Abstract
Great efforts of the scientific community are focused on the development of catalysts for the reduction of carbon dioxide (CO2) to useful molecules such as carbon monoxide, formic acid, methanol, ethanol, methane, ethylene, or acetate. Various metal porphyrin complexes were synthesized and studied to develop highly active and selective catalysts. While the substituents on the porphyrin core (the primary coordination sphere) determine the reactivity of the metal, the introduction of the secondary coordination is important for the binding and activation of CO2. In this review, selected examples of iron porphyrin catalysts with a secondary coordination sphere capable of stabilizing intermediates of the CO2 reduction process by hydrogen bonding are presented.
1. Introduction
The concentration of carbon dioxide (CO2) in the atmosphere is steadily increasing (Figure 1) [1] and its accumulation is causing many harmful climate changes such as global warming, mass loss of the land ice sheets in both Antarctica and Greenland, sea level rise, expansion of deserts, and more, all of which directly affect the sustainability of life on Earth. Natural consumption of CO2 is not enough to reduce CO2 levels in the atmosphere. Therefore, many efforts are focused on converting CO2 into useful molecules through chemical methods [2].
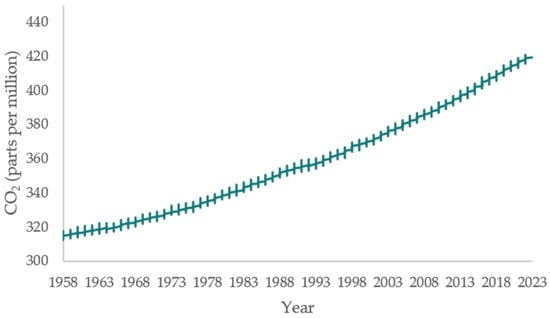
Figure 1.
The increase of CO2 level from 1958 to the present [1].
The problem in CO2 conversion is the very high thermodynamic stability of CO2 and electrocatalytic reduction showed promising results [3,4]. The conversion of CO2 into useful molecules consists of a combination of reduction and protonation, which leads to several different mono-carbon (C1) molecules such as carbon monoxide, formic acid, formaldehyde, methanol, and methane (Scheme 1) as well as multi-carbon (C2+) products such as ethylene, ethanol, etc. [2,5].
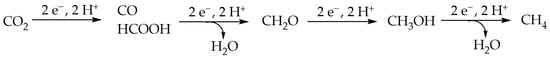
Scheme 1.
Possible C1 products of CO2 reduction.
The requirement for efficient catalysts is high activity and selectivity in CO2 reduction. In nature, there are various examples of small molecules activation and conversion by tetrapyrrole-based macrocycles, e.g., heme, the iron complex of porphyrin, which is very important for the binding, transport, storage, and activation of oxygen in various proteins and enzymes [6]; siroheme, the iron complex of isobacteriochlorin, which catalyzes the reduction of nitrite and sulfite [7]. Mimicking nature, scientists have developed numerous metal porphyrinoid complexes capable of electrocatalytically reducing CO2 [8]. The possibility of substitution of both meso- and β-positions of the porphyrin (Figure 2a), and complexation with different metals allows a wide range of catalyst modifications and the study of their effects on the efficiency and selectivity of CO2 reduction.
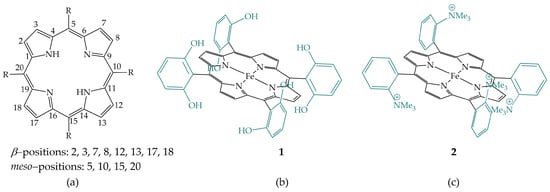
Figure 2.
(a) Porphyrin structure with denoted meso- and β-positions; (b) Iron 5,10,15,20-tetrakis(2′,6′-dihydroxyphenyl)-porphyrin; (c) Iron tetraphenylporphyrin with four trimethylanilinium groups.
Iron porphyrins show promising results for the reduction of CO2 to CO [9]. Many mechanistic studies have been carried out [4,10]. Iron porphyrins bind CO2 in its Fe(0) state, whereupon subsequent proton and electron transfer and release of CO occur. Through-structure substituent effect (the primary coordination sphere) stabilizes the reduced metal and determines the reactivity of the metal. The introduction of the secondary coordination sphere improves the stability of the CO2 reduction intermediates, which directly affects the selectivity and rate of reduction [11,12,13].
Savéant et al. made a systematic study of the substitution effects on electrochemical CO2 reduction catalyzed by iron porphyrins. The introduction of phenolic groups, a local proton source, in all ortho and ortho′ positions of the phenyl groups of porphyrin, leads to enhanced activity of iron porphyrin (Figure 2b) [14]. Their investigation of the effects of the primary coordination sphere by the introduction of electron-withdrawing and electron-donating substituents with successive phenyl perfluorination and the o,o′-methoxy substitution of iron tetraphenylporphyrin [15] as well as the effects of the secondary coordination sphere by the introduction of positive charges, the four trimethylanilinium groups, and negative charges, the four sulfonate groups, on the phenyl rings of iron tetraphenylporphyrin, led to the excellent performing catalyst 2, showing very high selectivity for the reduction of CO2 to CO with a maximum turnover frequency (TOF) of 106 s−1 (Figure 2c) [16].
Various strategies have been used for the secondary coordination sphere such as the introduction of local proton sources, hydrogen bond donors, cationic moieties, and bimetallic approach [13]. In this review, examples of iron porphyrin electrocatalysts with ligands that stabilize intermediates by hydrogen bonding are selected (Figure 3).
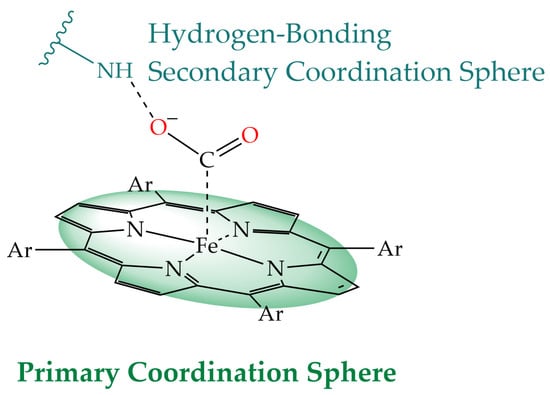
Figure 3.
Representation of primary and hydrogen-bonding secondary coordination sphere.
2. Hydrogen-Bonding Secondary Coordination Sphere Effect
As a general mechanism for CO2 reduction to CO by iron (III) tetraphenylporphyrin (FeTPP), FeTPP undergoes three reversible reductions that correspond to reductions from Fe(III) to Fe(II) to Fe(I) and to Fe(0), the active form that reacts with CO2 [17].
Iron porphyrins with hydrogen bonding groups in the secondary coordination sphere form hydrogen bonding interactions with at least two intermediates of reduction, an Fe(II)–CO22− and an Fe(II)–COOH species, which affect their stability and influence both the selectivity and the rate of CO2 reduction [18]. The Fe(II)–CO22− species is very basic and can be easily protonated by a weak acid to generate Fe(II)–COOH species. In the presence of a strong acid, protonation of Fe(II)–COOH species leads to –OH protonation, elimination of water, and formation of Fe(II)–CO. In the presence of a weak acid, C–protonation of the Fe(II)–COOH species occurs and formate is released. The rate-determining step is a bond cleavage of the Fe(II)–COOH species. Hydrogen-bonding residues are also able to form hydrogen bonds with external proton sources, which bring them close to the CO2 reduction intermediates and synergistically activate them. Iron porphyrins are functionalized with various hydrogen bonding groups such as amides, phenols, guanidines, triazoles, ureas, pyridines, amines or imidazoles.
2.1. Amides in the Secondary Coordination Sphere
Nichols et al. investigated the secondary sphere effects of positional isomers of iron porphyrins having pendant amide in the ortho or para position of the phenyl ring, proximal or distal to the porphyrin macrocycle (Figure 4) in dimethylformamide (DMF) in the presence of phenol (PhOH) as an acid source [19]. All four porphyrin derivatives reduce CO2 to CO. Compared to FeTPP, ortho derivatives 3 and 4 show an increase in CO2 reduction rate due to secondary sphere interactions, while secondary sphere effects are absent in para derivatives 5 and 6. Moreover, the effects are more pronounced in the porphyrin 4 when the amide group is distal to the porphyrin macrocycle.
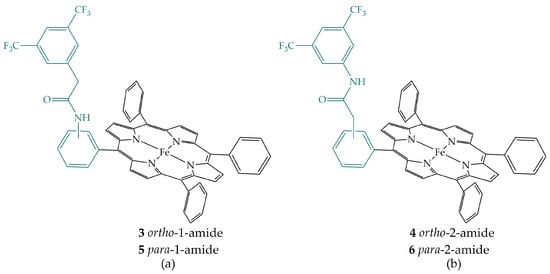
Figure 4.
(a) ortho- and para-1-amide iron porphyrins 3 and 5; (b) ortho- and para-2-amide iron porphyrins 4 and 6.
2.2. Phenols and Guanidines in the Secondary Coordination Sphere
Margarit et al. synthesized iron porphyrins having phenolic (7) and guanidyl (8) groups in the secondary coordination sphere (Figure 5), hanging above the iron porphyrin macrocycle, which reduce CO2 to CO with Faradaic efficiencies of more than 93% [20]. Computational studies of CO2 binding to the reduced iron porphyrin Fe(0), showed thermodynamically more stable binding within hangman cleft due to hydrogen bonding for both porphyrins compared to the other side of the porphyrin macrocycle. A stronger association is observed for porphyrin with the phenolic group, which is also consistent with a higher rate constant compared to that with the guanidyl group. Computational studies also showed that both complexes have hydrogen bonding of the hanging group to the porphyrin prior to CO2 binding [20]. The phenolic group binds to the iron and nitrogen of the pyrrole, while the guanidyl group binds to the α and β carbon atoms of the pyrrole and is thus further away from the metal center, implying that upon CO2 binding, the backbone of the hanging group must rotate to accommodate CO2.
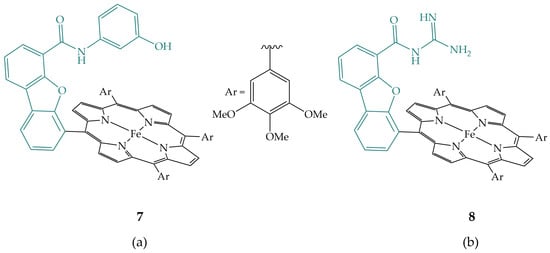
Figure 5.
Iron hangman porphyrins with (a) phenolic (7) and (b) guanidyl group (8).
Guo et al. synthesized porphyrin 9 (Figure 6), which also has a phenolic group hanging directly over the iron porphyrin macrocycle and investigated its electrocatalytic activity in CO2 reduction in comparison to its analogue, non-functionalized porphyrin 10 (Figure 6) [21]. Furthermore, the activity of porphyrins with two different counter ions, chloride (–Cl) and triflate (–OTf) was investigated. They were unable to obtain crystals suitable for X-ray structural analysis, but according to published data on various iron porphyrins, they assume that Fe(III) is incorporated in the center of the porphyrin macrocycle with a counter ion as an axial ligand as shown for the other porphyrin derivatives. Their studies showed that the chloride salt of porphyrin 9-Cl is much less active compared to porphyrin 10-Cl due to the hydrogen bonding of the axial chloride with the phenolic group. Switching to the triflate salt, 9-OTf is more active than both 9-Cl and 10-Cl. 10-OTf has the same activity as 10-Cl. Thus, iron porphyrin 9 shows an activity dependence on the counter ions and in addition to the positive secondary coordination sphere effect of the phenolic group in the CO2 reduction reaction, as shown above for the porphyrin 7, the phenolic group in porphyrin 9 also has an inhibitory effect, depending on the counter ion used.
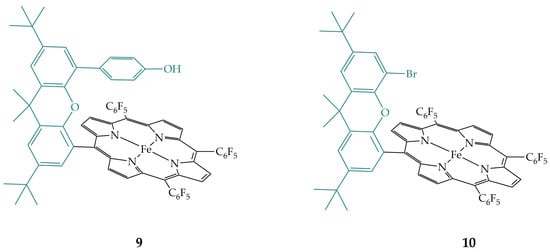
Figure 6.
Iron porphyrin with phenolic group 9 and its non-functionalized analogue, iron porphyrin 10.
In addition, Guo et al. synthesized porphyrin 11 (Figure 7) with the pendant guanidyl group and compared its electrocatalytic activity and selectivity for CO2 reduction with its non-functionalized analogue tetrakis(3,4,5-trimethoxyphenyl)porphyrin 12 (Figure 7) [22]. In acetonitrile with added water, both show the same selectivity for CO2 reduction to CO, but porphyrin 11 is much more active than its analogue 12, with a TOF value that is an order of magnitude higher. In aqueous solutions, porphyrin 11 is much more active and selective than porphyrin 12 with a Faradaic efficiency of 96%, while porphyrin 12 has a Faradaic efficiency of 65% for CO2 reduction to CO. The guanidyl group of porphyrin 11 is protonated in an aqueous solution; therefore, it can stabilize intermediates of CO2 reduction and act as a proton relay.
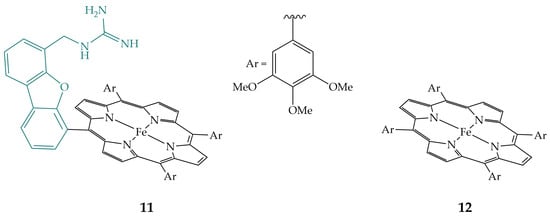
Figure 7.
Iron porphyrin with guanidyl group 11 and its non-functionalized analogue, iron porphyrin 12.
2.3. Triazoles in the Secondary Coordination Sphere
Sen et al. studied CO2 reduction with three iron porphyrins that differ in hydrogen bonding residues in the secondary coordination sphere with the same external acid, with porphyrin 13 having an amide, a hydrogen bond donor, while porphyrins 14 and 15 have hydrogen bond acceptors, 4-tert-butyltriazole and 4-methylcarboxylatetriazole groups, respectively, that are able to entrap water molecules (Figure 8) [23]. Porphyrins are derivatized in the ortho position of the phenyl ring. In addition, the pivaloyl groups of porphyrin 13 provide a hydrophobic environment, while the groups of porphyrins 14 and 15 provide a hydrophilic environment. All three porphyrins showed a reduction of CO2 to CO but with different values: 1 s−1, 4.2 s−1, and 2 × 103 s−1 for iron porphyrin 15, 14, and 13, respectively (with respect to the rate of porphyrin 15 set to 1). Their study showed that it is possible to affect CO2 reduction rates only by regulating electrostatics and hydrogen bonding interactions, while hydrophobicity does not have much effect on it.
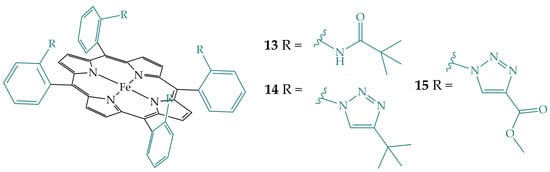
Figure 8.
Iron porphyrins with amide (13), 4-tert-butyltriazole (14), and 4-methylcarboxylatetriazole (15) groups.
2.4. Ureas in the Secondary Coordination Sphere
Gotico et al. designed iron porphyrin derivative 16, which has amide groups in the secondary coordination sphere, and compared its electrocatalytic activity with iron porphyrin derivative 17, which has urea groups, opening the possibility of multipoint hydrogen bonding interactions (Figure 9a) [24]. Both porphyrins are derivatized in the ortho position of all phenyl rings with an αβαβ configuration. They showed an improvement in electrocatalytic activity for both iron porphyrins 16 and 17 compared to FeTPP, while a stronger improvement was observed for porphyrin 17 with urea functionalities. A controlled experiment with FeTPP and the addition of external urea showed no enhancement, highlighting the importance of the pre-organized structure of the iron porphyrin derivative. DFT calculations confirmed two weak hydrogen bonds of the amide groups with CO2 in porphyrin 16 and four strong interactions with urea groups in porphyrin 17. The electrocatalytic activity was studied in DMF using water as a proton source, which turned out to be a better proton source than the more acidic trifluoroethanol (TFE) or phenol. Water acts in synergy with urea groups. Computational studies confirmed the possibility of complexed water molecules with hydrogen bonds between the CO2 and urea groups. Gotico et al. also studied the electrocatalytic activity of porphyrins with two urea groups in αα (18) and αβ (19) configurations (Figure 9b), where one side of the metal remains free after CO2 binding [25]. αα porphyrin 18 showed higher binding affinity towards CO2 but αβ porphyrin 19 showed higher electrocatalytic activity. In the latter complex, the approach of water is easier due to the αβ configuration, so the proton transfer process is faster.
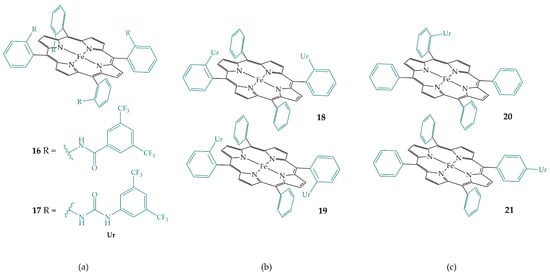
Figure 9.
(a) Iron porphyrins with four amide (16) and urea (17) groups with an αβαβ configuration; (b) Iron porphyrins with two urea groups with αα (18) and αβ (19) configurations; (c) Iron porphyrins with one urea group in ortho position (20), and para position (21).
In addition, Derrick et al. functionalized iron porphyrin with one urea group in the ortho position (porphyrin 20), and para position (porphyrin 21) of the porphyrin phenyl ring and investigated electrochemical CO2 reduction with bicarbonate as an additive (Figure 9c) [26]. Bicarbonate serves as a proton donor and its interaction with the urea group through hydrogen bonding increases its acidity and brings it close to the CO2-bonded intermediate, resulting in rapid proton transfer and an increase in the catalytic rate of electrochemical CO2 reduction. The increase is more pronounced for the ortho-functionalized porphyrin 20.
2.5. Amines in the Secondary Coordination Sphere
Liu et al. studied electrochemical CO2 reduction with simple iron porphyrins bearing an amino group in the ortho position, iron 5-(o-aminopheny1)-10,15,20-triphenylporphyrin (22) and the para position, iron 5-(p-aminopheny1)-10,15,20-triphenylporphyrin (23) (Figure 10a) [27]. In this study, as with the ortho iron porphyrin derivatives 2 and 3 mentioned above, the ortho-substituted porphyrin 22 showed better TOF, lower overpotential, and higher selectivity, compared to the para-substituted iron porphyrin 23. DFT calculations showed stabilization of Fe(II)–CO22− species forming hydrogen bond interactions with the amino group.
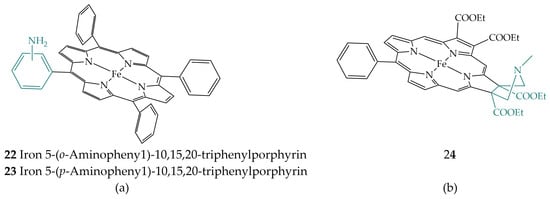
Figure 10.
(a) Iron 5-(o-Aminopheny1)-10,15,20-triphenylporphyrin (22) and iron 5-(p-Aminopheny1)-10,15,20-triphenylporphyrin (23); (b) Iron porphyrin with a rigid pendant amine group 24.
Amanullah et al. tuned the CO2 reduction to produce HCOOH. The reduction was catalyzed with iron porphyrinoid 24, which has a rigid pendant amine (Figure 10b), in DMF with water as the proton source [28]. In contrast to the above examples, iron porphyrinoid 24 can activate CO2 in its Fe(I) state, thereby lowering the overpotential of the process. The pendant amine is located near the active site and after its protonation, CO2 binding to Fe(I) is possible as well as stabilization of Fe(III)−COOH and Fe(II)−COOH species.
Guo et al. synthesized four porphyrins differing in the number of N,N,N-trimethylbenzylamine groups, porphyrin 25 with two groups in cis position, porphyrin 26 with two groups in trans position, porphyrin 27 with one group, and the non-functionalized analogue, porphyrin 28 (Figure 11) [29]. Porphyrin 25 showed the best electrocatalytic activity for CO2 reduction to CO in acetonitrile using phenol as a proton source, followed by porphyrin 26, porphyrin 27, and porphyrin 28. Therefore, functionalization of iron porphyrin with N,N,N-trimethylbenzylamine groups increased the activity compared to non-functionalized porphyrin. The cis-isomer has the best stabilization of the CO2-bonded intermediate by both electrostatic and hydrogen bonding and promotes better rate-determining C–O bond cleavage due to cooperative interactions with both an Fe(II)–COOH intermediate and phenol.
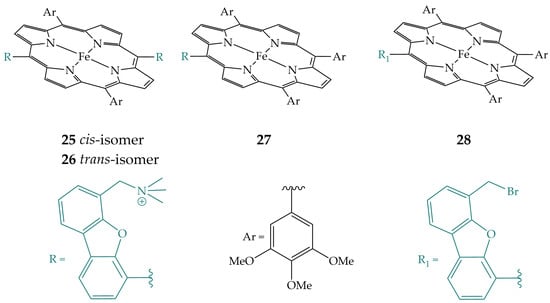
Figure 11.
Iron porphyrins with N,N,N-trimethylbenzylamine groups 25, 26, 27, and non-functionalized analogue, iron porphyrin 28.
2.6. Pyridines in the Secondary Coordination Sphere
Ramuglia et al. provided iron porphyrin (29) with pendant pyridine substituents (Figure 12) and compared its electrocatalytic activity with a non-functionalized tetramesitylporphyrin (30) in acetonitrile with the addition of the weak acids [30]. Porphyrin 29 showed an increased reduction rate compared to non-functionalized porphyrin 30, which can be attributed to the hydrogen bonding interactions of the pyridine substituents assisting in the proton transfer to the one-electron reduced CO2 adduct or stabilization of the Fe(II)–COOH species.
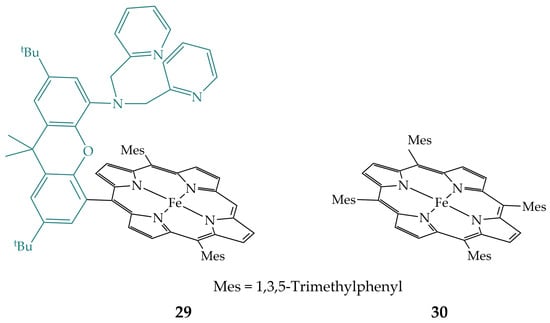
Figure 12.
Iron porphyrin with pendant pyridine substituents 29 and non-functionalized analogue 30.
2.7. Imidazoles in the Secondary Coordination Sphere
Narouz et al. synthesized a series of iron porphyrins with pendant imidazolium ligands in the ortho position (porphyrins 31 and 33) and para position (porphyrin 32) of the porphyrin phenyl rings (Figure 13) [31]. The imidazolium pendant ligands in porphyrins 31 and 32 could enhance the stabilization of the intermediates of CO2 reduction by both electrostatic and hydrogen bonding interactions, while porphyrin 33 has no hydrogen bonding interactions. In this way, both position tuning and the effects of electrostatic and hydrogen bonding interactions on the activity of the reduction reaction can be compared. The best performance was obtained with porphyrin 31 in both acetonitrile and aqueous media, followed by porphyrin 33 and porphyrin 32. Porphyrin 31 showed a 5-fold increase in CO2 binding affinity over porphyrin 33, a 20-fold increase over porphyrin 32, and a 25-fold increase in CO2 binding affinity compared to FeTPP, demonstrating the importance of the introduction of the secondary coordination sphere as well as proper positioning in the secondary coordination sphere. The TOF values are also much higher for porphyrin 31 compared to porphyrin 32 and FeTPP, an increase of several thousand, but only slightly higher than for porphyrin 33, indicating the dominance of the electrostatic effect in the catalytic enhancement. In addition, porphyrins 31 and 33 are more selective for CO2 reduction to CO compared to porphyrin 32.
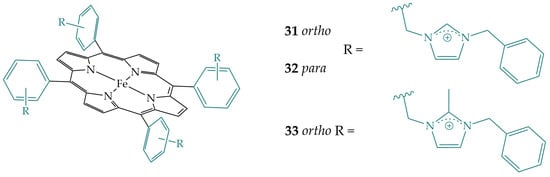
Figure 13.
Iron porphyrins with pendant imidazolium substituents.
2.8. Summary of Data for Iron Porphyrins
The iron porphyrins discussed above and their electrochemical properties are summarized in Table 1. The detailed description of the foot-of-the-wave (FOW) analysis and the estimation of the TOF values are described elsewhere [12,32]. Plotting the log TOF against the overpotential (η) estimated based on the catalytic parameters by the FOW analysis yields a catalytic Tafel plot (Figure 14). The compilation of catalytic Tafel plots for different porphyrins in one diagram allows a quick comparison of the catalytic activity with effective electrocatalysts located in the upper left part of the plot having large TOFmax at a low overpotential of the system.

Table 1.
Iron porphyrins, overpotential (η′)1, the standard potential of the reversible reduction peak of the active form of the catalyst ()1, log TOFmax values and Faradaic efficiency (FE) for CO production.
Figure 14.
Representation of a catalytic Tafel plot.
3. Conclusions
CO2 can be catalytically reduced to various products. The selectivity of the reaction is very important for the further use of these products. In this review, iron porphyrin electrocatalysts with hydrogen bonding residues in the secondary coordination sphere are presented. It is shown how a simple modification of iron porphyrin can improve the rate and selectivity of CO2 reduction compared to FeTPP by stabilizing reduction intermediates through hydrogen bonding as well as hydrogen bonding interactions with external proton sources. Nevertheless, the precise positioning, as well as the strength of hydrogen bonding interactions are very important. Further improvements are needed to increase electrocatalytic activity, reduce side products like molecular hydrogen, and tune the reduction to produce multi-carbon (C2+) products.
Author Contributions
Idea, conceptualization, literature search, writing—original draft preparation, visualization, A.B.; writing—review and editing, D.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Carbon Dioxide. National Oceanic and Atmospheric Administration. Available online: https://climate.nasa.gov/ (accessed on 6 March 2023).
- Kinzel, N.W.; Werlé, C.; Leitner, W. Transition Metal Complexes as Catalysts for the Electroconversion of CO2: An Organometallic Perspective. Angew. Chem. Int. Ed. 2021, 60, 11628–11686. [Google Scholar] [CrossRef]
- Pappijn, C.A.R.; Ruitenbeek, M.; Reyniers, M.-F.; Van Geem, K.M. Challenges and Opportunities of Carbon Capture and Utilization: Electrochemical Conversion of CO2 to Ethylene. Front. Energy Res. 2020, 8, 557466. [Google Scholar]
- Francke, R.; Schille, B.; Roemelt, M. Homogeneously Catalyzed Electroreduction of Carbon Dioxide−Methods, Mechanisms, and Catalysts. Chem. Rev. 2018, 118, 4631–4701. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, H.; Xiang, H.; Rasul, S.; Fontmorin, J.-M.; Izadi, P.; Roldan, A.; Taylor, R.; Feng, Y.; Banerji, L.; et al. How to Go Beyond C1 Products with Electrochemical Reduction of CO2. Sustain. Energy Fuels 2021, 5, 5893–5914. [Google Scholar] [CrossRef]
- Zhang, W.; Lai, W.; Cao, R. Energy-Related Small Molecule Activation Reactions: Oxygen Reduction and Hydrogen and Oxygen Evolution Reactions Catalyzed by Porphyrin- and Corrole-Based Systems. Chem. Rev. 2017, 117, 3717–3797. [Google Scholar] [CrossRef]
- Amanullah, S.; Saha, P.; Dey, A. Recent Developments in the Synthesis of Bio-Inspired Iron Porphyrins for Small Molecule Activation. Chem. Commun. 2022, 58, 5808–5828. [Google Scholar] [CrossRef] [PubMed]
- Dedić, D.; Dorniak, A.; Rinner, U.; Schöfberger, W. Recent Progress in (Photo-)-Electrochemical Conversion of CO2 With Metal Porphyrinoid-Systems. Front. Chem. 2021, 9, 685619. [Google Scholar] [CrossRef]
- Bhugun, I.; Lexa, D.; Savéant, J.-M. Catalysis of the Electrochemical Reduction of Carbon Dioxide by Iron(0) Porphyrins. Synergistic Effect of Lewis Acid Cations. J. Phys. Chem. 1996, 100, 19981–19985. [Google Scholar] [CrossRef]
- Lei, K.; Xia, B.Y. Electrocatalytic CO2 Reduction: From Discrete Molecular Catalysts to Their Integrated Catalytic Materials. Chem. Eur. J. 2022, 28, e202200141. [Google Scholar]
- Amanullah, S.; Saha, P.; Nayek, A.; Ahmed, M.E.; Dey, A. Biochemical and Artificial Pathways for the Reduction of Carbon Dioxide, Nitrite and the Competing Proton Reduction: Effect of 2nd Sphere Interactions in Catalysis. Chem. Soc. Rev. 2021, 50, 3755–3823. [Google Scholar] [CrossRef]
- Gotico, P.; Leibl, W.; Halime, Z.; Aukauloo, A. Shaping the Electrocatalytic Performance of Metal Complexes for CO2 Reduction. ChemElectroChem 2021, 8, 3472–3481. [Google Scholar] [CrossRef]
- Nichols, A.W.; Machan, C.W. Secondary-Sphere Effects in Molecular Electrocatalytic CO2 Reduction. Front. Chem. 2019, 7, 397. [Google Scholar] [CrossRef] [PubMed]
- Costentin, C.; Drouet, S.; Robert, M.; Savéant, J.-M. A Local Proton Source Enhances CO2 Electroreduction to CO by a Molecular Fe Catalyst. Science 2012, 338, 90–94. [Google Scholar] [CrossRef]
- Azcarate, I.; Costentin, C.; Robert, M.; Savéant, J.-M. Dissection of Electronic Substituent Effects in Multielectron−Multistep Molecular Catalysis. Electrochemical CO2-to-CO Conversion Catalyzed by Iron Porphyrins. J. Phys. Chem. C 2016, 120, 28951–28960. [Google Scholar] [CrossRef]
- Azcarate, I.; Costentin, C.; Robert, M.; Savéant, J.-M. Through-Space Charge Interaction Substituent Effects in Molecular Catalysis Leading to the Design of the Most Efficient Catalyst of CO2-to-CO Electrochemical Conversion. J. Am. Chem. Soc. 2016, 138, 16639–16644. [Google Scholar] [CrossRef]
- Hammouche, M.; Lexa, D.; Savéant, J.M. Catalysis of the Electrochemical Reduction of Carbon Dioxide by Iron(“0”) Porphyrins. J. Electroanal. Chem. 1988, 249, 347–351. [Google Scholar] [CrossRef]
- Mondal, B.; Rana, A.; Sen, P.; Dey, A. Intermediates Involved in the 2e−/2H+ Reduction of CO2 to CO by Iron(0) Porphyrin. J. Am. Chem. Soc. 2015, 137, 11214–11217. [Google Scholar] [CrossRef]
- Nichols, E.M.; Derrick, J.S.; Nistanaki, S.K.; Smith, P.T.; Chang, C.J. Positional Effects of Second-Sphere Amide Pendants on Electrochemical CO2 Reduction Catalyzed by Iron Porphyrins. Chem. Sci. 2018, 9, 2952–2960. [Google Scholar] [CrossRef]
- Margarit, C.G.; Schnedermann, C.; Asimow, N.G.; Nocera, D.G. Carbon Dioxide Reduction by Iron Hangman Porphyrins. Organometallics 2019, 38, 1219–1223. [Google Scholar] [CrossRef]
- Guo, K.; Li, X.; Lei, H.; Zhang, W.; Cao, R. Unexpected Effect of Intramolecular Phenolic Group on Electrocatalytic CO2 Reduction. ChemCatChem 2020, 12, 1591–1595. [Google Scholar] [CrossRef]
- Guo, H.; Liang, Z.; Guo, K.; Lei, H.; Wang, Y.; Zhang, W.; Cao, R. Iron Porphyrin with Appended Guanidyl Group for Significantly Improved Electrocatalytic Carbon Dioxide Reduction Activity and Selectivity in Aqueous Solutions. Chin. J. Catal. 2022, 43, 3089–3094. [Google Scholar] [CrossRef]
- Sen, P.; Mondal, B.; Saha, D.; Rana, A.; Dey, A. Role of 2nd Sphere H-bonding Residues in Tuning the Kinetics of CO2 Reduction to CO by Iron Porphyrin Complexes. Dalton Trans. 2019, 48, 5965–5977. [Google Scholar] [CrossRef] [PubMed]
- Gotico, P.; Boitrel, B.; Guillot, R.; Sircoglou, M.; Quaranta, A.; Halime, Z.; Leibl, W.; Aukauloo, A. Second-Sphere Biomimetic Multipoint Hydrogen-Bonding Patterns to Boost CO2 Reduction of Iron Porphyrins. Angew. Chem. Int. Ed. 2019, 58, 4504–4509. [Google Scholar] [CrossRef]
- Gotico, P.; Roupnel, L.; Guillot, R.; Sircoglou, M.; Leibl, W.; Halime, Z.; Aukauloo, A. Atropisomeric Hydrogen Bonding Control for CO2 Binding and Enhancement of Electrocatalytic Reduction at Iron Porphyrins. Angew. Chem. Int. Ed. 2020, 59, 22451–22455. [Google Scholar] [CrossRef] [PubMed]
- Derrick, J.S.; Loipersberger, M.; Nistanaki, S.K.; Rothweiler, A.V.; Head-Gordon, M.; Nichols, E.M.; Chang, C.J. Templating Bicarbonate in the Second Coordination Sphere Enhances Electrochemical CO2 Reduction Catalyzed by Iron Porphyrins. J. Am. Chem. Soc. 2022, 144, 11656–11663. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Fan, Y.-J.; Zhang, J.-L. Construction of Secondary Coordination Sphere Boosts Electrochemical CO2 Reduction of Iron Porphyrins. J. Porphyr. Phthaloc. 2020, 24, 465–472. [Google Scholar] [CrossRef]
- Amanullah, S.; Saha, P.; Dey, A. Activating the Fe(I) State of Iron Porphyrinoid with Second-Sphere Proton Transfer Residues for Selective Reduction of CO2 to HCOOH via Fe(III/II)−COOH Intermediate(s). J. Am. Chem. Soc. 2021, 143, 13579–13592. [Google Scholar] [CrossRef]
- Guo, K.; Li, X.; Lei, H.; Guo, H.; Jin, X.; Zhang, X.-P.; Zhang, W.; Apfel, U.-P.; Cao, R. Role-Specialized Division of Labor in CO2 Reduction with Doubly-Functionalized Iron Porphyrin Atropisomers. Angew. Chem. Int. Ed. 2022, 61, e202209602. [Google Scholar] [CrossRef]
- Ramuglia, A.R.; Budhija, V.; Ly, K.H.; Marquardt, M.; Schwalbe, M.; Weidinger, I.M. An Iron Porphyrin Complex with Pendant Pyridine Substituents Facilitates Electrocatalytic CO2 Reduction via Second Coordination Sphere Effects. ChemCatChem 2021, 13, 3934–3944. [Google Scholar] [CrossRef]
- Narouz, M.R.; De La Torre, P.; An, L.; Chang, C.J. Multifunctional Charge and Hydrogen-Bond Effects of Second- Sphere Imidazolium Pendants Promote Capture and Electrochemical Reduction of CO2 in Water Catalyzed by Iron Porphyrins. Angew. Chem. Int. Ed. 2022, 61, e202207666. [Google Scholar] [CrossRef]
- Costentin, C.; Robert, M.; Savéant, J.-M. Catalysis of the Electrochemical Reduction of Carbon Dioxide. Chem. Soc. Rev. 2013, 42, 2423–2436. [Google Scholar] [CrossRef] [PubMed]
- Gotico, P.; Halime, Z.; Aukauloo, A. Recent Advances in Metalloporphyrin-Based Catalyst Design Towards Carbon Dioxide Reduction: From Bio-Inspired Second Coordination Sphere Modifications to Hierarchical Architectures. Dalton Trans. 2020, 49, 2381–2396. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).