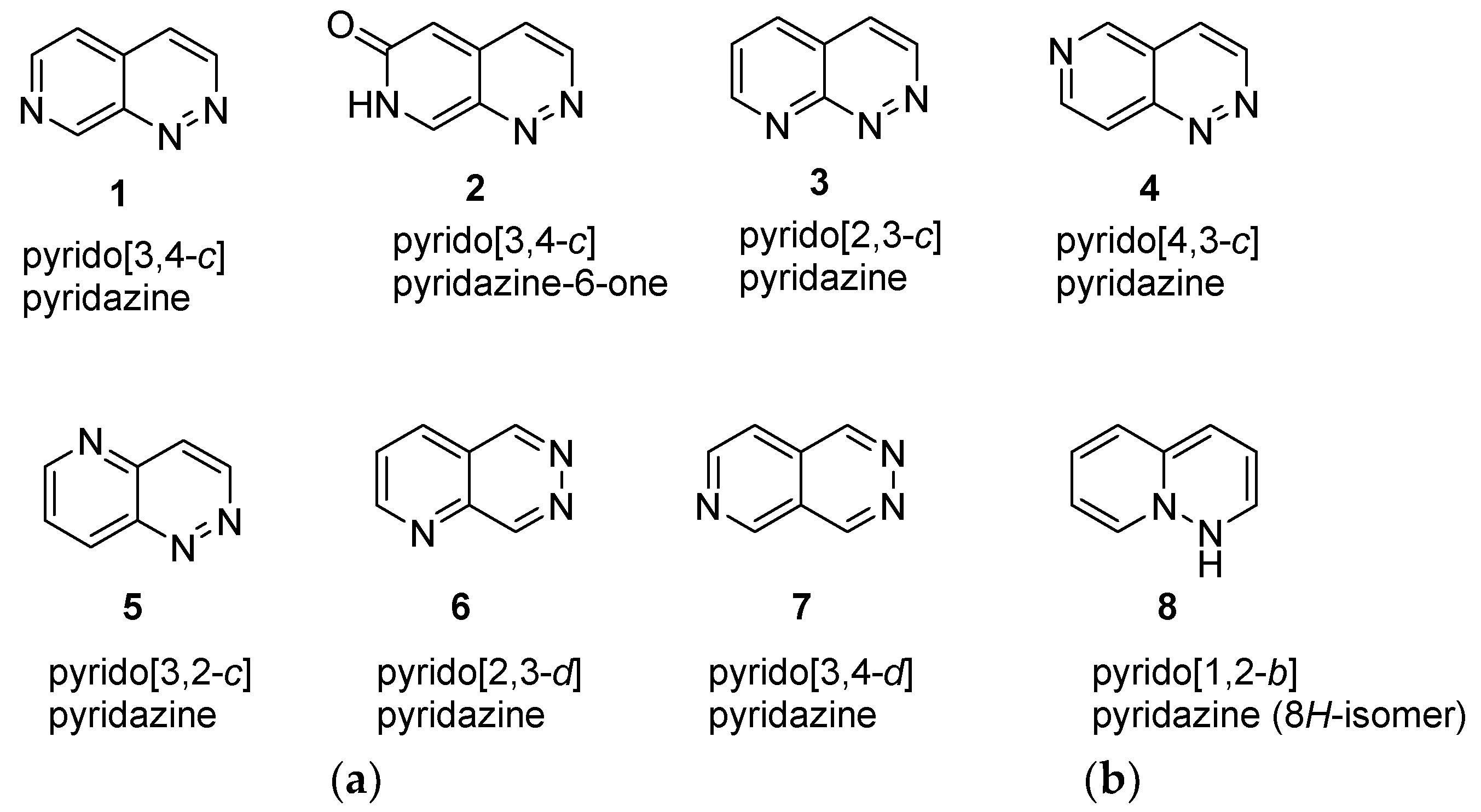
1. Introduction
2. Discussion
2.1. Bicyclic Pyrido[3,4-c]pyridazine Derivatives
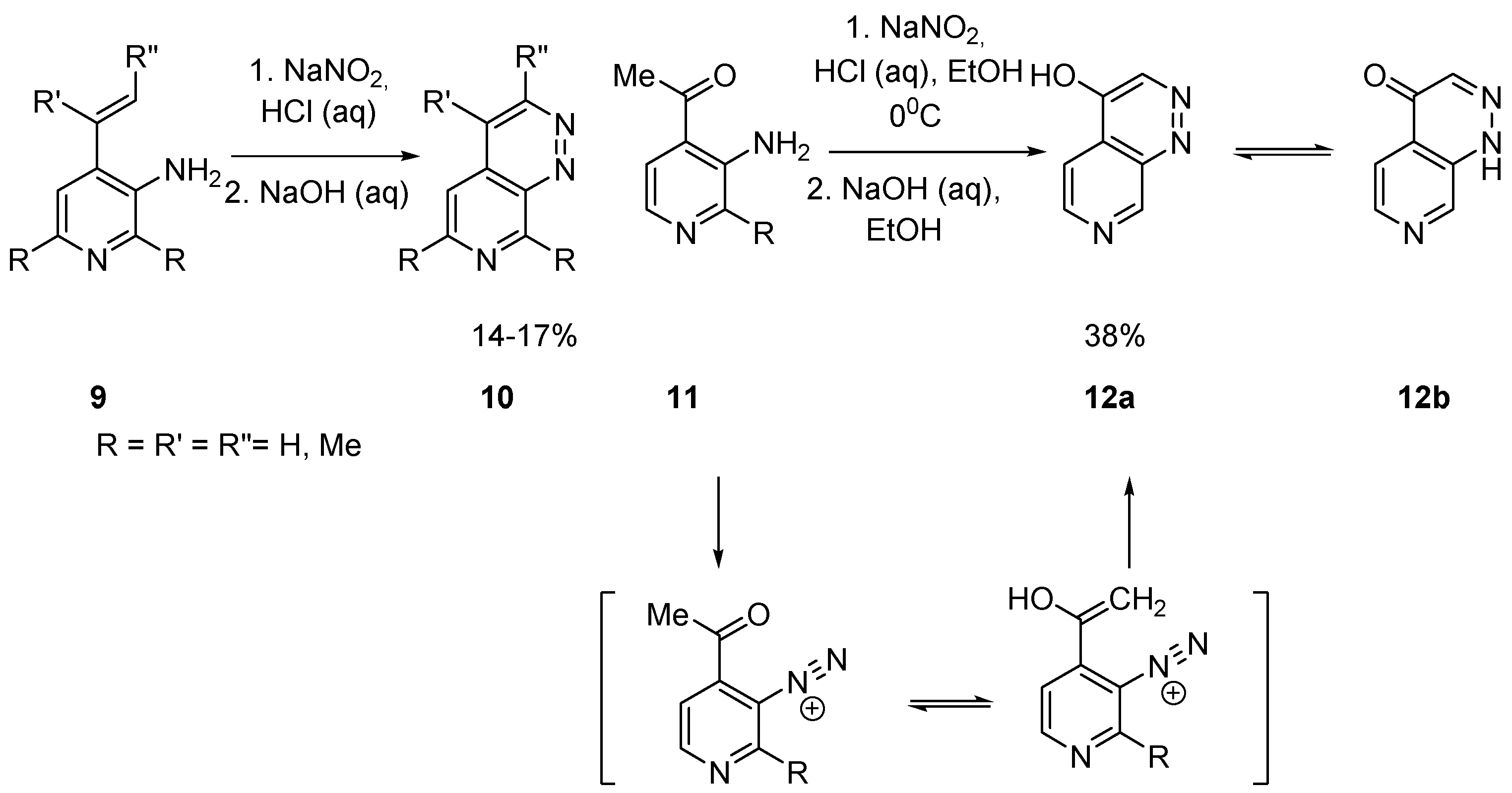
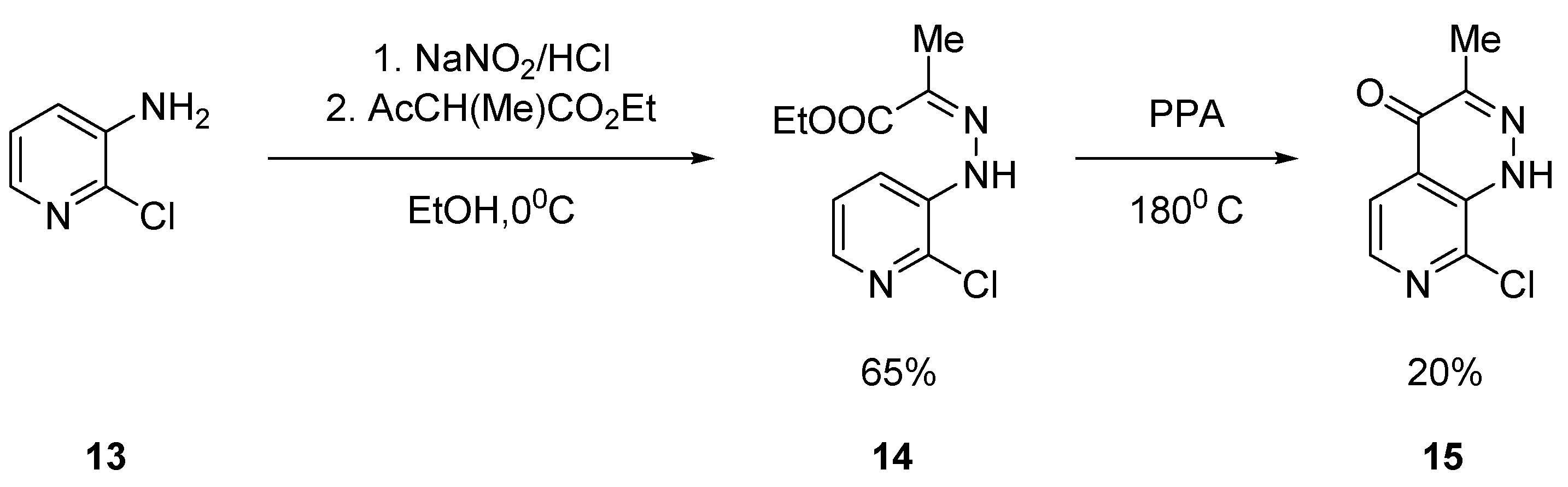
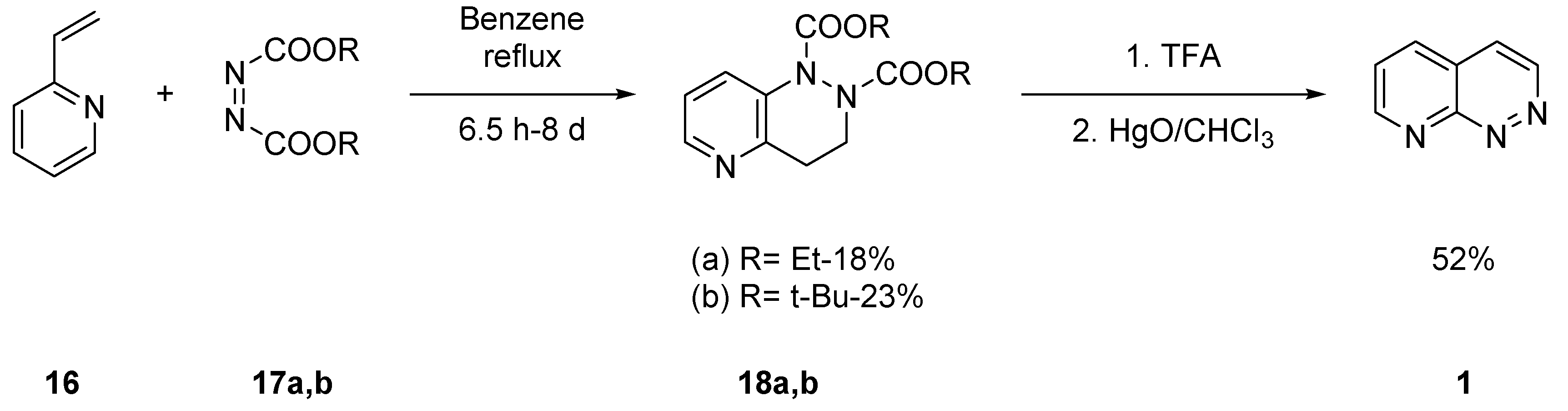
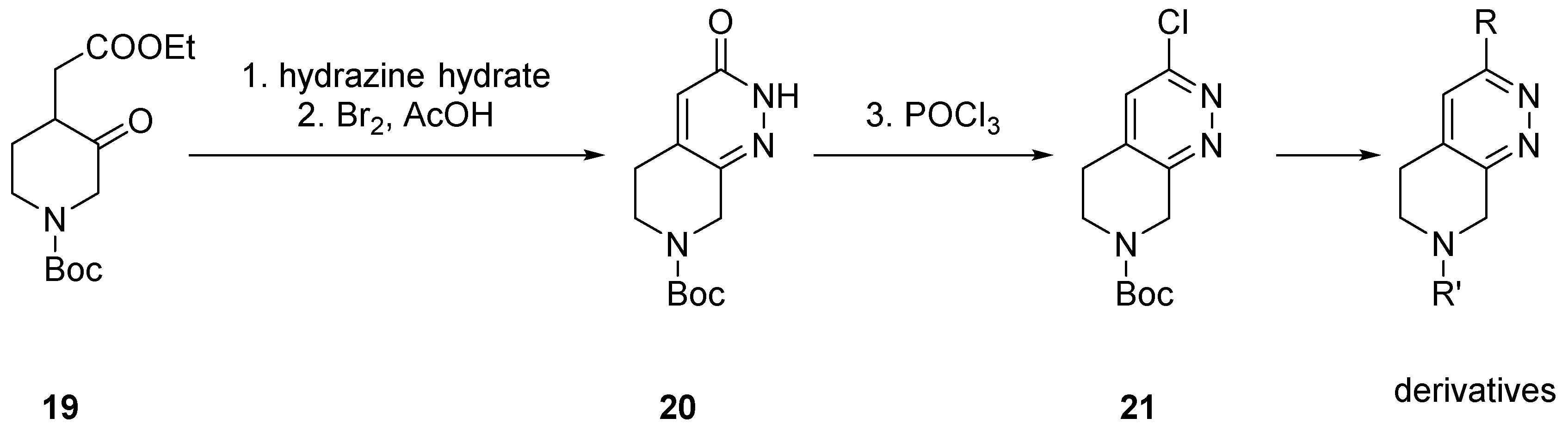
2.1.1. Starting from Pyridine Derivatives
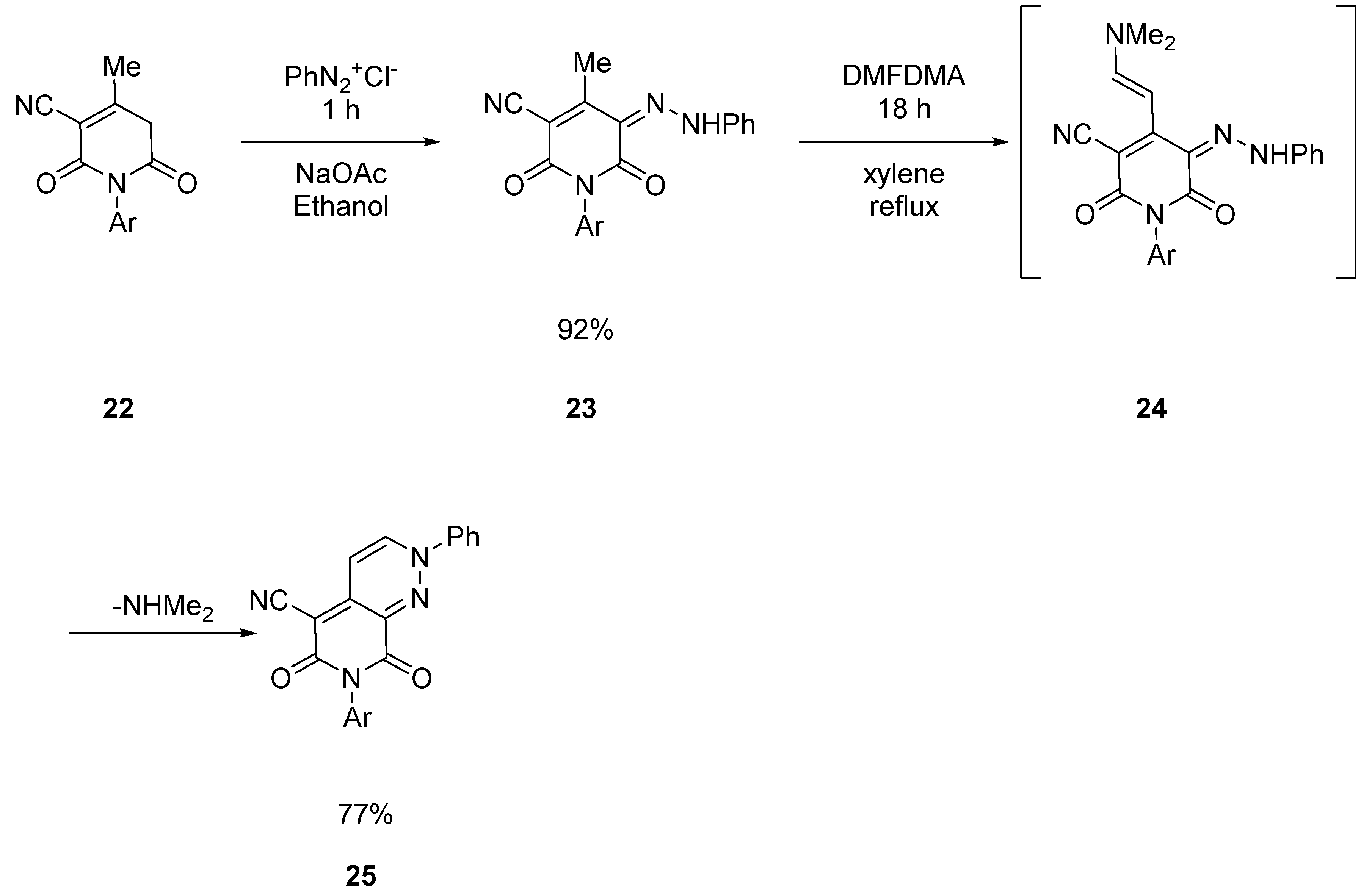
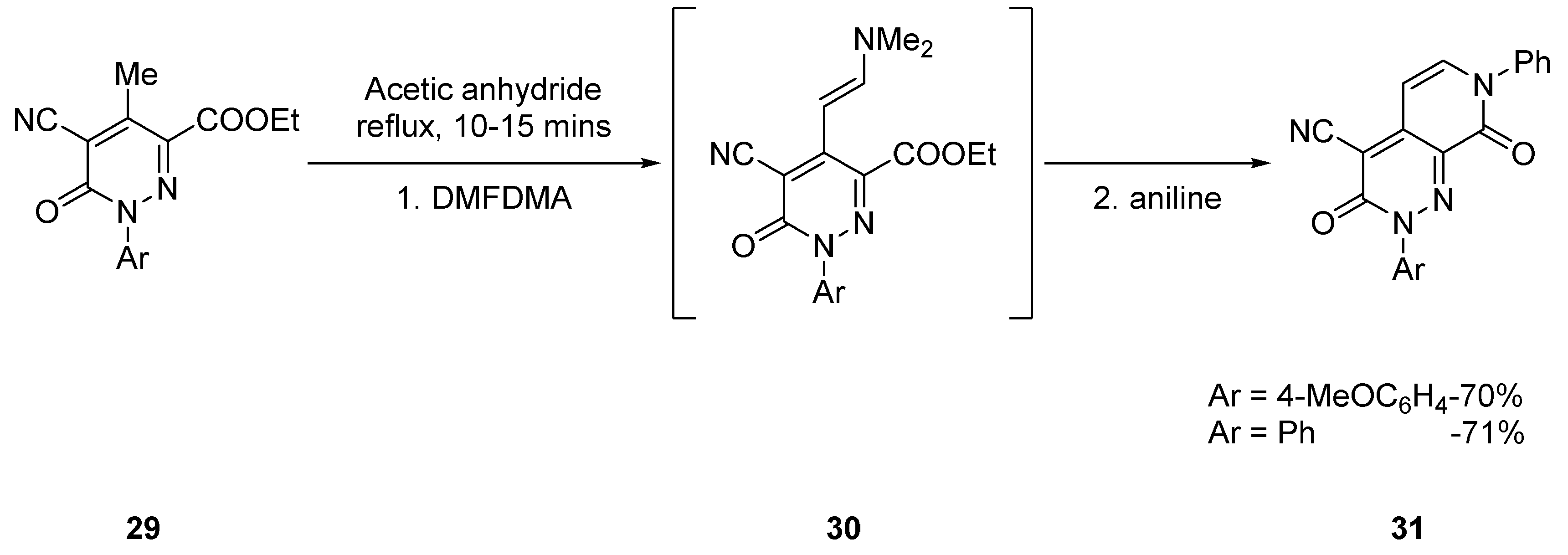
2.1.2. Starting from Pyridazine Derivatives
2.1.3. Starting from Other Building Blocks
2.2. Tricyclic Derivatives
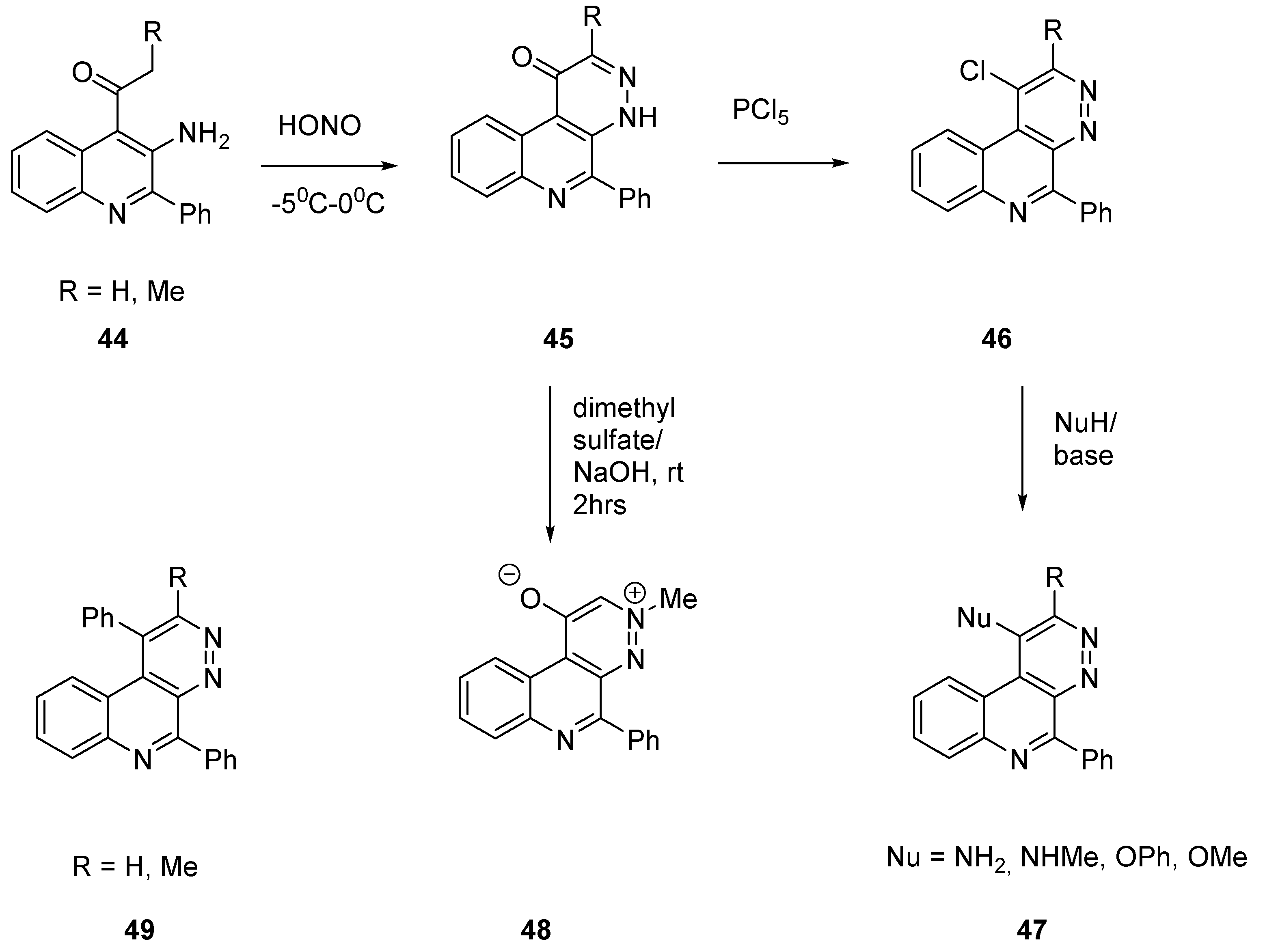
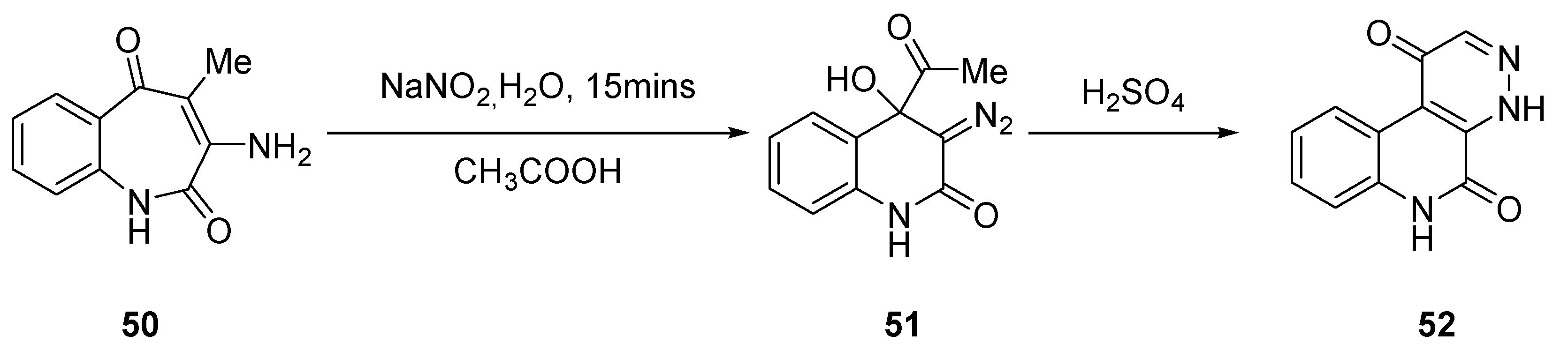
2.2.1. Benzo Fused Tricyclic Derivatives Starting from Quinolines
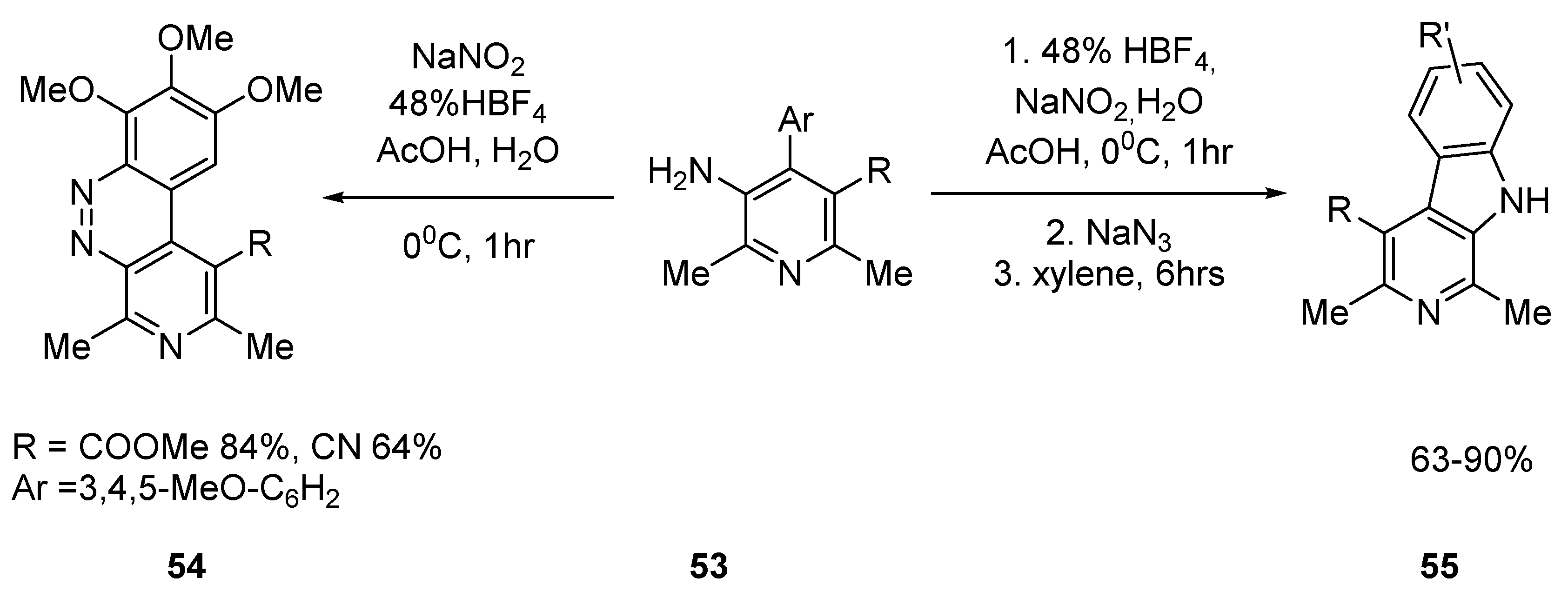
2.2.2. Benzo Fused Tricyclic Derivatives Starting from Pyridines
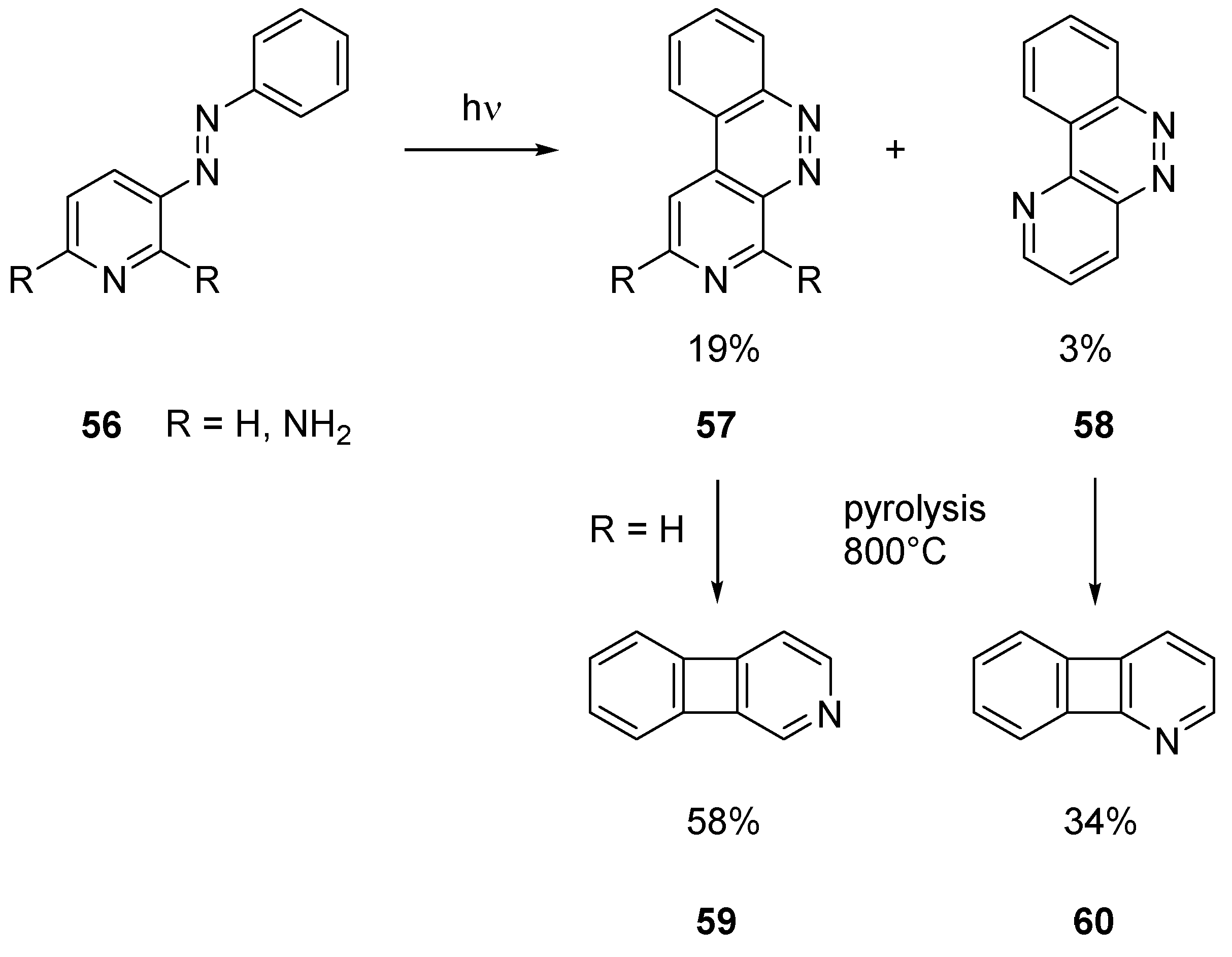
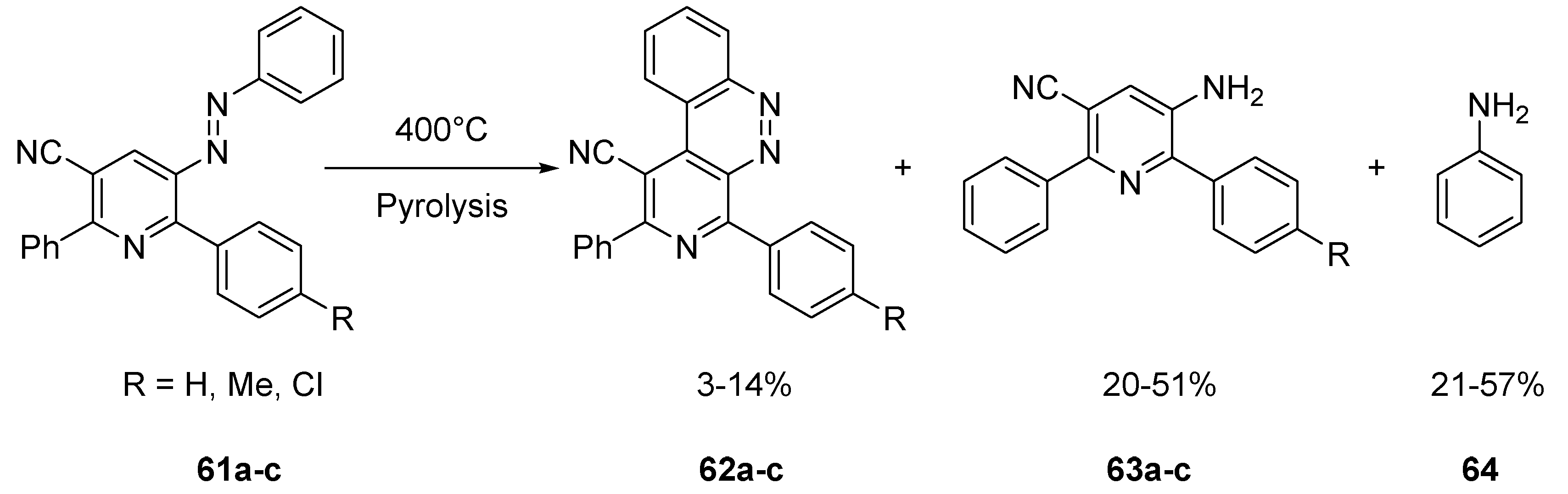
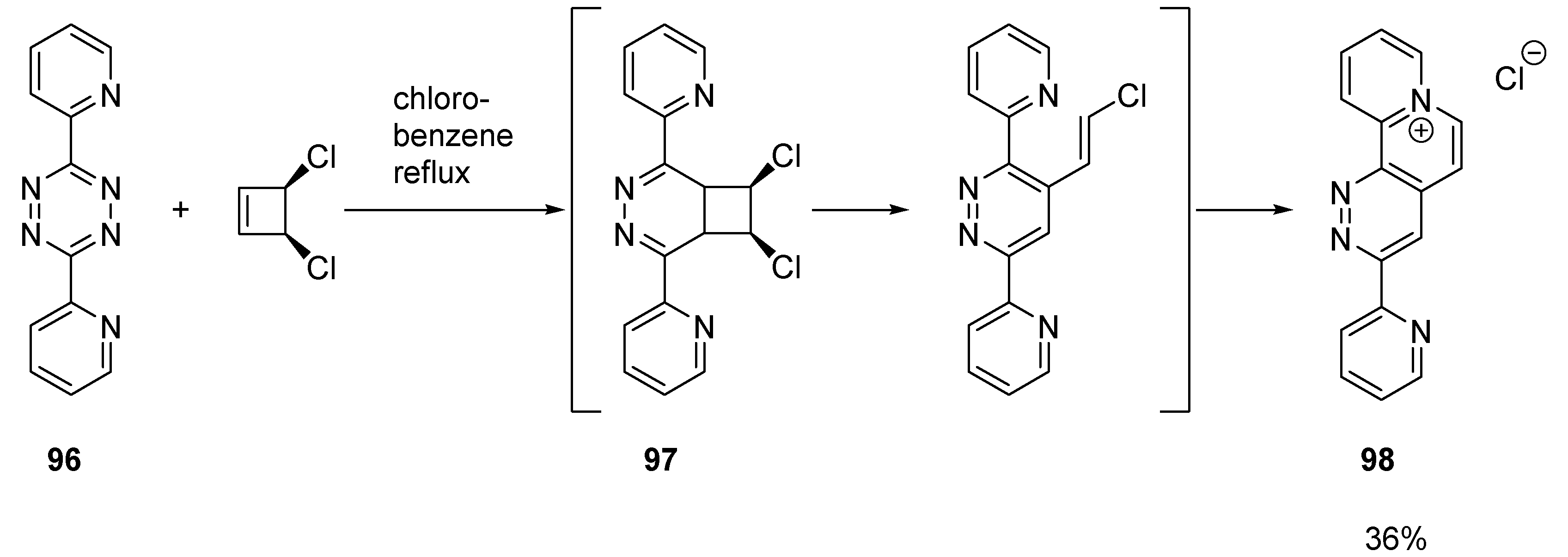
2.2.3. Benzo Fused Tricyclic Derivatives Starting from Cinnolines, Pyridazines or Tetrazines
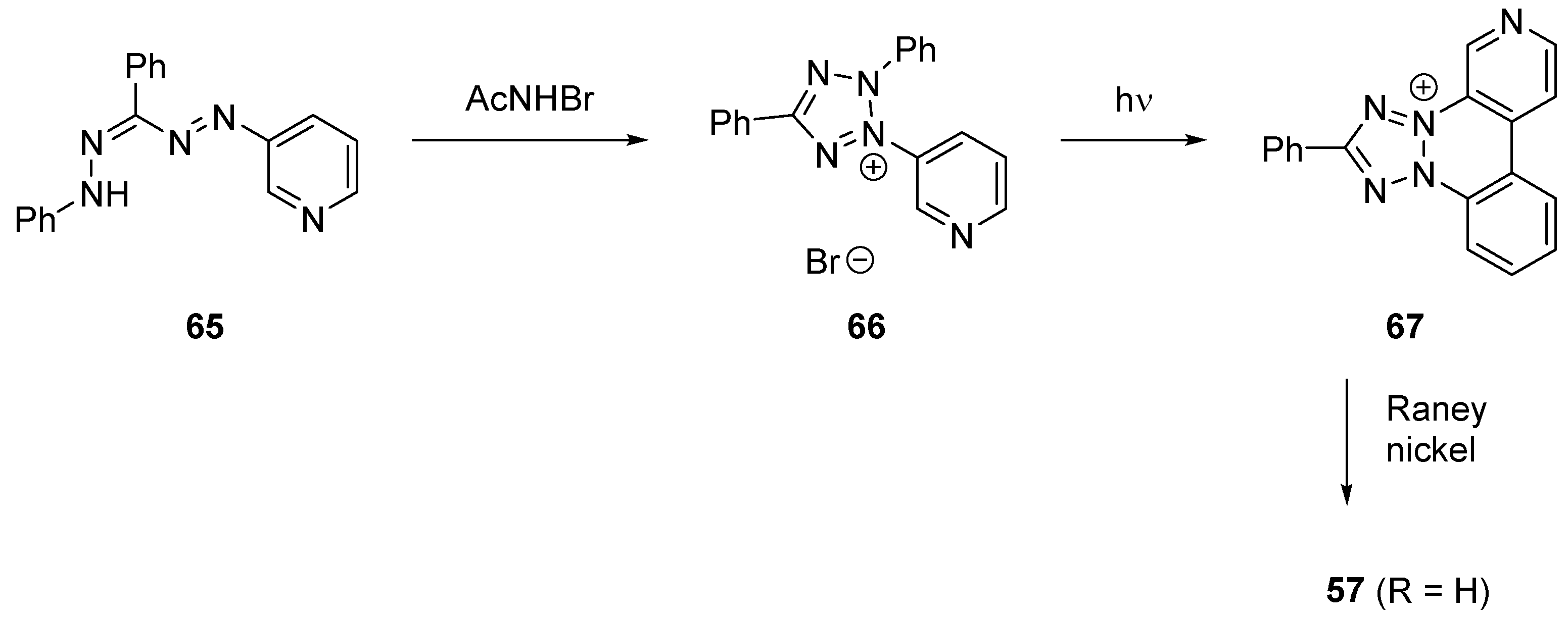
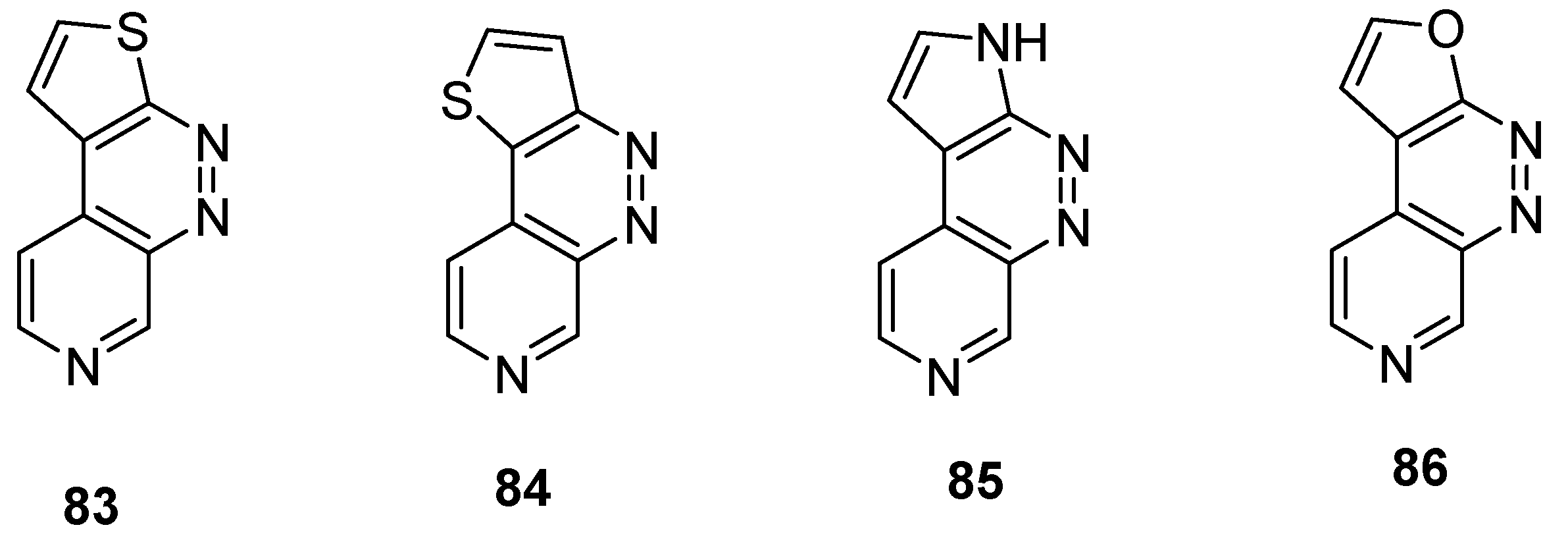
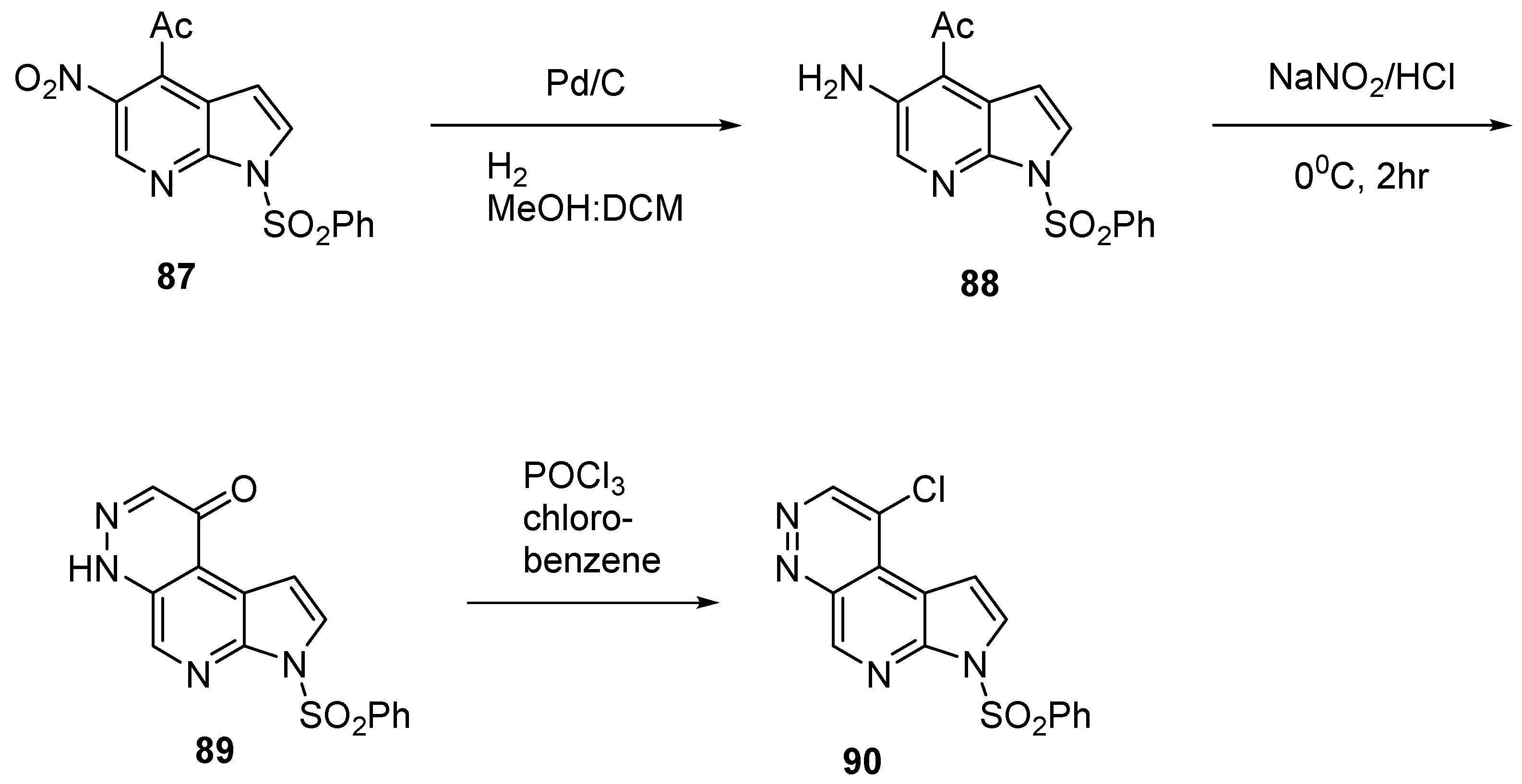
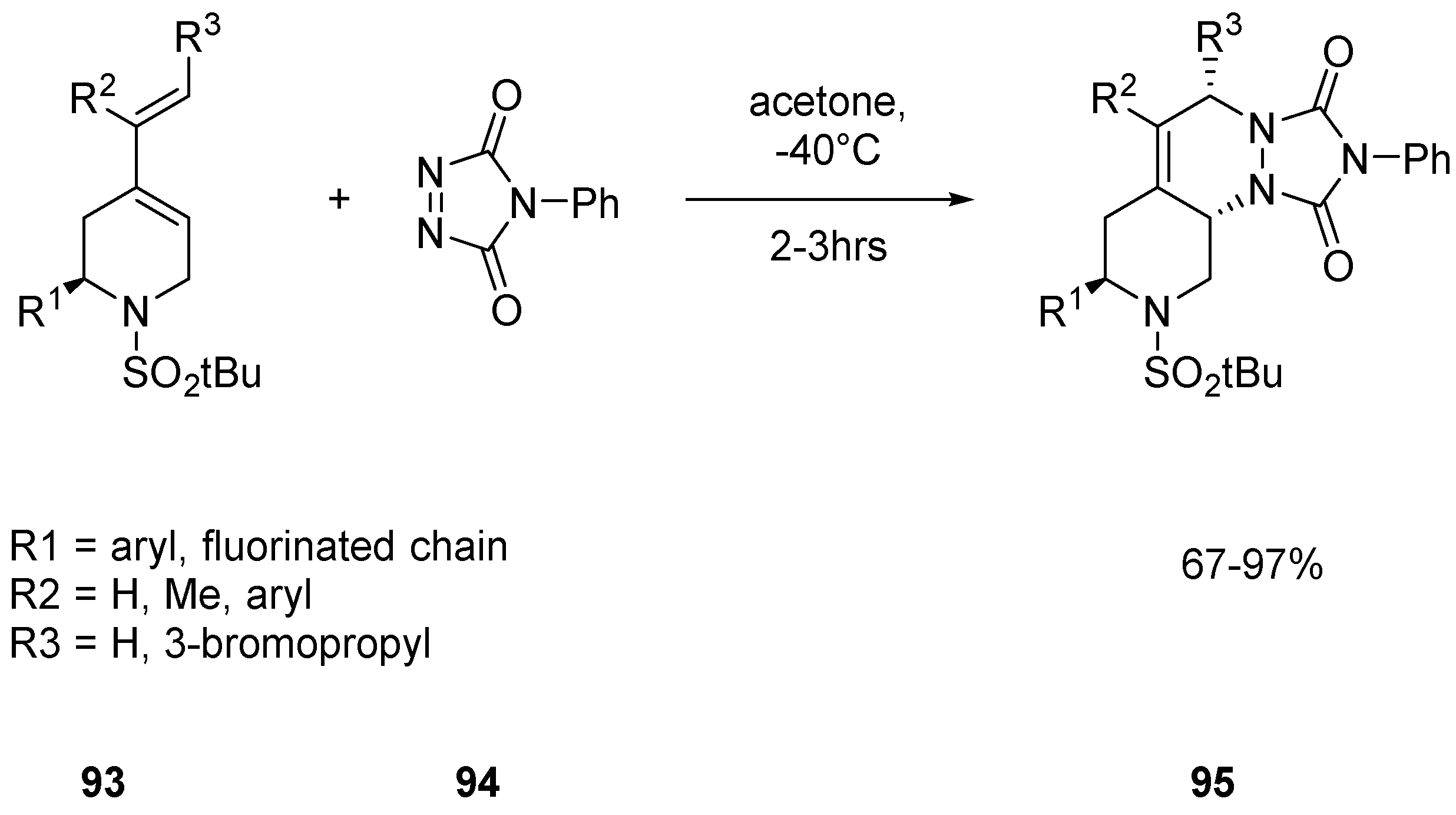
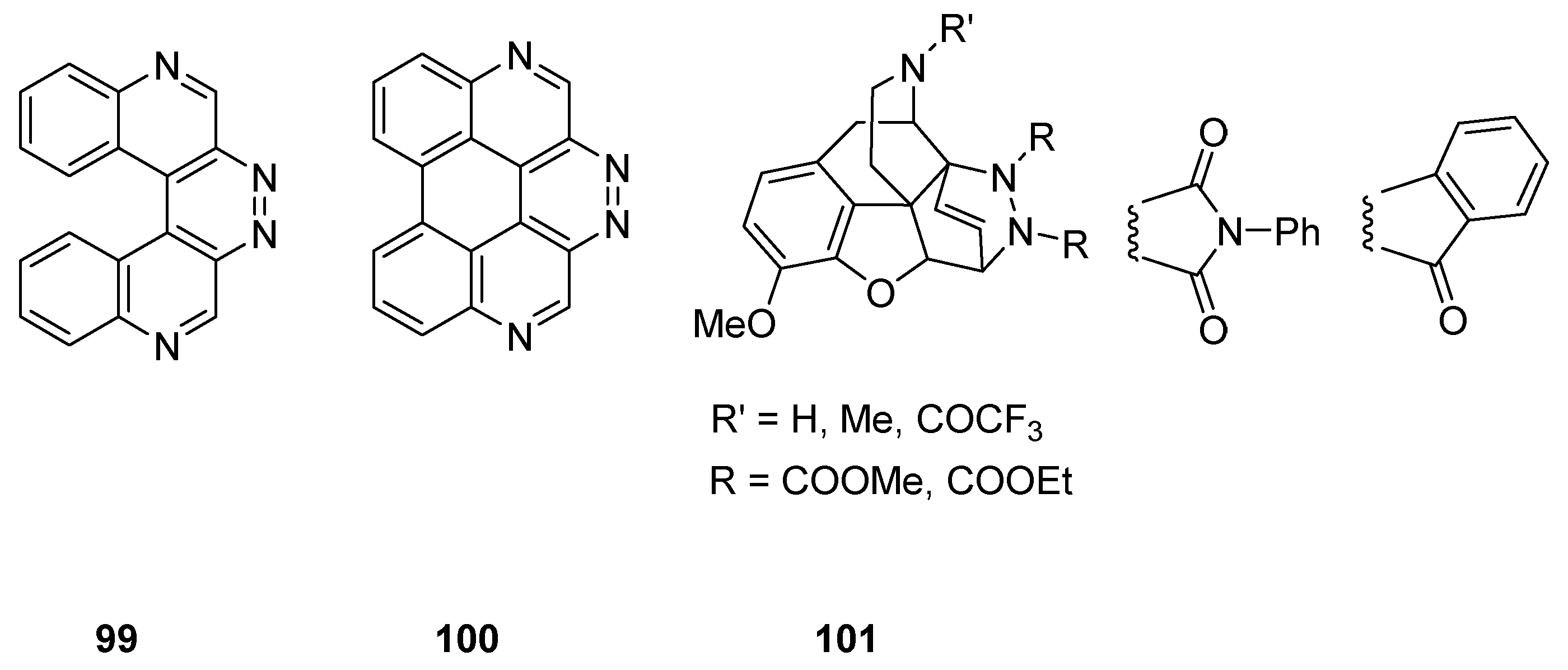
2.2.4. Heterocycle Fused Tricyclic Derivatives
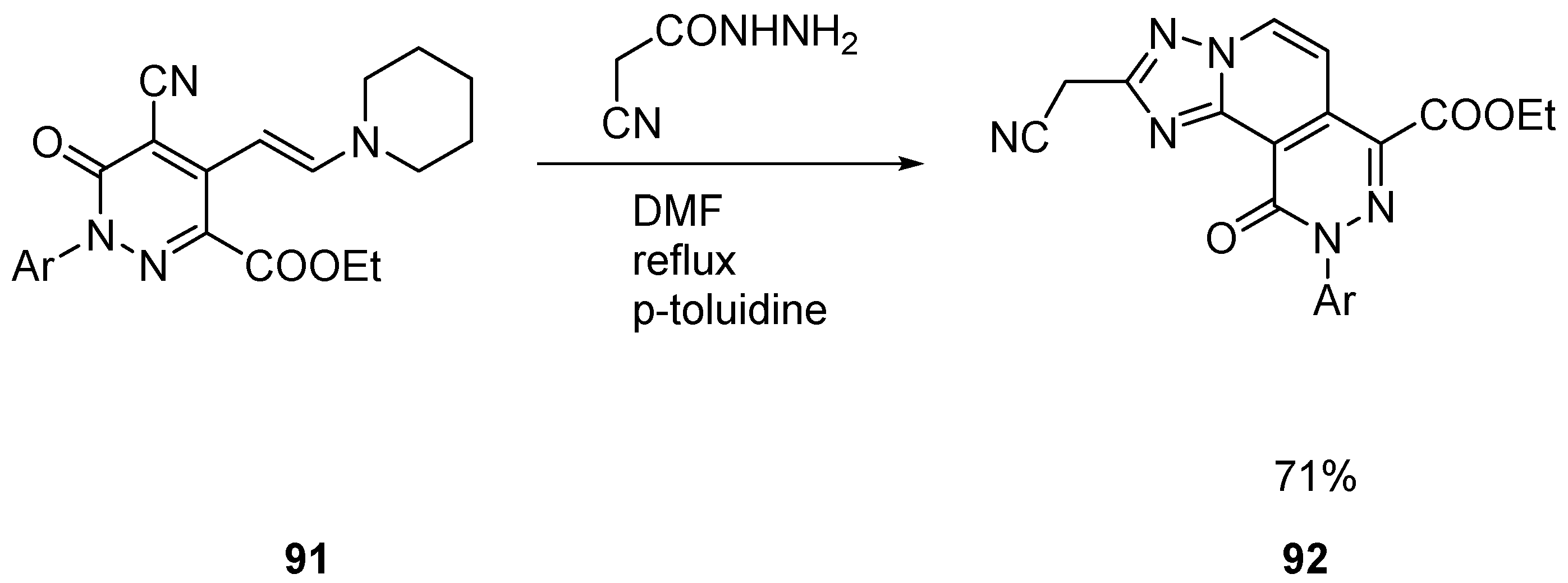
2.3. Polycyclic Derivatives
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walsh, C.T. Nature loves nitrogen heterocycles. Tetrahedron Lett. 2015, 56, 3075–3081. [Google Scholar] [CrossRef]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef] [PubMed]
- Pitt, W.R.; Parry, D.M.; Perry, B.G.; Groom, C.R. Heteroaromatic Rings of the Future. J. Med. Chem. 2009, 52, 2952–2963. [Google Scholar] [CrossRef] [PubMed]
- Wojcicka, A.; Nowicka-Zuchowska, A. Synthesis and Biological Activity of Pyridopyridazine Derivatives: A Mini Review. Mini-Rev. Org. Chem. 2018, 16, 3–11. [Google Scholar] [CrossRef]
- Thorimbert, S.; Botuha, C.; Passador, K. ‘Heteroaromatic Rings of the Future’: Exploration of Unconquered Chemical Space. Synthesis 2019, 51, 384–398. [Google Scholar] [CrossRef]
- Leach, A.G.; Kidley, N.J. Quantitatively Interpreted Enhanced Inhibition of Cytochrome P450s by Heteroaromatic Rings Containing Nitrogen. J. Chem. Inf. Model. 2011, 51, 1048–1063. [Google Scholar] [CrossRef]
- Graton, J.; le Questel, J.Y.; Maxwell, P.; Popelier, P. Hydrogen-Bond Accepting Properties of New Heteroaromatic Ring Chemical Motifs: A Theoretical Study. J. Chem. Inf. Model. 2016, 56, 322–334. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Shinkai, I. (Eds.) Product Class 18: Pyridopyridazines. In Science of Synthesis, 16: Category 2, Hetarenes and Related Ring Systems; Thieme: New York, NY, USA, 2004. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Elmenoufy, A.H.; Elagawany, M.; Ghoneim, M.M.; Moawad, A. “Pyridopyridazine”: A Versatile Nucleus in Pharmaceutical Field. J. Biosci. Med. 2015, 3, 59–66. [Google Scholar] [CrossRef][Green Version]
- Atkinson, C.M.; Biddle, B.N. Triazaphenanthrenes. Part VI. Further observations on the Widman-Stoermer and Borsche reactions. J. Chem. Soc. C 1966, 2053–2060. [Google Scholar] [CrossRef]
- Stockmann, V.; Primpke, S.; Fiksdahl, A. 7-Azacinnolin-4(1H)-one preparation and NMR studies of tautomery. J. Heterocycl. Chem. 2011, 48, 737–741. [Google Scholar] [CrossRef]
- Bundy, G.L. Heterocycle Carboxamides as Antiviral Agents (WO 02/04444); World Intellectual Property Organisation: Geneva, Switzerland, 2002. [Google Scholar]
- Kochhar, M.M. 8-chloro-4-hydroxy-3-methyl-pyrido[3,4-c]pyridazine. J. Heterocycl. Chem. 1969, 6, 977–978. [Google Scholar] [CrossRef]
- Jones, G.; Rafferty, P. A synthesis of annulated pyridazines by cycloaddition of azodicarboxylates to vinylpyridines. Tetrahedron Lett. 1978, 19, 2731–2734. [Google Scholar] [CrossRef]
- Jones, G.; Rafferty, P. The synthesis of annulated pyridazines by cycloaddition of azodicarboxylates to vinyl heterocycles. Tetrahedron 1979, 35, 2027–2033. [Google Scholar] [CrossRef]
- Gao, Y. Piperazinyl Oxoalkyl Tetrahydroisoquinolines and Related Analogues (WO 2007/106349); World Intellectual Property Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Zhao, L.; Jin, L.; Jin, Y. 5,6,7,8-Tetrahydro-2H-Pyridineo [3,4-c] Pyridine Synthesis Method of Azine-3-Ketones (CN 102924452 A); State Intellectual Property Office of the People’s Republic of China: Beijing, China, 2013. [Google Scholar]
- Ashton, W.T.; Caldwell, C.G.; Duffy, J.L.; Mathvink, R.J.; Wang, L.; Weber, A. 3-Amino-4-Phenylbutanoic Acid Derivatives as Dipeptidyl Peptidase Inhibitors for the Treatment or Prevention of Diabetes (WO 2004/069162); World Intellectual Property Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Wan, H.; Geng, M.; Yu Cheng, C. A Class of Compounds with Kinase Inhibitory Activity, Preparation Method and Uses (201610383073.2); State Intellectual Property Office of the People’s Republic of China: Beijing, China, 2016. [Google Scholar]
- Szabo, G.; Potor, A. Naphthyridine and Pyrido[3,4-c]pyridazine Derivatives as GABAa α5 Receptor Modulators (WO 2021/191838); World Intellectual Property Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Lange, U.; Ochse, M.; van der Kam, E.; van Bergeijk, J.; Turner, S.; Oellien, F.; Walleser, P.; Amberg, W.; Hornberger, W.; Geneste, H.; et al. Fused Heterocyclic Compounds as SlP Modulators (US 2017/0057966); United States Patent Application Publication: Ludwigshafen, Denmark, 2017. [Google Scholar]
- Al-Mousawi, S.M.; Abdelhamid, I.A.; Moustafa, M.S. Alkylazinylcarbonitriles as building blocks in organic synthesis: Synthesis of 3-amino-7-arylhyrazonothieno-7H-[3,4-c]-pyridine-4,6-diones and pyrido-[3,4-c]-pyridazine-5-carbonitrile. Arkivoc 2007, 2007, 213–221. [Google Scholar] [CrossRef]
- Barsy, M.A. Studies on Polyfunctionalised Heteroaromatics: Synthesis of Several New Cinnoline and Pyrido[3,4-c]pyridazine Derivatives. J. Chin. Chem. Soc. 2000, 47, 951–955. [Google Scholar] [CrossRef]
- El Rady, E.A.; Barsy, M.A. A Convenient and Facile Synthesis of Pyridine, Pyridazine, Pyrimido[4,5-c]pyridazine, Pyrido[3,4-c]pyridazine, 1,6-Naphthyridine and Phthalazine Derivatives. ChemInform 2006, 37, 243–248. [Google Scholar] [CrossRef]
- Elnaghi, M.H.; Hassan, M.E.; Elmaghraby, M.A.; Abdul Aziz, M.A. Studies on alkylheteroaromatics: A novel synthesis of pyrido[3,4-c]pyridazine derivatives. Sci. Technol. Bull. 1992, 13, 45–50. [Google Scholar]
- Al-Awadhi, H.; Al-Omran, F.; HElnagdi, M.; Infantes, L.; Foces-Foces, C.; Jagerovic, N.; Elguero, J. New synthetic approaches to condensed pyridazinones: Alkylpyridazinyl carbonitriles as building blocks for the synthesis of condensed pyridazinones. Tetrahedron 1995, 51, 12745–12762. [Google Scholar] [CrossRef]
- Laihia, K.; Kolehmainen, E.; Wegelius, E.; Miltsov, S.; Nikiforov, V.; Karavan, V. NMR Spectroscopic and X-ray Structural Characterization of Two Diaryl Derivatives of (4-Cyano-6-methyl-3,8-dioxo-2,3,5,6,7,8-hexahydropyrido[3,4-c] Pyridazin-6-yl)hydrazono-acetic acid Ethyl Ester. Heterocycl. Commun. 2004, 10, 35–42. [Google Scholar] [CrossRef]
- Darwish, E.S.; Abdelrahman, M.A.; Salaheldin, A.M. Enamines in Heterocyclic Synthesis: A Novel Simple and Efficient Route to Condensed Pyridazines. Z. Für Nat. B 2011, 66, 597–602. [Google Scholar] [CrossRef]
- Erian, A.W.; Elkholy, Y.M.; Al-arab, E.E.; Elnagdf, M.H. Polyfunctionally Substituted Heterocycles: Synthesis of New Polyfunctionally Substituted Phthalazines, Pyrido[3,4-c]pyridazines and Pyrazolo[3,4-c]pyridazines. Phosphorus Sulfur Silicon Relat. Elem. 1997, 122, 133–141. [Google Scholar] [CrossRef]
- Manhi, F.M.; Zayed, S.E.; Ali, F.A.; Elnagdi, M.H. Studies with Polyfunctionally Substituted Heterocycles: Novel Syntheses of Pyrido[4,3-d]pyridazines and of Pyrido[3,4-d]pyridazines. Collect. Czechoslov. Chem. Commun. 1992, 57, 1770–1774. [Google Scholar] [CrossRef]
- Elghandour, A.H.H.; Hussein, A.H.M.; Elnagdi, M.H.; Harb, A.F.A.; Metwally, S.A.M. Polyfunctionally Substituted Heterocycles: Synthesis of new polyfunctionally substituted phthalazines, thieno[3,4-d]pyridazines and pyrido [3,4-c]pyridazines. J. Fur Prakt. Chem. Chem.-Ztg. 1992, 334, 723–726. [Google Scholar] [CrossRef]
- Hussein, A.M.; Atalla, A.A.; El-dean, A.M.K. Novel Synthesis of Pyridopyridazine, Pyrrolopyridazine and Some Pyridazine Derivatives. Pol. J. Chem. 1995, 69, 1642. [Google Scholar]
- Hao, X. Cinnolines as Inhibitors of HPK 1 (WO 2021/004535); World Intellectual Property Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Berger, U.; Dannhardt, G.; Obergrusberger, R. Diels-Alder-Reaktionen mit cyclischen Iminen, 3. Mitt. Cycloaddition von Iminen an Tetrazine. Arch. Pharm. 1982, 315, 428–437. [Google Scholar] [CrossRef]
- Atkinson, C.M.; Mattocks, A.R. 733. Triazaphenanthrenes. Part II. Derivatives of 10-phenyl- 1:2:9-triazaphenanthrene. J. Chem. Soc. 1957, 3722–3726. [Google Scholar] [CrossRef]
- Atkinson, C.M.; Mattocks, A.R. 320. Triazaphenanthrenes. Part V. The site of quaternisation in some triazaphenanthrene methiodides. J. Chem. Soc. 1962, 1671–1678. [Google Scholar] [CrossRef]
- Cajipe, G.J.B.; Landen, G.; Semler, B.; Moore, H.W. Reaction of aminoquinones and related vinylogous amides with nitrous acid. Synthesis and chemistry of cyclic diazo ketones. J. Org. Chem. 1975, 40, 3874–3878. [Google Scholar] [CrossRef]
- Proshchenkova, V.; Shuvalov, V.Y.; Glyzdinskaya, L.V.; Fisyuk, A.S.; Chernenko, S.A.; Khvostov, M.V.; Tolstikova, T.G.; Vorontsova, M.; Sagitullina, G.P. Synthesis of 4-Ethoxycarbonyl(cyano)-β-Carbolines via Thermolysis of 4-Aryl-3(5)-Azidopyridine Derivatives and the Study of their Optical and Hypoglycemic Properties. Chem. Heterocycl. Compd. 2021, 57, 187–198. [Google Scholar] [CrossRef]
- Barton, J.; Walker, R. Synthesis of the monoazabiphenylenes. Tetrahedron Lett. 1975, 16, 569–572. [Google Scholar] [CrossRef]
- Iqbal, J.; Gupta, A.; Husain, A. Photochemistry of phenazopyridine hydrochloride. Pharmazie 2006, 61, 747–750. [Google Scholar] [PubMed]
- Al-Awadi, N.A.; Ibrahim, Y.A.; Elnagdi, M.; Adam, A.Y.; John, E. Gas-phase pyrolysis of arylazonicotinates and nicotinonitriles: Routes towards new aminopyridine and pyrido[3,4-c]cinnoline derivatives. J. Anal. Appl. Pyrolysis 2017, 124, 602–609. [Google Scholar] [CrossRef]
- Jerchel, D.; Fischer, H. 2.3-Azadiphenylen-tetrazoliumsalze und Triazaphenanthrene. Chem. Ber. 1956, 89, 563–570. [Google Scholar] [CrossRef]
- Ames, D.E.; Byrne, C.J.A. Cinnolines. Part XVII. Reactions of 4-chlorocinnoline-3-carbonitrile and preparation of 2,3-dihydro-3-imino-1H-pyrazolo[4,3-c]cinnolines. J. Chem. Soc. Perkin Trans. 1 1976, 6, 592. [Google Scholar] [CrossRef]
- Goerlitzer, K.; Behrje, H. Furo-, Pyrrolo- and Pyridazino(3,4-c)quinolines. Pharmazie 1996, 51, 528–534. [Google Scholar] [CrossRef]
- Goerlitzer, K.; Fabian, J.; Jones, P.G.; Frohberg, P.; Drutkowski, G. Pyridazino[3,4-c]quinolines and Pyridazino[4,5-c]quinolines—Synthesis and Investigation of Lipoxygenase Inhibition. Pharmazie 2002, 57, 362–371. [Google Scholar] [CrossRef]
- Seitz, G.; Kämpchen, T. Inverse” Diels-Alder-Additionen, 4. Mitt.: π-Elektronenreiche, benzokondensierte Heteroaromaten als „inverse” Dienophile. Arch. Der Pharm. 1976, 309, 679–681. [Google Scholar] [CrossRef]
- Pindur, U.; Kim, Y.S.; Schollmeyer, D. Cyclization Reactions of 2,2’-Bis-N-methylindolyl to Potential Protein Kinase C Inhibitors. Heterocycles 1994, 38, 2267. [Google Scholar] [CrossRef]
- MacBride, J.A.H. Synthesis of 2,7-diazabiphenylene by thermal extrusion of nitrogen from 2,7,9,10-tetra-azaphenanthrene. J. Chem. Soc. Chem. Commun. 1974, 9, 359b. [Google Scholar] [CrossRef]
- Kanoktanaporn, S.; MacBride, J.A.H. Preparation of ring-fused pyridazines by reduction of 3,3′-dinitro-4,4′-bipyridyl and 3,3′-dinitro-4,4′-biquinolyl. J. Chem. Soc. Perkin Trans. 1 1978, 1126–1131. [Google Scholar] [CrossRef]
- Kanoktanaporn, S.; MacBride, J.A.A.; King, T.J. Biphenylenes and Heterocyclic Analogs of Biphenylene. Part II. Preparation of f 2,7-Diazabiphenylene, its Quaternary Salts, and an Unstable Angular Dibenzo-derivative; X-ray Crystal Structure of 2-Allyl-2-azonia-7-azabiphenylene bromi. J. Chem. Res. Synop. 1980, 11, 2911–2940. [Google Scholar] [CrossRef]
- Kaczmarek, U. Bipyridines. XIX. Synthesis of some novel 2,7,9,10-Tetraazaphenanthrene derivatives. J. Fuer Prakt. Chem. 1987, 329, 592–598. [Google Scholar] [CrossRef]
- MacBride, J.; Wright, P.H.; Wakefield, B.J. Synthesis of 1.6-Diazabiphenylene by Flash Vacuum Pyrolysis of 2,5,9,10-tetra-azaphenanthrene; NMR Evidence for Rehybridisation in Biphenylenes. Tetrahedron Lett. 1981, 22, 4545–4548. [Google Scholar] [CrossRef]
- Stockmann, V.; Fiksdahl, A. Preparation of new pyrido[3,4-b]thienopyrroles and pyrido[4,3-e]thienopyridazines. Tetrahedron 2008, 64, 7626–7632. [Google Scholar] [CrossRef]
- Stockmann, V.; Eriksen, K.L.; Fiksdahl, A. Preparation of novel pyridine-fused tris-heterocycles; pyrido[4,3-e]pyrrolo-/pyrido[4,3-e]furano[2,3-c]pyridazines and pyrido[3,4-b]pyrrolo[3,2-d]pyrrole. Tetrahedron 2008, 64, 11180–11184. [Google Scholar] [CrossRef]
- Barawkar, D.; Bende, T.; Zahler, R.; Bandyopadhyay, A.; Sarangthem, R.S.; Doshi, J.; Waman, Y.; Jadhav, R.; Singh, U.P. Substituted Fused Tricyclic Compounds, Compositions and Medicinal Applications Thereof (WO 2012/127506); World Intellectual Property Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Llobat, A.; Escorihuela, J.; Sedgwick, D.M.; Rodenes, M.; Román, R.; Soloshonok, V.A.; Han, J.; Medio-Simón, M.; Fustero, S. The Ruthenium-Catalyzed Domino Cross Enyne Metathesis/Ring-Closing Metathesis in the Synthesis of Enantioenriched Nitrogen-Containing Heterocycles. Eur. J. Org. Chem. 2020, 4193–4207. [Google Scholar] [CrossRef]
- Elix, J.A.; Sterns, M.; Wilson, W.S.; Warrener, R.N. Preparation and X-ray structure of the 8a-azonia-3,4-diazaphenanthrene cation. J. Chem. Soc. D Chem. Commun. 1971, 9, 426b. [Google Scholar] [CrossRef]
- Merz, H.; Pook, K.H. Reaktionen des thebains mit azodicarbonsäureestern. Tetrahedron 1970, 26, 1727–1741. [Google Scholar] [CrossRef]
- Giger, R.; Rubinstein, R.; Ginsburg, D. Synthesis and reactions of the Diels-Alder adduct of thebaine with 4-phenyl-1,2,4-triazoline-3,5-dione. Tetrahedron 1973, 29, 2387–2391. [Google Scholar] [CrossRef]
- Marton, J.; Szabó, Z.; Csorvássy, I.; Simon, C.; Hosztafi, S.; Makleit, S. Reaction of morphinan-6,8-dienes with azadienophiles. Tetrahedron 1996, 52, 2449–2464. [Google Scholar] [CrossRef]
- Csutoras, C.; Berenyi, S.; Czako, B.; Makleit, S. Syntheses and transformations of novel nitrogen and sulfur containing morphinanedienes. Mon. Fur Chem. Chem. Mon. 1997, 128, 1267–1273. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alegbejo Price, T.O.; Emery, F.d.S.; Dehaen, W. Synthetic Pathways to Pyrido[3,4-c]pyridazines and Their Polycyclic Derivatives. Organics 2022, 3, 430-445. https://doi.org/10.3390/org3040028
Alegbejo Price TO, Emery FdS, Dehaen W. Synthetic Pathways to Pyrido[3,4-c]pyridazines and Their Polycyclic Derivatives. Organics. 2022; 3(4):430-445. https://doi.org/10.3390/org3040028
Chicago/Turabian StyleAlegbejo Price, Temitayo Omowumi, Flavio da Silva Emery, and Wim Dehaen. 2022. "Synthetic Pathways to Pyrido[3,4-c]pyridazines and Their Polycyclic Derivatives" Organics 3, no. 4: 430-445. https://doi.org/10.3390/org3040028
APA StyleAlegbejo Price, T. O., Emery, F. d. S., & Dehaen, W. (2022). Synthetic Pathways to Pyrido[3,4-c]pyridazines and Their Polycyclic Derivatives. Organics, 3(4), 430-445. https://doi.org/10.3390/org3040028






