Journal Description
Organics
Organics
is an international, peer-reviewed, open access journal on organic chemistry published quarterly online by MDPI.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within Scopus, ESCI (Web of Science), CAPlus / SciFinder, and other databases.
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 36.3 days after submission; acceptance to publication is undertaken in 5.2 days (median values for papers published in this journal in the first half of 2025).
- Recognition of Reviewers: APC discount vouchers, optional signed peer review, and reviewer names published annually in the journal.
- Journal Cluster of Chemical Reactions and Catalysis: Catalysts, Chemistry, Electrochem, Inorganics, Molecules, Organics, Oxygen, Photochem, Reactions, Sustainable Chemistry.
Impact Factor:
1.6 (2024);
5-Year Impact Factor:
1.8 (2024)
Latest Articles
Recent Advances in the Synthesis of 4H-Benzo[d][1,3]oxathiin-4-ones and 4H-Benzo[d][1,3]dioxin-4-ones
Organics 2025, 6(4), 48; https://doi.org/10.3390/org6040048 - 24 Oct 2025
Abstract
►
Show Figures
4H-Benzo[d][1,3]oxathiin-4-ones and 4H-benzo[d][1,3]dioxin-4-ones, as important classes of sulfur- or oxygen-containing heterocyclic compounds, possess significant application potential in the fields of pharmaceutical chemistry, agriculture, and the food industry due to their distinctive structural characteristics and diverse
[...] Read more.
4H-Benzo[d][1,3]oxathiin-4-ones and 4H-benzo[d][1,3]dioxin-4-ones, as important classes of sulfur- or oxygen-containing heterocyclic compounds, possess significant application potential in the fields of pharmaceutical chemistry, agriculture, and the food industry due to their distinctive structural characteristics and diverse biological activities. In recent years, efficient synthetic strategies for these compounds have witnessed remarkable progress. This review summarizes significant advancements in the construction of these heterocycles from 2012 to the present.
Full article
Open AccessArticle
Efficient Degradation of Cis-Polyisoprene by GQDs/g-C3N4 Nanoparticles Under UV Light Irradiation
by
Cilong Chen, Jinrui Liu, Bangsen Li, Dashuai Zhang, Peisong Zhang, Jianjun Shi and Zaifeng Shi
Organics 2025, 6(4), 47; https://doi.org/10.3390/org6040047 - 14 Oct 2025
Abstract
►▼
Show Figures
Rubber material with high elasticity and viscoelasticity has become the most widely used universal material, and the study of the aging failure mechanism of rubber has been meaningful research in the polymer materials field. Cis-polyisoprene was employed to analyze the mechanism of
[...] Read more.
Rubber material with high elasticity and viscoelasticity has become the most widely used universal material, and the study of the aging failure mechanism of rubber has been meaningful research in the polymer materials field. Cis-polyisoprene was employed to analyze the mechanism of oxidative degradation under artificial UV irradiation, and the GQDs/g-C3N4 photocatalysis with a 2D layered structure prepared by the method of microwave-assisted polymerization enabled to accelerate the degradation procedure. The results showed that the oxidation of cis-polyisoprene occurred during the irradiation for 3 days and the structure of cis-polyisoprene changed. The α-H of the double bond was attacked by oxygen to form hydroperoxide. Then, aldehydes and ketones generated as the addition reaction of double bonds occurred. The content of the hydrogen of C=C reduced, and the oxidative degradation was dominant at the initial aging stage. The crosslinking reaction was dominant at the final aging stage and the average molecular weight decreased from 15.49 × 104 to 8.78 × 104. The GQDs could promote the charge transfer and the photodegradation efficiency and inhibit the electron–hole recombination. The light capture ability of GQDs was improved after compositing with g-C3N4. The free radicals ·O22− generated after adding GQDs/g-C3N4 nanoparticles, and the molecular weight of cis-polyisoprene decreased to 5.79 × 104, with the photocatalytic efficiency increasing by 20%. This work provided academic bases and reference values for the application of photocatalysts in the field of natural rubber degradation and rubber wastewater treatment.
Full article

Figure 1
Open AccessReview
Synthetic Routes and Bioactivity Profiles of the Phenothiazine Privileged Scaffold
by
Aigul E. Malmakova and Alan M. Jones
Organics 2025, 6(4), 46; https://doi.org/10.3390/org6040046 - 10 Oct 2025
Abstract
►▼
Show Figures
This review offers a focused overview of the strategies used to build and modify phenothiazine (PTZ) derivatives. It covers both classical synthetic approaches and advances reported since 2014, including transition metal-catalyzed transformations and greener techniques, such as electrosynthesis, microwave-assisted reactions, and ultrasound-promoted methods.
[...] Read more.
This review offers a focused overview of the strategies used to build and modify phenothiazine (PTZ) derivatives. It covers both classical synthetic approaches and advances reported since 2014, including transition metal-catalyzed transformations and greener techniques, such as electrosynthesis, microwave-assisted reactions, and ultrasound-promoted methods. Each strategy is evaluated with respect to efficiency, scalability, and sustainability. In parallel, the review surveys the diverse bioactivity profiles of PTZ derivatives, ranging from antipsychotic, anticancer, and antimicrobial activities to emerging applications in photodynamic therapy and neuroprotection. By correlating synthetic accessibility with biological potential, this review provides an integrated perspective that highlights advances achieved since 2014 and outlines future opportunities for rational PTZ design and applications.
Full article

Graphical abstract
Open AccessArticle
The Song Remains the Same, but the Enzymes Don’t: Imidazolium ILs as Potential Disruptors of Fatty Acid Metabolism
by
Savina Stoyanova and Milen G. Bogdanov
Organics 2025, 6(4), 45; https://doi.org/10.3390/org6040045 - 2 Oct 2025
Abstract
►▼
Show Figures
This study examined twenty-eight N-methylimidazolium ionic liquids (ILs) with various substituents and anions to assess their impact on the activity of Carnitine Acetyltransferase (CAT), an indispensable enzyme in human metabolism. In vitro experiments demonstrated that these compounds inhibited CAT in a concentration-dependent
[...] Read more.
This study examined twenty-eight N-methylimidazolium ionic liquids (ILs) with various substituents and anions to assess their impact on the activity of Carnitine Acetyltransferase (CAT), an indispensable enzyme in human metabolism. In vitro experiments demonstrated that these compounds inhibited CAT in a concentration-dependent manner, with IC50 values ranging from 0.93 to 30.8 mM. Structural analysis of the ILs revealed the following structure–activity relationships: (i) the length of the hydrocarbon chain at N3 markedly affects CAT activity, with longer chains resulting in stronger inhibition; (ii) the degree of unsaturation and the presence of polar groups are not essential for increased activity; (iii) the effect of the anion aligns with the Hofmeister series. One of the most potent compounds, 1-decyl-3-methylimidazolium bromide [C10C1im]Br, was identified as a mixed inhibitor of CAT with a Ki of 0.77 mM. These findings raise concerns about the biocompatibility of commonly used imidazolium ILs, as they may interfere with fatty acid oxidation by inhibiting their cellular transport.
Full article

Figure 1
Open AccessArticle
Influence of MW Irradiation on the Reaction Between (2R,7R,11S,16S)-1,8,10,17-tetraazapentacyclo[8.8.1.1.8,170.2,70.11,16]icosane and p-Substituted Phenols
by
Diego Quiroga, Jaime Ríos-Motta and Augusto Rivera
Organics 2025, 6(4), 44; https://doi.org/10.3390/org6040044 - 2 Oct 2025
Abstract
►▼
Show Figures
4,4′-substituted-2,2′-((hexahydro-1H-benzo[d]imidazole-1,3(2H)-diyl)bis(methylene))bisphenols (1a–d) and 2,6-bis{[3-(2-hydroxy-5-substitutedbenzyl)octahydro-1H-benzimidazol-1-yl]methyl}-4-substitutedphenols (2a–b) were synthesized via microwave (MW) irradiation of aminal (2R,7R,11S,16S
[...] Read more.
4,4′-substituted-2,2′-((hexahydro-1H-benzo[d]imidazole-1,3(2H)-diyl)bis(methylene))bisphenols (1a–d) and 2,6-bis{[3-(2-hydroxy-5-substitutedbenzyl)octahydro-1H-benzimidazol-1-yl]methyl}-4-substitutedphenols (2a–b) were synthesized via microwave (MW) irradiation of aminal (2R,7R,11S,16S)-1,8,10,17-tetraazapentacyclo[8.8.1.1.8,170.2,70.11,16]icosane 2 with p-substituted phenols. Microwave (MW) irradiation improved reaction rates and yields at 80 °C. Compounds 1a–d were racemic, and 2a–b were diastereomeric. NMR spectra revealed key signals for the perhydrobenzimidazole fragment, aromatic rings, and aminal carbons. Differences in the 13C NMR spectra highlighted structural variations, such as distinct carbonyl and methoxyl signals in 2d. MW irradiation at higher temperatures (100–120 °C) reduced yields of 1, especially for phenols with methyl (Me) and methoxy (OMe) groups, suggesting a shift toward the formation of compound 2. Additionally, higher temperatures led to polymerization byproducts, emphasizing the impact of MW energy on reaction pathways. These results provide valuable insights for designing molecules with potential applications in materials science and medicinal chemistry.
Full article

Figure 1
Open AccessArticle
Degrees of Sulfonation: Mapping the Reactivity Landscape of Acridine and Acridone
by
Péter Kisfaludi, Sára Spátay, Péter Huszthy and Ádám Golcs
Organics 2025, 6(3), 43; https://doi.org/10.3390/org6030043 - 12 Sep 2025
Abstract
Although sulfonated acridines and acridones are valuable scaffolds in diagnostics and materials science, to our best knowledge, there is no comprehensive study that addresses how the degree of sulfonation depends on reaction parameters. To fill this gap, we investigated the sulfonation behavior of
[...] Read more.
Although sulfonated acridines and acridones are valuable scaffolds in diagnostics and materials science, to our best knowledge, there is no comprehensive study that addresses how the degree of sulfonation depends on reaction parameters. To fill this gap, we investigated the sulfonation behavior of unsubstituted acridine and acridone under classical conditions, using sulfuric acid, oleum, and chlorosulfonic acid. A factorial experimental design was applied to systematically evaluate the influence of temperature and reagent excess on the extent of sulfonation, while keeping the reaction time constant. Products were analyzed by HPLC–MS/MS to determine the degree of sulfonation and its distribution. Regioselectivity and product isolation were not addressed in this study. Our results provide a foundational dataset for controlling sulfonation level for these heterocycles and can help future synthetic applications where defined sulfonation patterns are desired.
Full article
(This article belongs to the Special Issue Chemistry of Heterocyclic Compounds)
►▼
Show Figures

Graphical abstract
Open AccessArticle
Fast and Efficient Synthesis of Fluoro Phenyl 1,2,3-Triazoles via Click Chemistry with Ultrasound Irradiation and Their Biological Efficacy Against Candida albicans
by
Elisa Leyva, Johana Aguilar, Silvia E. Loredo-Carrillo and Ismael Acosta-Rodríguez
Organics 2025, 6(3), 42; https://doi.org/10.3390/org6030042 - 8 Sep 2025
Abstract
►▼
Show Figures
Several fluoro phenyl triazoles were synthesized using click chemistry between fluoro phenyl azides and phenyl acetylene. Under ultrasound irradiation, this synthetic procedure was performed with Cu (I) in the presence of 1,10-phenanthroline. It is fast with high yields of target compounds. In addition,
[...] Read more.
Several fluoro phenyl triazoles were synthesized using click chemistry between fluoro phenyl azides and phenyl acetylene. Under ultrasound irradiation, this synthetic procedure was performed with Cu (I) in the presence of 1,10-phenanthroline. It is fast with high yields of target compounds. In addition, fluoro phenyl triazoles were evaluated against Candida albicans. The inhibition percentage of yeast growth was investigated using different concentrations of triazoles. Compounds containing a fluorine atom in 2, 4, 2,6, and 2,4,6 positions inhibited a higher percentage of yeast growth. All of the triazoles showed inhibition of the yeast–mycelium transition, which was related to pathogenicity of yeast strain C. albicans.
Full article

Figure 1
Open AccessReview
Synthesis and Biological Activity of 5-Substituted-2,4-dihydro-1,2,4-triazole-3-thiones and Their Derivatives
by
Abdukhakim A. Ziyaev, Sobirdjan A. Sasmakov, Turdibek T. Toshmurodov, Jaloliddin M. Abdurakhmanov, Saidazim A. Ikramov, Shukhrat Sh. Khasanov, Oybek N. Ashirov, Mavluda A. Ziyaeva and Dilrabo B. Begimqulova
Organics 2025, 6(3), 41; https://doi.org/10.3390/org6030041 - 4 Sep 2025
Abstract
►▼
Show Figures
Derivatives of 1,2,4-triazole-3-thione exhibit a variety of biological activities, including antimicrobial (e.g., compounds 31d–k, 32d, 36f), antitumor (e.g., 71, 77a–c, 82g, 94h), anti-inflammatory, analgesic (100a, 102, 105), antidiabetic,
[...] Read more.
Derivatives of 1,2,4-triazole-3-thione exhibit a variety of biological activities, including antimicrobial (e.g., compounds 31d–k, 32d, 36f), antitumor (e.g., 71, 77a–c, 82g, 94h), anti-inflammatory, analgesic (100a, 102, 105), antidiabetic, and antioxidant (104, 138) activity. These compounds can be efficiently synthesized by classical methods (e.g., cyclization of thiosemicarbazides) and/or modern “green” approaches, which allow for obtaining target compounds in high yields (up to 96%). The presence of electron-donating groups (e.g., -OH, -OCH3) enhances antimicrobial and antitumor activity. Substituents in the aromatic ring (e.g., NO2, Cl) affect the ability to bind to biological targets such as DNA or enzymes. 1,2,4-triazole-3-thiones can also be used as fungicides and herbicides (e.g., 131), demonstrating high efficiency against phytopathogens. Thus, 1,2,4-triazole-3-thione derivatives are multifunctional compounds with high potential for the development of new drugs and agrochemicals. Their further study and modification can lead to the creation of more effective and safer drugs.
Full article

Figure 1
Open AccessArticle
Microwave-Assisted Catalytic Transfer Hydrogenation of Chalcones: A Green, Fast, and Efficient One-Step Reduction Using Ammonium Formate and Pd/C
by
Wender Alves Silva, Sayuri Cristina Santos Takada, Felipe Marques Nogueira and Luiz Arthur Ramos Almeida
Organics 2025, 6(3), 40; https://doi.org/10.3390/org6030040 - 3 Sep 2025
Abstract
►▼
Show Figures
Catalytic transfer hydrogenation (CTH) and microwave-assisted organic synthesis (MAOS) have each advanced the sustainability of reduction chemistry; however, their combined application to conjugated enones remains largely unexplored. To the best of our knowledge, no unified protocol has been reported for the rapid, one-pot
[...] Read more.
Catalytic transfer hydrogenation (CTH) and microwave-assisted organic synthesis (MAOS) have each advanced the sustainability of reduction chemistry; however, their combined application to conjugated enones remains largely unexplored. To the best of our knowledge, no unified protocol has been reported for the rapid, one-pot conversion of chalcones into saturated alcohols under microwave irradiation. Herein, we report a concise and green method that integrates MAOS with Pd/C-catalyzed CTH, employing inexpensive ammonium formate in ethanol. In contrast to state-of-the-art hydrogenations that require pressurized H2 or costly metal complexes, our strategy (i) achieves complete conversion within 20 min at 60 °C, (ii) tolerates both electron-rich and electron-poor substrates, (iii) reduces nitro-substituted chalcones in a single step, and (iv) consumes < 0.005 kWh per reaction—an approximately 250-fold energy saving relative to conventional procedures. These results position microwave-driven CTH as a scalable alternative for synthesizing pharmacologically relevant saturated alcohol scaffolds from readily available chalcones.
Full article

Graphical abstract
Open AccessArticle
Synthesis, Purification, Characterization, and ABTS Antioxidant Evaluation of Novel Azo Dyes
by
Jeremy A. Rodríguez-Vargas, Sebastián H. Díaz-Rodríguez, Víctor G. Vergara-Rodríguez, Ángel Vidal-Rosado, Cristtian Rivera-Torres, Alejandra Ríos-Rodríguez, Martín Rodríguez-Del Valle, Daliana Agosto-Disdier, Marielys Torres-Díaz, Kai H. Griebenow and Raúl R. Rodríguez-Berríos
Organics 2025, 6(3), 39; https://doi.org/10.3390/org6030039 - 2 Sep 2025
Abstract
►▼
Show Figures
The search for bioactive compounds with antioxidant properties is critical in combating oxidative stress-related diseases and advancing novel therapeutic agents. Azo dyes, traditionally used in textiles, food, and cosmetics, have recently attracted attention due to their emerging biological activities, including antioxidant potential. In
[...] Read more.
The search for bioactive compounds with antioxidant properties is critical in combating oxidative stress-related diseases and advancing novel therapeutic agents. Azo dyes, traditionally used in textiles, food, and cosmetics, have recently attracted attention due to their emerging biological activities, including antioxidant potential. In this study, we synthesized and characterized 267 azo dyes derived from natural phenolic cores such as salicylic acid, syringol, and 5,6,7,8-tetrahydro-2-naphthol. Eighteen of these compounds are novel. Structural characterization was performed using NMR, UV-Vis, IR spectroscopy, and mass spectrometry. Antioxidant activity was assessed using in vitro assays with ABTS radical scavenging method. SAR analysis revealed that dyes derived from syringol and 5, 6, 7, 8-tetrahydro-2-naphthol showed the most consistent and potent antioxidant activity. Notably, azo dyes bearing fluoro and nitro substituents in the para position exhibited the lowest IC50 values, highlighting the influence of electron-withdrawing groups and substitution patterns on antioxidant behavior. This work establishes a precedent for SAR-driven evaluation of azo dyes using ABTS and supports their further exploration as functional antioxidant agents in medicinal chemistry.
Full article

Figure 1
Open AccessArticle
Mechanochemical Synthesis, Spectroscopic Characterization and Molecular Structure of Piperidine–Phenytoin Salt
by
María Isabel Amil-Miranda, Armando Pineda-Contreras, Francisco Javier Martínez-Martínez, Marcos Flores-Álamo, Hector García-Ortega and Juan Saulo González-González
Organics 2025, 6(3), 38; https://doi.org/10.3390/org6030038 - 22 Aug 2025
Abstract
Phenytoin is an anticonvulsant drug that suffers from low aqueous solubility. The formation of phenytoin salts is a strategy employed to address this issue. A phenytoin–piperidine salt (PPD–PNT) was synthesized by solvent-assisted grinding and characterized by infrared (IR) spectroscopy, 1H and 13
[...] Read more.
Phenytoin is an anticonvulsant drug that suffers from low aqueous solubility. The formation of phenytoin salts is a strategy employed to address this issue. A phenytoin–piperidine salt (PPD–PNT) was synthesized by solvent-assisted grinding and characterized by infrared (IR) spectroscopy, 1H and 13C Nuclear Magnetic Resonance (NMR), and powder and single crystal X-ray diffraction. The IR and NMR spectra obtained differed from those of the starting compounds, showing shifts in the N-H and C=O group signals, as well as the appearance of NH+ signals, indicating proton transfer and salt formation. Powder X-ray diffraction confirmed the formation of a new solid phase corresponding to the salt. Single crystal X-ray diffraction showed the molecular structure of the PPD–PNT salt.
Full article
(This article belongs to the Special Issue Chemistry of Heterocyclic Compounds)
►▼
Show Figures

Graphical abstract
Open AccessArticle
Synthetic Approaches to Steroidal Thiosemicarbazones, 1,3,4-Thia(selena)diazolines, and Oxalate-Linked Dimers
by
Luis A. Méndez-Delgado, Mónica Martínez-Montiel, Alma Fuentes-Aguilar, Socorro Meza-Reyes, Sara Montiel-Smith, José Luis Vega-Baez, José M. Padrón and Penélope Merino-Montiel
Organics 2025, 6(3), 37; https://doi.org/10.3390/org6030037 - 22 Aug 2025
Abstract
►▼
Show Figures
A total of 24 novel steroidal derivatives were synthesized, including 1,3,4-thia(selena)diazolines and structurally unique spirothiadiazolines, obtained through intramolecular cyclization under standard acetylation conditions. This strategy was further extended to the construction of a novel dimeric compound bearing a thiadiazoline linker. Seleno- and thiosemicarbazone
[...] Read more.
A total of 24 novel steroidal derivatives were synthesized, including 1,3,4-thia(selena)diazolines and structurally unique spirothiadiazolines, obtained through intramolecular cyclization under standard acetylation conditions. This strategy was further extended to the construction of a novel dimeric compound bearing a thiadiazoline linker. Seleno- and thiosemicarbazone precursors were derived from various functionalized steroidal monomers and dimers via straightforward synthetic protocols. Key intermediates included aldehyde 7 and ketones 16, 19, and 24. Rotameric equilibria were observed in certain thiosemicarbazones, attributed to partial double-bond character in the N–CS bond. Cyclization yielded heterocyclic systems as epimeric mixtures, and in some cases, inseparable mixtures of isomers were obtained due to low diastereoselectivity. Full structural elucidation of epimeric pairs was achieved using 2D NMR and IR spectroscopy, with compounds 2, 3, 5, 11, 17, 27, 28a, and 28b further confirmed by single-crystal X-ray diffraction. Preliminary antiproliferative assays against human cancer cell lines revealed GI50 values below 10 µM for compounds 21, 22, and 27.
Full article
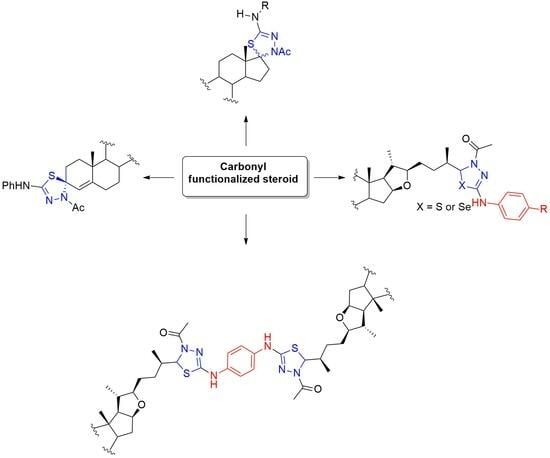
Graphical abstract
Open AccessArticle
Investigation on Porous Carbon-Loaded MnO for Removing Hexavalent Chromium from Aqueous Solution
by
Liping Wang and Mingyu Zhang
Organics 2025, 6(3), 36; https://doi.org/10.3390/org6030036 - 12 Aug 2025
Abstract
►▼
Show Figures
Porous carbon-loaded MnO was prepared via a combination of the sol–gel method and the chemical blow molding method using polyvinylpyrrolidone (PVP) and manganese nitrate as starting materials. SEM, EDX, TEM, FTIR, XRD, XPS, nitrogen adsorption–desorption, and elemental analysis were used to assess its
[...] Read more.
Porous carbon-loaded MnO was prepared via a combination of the sol–gel method and the chemical blow molding method using polyvinylpyrrolidone (PVP) and manganese nitrate as starting materials. SEM, EDX, TEM, FTIR, XRD, XPS, nitrogen adsorption–desorption, and elemental analysis were used to assess its physical and chemical characteristics. Furthermore, the adsorption property of porous carbon-loaded MnO for hexavalent chromium (Cr(VI)) in polluted water was investigated in detail. The results demonstrated that large numbers of MnO nanoparticles were evenly mounted on the surfaces of carbon walls, with a uniform distribution of C, N, and O elements. The BET surface area was 46.728 m2/g, and the pore sizes of porous carbon ranged from 2 nm to 10 nm. Additionally, abundant surface functional groups were found in porous carbon-loaded MnO, a result consistent with XPS data and applicable to the adsorption of heavy metals from aqueous solutions containing Cr(VI). The Freundlich model fitted the adsorption isotherm well, and the pseudo−second−order model precisely matched the adsorption kinetics. According to the study results, the adsorption was multilayer, and the adsorption process involved an endothermic reaction. These results indicate that this is a feasible way to synthesize a high−efficiency adsorbent for the removal of harmful heavy−metal ions from wastewater.
Full article

Figure 1
Open AccessArticle
Profiling of Disubstituted Chloroacetamides’ Potential Biological Activity by Liquid Chromatography
by
Suzana Apostolov, Dragana Mekić, Marija Mitrović, Slobodan Petrović and Gyöngyi Vastag
Organics 2025, 6(3), 35; https://doi.org/10.3390/org6030035 - 4 Aug 2025
Abstract
►▼
Show Figures
Modern agriculture relies heavily on the use of pesticides, with one-third of them being herbicides. Chloroacetamides are the most widely used herbicides because of their high effectiveness, but their extensive use poses environmental challenges and threatens the health of living organisms due to
[...] Read more.
Modern agriculture relies heavily on the use of pesticides, with one-third of them being herbicides. Chloroacetamides are the most widely used herbicides because of their high effectiveness, but their extensive use poses environmental challenges and threatens the health of living organisms due to toxicity risks. Since the pharmacokinetic behavior and toxicity of a compound are influenced by its lipophilicity, this essential physicochemical parameter for disubstituted chloroacetamides was determined in silico and experimentally through thin-layer chromatography on reversed phases (RPTLC C18/UV254s) in mixtures of water and distinct organic modifiers. The pharmacokinetic profile of chloroacetamides was analyzed by using the BOILED-Egg model. The correlation between the obtained chromatographic parameters and software-based lipophilicity, pharmacokinetic, and ecotoxicity predictors of the studied chloroacetamides was assessed by using linear regression, but more comprehensive insight was obtained through multivariate methods—Cluster Analysis and Principal Component Analysis. It was observed that the total number of carbon atoms in the structure of their molecules, along with the type of hydrocarbon substituents, are the most important factors affecting lipophilicity, pharmacokinetics, and potential toxicity to non-target organisms.
Full article

Figure 1
Open AccessArticle
Limitations of Frontier Orbital and Charge Approaches in the Description of Electrophilic Aromatic Substitution
by
Lucia Emanuele and Maurizio D’Auria
Organics 2025, 6(3), 34; https://doi.org/10.3390/org6030034 - 1 Aug 2025
Abstract
►▼
Show Figures
DFT calculations at the B3LYP/aug-cc-pVDZ level of theory on some aromatic substrates showed that in the HOMO (Highest Occupied Molecular Orbital) of nitrobenzene, the atomic coefficients are not in agreement with the meta-directing behavior of this compound. The atomic coefficients are the same
[...] Read more.
DFT calculations at the B3LYP/aug-cc-pVDZ level of theory on some aromatic substrates showed that in the HOMO (Highest Occupied Molecular Orbital) of nitrobenzene, the atomic coefficients are not in agreement with the meta-directing behavior of this compound. The atomic coefficients are the same in the ortho and in the meta positions. The HOMO (or NHOMO (Next Occupied Molecular Orbital) in the case of benzaldehyde) is not in agreement with the experimental results when deactivating, meta-orienting compounds are considered. Mulliken charges sometimes are not able to explain the observed reactivity. Hirshfeld charges allow us to predict the orientation of the attack of an electrophile on the aromatic ring, with the exception of nitrobenzene. Both HOMO atomic coefficients and charges are in agreement with the experimental results when deactivating, ortho-para orienting, and activating compounds are tested.
Full article

Figure 1
Open AccessReview
PANI-Based Thermoelectric Materials
by
Mengran Chen, Dongmei Xie, Hongqing Zhou and Pengan Zong
Organics 2025, 6(3), 33; https://doi.org/10.3390/org6030033 - 22 Jul 2025
Cited by 1
Abstract
►▼
Show Figures
Polyaniline (PANI) based thermoelectric materials have attracted much attention in flexible energy harvesting devices due to their unique molecular structure, excellent chemical stability, and low cost. However, the intrinsic thermoelectric performance of intrinsic PANI makes it difficult to meet the needs of practical
[...] Read more.
Polyaniline (PANI) based thermoelectric materials have attracted much attention in flexible energy harvesting devices due to their unique molecular structure, excellent chemical stability, and low cost. However, the intrinsic thermoelectric performance of intrinsic PANI makes it difficult to meet the needs of practical applications due to its low electronic transport properties. This review focuses on the preparation methods and key strategies for developing high-performance PANI-based thermoelectric materials. It aims to comprehensively update knowledge regarding synthesis methods, microstructures, thermoelectric properties, and underlying mechanisms. The overall goal is to provide timely insights to promote the development of high-performance PANI-based thermoelectric materials.
Full article

Graphical abstract
Open AccessReview
Engineering Nascent Disentangled Ultra-High-Molecular-Weight Polyethylene Based on Heterogeneous Catalytic Polymerization
by
Lei Li
Organics 2025, 6(3), 32; https://doi.org/10.3390/org6030032 - 21 Jul 2025
Abstract
►▼
Show Figures
Ultra-high-molecular-weight polyethylene (UHMWPE) is a pivotal material in engineering and biomedical applications due to its exceptional mechanical strength, wear resistance, and impact performance. However, its extreme melt viscosity, caused by extensive chain entanglements, severely limits processability via conventional melt-processing techniques. Recent advances in
[...] Read more.
Ultra-high-molecular-weight polyethylene (UHMWPE) is a pivotal material in engineering and biomedical applications due to its exceptional mechanical strength, wear resistance, and impact performance. However, its extreme melt viscosity, caused by extensive chain entanglements, severely limits processability via conventional melt-processing techniques. Recent advances in catalytic synthesis have enabled the production of disentangled UHMWPE (dis-UHMWPE), which exhibits enhanced processability while retaining superior mechanical properties. Notably, heterogeneous catalytic systems, utilizing supported fluorinated bis (phenoxy-imine) titanium (FI) catalysts, polyhedral oligomeric silsesquioxanes (POSS)-modified Z-N catalysts, and other novel catalysts, have emerged as promising solutions, combining structural control with industrial feasibility. Moreover, optimizing polymerization conditions further enhances chain disentanglement while maintaining ultra-high molecular weights. These systems utilize nanoscale supports and ligand engineering to spatially isolate active sites, tailor the chain propagation/crystallization kinetics, and suppress interchain entanglement during polymerization. Furthermore, characterization techniques such as melt rheology and differential scanning calorimetry (DSC) provide critical insights into chain entanglement, revealing distinct reorganization kinetics and bimodal melting behavior in dis-UHMWPE. This development of hybrid catalytic systems opens up new avenues for solid-state processing and industrial-scale production. This review highlights recent advances concerning interaction between catalyst design, polymerization control, and material performance, ultimately unlocking the full potential of UHMWPE for next-generation applications.
Full article

Figure 1
Open AccessArticle
Synthesis of Cannabigerol and Cannabigerol Derivatives
by
Juan F. Ortuño, Alessio Ghisolfi, Raquel Almansa, Olga Soares do Rego Barros, Ana Sirvent, José M. Sansano and Francisco Foubelo
Organics 2025, 6(3), 31; https://doi.org/10.3390/org6030031 - 16 Jul 2025
Abstract
►▼
Show Figures
The synthesis of cannabigerol—a cannabinoid with significant pharmaceutical potential—is described. The synthesis involves four stages. In the first step, (E)-non-3-en-2-one reacts with dimethyl malonate to yield a cyclic enone, which is subsequently oxidized with bromine to produce the olivetol ester. This ester then
[...] Read more.
The synthesis of cannabigerol—a cannabinoid with significant pharmaceutical potential—is described. The synthesis involves four stages. In the first step, (E)-non-3-en-2-one reacts with dimethyl malonate to yield a cyclic enone, which is subsequently oxidized with bromine to produce the olivetol ester. This ester then undergoes an alumina-catalyzed coupling reaction with geraniol, followed by ester hydrolysis to obtain cannabigerol. By modifying the chain length of the enone in the initial step and employing allylic alcohols other than geraniol, a range of cannabigerol derivatives can be synthesized, including the natural product cannabigerovarin.
Full article

Figure 1
Open AccessArticle
Density Functional Theory Study on Mechanism and Selectivity of Nickel-Catalyzed Hydroboration of Vinylarenes
by
Jingwei Wu, Yongzhu Zhou, Lei Zhang, Jie Zhang, Pei Song, Xiaoling Wang and Cuihong Wang
Organics 2025, 6(3), 30; https://doi.org/10.3390/org6030030 - 11 Jul 2025
Abstract
►▼
Show Figures
Density functional theory calculations were performed to elucidate the mechanistic details and origins of the selectivity of the nickel-catalyzed hydroboration of vinylarenes using B2pin2/MeOH. The catalytic cycles involved four sequential elementary steps: hydronickelation, anion exchange, transmetalation, and reductive elimination.
[...] Read more.
Density functional theory calculations were performed to elucidate the mechanistic details and origins of the selectivity of the nickel-catalyzed hydroboration of vinylarenes using B2pin2/MeOH. The catalytic cycles involved four sequential elementary steps: hydronickelation, anion exchange, transmetalation, and reductive elimination. Kinetic analyses identified hydronickelation as the rate-determining step with an activation barrier of 19.8 kcal/mol, while transmetalation proceeded through a stepwise mechanism characterized by two distinct transition states. Comprehensive analyses of the relevant transition structures and energetics demonstrated that the observed R-enantioselectivity (94% ee) originated from favorable nonbonding interactions. Lastly, our calculations suggested that the Markovnikov regioselectivity was predominantly governed by steric factors rather than electronic effects.
Full article

Figure 1
Open AccessArticle
Complementary Synthesis of Anti- and Syn-Hydroxymethyl 1,3-Diols via Regioselective Ring Opening of TIPS-Protected 2,3-Epoxy Alcohols: Toward Polypropionate Fragments
by
Raúl R. Rodríguez-Berríos and José A. Prieto
Organics 2025, 6(3), 29; https://doi.org/10.3390/org6030029 - 10 Jul 2025
Abstract
►▼
Show Figures
Hydroxymethyl 1,3-diol motifs are common structural motifs in natural products, particularly in polypropionates with important therapeutic potential. However, general and complementary methods for their regio- and diastereoselective synthesis remain limited. In this study, we expanded a second-generation epoxide-based methodology involving the regioselective cleavage
[...] Read more.
Hydroxymethyl 1,3-diol motifs are common structural motifs in natural products, particularly in polypropionates with important therapeutic potential. However, general and complementary methods for their regio- and diastereoselective synthesis remain limited. In this study, we expanded a second-generation epoxide-based methodology involving the regioselective cleavage of TIPS-monoprotected cis- and trans-2,3-epoxy alcohols using alkenyl Grignard reagents. Regioselective ring opening of cis-epoxides provided anti-1,3-diols, while trans-epoxides afforded the corresponding syn-1,3-diols. The use of cis-propenylmagnesium bromide and vinyl Grignard reagents enabled direct access to cis- and terminal homoallylic 1,3-diols, respectively, with moderate to good yields (46–88%) and excellent regioselectivities (95:5). In contrast, reactions with trans-propenyl Grignard reagent led to partial alkene isomerization, limiting their synthetic utility. To address this, a complementary two-step approach employing propynyl alanate addition followed by sodium/ammonia reduction was incorporated, providing access to trans-homoallylic 1,3-diols with high diastereoselectivity. All 1,3-diols were characterized by NMR spectroscopy, confirming regioselective epoxide opening. These combined strategies offer a practical and modular platform for the synthesis of syn- and anti-hydroxymethylated 1,3-diols and their application to the construction of polypropionate-type fragments, supporting future efforts in the total synthesis of polyketide natural products.
Full article
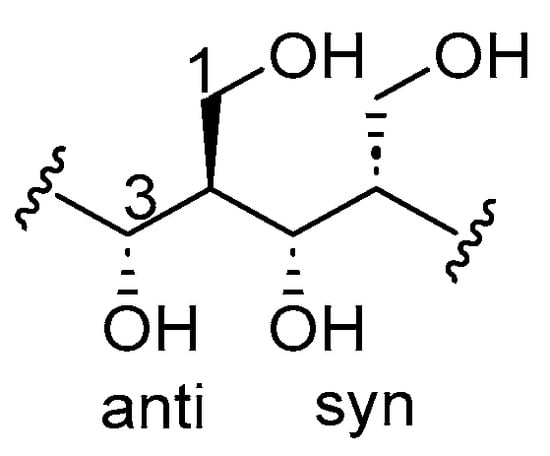
Figure 1
Highly Accessed Articles
Latest Books
E-Mail Alert
News
Topics
Topic in
Atoms, Crystals, Molecules, Organics, Symmetry, Inorganics
Advances in Molecular Symmetry and Chirality Research
Topic Editors: Ralph N. Salvatore, Guzman Gil-RamirezDeadline: 31 March 2026

Conferences
Special Issues
Special Issue in
Organics
Synthesis and Evaluation of Biological Activity of Podophyllotoxin and Its Analogues as Anticancer Drug Candidates
Guest Editor: Zbigniew CzarnockiDeadline: 30 November 2025
Special Issue in
Organics
Organic Supramolecular Chemistry of Natural Products
Guest Editor: Ruilong ShengDeadline: 31 December 2025
Special Issue in
Organics
Recent Advances in Selective Oxidation
Guest Editors: Lei Liu, Xigong LiuDeadline: 28 February 2026
Special Issue in
Organics
Advanced Oxidation Processes for Efficient Removal of Organic Pollutants in Water Treatment
Guest Editor: Yan WangDeadline: 10 June 2026
Topical Collections
Topical Collection in
Organics
Advanced Research Papers in Organics
Collection Editors: Wim Dehaen, Michal Szostak, Huaping Xu








