Exploration of the Divergent Outcomes for the Nenitzescu Reaction of Piperazinone Enaminoesters
Abstract
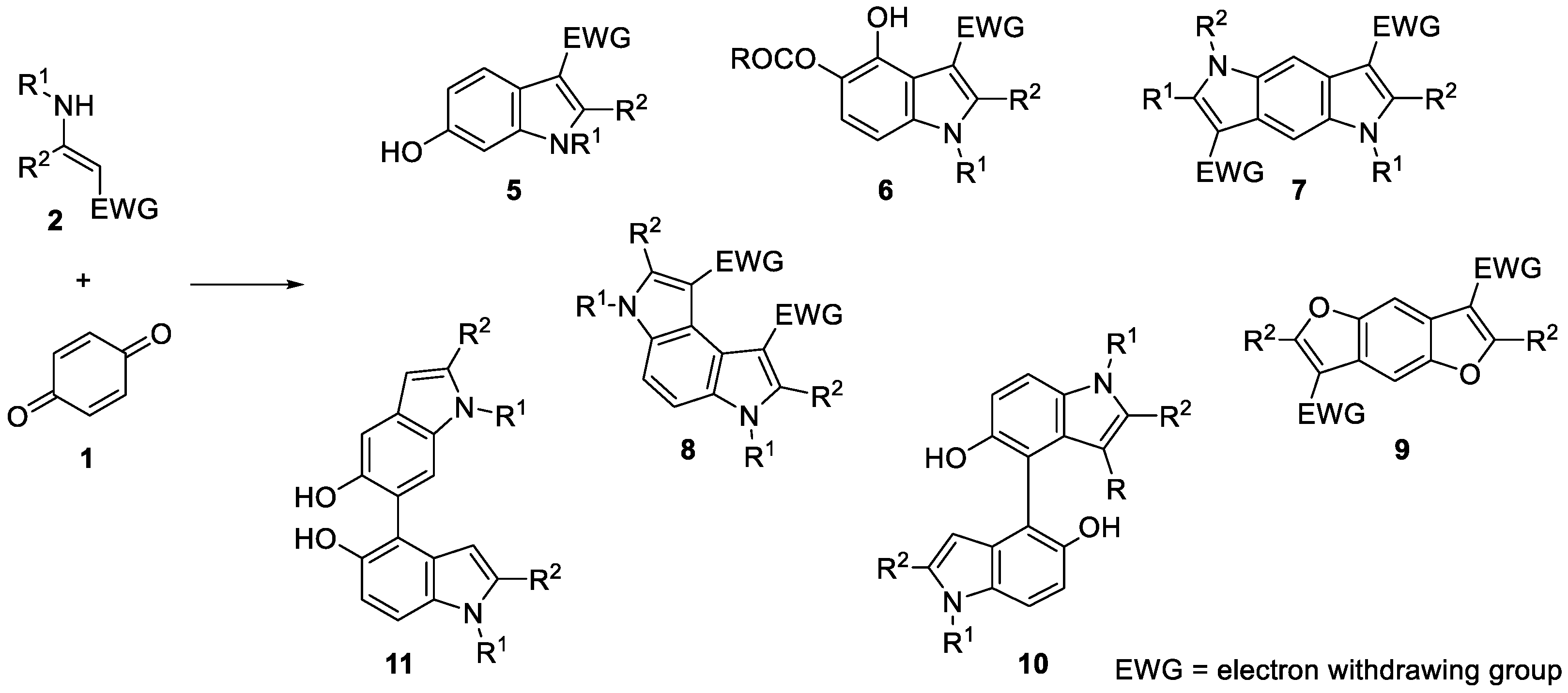
:1. Introduction
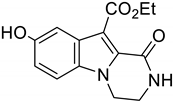
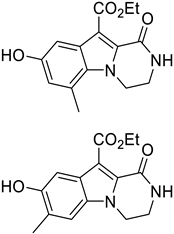
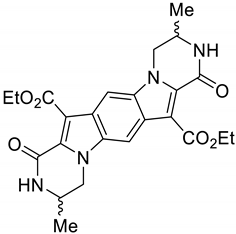
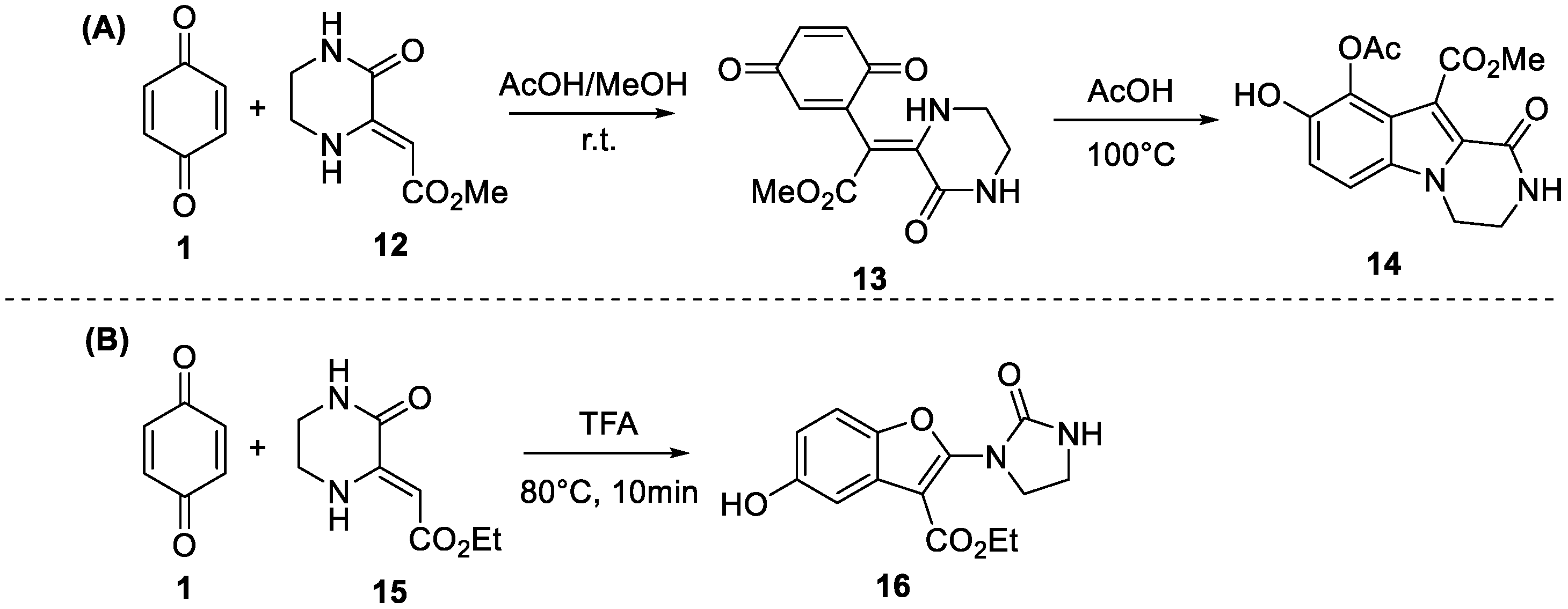
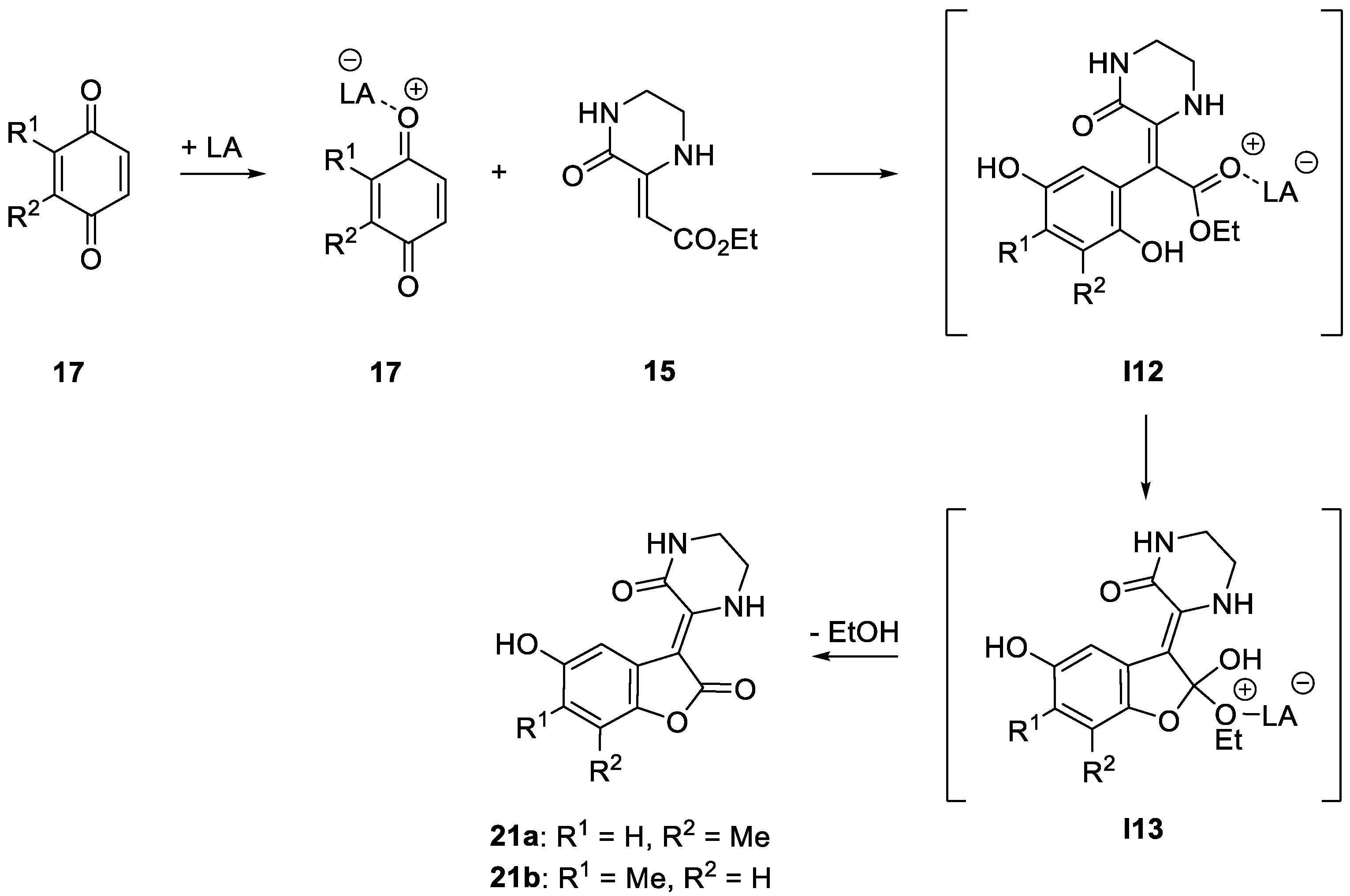
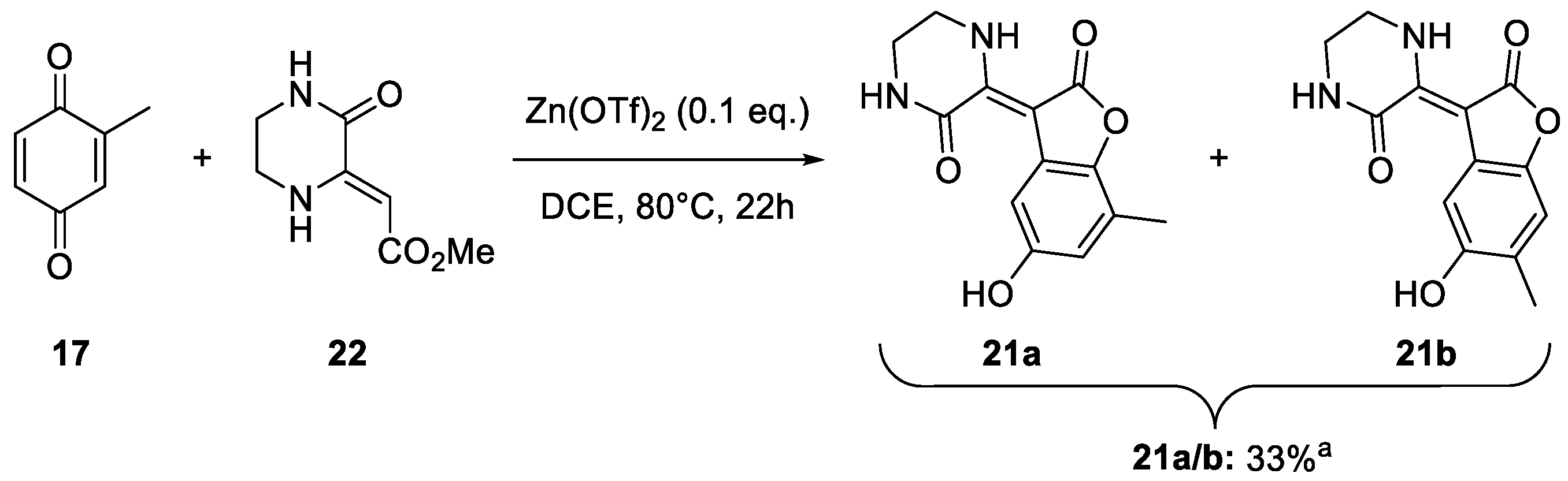
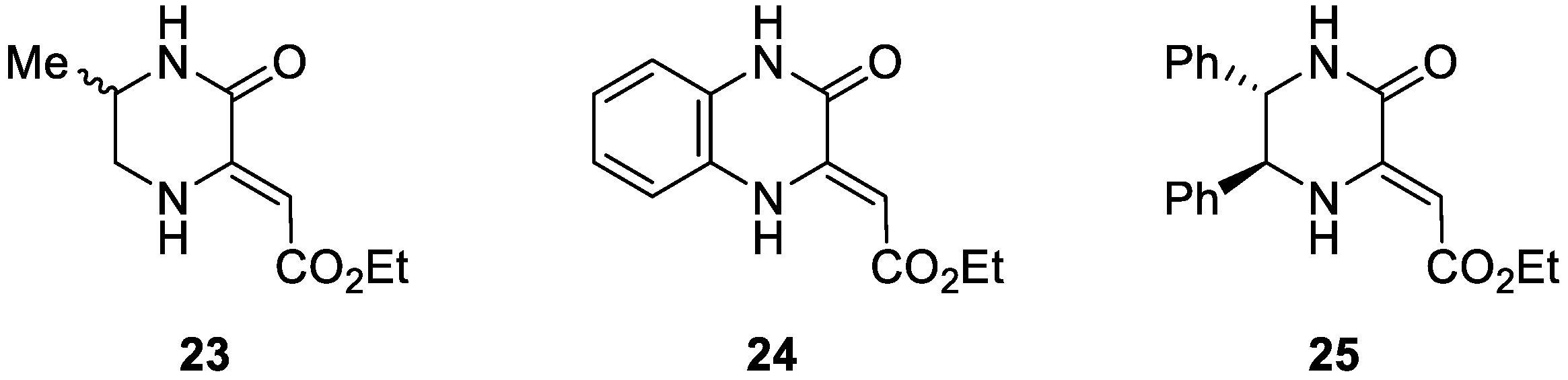
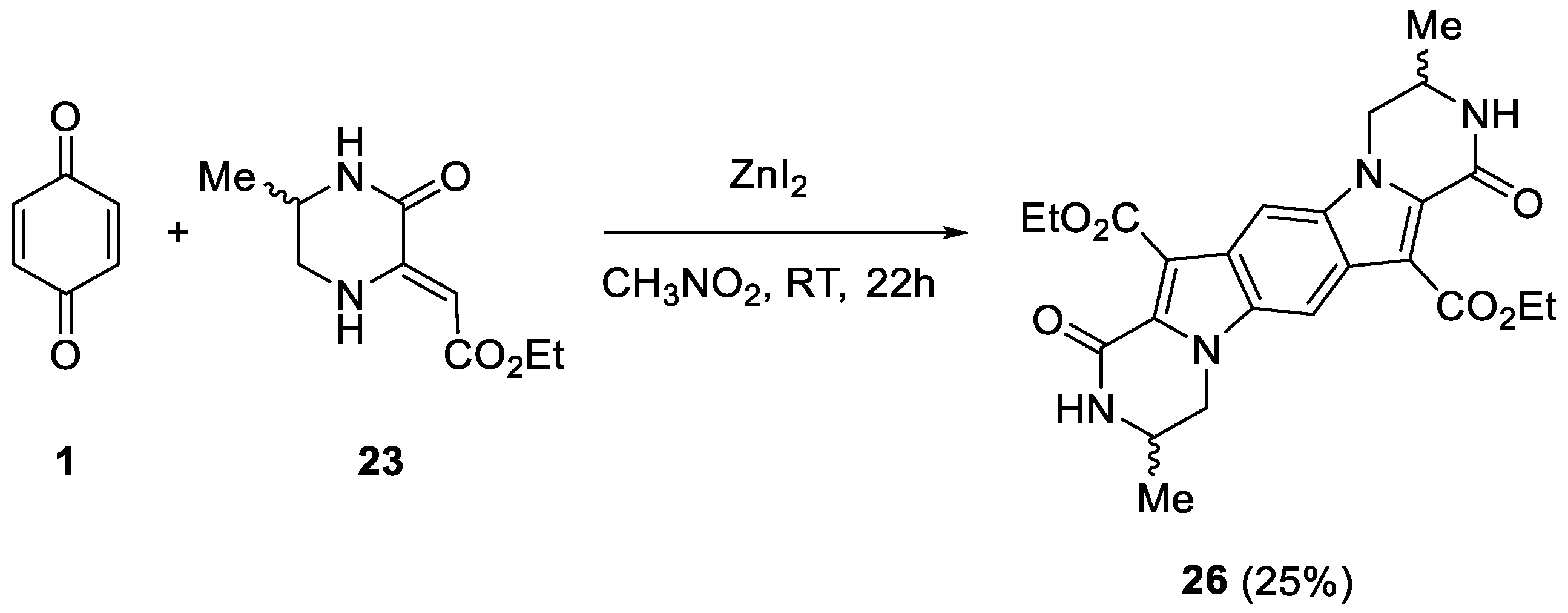
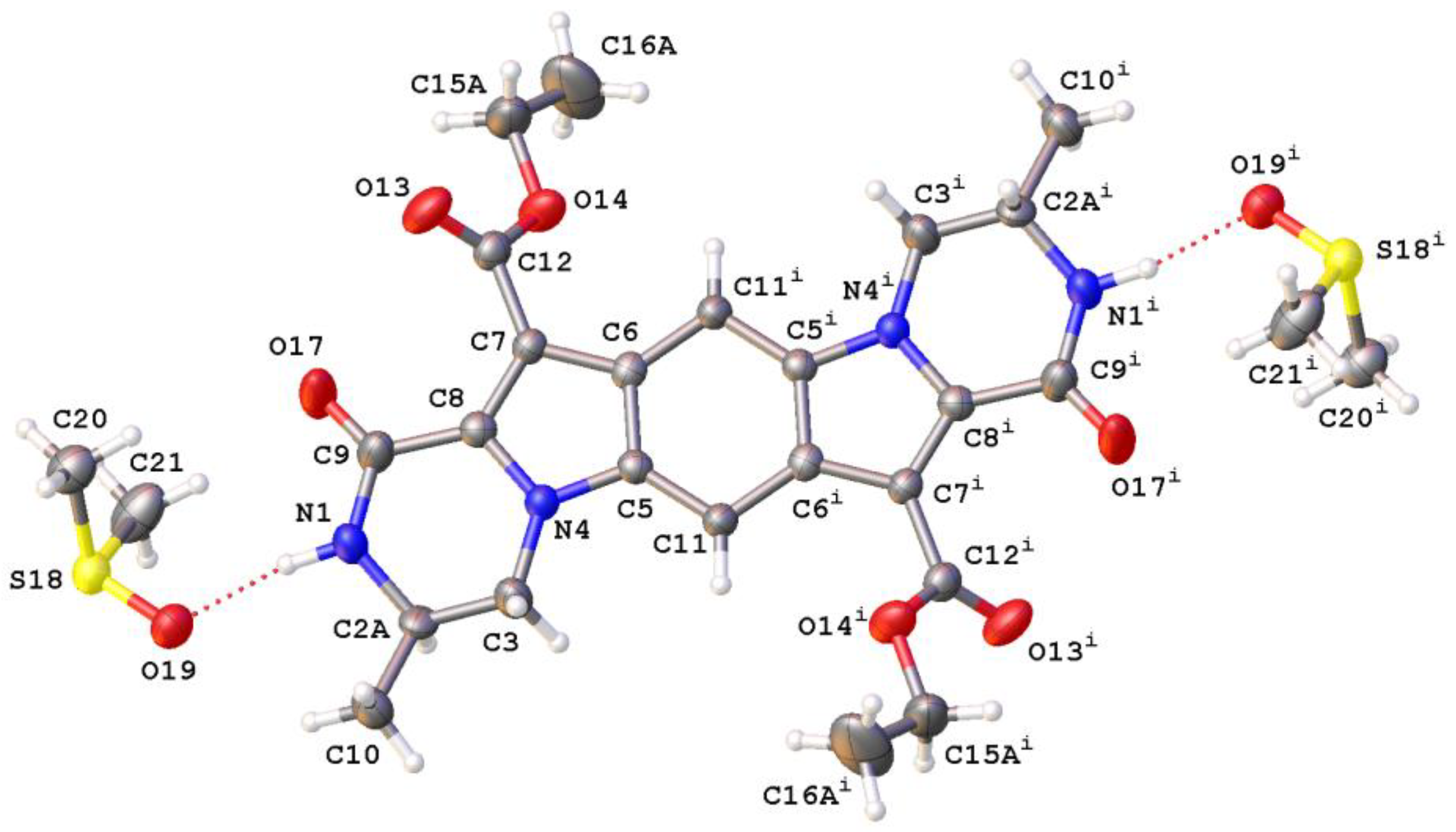
2. Results and Discussion
3. Conclusions
4. Experimental Section
4.1. Materials and Methods
4.2. Synthesis of Piperazinone Enaminoesters
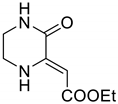

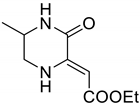


4.3. qNMR Optimization Study
4.4. Synthesis of Reaction Products

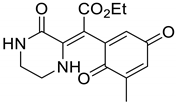
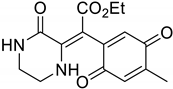

Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dumitrascu, F.; Ilies, M.A. Recent Advances in the Nenitzescu Indole Synthesis (1990–2019). Adv. in Heterocycl. Chem. 2021, 133, 65–177. [Google Scholar] [CrossRef]
- Singh, R.; Prakash, R.; Dehaen, W. Polycyclic Heterocycles by Condensation of 1,4-Benzoquinone Analogs and Nucleophiles. Adv.in Heterocycl. Chem. 2021, 135, 319–410. [Google Scholar] [CrossRef]
- Blaising, J.; Polyak, S.J.; Pécheur, E.I. Arbidol as a Broad-Spectrum Antiviral: An Update. Antiviral Res. 2014, 107, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Vigerelli, H.; Sciani, J.M.; Pereira, P.M.C.; Lavezo, A.A.; Silva, A.C.R.; Collaço, R.C.O.; Rocha, T.; Bueno, T.C.; Pimenta, D.C. Bufotenine, a Tryptophan-Derived Alkaloid, Suppresses the Symptoms and Increases the Survival Rate of Rabies-Infected Mice: The Development of a Pharmacological Approach for Rabies Treatment. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26, e20190050. [Google Scholar] [CrossRef] [PubMed]
- Fink, S.L.; Vojtech, L.; Wagoner, J.; Slivinski, N.S.J.; Jackson, K.J.; Wang, R.; Khadka, S.; Luthra, P.; Basler, C.F.; Polyak, S.J. The Antiviral Drug Arbidol Inhibits Zika Virus. Sci. Rep. 2018, 8, 2–10. [Google Scholar] [CrossRef] [Green Version]
- Boriskin, Y.S.; Pécheur, E.I.; Polyak, S.J. Arbidol: A Broad-Spectrum Antiviral That Inhibits Acute and Chronic HCV Infection. Virol. J. 2006, 3, 56. [Google Scholar] [CrossRef] [Green Version]
- Chai, H.; Zhao, Y.; Zhao, C.; Gong, P. Synthesis and In Vitro Anti-Hepatitis B Virus Activities of Some Ethyl 6-Bromo-5-Hydroxy-1H-Indole-3-Carboxylates. Bioorganic Med. Chem. 2006, 14, 911–917. [Google Scholar] [CrossRef]
- Pécheur, E.-I.; Borisevich, V.; Halfmann, P.; Morrey, J.D.; Smee, D.F.; Prichard, M.; Mire, C.E.; Kawaoka, Y.; Geisbert, T.W.; Polyak, S.J. The Synthetic Antiviral Drug Arbidol Inhibits Globally Prevalent Pathogenic Viruses. J. Virol. 2016, 90, 3086–3092. [Google Scholar] [CrossRef] [Green Version]
- Amani, B.; Amani, B.; Zareei, S.; Zareei, M. Efficacy and Safety of Arbidol (Umifenovir) in Patients with COVID-19: A Systematic Review and Meta-Analysis. Immun. Inflamm. Dis. 2021, 9, 1197–1208. [Google Scholar] [CrossRef]
- Zhu, Z.; Lu, Z.; Xu, T.; Chen, C.; Yang, G.; Zha, T.; Lu, J.; Xue, Y. Arbidol Monotherapy Is Superior to Lopinavir/Ritonavir in Treating COVID-19. J. Infect. 2020, 81, e21–e23. [Google Scholar] [CrossRef]
- Lucas, S. The Pharmacology of Indomethacin. Headache 2016, 56, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Fukai, T.; Kaitou, K.; Terada, S. Antimicrobial Activity of 2-Arylbenzofurans from Morus Species against Methicillin-Resistant Staphylococcus Aureus. Fitoterapia 2005, 76, 708–711. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.X.; Yang, Y.; Zhang, T.; Chen, R.Y.; Yu, D.Q. Bioactive 2-Arylbenzofuran Derivatives from Morus Wittiorum. Fitoterapia 2010, 81, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Zelová, H.; Hanáková, Z.; Čermáková, Z.; Šmejkal, K.; Dalĺ Acqua, S.; Babula, P.; Cvačka, J.; Hošek, J. Evaluation of Anti-Inflammatory Activity of Prenylated Substances Isolated from Morus Alba and Morus Nigra. J. Nat. Prod. 2014, 77, 1297–1303. [Google Scholar] [CrossRef]
- Salomé, C.; Narbonne, V.; Ribeiro, N.; Thuaud, F.; Serova, M.; De Gramont, A.; Faivre, S.; Raymond, E.; Désaubry, L. Benzofuran Derivatives as a Novel Class of Inhibitors of MTOR Signaling. Eur. J. Med. Chem. 2014, 74, 41–49. [Google Scholar] [CrossRef]
- Durán, N.; Justo, G.Z.; Ferreira, C.V.; Melo, P.S.; Cordi, L.; Martins, D. Violacein: Properties and Biological Activities. Biotechnol. Appl. Biochem. 2007, 48, 127. [Google Scholar] [CrossRef]
- Salomé, C.; Ribeiro, N.; Chavagnan, T.; Thuaud, F.; Serova, M.; De Gramont, A.; Faivre, S.; Raymond, E.; Désaubry, L. Benzofuran Derivatives as Anticancer Inhibitors of MTOR Signaling. Eur. J. Med. Chem. 2014, 81, 181–191. [Google Scholar] [CrossRef]
- Page, R.L.; Hamad, B.; Kirkpatrick, P. Dronedarone. Nat. Rev. Drug Discov. 2009, 8, 769–770. [Google Scholar] [CrossRef]
- Singh, R.; Horsten, T.; Prakash, R.; Dey, S.; Dehaen, W. Application of the Meerwein Reaction of 1,4-Benzoquinone to a Metal-Free Synthesis of Benzofuropyridine Analogues. Beilstein J. Org. Chem. 2021, 17, 977–982. [Google Scholar] [CrossRef]
- Winant, P.; Dehaen, W. A Visible-Light-Induced, Metal-Free Bis-Arylation of 2,5-Dichlorobenzoquinone. Beilstein J. Org. Chem. 2021, 17, 2315–2320. [Google Scholar] [CrossRef]
- Singh, R.; Bhatia, H.; Prakash, P.; Debroye, E.; Dey, S.; Dehaen, W. Tandem Nenitzescu Reaction/Nucleophilic Aromatic Substitution to Form Novel Pyrido Fused Indole Frameworks. European J. Org. Chem. 2021, 2021, 4865–4875. [Google Scholar] [CrossRef]
- Van Hoof, M.; Bynens, L.; Daelemans, B.; González, M.C.R.; Van Meervelt, L.; De Feyter, S.; Dehaen, W. Octahydropyrimido[4,5- g]Quinazoline-5,10-Diones: Their Multicomponent Synthesis, Self-Assembly on Graphite and Electrochemistry. Chem. Commun. 2022, 58, 7686–7689. [Google Scholar] [CrossRef] [PubMed]
- Horsten, T.; Alegbejo Price, T.O.; Van Meervelt, L.; Emery, F.D.S.; Dehaen, W. 2-Imidazolidinone Benzofurans as Unexpected Outcome of the Lewis Acid Mediated Nenitzescu Reaction. New J. Chem. 2022, 46, 2028–2032. [Google Scholar] [CrossRef]
- Allen, G.R. The Synthesis of 5-Hydroxyindoles by the Nenitzescu Reaction. In Organic Reactions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; Volume 20, pp. 337–454. [Google Scholar]
- Granik, V.G.; Lyubchanskaya, V.M.; Mukhanova, T.I. The Nenitzescu Reaction (Review). Pharm. Chem. J. 1993, 27, 413–438. [Google Scholar] [CrossRef]
- Patil, S.; Patil, R.; Miller, D. Synthetic Applications of the Nenitzescu Reaction to Biologically Active 5-Hydroxyindoles. Curr. Org. Chem. 2008, 12, 691–717. [Google Scholar] [CrossRef]
- Allen, G.R.; Pidacks, C.; Weiss, M.J. The Mitomycin Antibiotics. Synthetic Studies. XIV. The Nenitzescu Indole Synthesis. Formation of Isomeric Indoles and Reaction Mechanism. J. Am. Chem. Soc. 1966, 88, 2536–2544. [Google Scholar] [CrossRef] [PubMed]
- Rǎileanu, D.; Palaghitǎ, M.; Nenitzescu, C.D. Nenitzescu Synthesis of 5-Hydroxyindoles—II. Tetrahedron 1971, 27, 5031–5047. [Google Scholar] [CrossRef]
- Raileanu, D.; Nenitzescu, C.D. Synthesis of 5-hydroxyindoles. I. derivatives of 2-phenyl-k-hydroxyindole. Rev. Roum. Chim. 1965, 10, 339. [Google Scholar]
- Velezheva, V.S.; Kornienko, A.G.; Topilin, S.V.; Turashev, A.D.; Peregudov, A.S.; Brennan, P.J. Lewis Acid Catalyzed Nenitzescu Indole Synthesis. J. Heterocycl. Chem. 2006, 43, 873–879. [Google Scholar] [CrossRef]
- Mukhanova, T.I.; Alekseeva, L.M.; Kuleshova, E.F.; Granik, V.G.; Chemical, C. Synthesis of 3-Acyl-5-Hydroxyindoles and 3-Acyl-5-Hydroxybenzofurans. Influence of Solvent on the Course of the Nenitzescu Reaction. Pharm. Chem. J. 1993, 27, 136–142. [Google Scholar] [CrossRef]
- Lyubchanskaya, V.M.; Savina, S.A.; Alekseeva, L.M.; Shashkov, A.S.; Chernyshev, V.V.; Granik, V.G. The First Example of the Synthesis of 1-Aminoindole Derivatives by the Nenitzescu Reaction. Russ. Chem. Bull. 2004, 53, 2834–2839. [Google Scholar] [CrossRef]
- Kuckländer, U.; Hühnermann, W. Beobachtungen Zum Mechanismus Der Nenitzescu-Reaktion Synthese von 6-Hydroxy-indol-Derivaten. Arch. Pharm. (Weinheim) 1979, 312, 515–526. [Google Scholar] [CrossRef]
- Lyubchanskaya, V.M.; Panisheva, E.K.; Savina, S.A.; Alekseeva, L.M.; Shashkov, A.S.; Granik, V.G. Quinoneimines in the Nenitzescu Reaction. Russ. Chem. Bull. 2005, 54, 1690–1699. [Google Scholar] [CrossRef]
- Kuckländer, U. Beobachtungen Zum Mechanismus Der Nenitzescu-Reaktion. Tetrahedron 1972, 28, 5251–5259. [Google Scholar] [CrossRef]
- Kuckländer, U. Beobachtungen Zum Mechanismus Der Nenitzescu-Reaktion-III Acylwanderungen-I. Tetrahedron 1975, 31, 1631–1639. [Google Scholar] [CrossRef]
- Kuckländer, U. Zum Mechanismus Der Nenitzescu-Reaktion. IV. Synthese Von Benzindol-Derivaten. Ann. (Justus Liebigs) Chem. 1978, 1631, 129–139. [Google Scholar] [CrossRef]
- Schols, D.; Ruchko, E.A.; Lavrenov, S.N.; Kachala, V.V.; Nawrozkij, M.B.; Babushkin, A.S. Structural Analogs of Umifenovir 2. The Synthesis and AntiHIV Activity Study of New Regioisomeric (Trans-2-Phenylcyclopropyl)-1H-Indole Derivatives. Chem. Heterocycl. Compd. 2015, 51, 978–983. [Google Scholar] [CrossRef]
- Kuckländer, U. Beobachtungen Zum Mechanismus Der Nenitzescu-Reaktion-II. Tetrahedron 1973, 29, 921–927. [Google Scholar] [CrossRef]
- Mukhanova, T.I.; Panisheva, E.K.; Lyubchanskaya, V.M.; Alekseeva, L.M.; Sheinker, Y.N.; Granik, V.G. β-(Benzofur-2-Yl)- and (Indol-2-Yl)Enamines in the Nenitzescu Reaction. Tetrahedron 1997, 53, 177–184. [Google Scholar] [CrossRef]
- Shvedov, V.I.; Panisheva, E.K.; Vlasova, T.F.; Grinev, A.N. Synthesis and Aminomethylation of 6-Hydroxyindoles. Chem. Heterocycl. Compd. 1973, 9, 1225–1227. [Google Scholar] [CrossRef]
- Kuckländer, U.; Edoho, J. Zur Entstehung von Pyrrolo [2,3-f] Indolen Bei Der Nenitzescu- Reaktion. Arch. Pharm. (Weinheim). 1980, 313, 582–587. [Google Scholar] [CrossRef]
- Horsten, T. Synthesis and Applications of 1,2- and 4,5-Annulated Indoles. Available online: https://lirias.kuleuven.be/handle/123456789/683665 (accessed on 10 December 2021).
- Parr, R.W.; Reiss, J.A. An Application of the Nenitszescu Reaction to the Synthesis of 1,2-Annulated Indoles and Benz[g]Indoles. Aust. J. Chem. 1984, 37, 1263–1270. [Google Scholar] [CrossRef]
- Hardeland, R.; Pandi-Perumal, S.R.; Cardinali, D.P. Melatonin. Int. J. Biochem. Cell Biol. 2006, 38, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Panisheva, E.K.; Alekseeva, L.M.; Shashkov, A.S.; Granik, V.G. Condensation of P-Benzoquinone with Anilides of β-Aminocrotonic Acid. Chem. Heterocycl. Compd. 2003, 39, 1013–1017. [Google Scholar] [CrossRef]
- Mikerova, N.I.; Panisheva, E.K. Formation of Substituted Benzofuranones-2 by a Nenitzescu Reaction. Khimiya Geterotsiklicheskikh Soedin. 1989, 10, 1429–1430. [Google Scholar] [CrossRef]
- Chun, Y.S.; Ryu, K.Y.; Kim, J.H.; Shin, H.; Lee, S.G. Tandem Blaise-Nenitzescu Reaction: One-Pot Synthesis of 5-Hydroxy-α-(Aminomethylene)Benzofuran-2(3H)-Ones from Nitriles. Org. Biomol. Chem. 2011, 9, 1317–1319. [Google Scholar] [CrossRef]
- Mbala, B.M.; Jacobs, J.; Claes, P.; Mudogo, V.; De Kimpe, N. Investigation towards an Efficient Synthesis of Benzo[g]Isoquinoline-1,5, 10(2H)-Triones. Tetrahedron 2011, 67, 8747–8756. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals, 8th Edition; Butterworth-Heinemann: Oxford, UK, 2017; Chapter 3; ISBN 9780128054574. [Google Scholar]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef] [Green Version]
- Agilent Technologies UK Ltd. CrysAlisPro version 1.171.40.25a; Agilent Technologies UK Ltd.: Yarnton, UK, 2012. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated speca-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Fan, M.J.; Li, G.Q.; Liang, Y.M. DABCO Catalyzed Reaction of Various Nucleophiles with Activated Alkynes Leading to the Formation of Alkenoic Acid Esters, 1,4-Dioxane, Morpholine, and Piperazinone Derivatives. Tetrahedron 2006, 62, 6782–6791. [Google Scholar] [CrossRef]
- Patrick, J.B.; Saunders, E.K. Studies on the Nenitzescu Synthesis of 5-Hydroxyindoles. Tetrahedron Lett. 1979, 20, 4009–4012. [Google Scholar] [CrossRef]


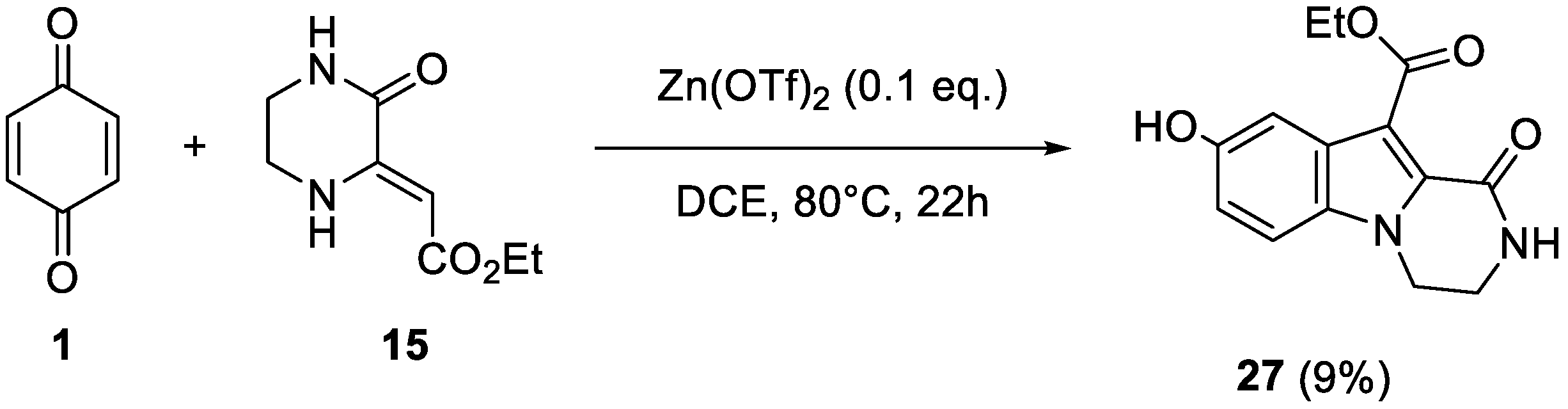
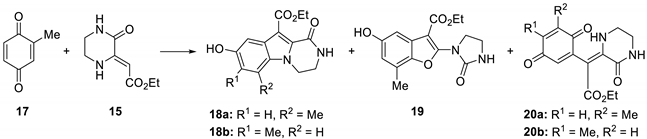
| Entry | Solvent | Additive (eq.) | SM 17 (eq.) | Time | T (°C) | Yield a (%) | ||
|---|---|---|---|---|---|---|---|---|
| 18a/b | 19 | 20a/b | ||||||
| 1 | ACN | BF3·OEt2 (1.2) | 2 | 3 h | r.t. | 18a (<5) | 19 (55) | 20a (<5) |
| 2 | Et2O | BF3·OEt2 (1.2) | 2 | 22 h | r.t. | 18a (7) | 19 (45) | 20b (10) |
| 3 | THF | BF3·OEt2 (1.2) | 2 | 22 h | r.t. | 18a (<5) | 19 (64) | - |
| 4 | DCM | BF3·OEt2 (1.2) | 2 | 22 h | r.t. | 18a (8); 18b (<5) | 19 (51) | 20a (11) |
| 5 | DCM | TFA (1.2) | 2 | 22 h | r.t. | 18a (6) | 19 (20) | 20a (13); 20b b |
| 6 | DCM | TfOH (1.2) | 2 | 22 h | r.t. | 18a (<5); 18b (6) | 19 (15) | 20a (15) |
| 7 | DCM | ZnCl2 (1.2) | 2 | 22 h | r.t. | 18a (<5); 18b (9) | 19 (<5) | 20a (41); 20b (19) |
| 8 | DCM | CuCl2 (1.2) | 2 | 22 h | r.t. | - | 19 (22) | 20b (45) |
| 10 | DCM | FeCl3 (1.2) | 2 | 22 h | r.t. | 18a (<5) | 19 (19) | 20a (13); 20b (15) |
| 11 | DCM | In(OTf)3 (1.2) | 2 | 22 h | r.t. | 18a (<5); 18b (5) | 19 (23) | b |
| 12 c | DCM | Sc(OTf)3 (1.2) | 2 | 22 h | r.t. | / | / | / |
| 13 | DCM | ZnCl2 (0.1) | 2 | 22 h | r.t. | 18a (<5); 18b (10) | - | 20a (44); 20b (20) |
| 14 | DCM | ZnCl2 (0.1) | 2 | 22 h | 40 | 18a (6); 18b (13) | - | 20a (47); 20b (21) |
| 15 | DCM | ZnI2 (0.1) | 2 | 22 h | 40 | 18a (11); 18b (10) | - | 20a (46); 20b (21) |
| 16 | DCM | ZnBr2 (0.1) | 2 | 22 h | 40 | 18a (6); 18b (12) | - | 20a (45); 20b (19) |
| 17 d | DCM | Zn(OTf)2 (0.1) | 2 | 22 h | 40 | 18a (<5); 18b (<5) | - | 20a (32); 20b (16) |
| 18 | DCM | ZnCl2 (0.1) | 2 | 95 h | 40 | 18a (7); 18b (14) | - | 20a (31); 20b (12) |
| 19 | DCM | ZnI2 (0.1) | 2 | 95 h | 40 | 18a (12); 18b (13) | - | 20a (26); 20b (9) |
| 20 | ACN | ZnCl2 (0.1) | 2 | 22 h | r.t. | 18a (9); 18b (8) | - | 20a (36); 20b (15) |
| 21 | EtOAc | ZnCl2 (0.1) | 2 | 22 h | r.t. | 18a (5); 18b (12) | - | 20a (43); 20b (22) |
| 22 | CH3NO2 | ZnCl2 (0.1) | 2 | 22 h | r.t. | 18a (15); 18b (12) | - | 20a (41); 20b (23) |
| 23 | DMF | ZnCl2 (0.1) | 2 | 22 h | r.t. | 18a (<5); 18b (6) | - | 20a (36); 20b (32) |
| 24 | ACN | ZnI2 (0.1) | 2 | 22 h | r.t. | 18a (11); 18b (7) | - | 20a (52); 20b (17) |
| 25 | EtOAc | ZnI2 (0.1) | 2 | 22 h | r.t. | 18a (8); 18b (9) | - | 20a (56); 20b (21) |
| 26 | CH3NO2 | ZnI2 (0.1) | 2 | 22 h | r.t. | 18a (17); 18b (9) | - | 20a (43); 20b (22) |
| 27 | DMF | ZnI2 (0.1) | 2 | 22 h | r.t. | 18a (<5); 18b (<5) | - | b |
| 28 | DMSO | ZnI2 (0.1) | 2 | 22 h | r.t. | 18a (<5); 18b (<5) | - | 20a (49); 20b (27) |
| 29 | CH3NO2 | ZnI2 (0.1) | 2 | 7 d | r.t. | 18a (16); 18b (10) | 19 (<5) | 20a (24); 20b (9) |
| 30 d | CH3NO2 | ZnI2 (0.1) | 2 | 116 h | 40 | 18a (15); 18b (12) | 19(<5) | 20a (7) |
| 31 d | CH3NO2 | ZnI2 (0.1) | 2 | 9 h | 80 | 18a (12); 18b (10) | 19 (8) | 20a (6); 20b (<5) |
| 32 d | CH3NO2 | ZnI2 (0.5) | 2 | 22 h | r.t. | 18a (5); 18b (8) | 19 (<5) | 20a (17); 20b (<5) |
| 33 d | CH3NO2 | ZnI2 (1) | 2 | 22 h | r.t. | 18a (6); 18b (9) | 19 (15) | - |
| 34 d | DCE | ZnI2 (0.1) | 1 | 48 h | 80 | 18a (<5); 18b (6) | 19 (<5) | 20a (<5) |
| 35 d | DCE | ZnI2 (0.1) | 2 | 48 h | 80 | 18a (7); 18b (9) | 19 (<5) | 20a (<5); 20b (<5) |

| Entry | Solvent | Additive | SM 17 (eq.) | T (°C) | Yield a (%) | |||
|---|---|---|---|---|---|---|---|---|
| 21a/b | 18a/b | 19 | 20a/b | |||||
| 36 | DCM | Sc(OTf)3 | 2 | r.t. | 21a (<5) | 18a (6); 18b (<5) | - | 20a (51); 20b (14) |
| 37 | CH3NO2 | Sc(OTf)3 | 2 | r.t. | 21a (<5) | 18a (8); 18b (<5) | - | 20a (33); 20b b |
| 38 | DCE | Sc(OTf)3 | 2 | 80 | 21a/b (11) | 18a (7); 18b (<5) | 19 (<5) | 20a (15); 20b (8) |
| 39 c | DCE | Sc(OTf)3 | 1 | 80 | 21a/b (22) | 18a (6); 18b (<5) | - | 20a (<5); 20b (<5) |
| 40 c | DCE | Zn(OTf)2 | 1 | 80 | 21a/b (22) | 18a (<5); 18b (<5) | - | 20a (<5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hermans, R.; Van Hoof, M.; Van Meervelt, L.; Dehaen, W. Exploration of the Divergent Outcomes for the Nenitzescu Reaction of Piperazinone Enaminoesters. Organics 2023, 4, 146-163. https://doi.org/10.3390/org4020012
Hermans R, Van Hoof M, Van Meervelt L, Dehaen W. Exploration of the Divergent Outcomes for the Nenitzescu Reaction of Piperazinone Enaminoesters. Organics. 2023; 4(2):146-163. https://doi.org/10.3390/org4020012
Chicago/Turabian StyleHermans, Rebecca, Max Van Hoof, Luc Van Meervelt, and Wim Dehaen. 2023. "Exploration of the Divergent Outcomes for the Nenitzescu Reaction of Piperazinone Enaminoesters" Organics 4, no. 2: 146-163. https://doi.org/10.3390/org4020012
APA StyleHermans, R., Van Hoof, M., Van Meervelt, L., & Dehaen, W. (2023). Exploration of the Divergent Outcomes for the Nenitzescu Reaction of Piperazinone Enaminoesters. Organics, 4(2), 146-163. https://doi.org/10.3390/org4020012







