Synthesis of an Electrodeficient Dipyridylbenzene-like Terdentate Ligand: Cyclometallating Ligand for Highly Emitting Iridium(III) and Platinum(II) Complexes
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Consideration
2.2. Absorption and Emission Spectroscopies
2.3. Experimental Procedures
3. Results
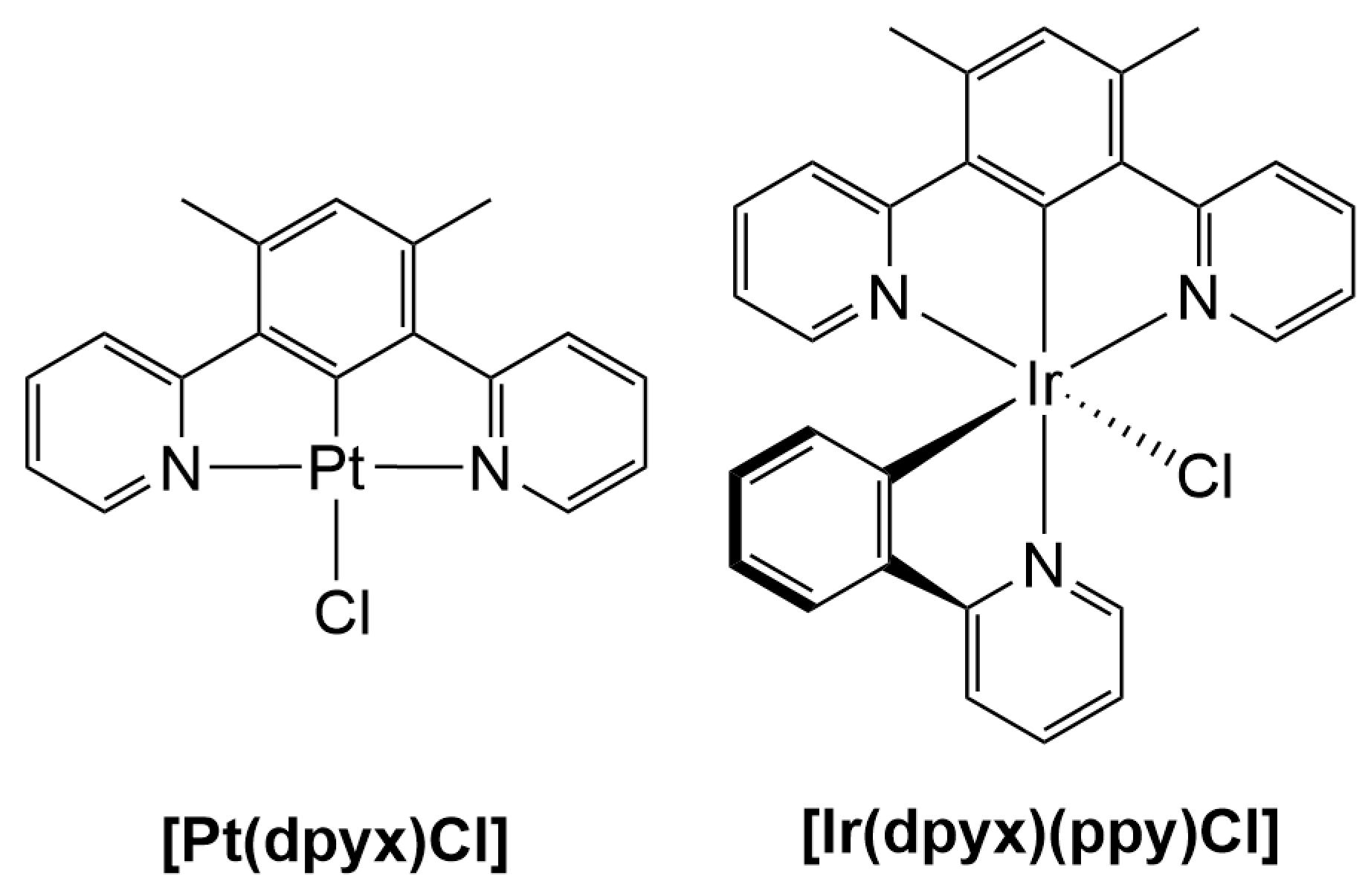
3.1. Synthesis
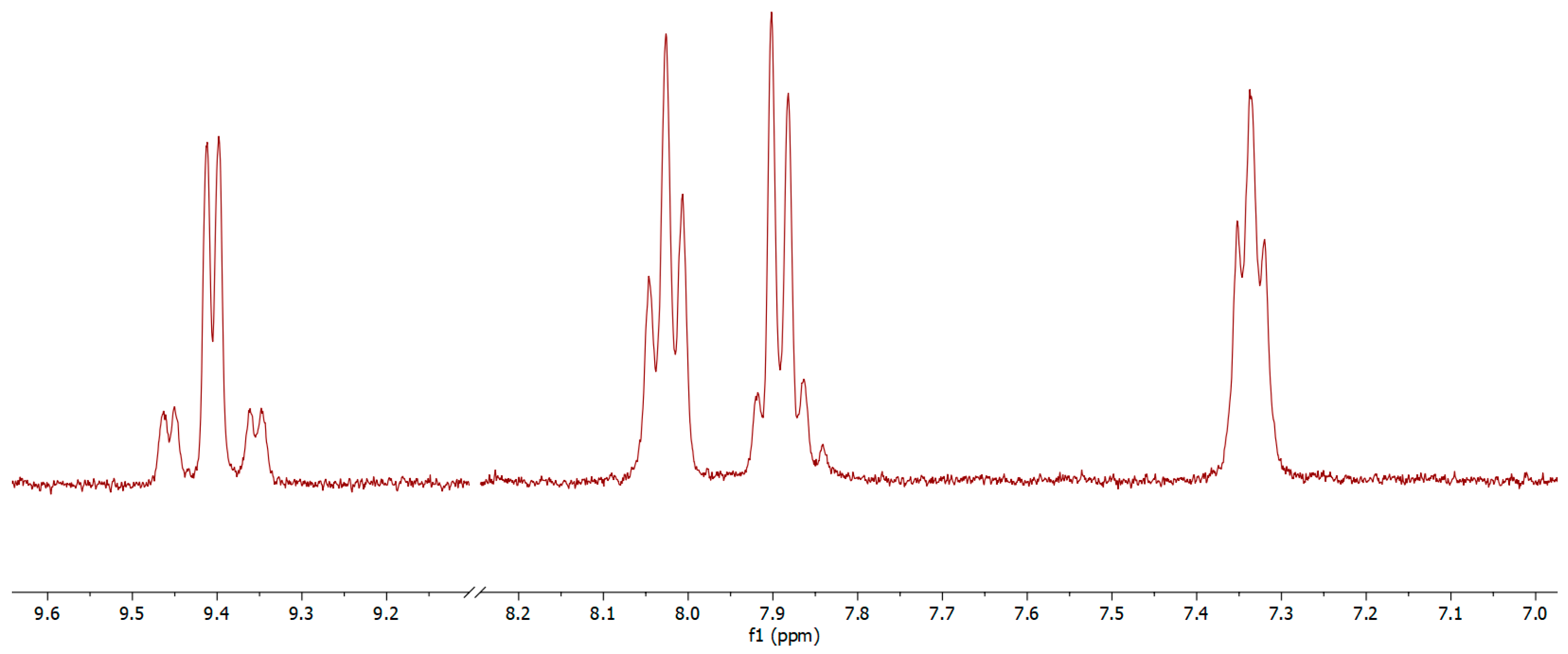
3.2. Characterisation
3.3. X-ray Single Crystal Diffraction
3.4. Absorption and Emission Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, T.-Y.; Wu, J.; Wu, Z.-G.; Zheng, Y.-X.; Zuo, J.-L.; Pan, Y. Rational Design of Phosphorescent Iridium(III) Complexes for Emission Color Tunability and Their Applications in OLEDs. Coord. Chem. Rev. 2018, 374, 55–92. [Google Scholar] [CrossRef]
- Pashaei, B.; Karimi, S.; Shahroosvand, H.; Abbasi, P.; Pilkington, M.; Bartolotta, A.; Fresta, E.; Fernandez-Cestau, J.; Costa, R.D.; Bonaccorso, F. Polypyridyl Ligands as a Versatile Platform for Solid-State Light-Emitting Devices. Chem. Soc. Rev. 2019, 48, 5033–5139. [Google Scholar] [CrossRef] [PubMed]
- Shon, J.H.; Teets, T.S. Molecular Photosensitizers in Energy Research and Catalysis: Design Principles and Recent Developments. ACS Energy Lett. 2019, 4, 558–566. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, L.; Li, F.; Yu, M.; Liu, Z.; Yi, T.; Huang, C. Aggregation-Induced Phosphorescent Emission (AIPE) of Iridium(Iii) Complexes. Chem. Commun. 2008, 3, 685–687. [Google Scholar] [CrossRef] [PubMed]
- Solomatina, A.I.; Slobodina, A.D.; Ryabova, E.V.; Bolshakova, O.I.; Chelushkin, P.S.; Sarantseva, S.V.; Tunik, S.P. Blood-Brain Barrier Penetrating Luminescent Conjugates Based on Cyclometalated Platinum(II) Complexes. Bioconjug. Chem. 2020, 31, 2628–2637. [Google Scholar] [CrossRef]
- Costa, R.D.; Ortí, E.; Bolink, H.J.; Graber, S.; Schaffner, S.; Neuburger, M.; Housecroft, C.E.; Constable, E.C. Archetype Cationic Iridium Complexes and Their Use in Solid-State Light-Emitting Electrochemical Cells. Adv. Funct. Mater. 2009, 19, 3456–3463. [Google Scholar] [CrossRef]
- Meier, S.B.; Tordera, D.; Pertegàs, A.; Roldàn-Carmona, C.; Ortì, E.; Bolink, H.J. Light-Emitting Electrochemical Cells: Recent Progress and Future Prospects. Mater. Today 2014, 17, 217–223. [Google Scholar] [CrossRef]
- Salehi, A.; Fu, X.; Shin, D.-H.; So, F. Recent Advances in OLED Optical Design. Adv. Funct. Mater. 2019, 29, 1808803. [Google Scholar] [CrossRef]
- Zhao, J.H.; Hu, Y.X.; Lu, H.Y.; Lü, Y.L.; Li, X. Progress on Benzimidazole-Based Iridium(III) Complexes for Application in Phosphorescent OLEDs. Org. Electron. 2017, 41, 56–72. [Google Scholar] [CrossRef]
- Choy, W.C.H.; Chan, W.K.; Yuan, Y. Recent Advances in Transition Metal Complexes and Light-Management Engineering in Organic Optoelectronic Devices. Adv. Mater. 2014, 26, 5368–5399. [Google Scholar] [CrossRef]
- Lo, K.K.-W. Luminescent Rhenium(I) and Iridium(III) Polypyridine Complexes as Biological Probes, Imaging Reagents, and Photocytotoxic Agents. Acc. Chem. Res. 2015, 48, 2985–2995. [Google Scholar] [CrossRef]
- Yuan, Y.J.; Yu, Z.T.; Chen, D.Q.; Zou, Z.G. Metal-Complex Chromophores for Solar Hydrogen Generation. Chem. Soc. Rev. 2017, 46, 603–631. [Google Scholar] [CrossRef]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W.C. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef] [Green Version]
- Silvestroni, L.; Accorsi, G.; Armaroli, N.; Balzani, V.; Bergamini, G.; Campagna, S.; Cardinali, F.; Chiorboli, C.; Indelli, M.T.; Kane-Maguire, N.A.P.; et al. Photochemistry and Photophysics of Coordination Compounds I; Balzani, V., Campagna, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 281, ISBN 9783642255281. [Google Scholar]
- Barbieri, A.; Barigelletti, F.; Cheng, E.C.-C.; Flamigni, L.; Gunnlaugsson, T.; Kirgan, R.A.G.; Kumaresan, D.; Leonard, J.P.; Nolan, C.B.; Rillema, D.P.; et al. Photochemistry and Photophysics of Coordiantion Compounds II; Topics in current Chemistry; Campagna, S., Balzani, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 3-540-08986-1. [Google Scholar]
- Volman, D.H.; Hammond, G.S.; Neckers, D.C.; Maestri, M.; Balzani, V.; Deuschel-Cornioley, C.; Von Zelewsky, A. Photochemistry and Luminescence of Cyclometalated Complexes. Adv. Photochem. 1992, 17. [Google Scholar] [CrossRef]
- Gildea, L.F.; Williams, J.A.G. Iridium and Platinum Complexes for OLEDs. In Organic Light-Emitting Diodes (OLEDs); Buckley, A., Ed.; Woodhead Publishing: Sawston, UK, 2013; pp. 77–113. ISBN 978-0-85709-425-4. [Google Scholar]
- Zanoni, K.P.S.; Coppo, R.L.; Amaral, R.C.; Murakami Iha, N.Y. Ir(III) Complexes Designed for Light-Emitting Devices: Beyond the Luminescence Color Array. Dalton Trans. 2015, 44, 14559–14573. [Google Scholar] [CrossRef] [PubMed]
- Housecroft, C.E.; Constable, E.C. Over the LEC Rainbow: Colour and Stability Tuning of Cyclometallated Iridium(III) Complexes in Light-Emitting Electrochemical Cells. Coord. Chem. Rev. 2017, 350, 155–177. [Google Scholar] [CrossRef] [Green Version]
- Kalinowski, J.; Fattori, V.; Cocchi, M.; Williams, J.A.G. Light-Emitting Devices Based on Organometallic Platinum Complexes as Emitters; Le Bozec, H., Guerchais, V., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2011; Volume 255. [Google Scholar]
- Baranoff, E.; Yum, J.-H.; Jung, I.; Vulcano, R.; Grätzel, M.; Nazeeruddin, M.K. Cyclometallated Iridium Complexes as Sensitizers for Dye-Sensitized Solar Cells. Chem. Asian J. 2010, 5, 496–499. [Google Scholar] [CrossRef]
- Guo, H.; Ji, S.; Wu, W.W.; Shao, J.; Zhao, J. Long-Lived Emissive Intra-Ligand Triplet Excited States (3IL): Next Generation Luminescent Oxygen Sensing Scheme and a Case Study with Red Phosphorescent Diimine Pt(II) Bis(Acetylide) Complexes Containing Ethynylated Naphthalimide or Pyrene Subunits. Analyst 2010, 135, 2832–2840. [Google Scholar] [CrossRef]
- Medina-Rodríguez, S.; Denisov, S.A.; Cudré, Y.; Male, L.; Marín-Suárez, M.; Fernández-Gutiérrez, A.; Fernández-Sánchez, J.F.; Tron, A.; Jonusauskas, G.; McClenaghan, N.D.; et al. High Performance Optical Oxygen Sensors Based on Iridium Complexes Exhibiting Interchromophore Energy Shuttling. Analyst 2016, 141, 3090–3097. [Google Scholar] [CrossRef] [Green Version]
- Lanoë, P.-H.; Le Bozec, H.; Williams, J.A.G.; Fillaut, J.-L.; Guerchais, V. Cyclometallated Platinum(II) Complexes Containing Pyridyl-Acetylide Ligands: The Selective Influence of Lead Binding on Luminescence. Dalton Trans. 2010, 39, 707–710. [Google Scholar] [CrossRef] [Green Version]
- Lanoë, P.-H.; Fillaut, J.-L.; Toupet, L.; Williams, J.A.G.; Le Bozec, H.; Guerchais, V. Cyclometallated Platinum(II) Complexes Incorporating Ethynyl-Flavone Ligands: Switching between Triplet and Singlet Emission Induced by Selective Binding of Pb2+ Ions. Chem. Commun. 2008, 4333–4335. [Google Scholar] [CrossRef] [PubMed]
- Lanoë, P.-H.; Fillaut, J.-L.; Guerchais, V.; Le Bozec, H.; Williams, J.a.G. Metal Cation Induced Modulation of the Photophysical Properties of a Platinum(II) Complex Featuring a Dipicolylanilino–Acetylide Ligand. Eur. J. Inorg. Chem. 2011, 2011, 1255–1259. [Google Scholar] [CrossRef]
- Ortega-Forte, E.; Hernández-García, S.; Vigueras, G.; Henarejos-Escudero, P.; Cutillas, N.; Ruiz, J.; Gandía-Herrero, F. Potent Anticancer Activity of a Novel Iridium Metallodrug via Oncosis. Cell. Mol. Life Sci. 2022, 79, 510. [Google Scholar] [CrossRef]
- Shen, J.; Rees, T.W.; Ji, L.; Chao, H. Recent Advances in Ruthenium(II) and Iridium(III) Complexes Containing Nanosystems for Cancer Treatment and Bioimaging. Coord. Chem. Rev. 2021, 443, 214016. [Google Scholar] [CrossRef]
- Caporale, C.; Massi, M. Cyclometalated Iridium(III) Complexes for Life Science. Coord. Chem. Rev. 2018, 363, 71–91. [Google Scholar] [CrossRef] [Green Version]
- Chung, C.Y.-S.; Yam, V.W.-W. Induced Self-Assembly of Platinum(II) Alkynyl Complexes through Specific Interactions between Citrate and Guanidinium for Proof-of-Principle Detection of Citrate and an Assay of Citrate Lyase. Chemistry 2014, 20, 13016–13027. [Google Scholar] [CrossRef]
- Tu, T.; Fang, W.; Bao, X.; Li, X.; Dötz, K.H. Visual Chiral Recognition through Enantioselective Metallogel Collapsing: Synthesis, Characterization, and Application of Platinum-Steroid Low-Molecular-Mass Gelators. Angew. Chem. Int. Ed. 2011, 50, 6601–6605. [Google Scholar] [CrossRef]
- Li, K.; Zou, T.; Chen, Y.; Guan, X.; Che, C. Pincer-Type Platinum(II) Complexes Containing N-Heterocyclic Carbene (NHC) Ligand: Structures, Photophysical and Anion-Binding Properties, and Anticancer Activities. Chem. Eur.J. 2015, 21, 7441–7453. [Google Scholar] [CrossRef]
- Di Bella, C.S.; Dragonetti, M.; Pizzotti, D.; Roberto, F.; Tessore, R.; Ugo, M.G.; Humphrey, M.P.; Cifuentes, M.; Samoc, L.; Murphy, J.A.G.; et al. Molecular Organometallic Materials for Optics. Top. Organomet. Chem. 2010, 37, 179. [Google Scholar] [CrossRef]
- Rausch, A.F.; Murphy, L.; Williams, J.A.G.; Yersin, H. Improving the Performance of Pt(II) Complexes for Blue Light Emission by Enhancing the Molecular Rigidity. Inorg. Chem. 2012, 51, 312–319. [Google Scholar] [CrossRef]
- Congrave, D.G.; Hsu, Y.-T.; Batsanov, A.S.; Beeby, A.; Bryce, M.R. Sky-Blue Emitting Bridged Diiridium Complexes: Beneficial Effects of Intramolecular π–π Stacking. Dalton Trans. 2018, 47, 2086–2098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Culham, S.; Lanoë, P.-H.; Whittle, V.L.; Durrant, M.C.; Williams, J.A.G.A.G.; Kozhevnikov, V.N. Highly Luminescent Dinuclear Platinum(II) Complexes Incorporating Bis-Cyclometallating Pyrazine-Based Ligands: A Versatile Approach to Efficient Red Phosphors. Inorg. Chem. 2013, 52, 10992–11003. [Google Scholar] [CrossRef] [Green Version]
- Lanoë, P.-H.; Tong, C.M.; Harrington, R.W.; Probert, M.R.; Clegg, W.; Williams, J.A.G.; Kozhevnikov, V.N. Ditopic Bis-Terdentate Cyclometallating Ligands and Their Highly Luminescent Dinuclear Iridium(III) Complexes. Chem. Commun. 2014, 50, 6831–6936. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim-Ouali, M.; Dumur, F. Recent Advances on Metal-Based near-Infrared and Infrared Emitting OLEDs. Molecules 2019, 24, 1412. [Google Scholar] [CrossRef] [Green Version]
- Tamura, Y.; Hisamatsu, Y.; Kumar, S.; Itoh, T.; Sato, K.; Kuroda, R.; Aoki, S. Efficient Synthesis of Tris-Heteroleptic Iridium(III) Complexes Based on the Zn2+-Promoted Degradation of Tris-Cyclometalated Iridium(III) Complexes and Their Photophysical Properties. Inorg. Chem. 2017, 56, 812–833. [Google Scholar] [CrossRef]
- Lepeltier, M.; Graff, B.; Lalevée, J.; Wantz, G.; Ibrahim-Ouali, M.; Gigmes, D.; Dumur, F. Heteroleptic Iridium (III) Complexes with Three Different Ligands: Unusual Triplet Emitters for Light-Emitting Electrochemical Cells. Org. Electron. Phys. Mater. Appl. 2016, 37, 24–34. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Q.; He, H.; Wang, L.; Zhang, J. Tuning the Electronic and Phosphorescence Properties of Blue-Emitting Iridium(Iii) Complexes through Different Cyclometalated Ligand Substituents: A Theoretical Investigation. Dalton Trans. 2015, 44, 8577–8589. [Google Scholar] [CrossRef] [PubMed]
- Huckaba, A.J.; Cao, B.; Hollis, T.K.; Valle, H.U.; Kelly, J.T.; Hammer, N.I.; Oliver, A.G.; Webster, C.E. Platinum CCC-NHC Benzimidazolyl Pincer Complexes: Synthesis, Characterization, Photostability, and Theoretical Investigation of a Blue-Green Emitter. Dalton Trans. 2013, 42, 8820–8826. [Google Scholar] [CrossRef]
- Darmawan, N.; Yang, C.-H.; Mauro, M.; Raynal, M.; Heun, S.; Pan, J.; Buchholz, H.; Braunstein, P.; De Cola, L. Efficient Near-UV Emitters Based on Cationic Bis-Pincer Iridium(III) Carbene Complexes. Inorg. Chem. 2013, 52, 10756–10765. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, S.W.; Zhang, M.; Wu, C.; Li, W.; Wu, Y.; Yang, C.; Kang, F.; Meng, H.; Wei, G. Highly Efficient Phosphorescent Blue-Emitting [3+2+1] Coordinated Iridium(III) Complex for OLED Application. Front. Chem. 2021, 9, 758357. [Google Scholar] [CrossRef]
- Sivasubramaniam, V.; Brodkorb, F.; Hanning, S.; Loebl, H.P.; van Elsbergen, V.; Boerner, H.; Scherf, U.; Kreyenschmidt, M. Fluorine Cleavage of the Light Blue Heteroleptic Triplet Emitter FIrpic. J. Fluor. Chem. 2009, 130, 640–649. [Google Scholar] [CrossRef]
- Williams, J.A.G.; Beeby, A.; Davies, E.S.; Weinstein, J.A.; Wilson, C. An Alternative Route to Highly Luminescent Platinum(II) Complexes: Cyclometalation with N ∧ C ∧ N-Coordinating Dipyridylbenzene Ligands. Inorg. Chem. Commun. 2003, 42, 8609–8611. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Lan, Y.; Ma, D.; Song, X.; Duan, L. Fluorine-Free, Highly Efficient, Blue-Green and Sky-Blue-Emitting Cationic Iridium Complexes and Their Use for Efficient Organic Light-Emitting Diodes. J. Mater. Chem. C 2018, 6, 1509–1520. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Pan, F.; He, L.; Duan, L. Cationic Iridium Complexes with 5-Phenyl-1 H -1,2,4-Triazole Type Cyclometalating Ligands: Toward Blue-Shifted Emission. Inorg. Chem. 2019, 58, 12132–12145. [Google Scholar] [CrossRef]
- Henwood, A.F.; Pal, A.K.; Cordes, D.B.; Slawin, A.M.Z.; Rees, T.W.; Momblona, C.; Babaei, A.; Pertegás, A.; Ortí, E.; Bolink, H.J.; et al. Blue-Emitting Cationic Iridium(III) Complexes Featuring Pyridylpyrimidine Ligands and Their Use in Sky-Blue Electroluminescent Devices. J. Mater. Chem. C 2017, 5, 9638–9650. [Google Scholar] [CrossRef] [Green Version]
- Constable, E.C.; Henney, R.P.G.; Leese, T.A. Cyclometallation Reactions of 6-Phenyl-2,2’-Bipyridine; a Potential C,N,N-Donor Analogue of 2,2’: 6’,2"-Terpyridine. Crystal and Molecular Structure of Dichlorobis(6-Phenyl-2,2’-Bipyridine)Ruthenium(II). J. Chem. Soc. Dalt. Trans. 1990, 443–449. [Google Scholar] [CrossRef]
- Daniels, R.E.; Culham, S.; Hunter, M.; Durrant, M.C.; Probert, M.R.; Clegg, W.; Williams, J.A.G.; Kozhevnikov, V.N. When Two Are Better than One: Bright Phosphorescence from Non-Stereogenic Dinuclear Iridium(III) Complexes. Dalton Trans. 2016, 45, 6949–6962. [Google Scholar] [CrossRef] [Green Version]
- Williams, J.A.G.; Develay, S.; Rochester, D.L.; Murphy, L. Optimising the Luminescence of Platinum(II) Complexes and Their Application in Organic Light Emitting Devices (OLEDs). Coord. Chem. Rev. 2008, 252, 2596–2611. [Google Scholar] [CrossRef]
- Whittle, V.L.; Williams, J.A.G. A New Class of Iridium Complexes Suitable for Stepwise Incorporation into Linear Assemblies: Synthesis, Electrochemistry, and Luminescence. Inorg. Chem. 2008, 47, 6596–6607. [Google Scholar] [CrossRef]
- Brouwer, A.M. Standards for Photoluminescence Quantum Yield Measurements in Solution (IUPAC Technical Report)*. Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar] [CrossRef] [Green Version]
- Błachut, D.; Wojtasiewicz, K.; Czarnocki, Z. Some Pyridine Derivatives as “Route-Specific Markers” in 4-Methoxyamphetamine (PMA) Prepared by the Leuckart Method: Studies on the Role of the Aminating Agent in Their Distribution in the Final Product. Forensic Sci. Int. 2005, 152, 157–173. [Google Scholar] [CrossRef]
- Wilkinson, A.J.; Puschmann, H.; Howard, J.A.K.; Foster, C.E.; Williams, J.A.G. Luminescent Complexes of Iridium(III) Containing N^C^N-Coordinating Terdentate Ligands. Inorg. Chem. 2006, 45, 8685–8699. [Google Scholar] [CrossRef]
- Anderson, G.K.; Lin, M.; Sen, A.; Gretz, E. Reagents for Transition Metal Complexes and Oroganometallic Synthesis. In Inorganic Syntheses; Angelici, R.J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1990; Volume 28, pp. 1–463. [Google Scholar]
- Kumaresan, D.; Shankar, K.; Vaidya, S.; Balzani, V.; Campagna, S.; Williams, J.A.G.; Francesco, P.; Bergamini, G.; Balzani, V.; Indelli, M.; et al. Photochemistry and Photophysics of Coordination Compounds: Platinum. In Photochemistry and Photophysics of Coordination Compounds II; Balzani, V., Campagna, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 281, pp. 205–268. ISBN 978-3-540-73348-5. [Google Scholar]
- Li, G.; Congrave, D.G.; Zhu, D.; Su, Z.; Bryce, M.R. Recent Advances in Luminescent Dinuclear Iridium(III) Complexes and Their Application in Organic Electroluminescent Devices. Polyhedron 2018, 140, 146–157. [Google Scholar] [CrossRef]
- Williams, J.A.G. The Coordination Chemistry of Dipyridylbenzene: N-Deficient Terpyridine or Panacea for Brightly Luminescent Metal Complexes? Chem. Soc. Rev. 2009, 38, 1783–1801. [Google Scholar] [CrossRef] [PubMed]
- Flamigni, L.; Barbieri, A.; Sabatini, C.; Ventura, B.; Barigelletti, F. Photochemistry and Photophysics of Coordination Compounds: Iridium. In Photochemistry and Photophysics of Coordination Compounds II; Springer: Berlin/Heidelberg, Germany, 2007; pp. 143–203. [Google Scholar]
- Wilkinson, A.J.; Goeta, A.E.; Foster, C.E.; Williams, J.A.G. Synthesis and Luminescence of a Charge-Neutral, Cyclometalated Iridium(III) Complex Containing NCN- and CNC-Coordinating Terdentate Ligands. Inorg. Chem. 2004, 43, 6513–6515. [Google Scholar] [CrossRef]
- Brulatti, P.; Gildea, R.J.; Howard, J.A.K.; Fattori, V.; Cocchi, M.; Williams, J.A.G. Luminescent Iridium(III) Complexes with N^C^N-Coordinated Terdentate Ligands: Dual Tuning of the Emission Energy and Application to Organic Light-Emitting Devices. Inorg. Chem. 2012, 51, 3813–3826. [Google Scholar] [CrossRef] [PubMed]
- Schulze, B.; Friebe, C.; Jäger, M.; Görls, H.; Birckner, E.; Winter, A.; Schubert, U.S. Pt(II) Phosphors with Click-Derived 1,2,3-Triazole-Containing Tridentate Chelates. Organometallics 2018, 37, 145–155. [Google Scholar] [CrossRef]
- Rausch, A.F.; Homeier, H.H.H.; Yersin, H. Organometallic Pt(II) and Ir(III) Triplet Emitters for OLED Applications and the Role of Spin–Orbit Coupling: A Study Based on High-Resolution Optical Spectroscopy; Lees, A.J., Ed.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Scattergood, P.A.; Ranieri, A.M.; Charalambou, L.; Comia, A.; Ross, D.A.W.; Rice, C.R.; Hardman, S.J.O.; Heully, J.-L.; Dixon, I.M.; Massi, M.; et al. Unravelling the Mechanism of Excited-State Interligand Energy Transfer and the Engineering of Dual Emission in [Ir(C^N)2 (N^N)]+ Complexes. Inorg. Chem. 2020, 59, 1785–1803. [Google Scholar] [CrossRef]
- Obara, S.; Itabashi, M.; Okuda, F.; Tamaki, S.; Tanabe, Y.; Ishii, Y.; Nozaki, K.; Haga, M. Highly Phosphorescent Iridium Complexes Containing Both Tridentate Bis(Benzimidazolyl)-Benzene or -Pyridine and Bidentate Phenylpyridine: Synthesis, Photophysical Properties, and Theoretical Study of Ir-Bis(Benzimidazolyl)Benzene Complex. Inorg. Chem. 2006, 45, 8907–8921. [Google Scholar] [CrossRef] [PubMed]
- Lees, A.J. The Luminescence Rigidochromic Effect Exhibited by Organometailic Complexes: Rationale and Applications. Comments Inorg. Chem. A J. Crit. Discuss. Curr. Lit. 1995, 17, 319–346. [Google Scholar] [CrossRef]
- Murphy, L.; Brulatti, P.; Fattori, V.; Cocchi, M.; Williams, J. a G. Blue-Shifting the Monomer and Excimer Phosphorescence of Tridentate Cyclometallated Platinum(II) Complexes for Optimal White-Light OLEDs. Chem. Commun. (Camb) 2012, 48, 5817–5819. [Google Scholar] [CrossRef] [PubMed]

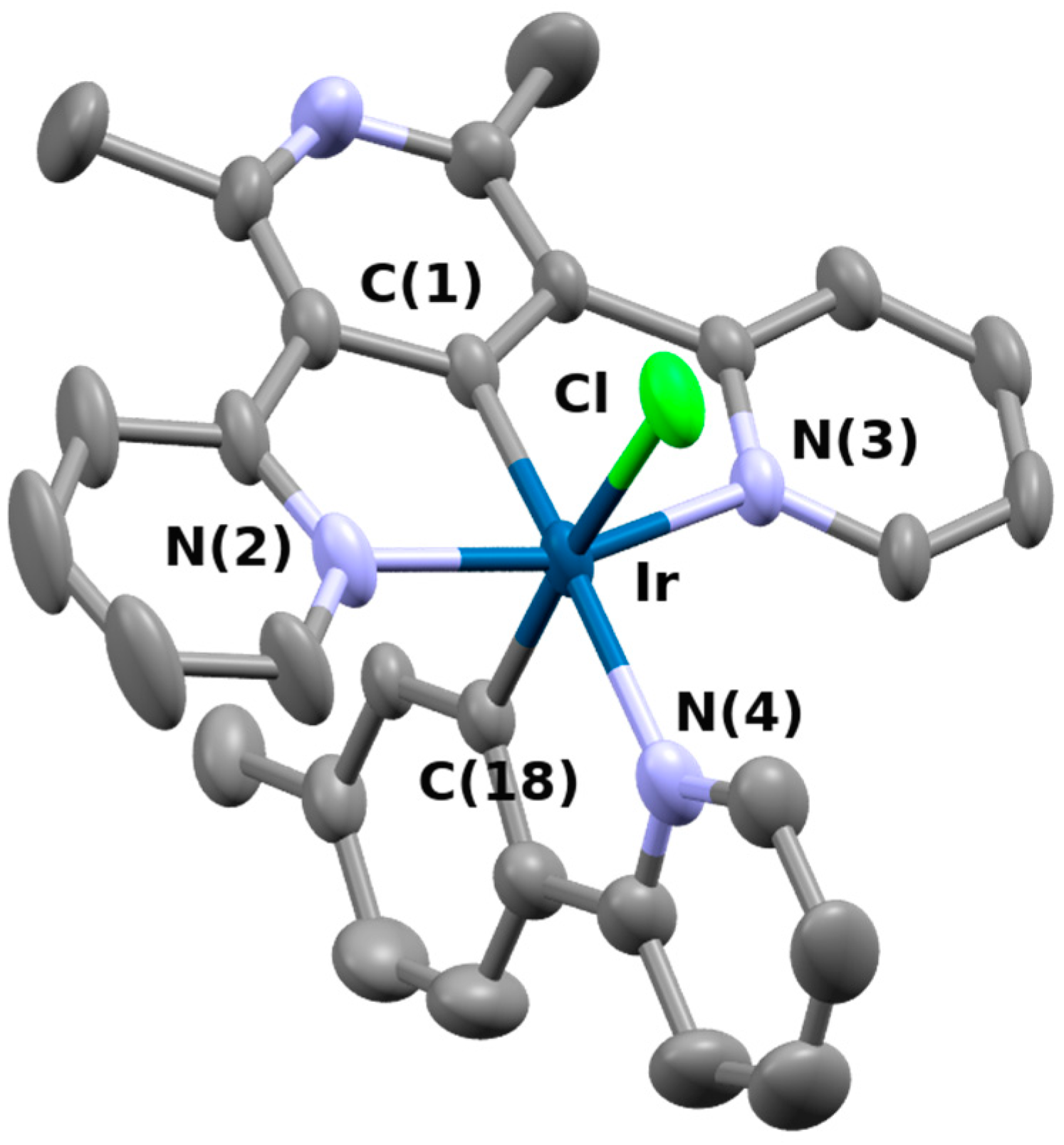

| Bond Length (Å) | Angles (°) | ||
|---|---|---|---|
| Ir−C(1) | 1.921(6) | C(1)−Ir−N(2) | 80.4(2) |
| Ir−N(2) | 2.043(6) | C(1)-Ir−N(3) | 80.0(2) |
| Ir−N(3) | 2.053(5) | C(1)−Ir−N(4) | 174.2(2) |
| Ir−N(4) | 2.158(5) | C(1)−Ir−C(18) | 94.8(2) |
| Ir−C(18) | 2.005(4) | C(1)−Ir−Cl | 92.7(2) |
| Ir−Cl | 2.462(2) | N(3)−Ir−C1 | 91.5(1) |
| N(2)-Ir−C1 | 88.1(1) | ||
| N(4)−Ir−Cl | 93.0(1) | ||
| C(18)−Ir−Cl | 172.1(1) | ||
| N(2)−Ir−N(3) | 160.3(2) |
| Complexes | λabs [nm] (ε 103 [M−1 cm−1]) | λem [nm] | Φ (Air) | τ [µs] (Air) | kr × 105 [s−1] 2 | Σknr × 105 [s−1] 2 | k[O2] × 109 [M−1 s−1] 3 | λem [nm] 77 K | τ [µs] 77 K |
|---|---|---|---|---|---|---|---|---|---|
| [Ir(dpyx)(ppy)Cl] 1 [57] | 258 (39.7), 285 (37.0), 353 (6.2), 369 (7.8), 399 (10.0), 417 (11.3), 455 (3.6), 492 (1.3), | 508 | 0.76 (0.02) | 1.6 (<0.10) | 4.8 | 1.5 | 4.9 | - | - |
| [Pt(dpx)Cl] [65] | - | 493 *, 524, 560 | 0.49 | 3.4 | 1.4 | 1.4 | - | - | - |
| 7 | 262 (11.9), 293 (75.5), 314 (29.4), 327 (34.6), 339 (41.0) 349 (23.3), 368 (26.7), 403 (2.9), 440 (0.8) | 470 *, 502, 533 | 0.18 (0.11) | 1.6 (1.02) | 1.1 | 5.1 | 0.2 | 463 *, 498, 538 | 2.3 |
| 8 | 254 (32.1), 280 (34.5), 400 (6.6), 440 (1.9), 484 (0.7) | 505 *, 528 | 0.61 (0.10) | 0.87 (0.12) | 7.0 | 4.5 | 2.6 | 500 *, 538, 585 | 4.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanoë, P.-H.; Philouze, C.; Loiseau, F. Synthesis of an Electrodeficient Dipyridylbenzene-like Terdentate Ligand: Cyclometallating Ligand for Highly Emitting Iridium(III) and Platinum(II) Complexes. Organics 2023, 4, 403-416. https://doi.org/10.3390/org4030029
Lanoë P-H, Philouze C, Loiseau F. Synthesis of an Electrodeficient Dipyridylbenzene-like Terdentate Ligand: Cyclometallating Ligand for Highly Emitting Iridium(III) and Platinum(II) Complexes. Organics. 2023; 4(3):403-416. https://doi.org/10.3390/org4030029
Chicago/Turabian StyleLanoë, Pierre-Henri, Christian Philouze, and Frédérique Loiseau. 2023. "Synthesis of an Electrodeficient Dipyridylbenzene-like Terdentate Ligand: Cyclometallating Ligand for Highly Emitting Iridium(III) and Platinum(II) Complexes" Organics 4, no. 3: 403-416. https://doi.org/10.3390/org4030029
APA StyleLanoë, P. -H., Philouze, C., & Loiseau, F. (2023). Synthesis of an Electrodeficient Dipyridylbenzene-like Terdentate Ligand: Cyclometallating Ligand for Highly Emitting Iridium(III) and Platinum(II) Complexes. Organics, 4(3), 403-416. https://doi.org/10.3390/org4030029








