An Overview of Pyrazole-Tetrazole-Based Hybrid Compounds: Synthesis Methods, Biological Activities and Energetic Properties
Abstract
:1. Introduction
2. General Information on Pyrazoles
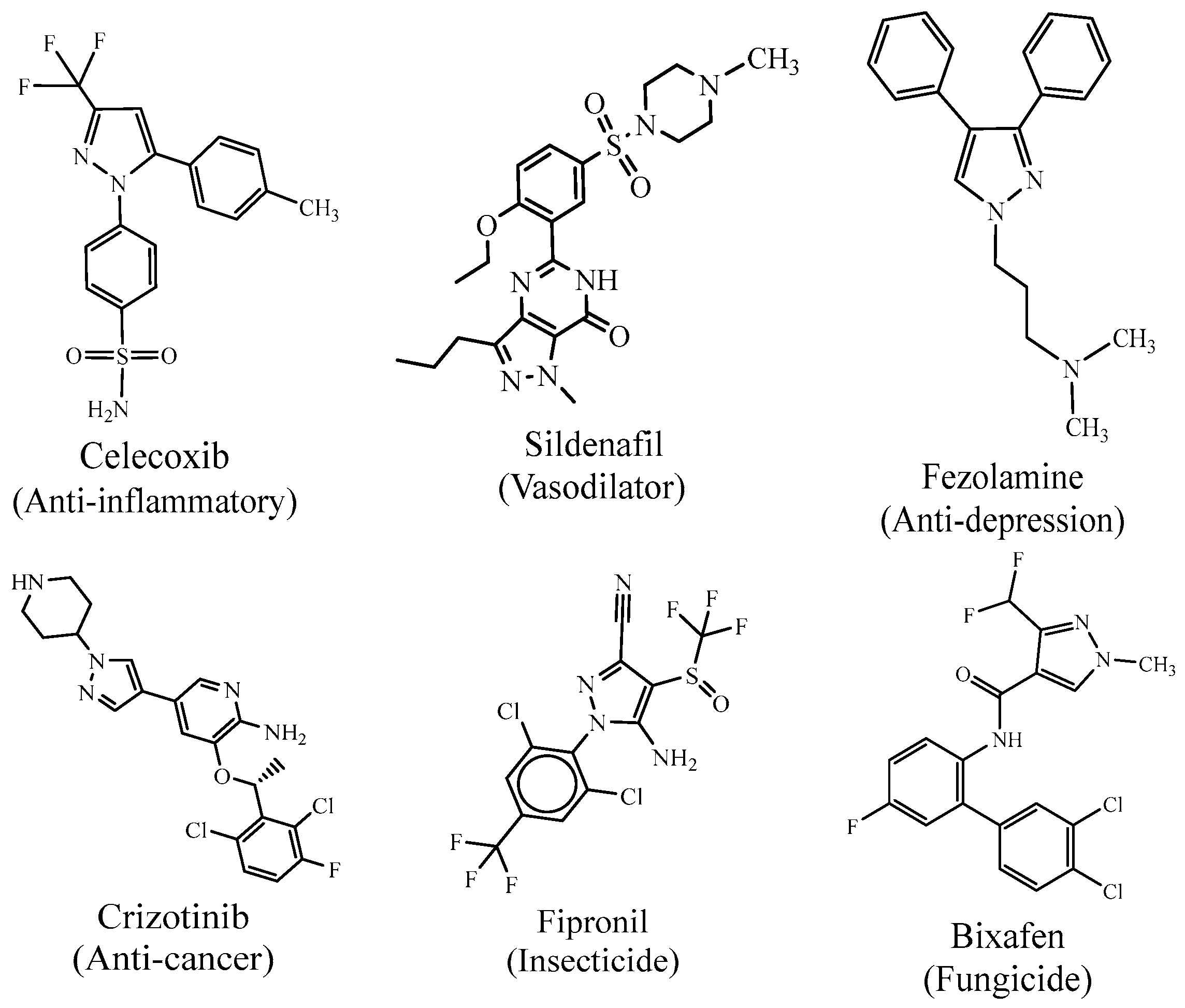
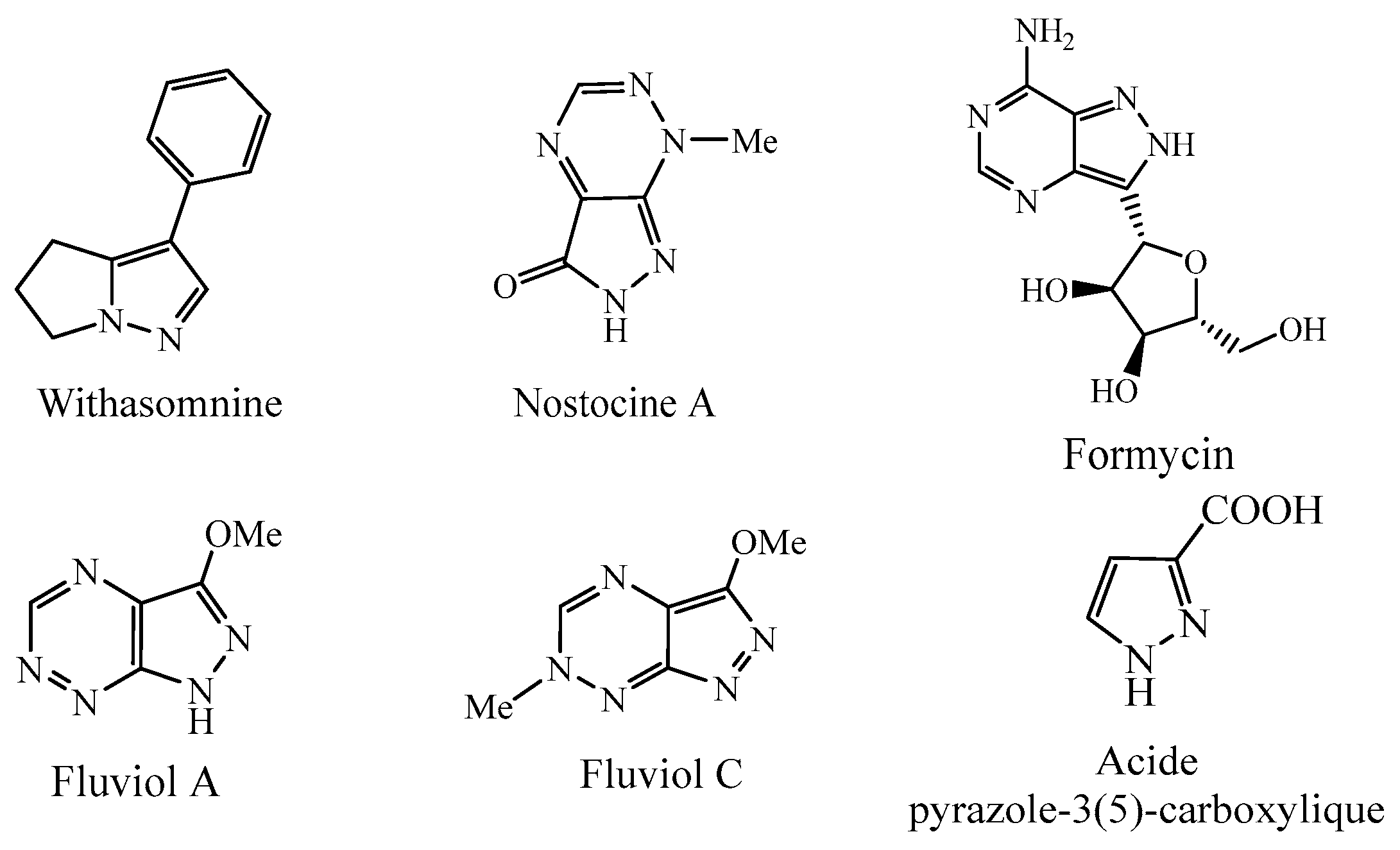
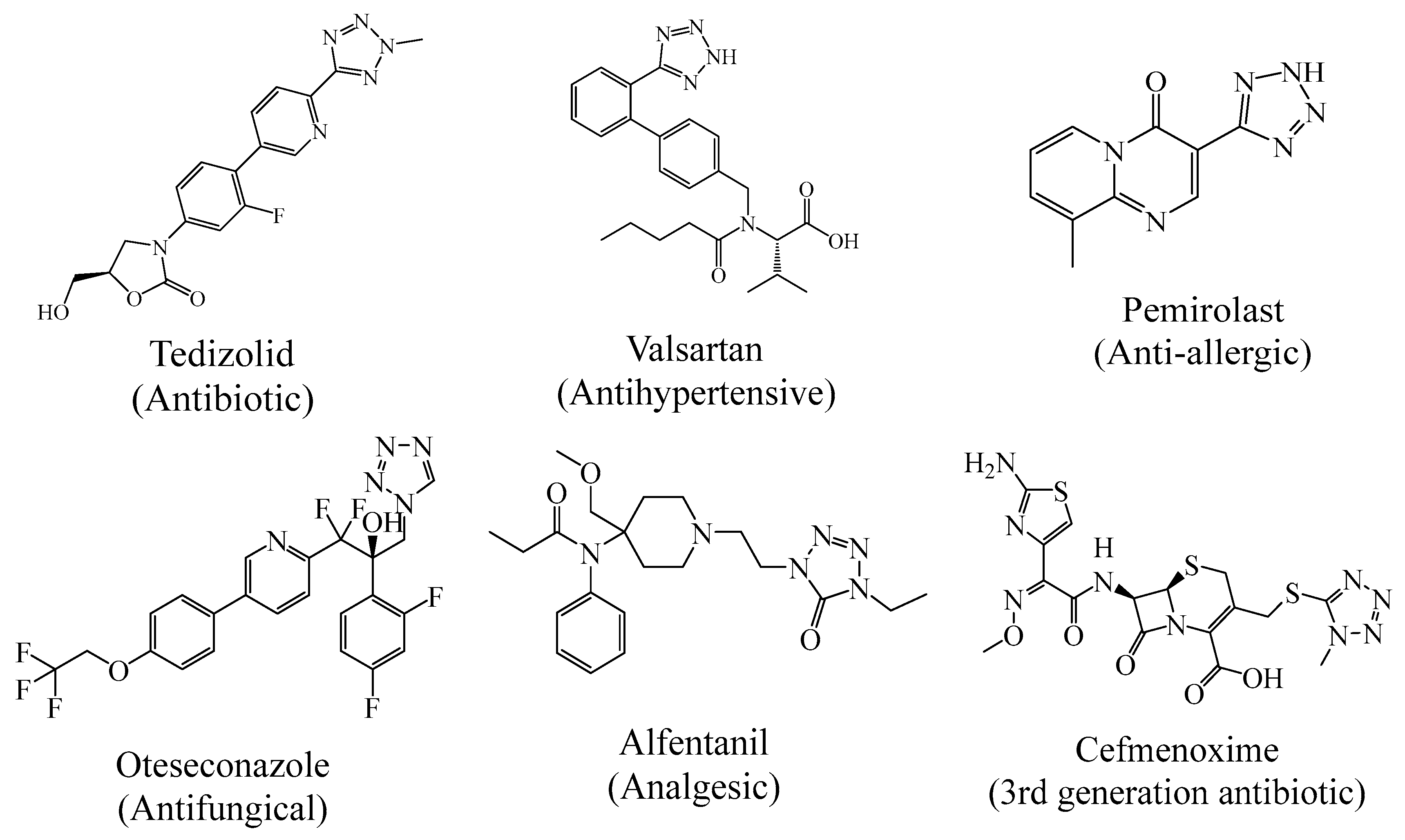
2.1. Biological Activities
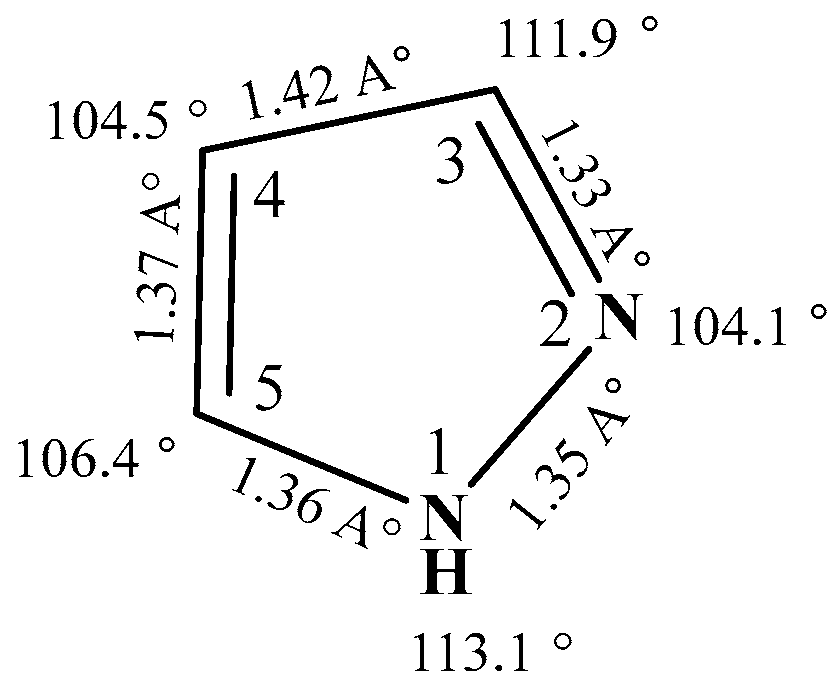
2.2. Structure and Physicochemical Properties
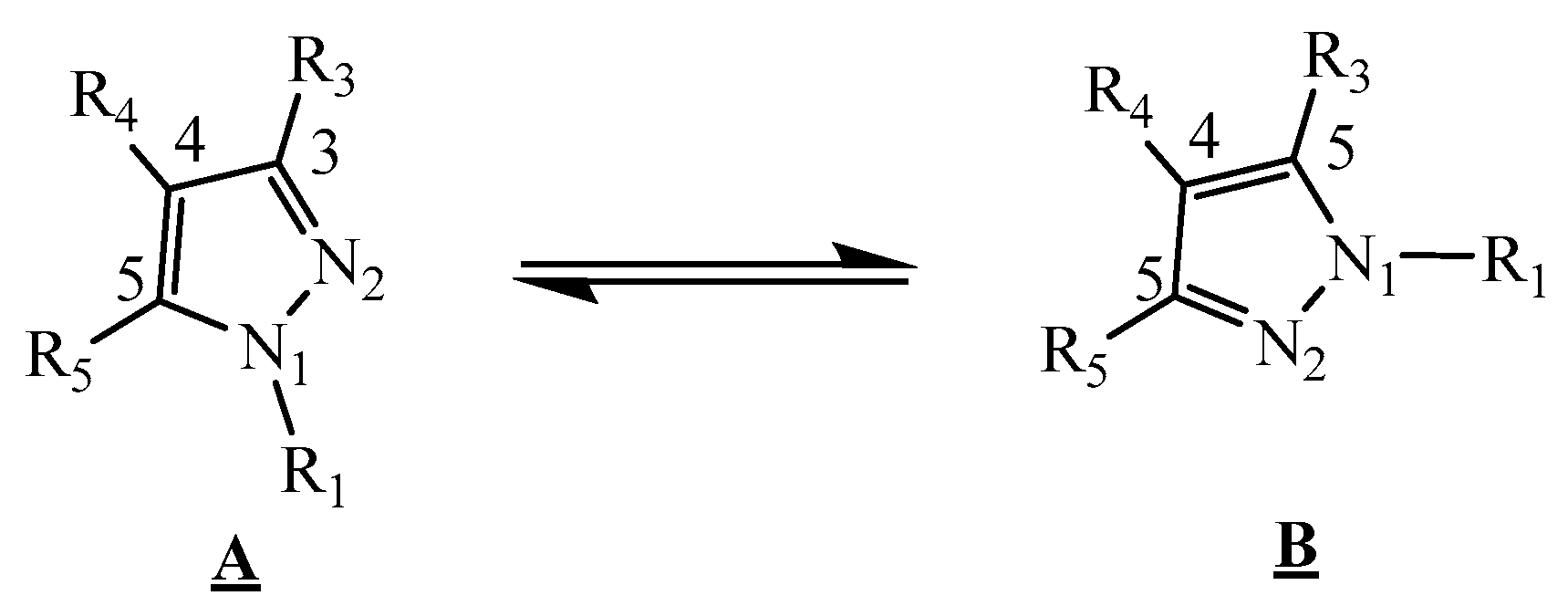
2.3. Pyrazole Reactivity
3. General Information on Tetrazoles
3.1. Biological Activities
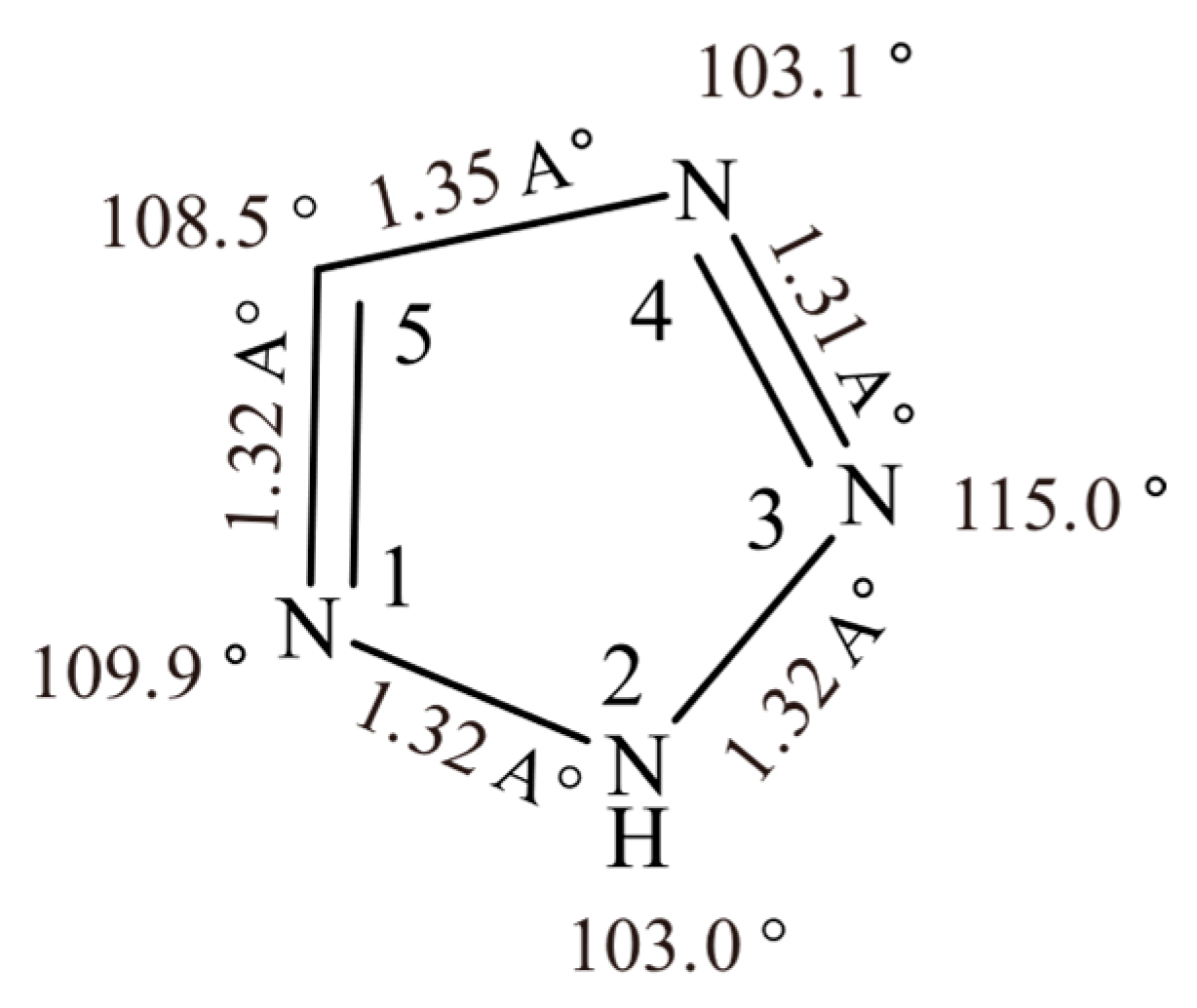
3.2. Structure and Physicochemical Properties
3.3. Tetrazole Reactivity
3.3.1. Reactions in the C5 Position
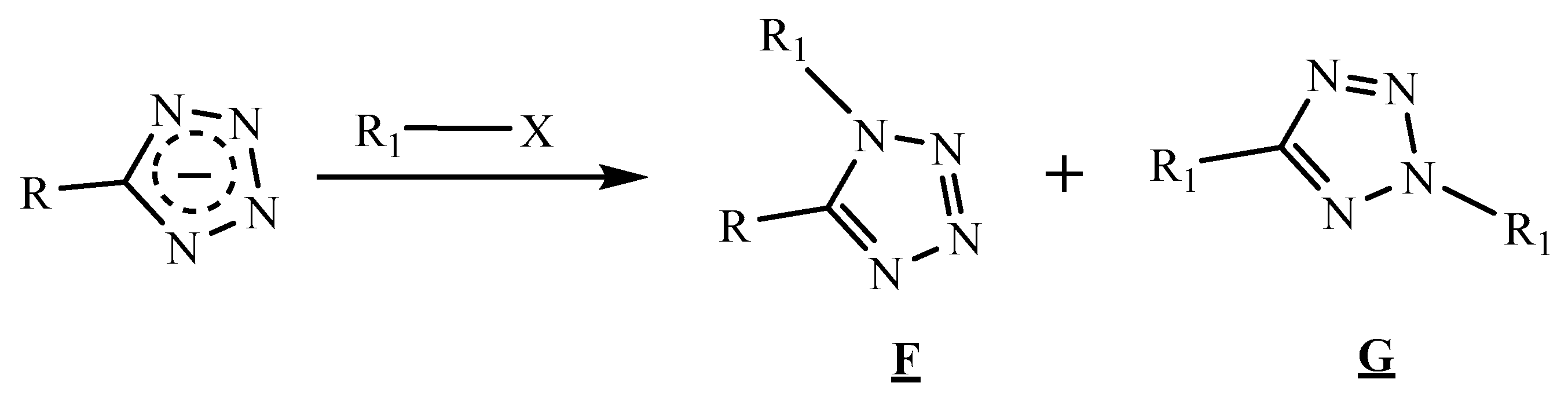
3.3.2. Reactions in the N1 and N2 Positions
4. Synthesis of Pyrazole−Tetrazole Hybrid Compounds and Their Applications
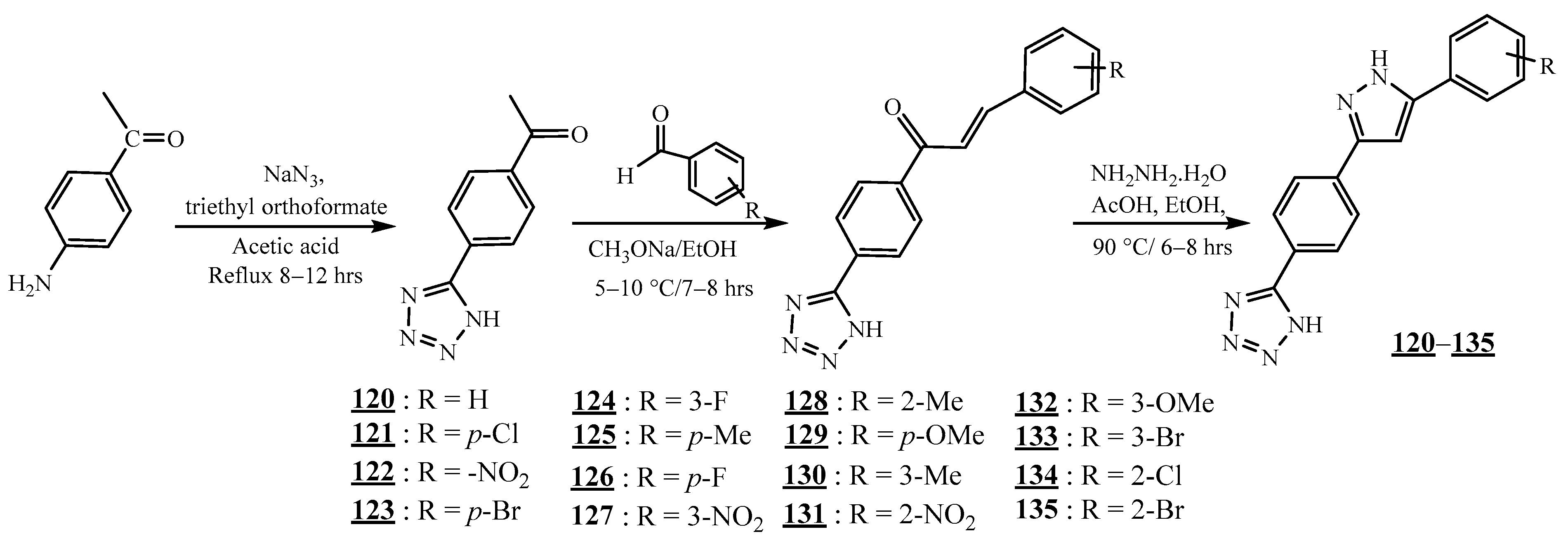
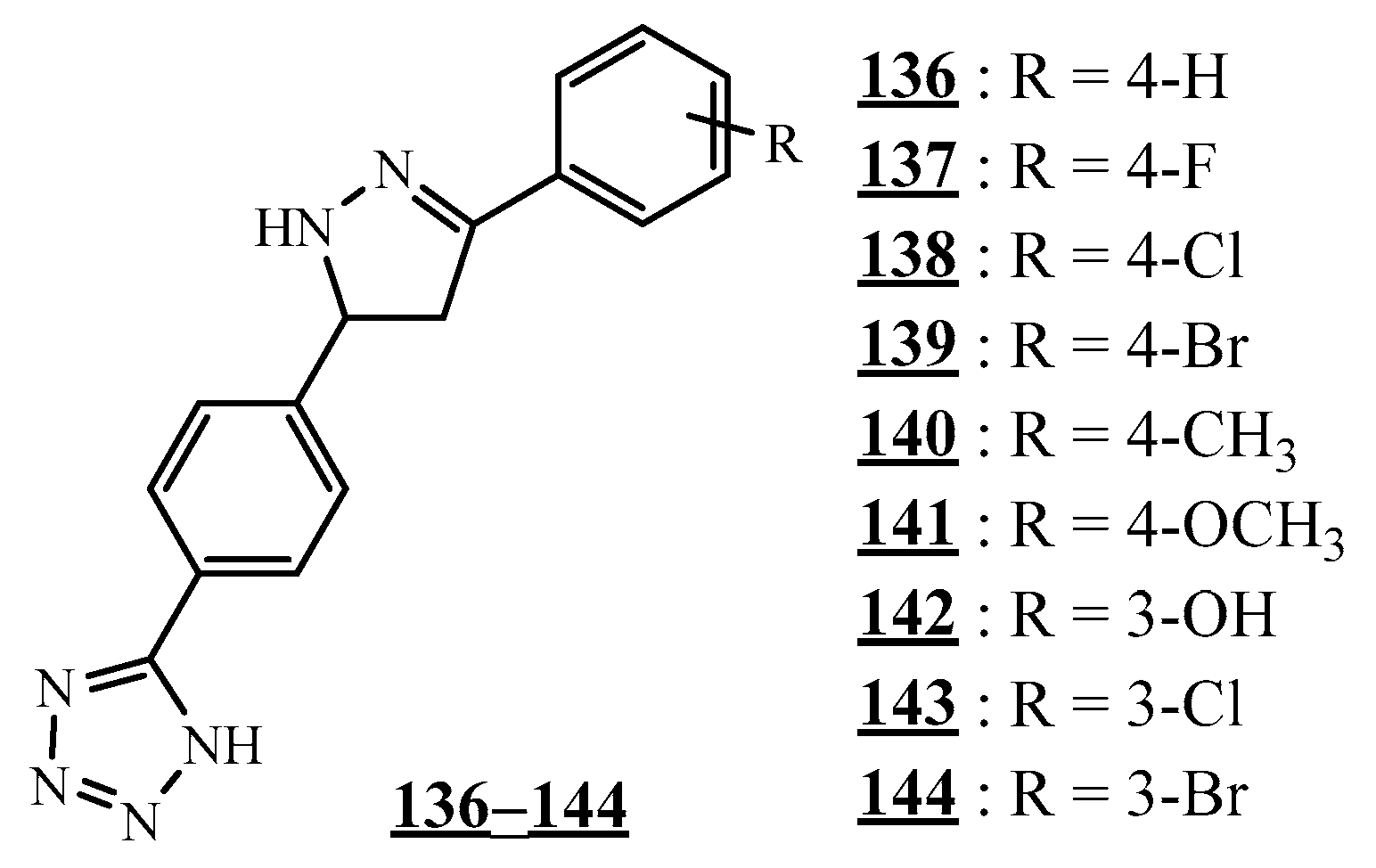
4.1. Carbon−Carbon Junction
4.2. Phenyl Junction
4.3. O-Alkyl Junction
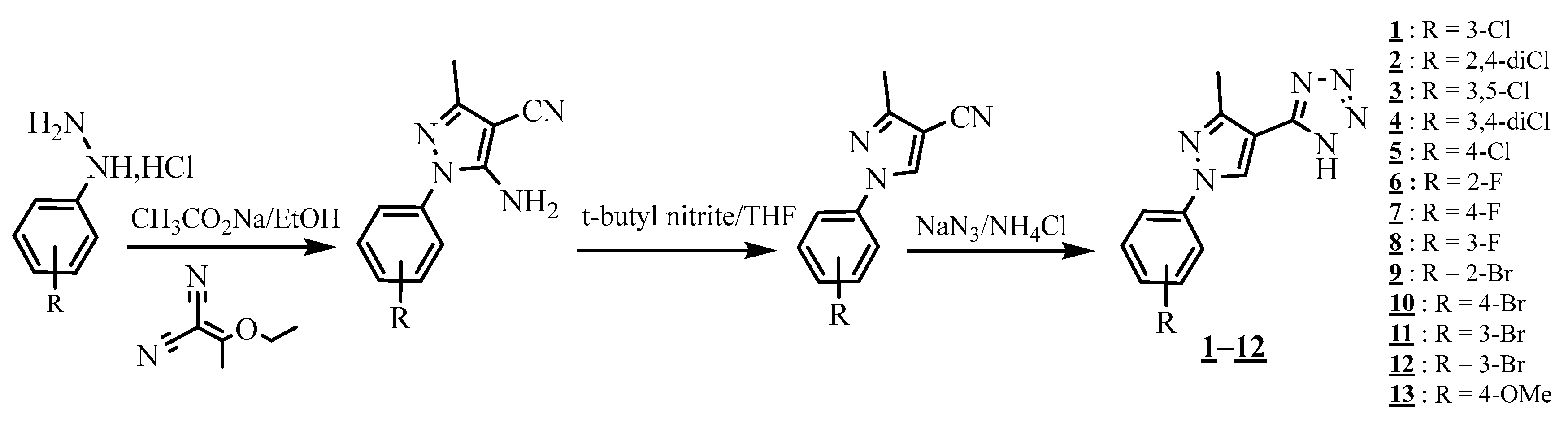
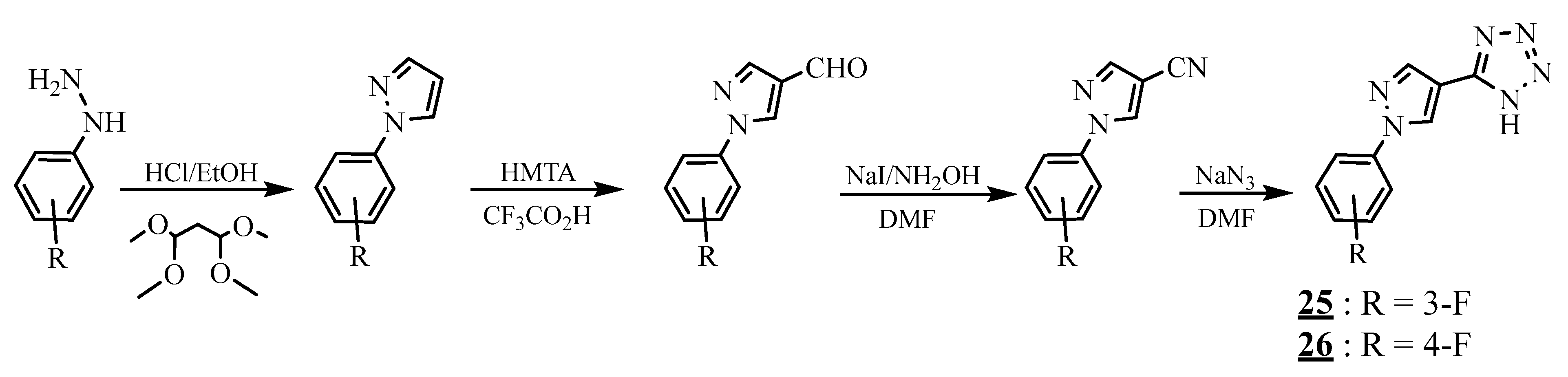
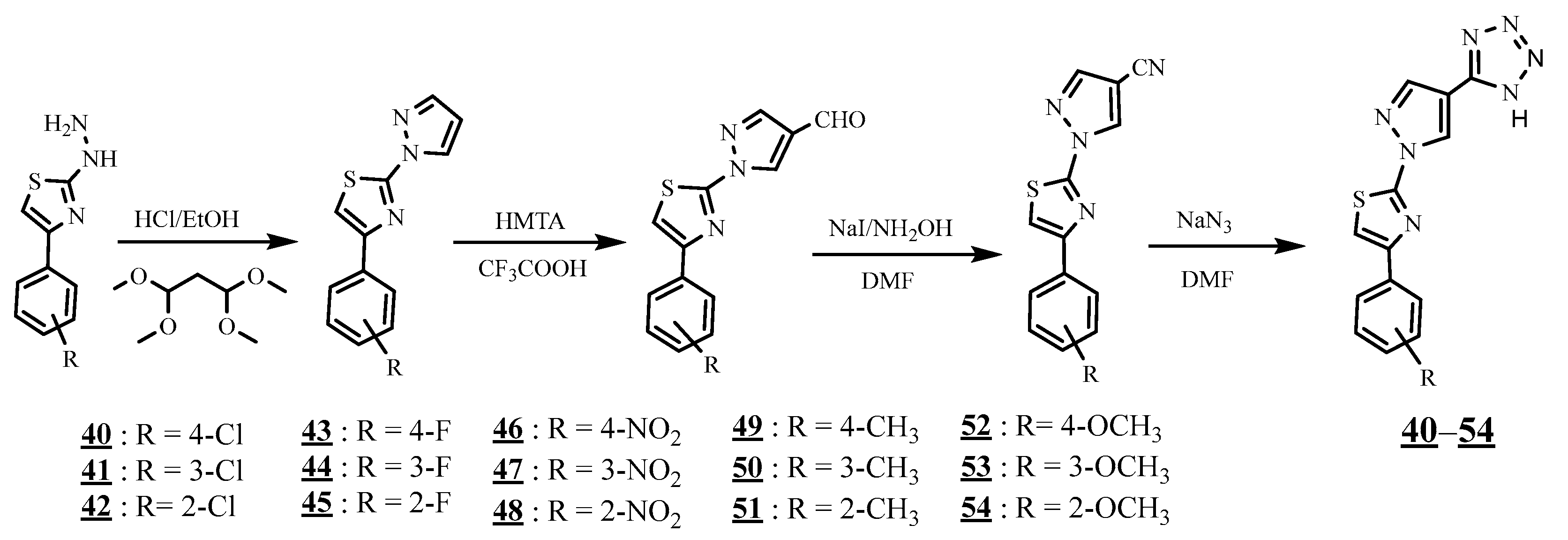
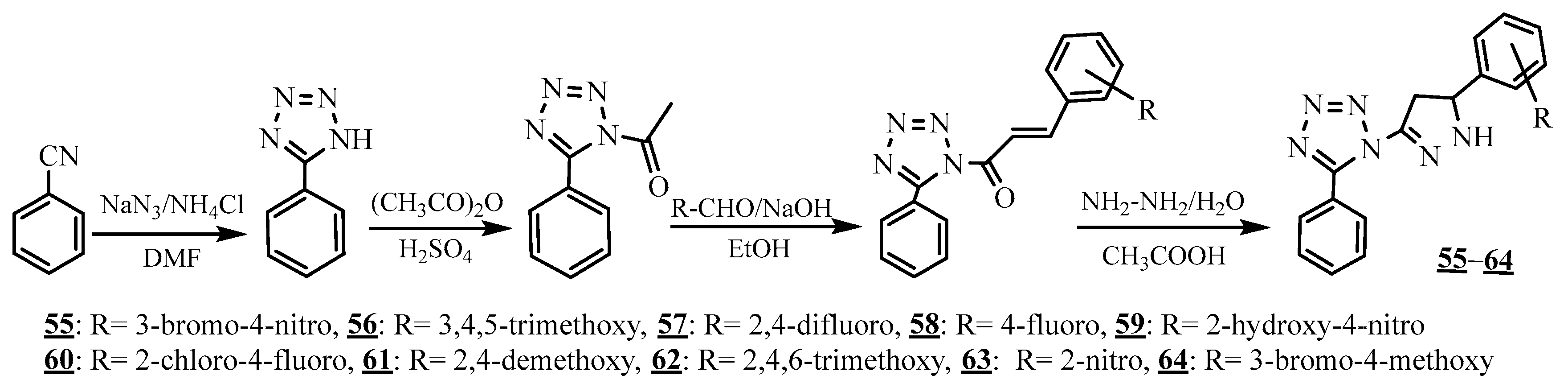
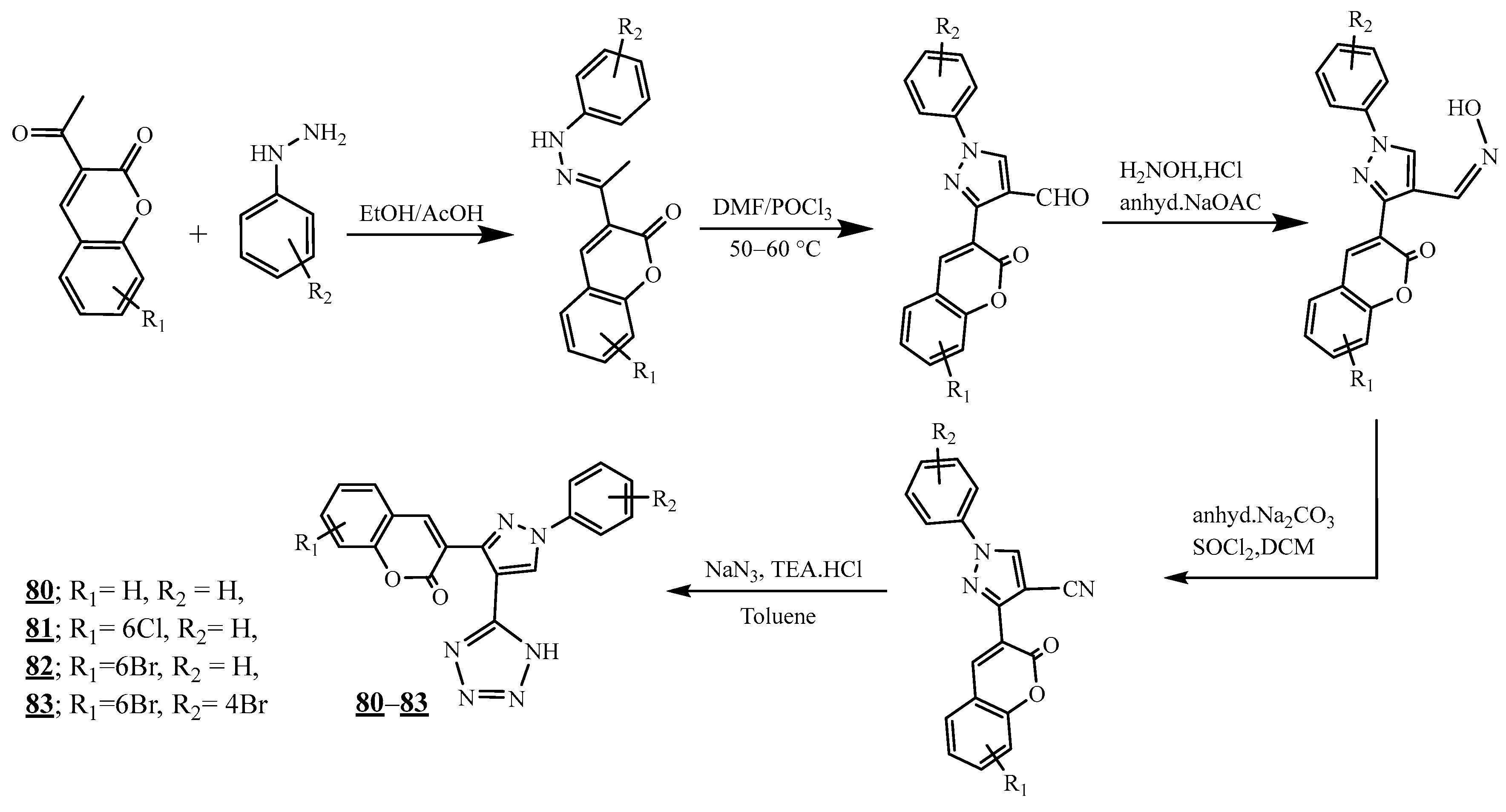
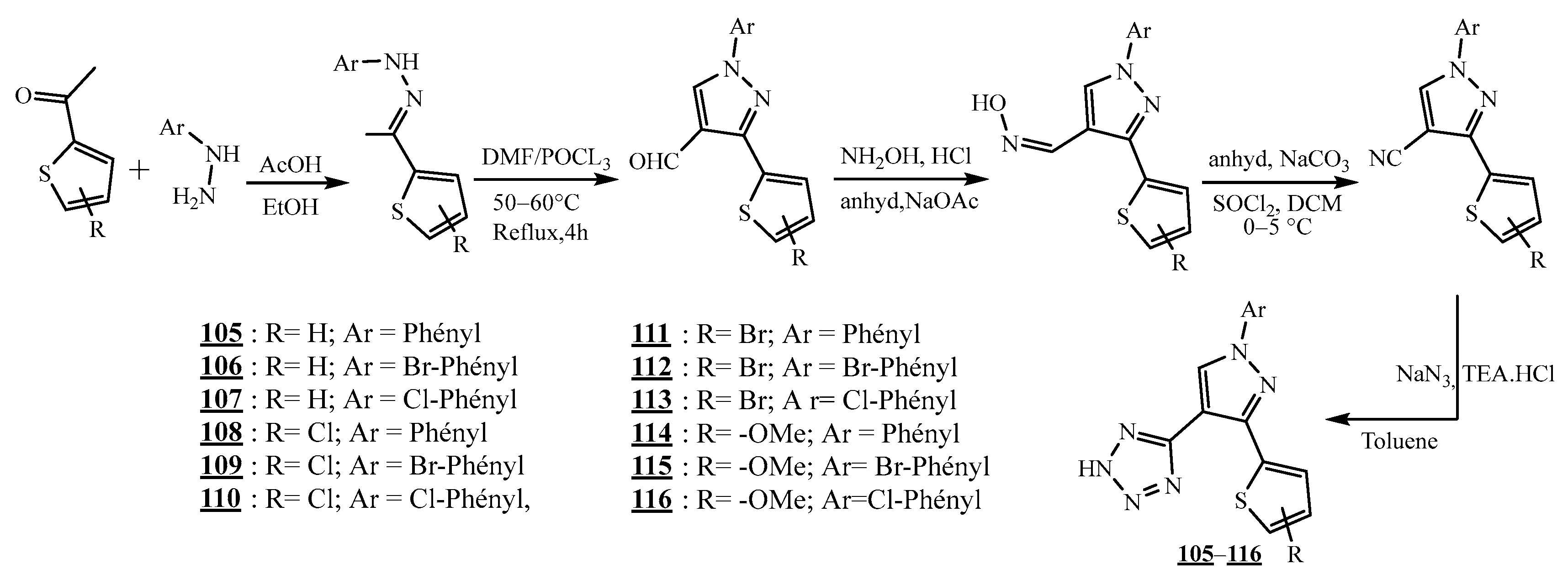
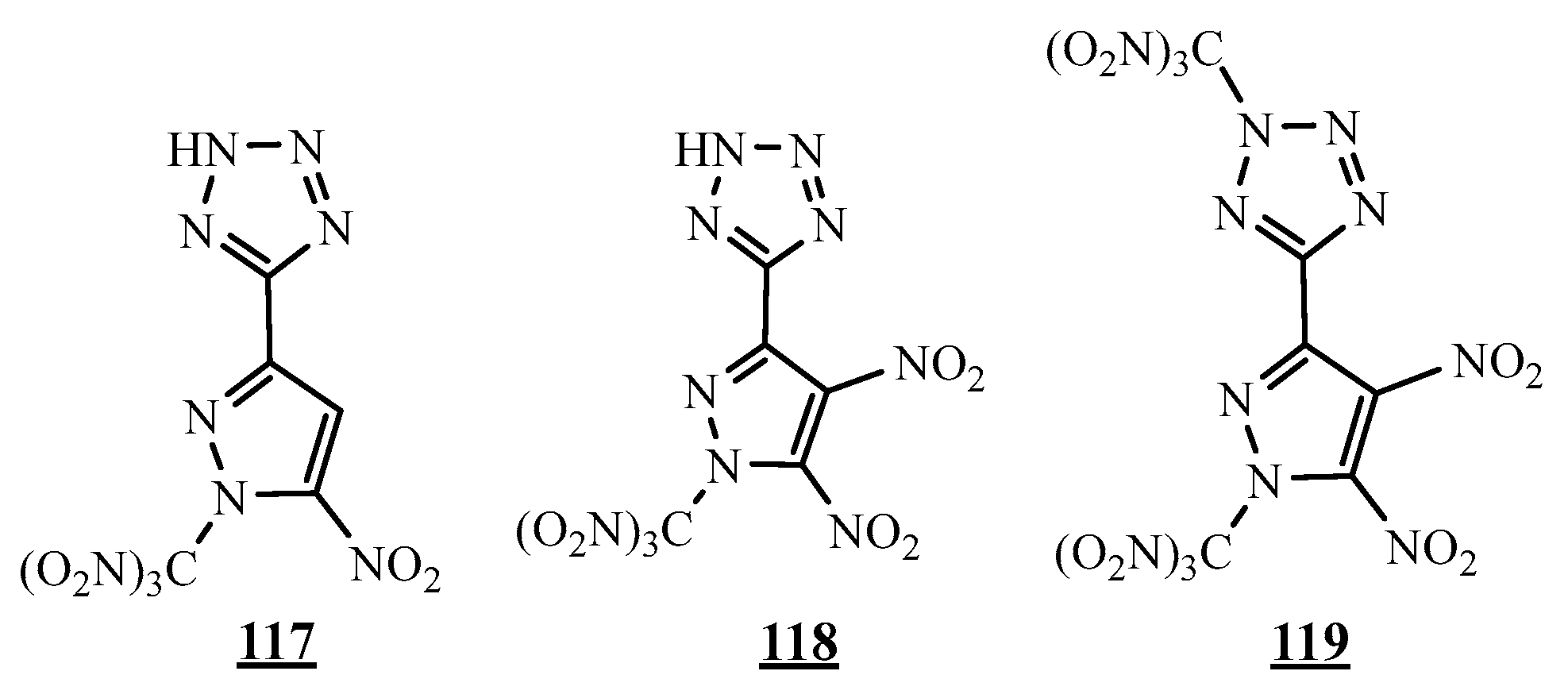
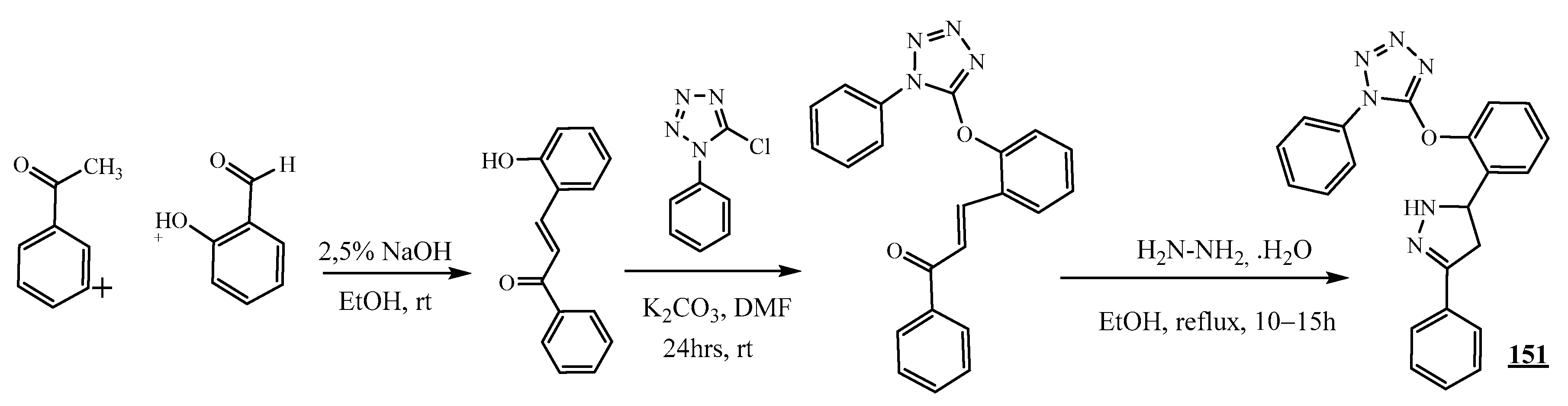
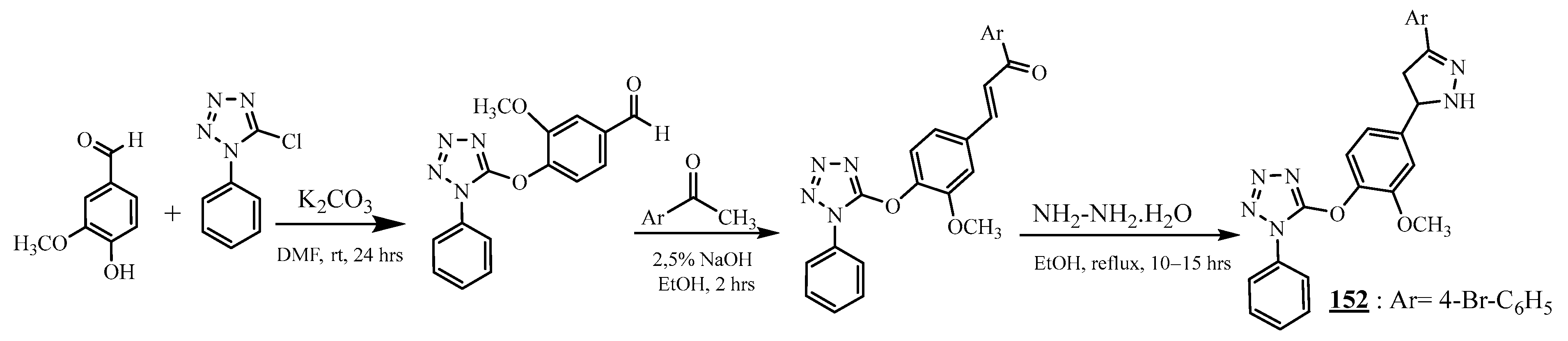
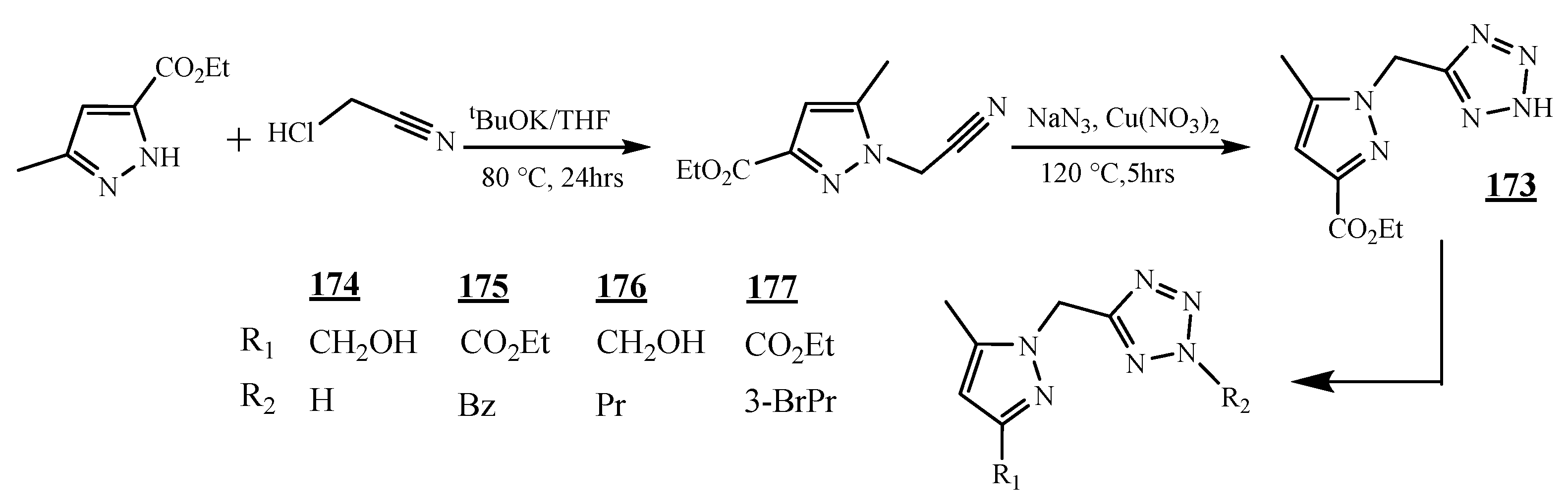
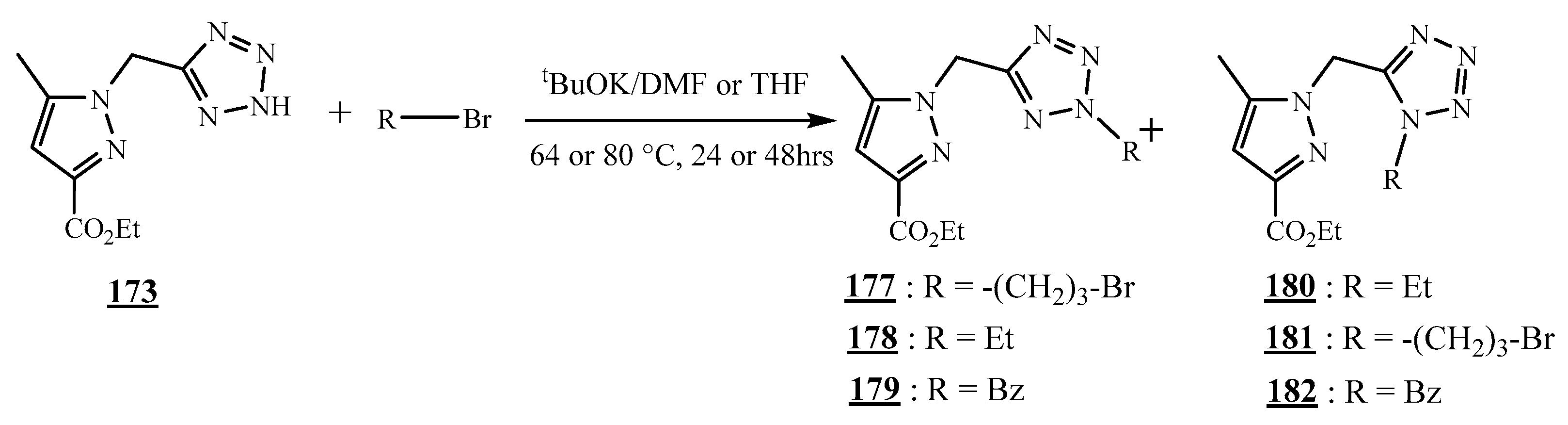
4.4. Carbon-Azote Junction
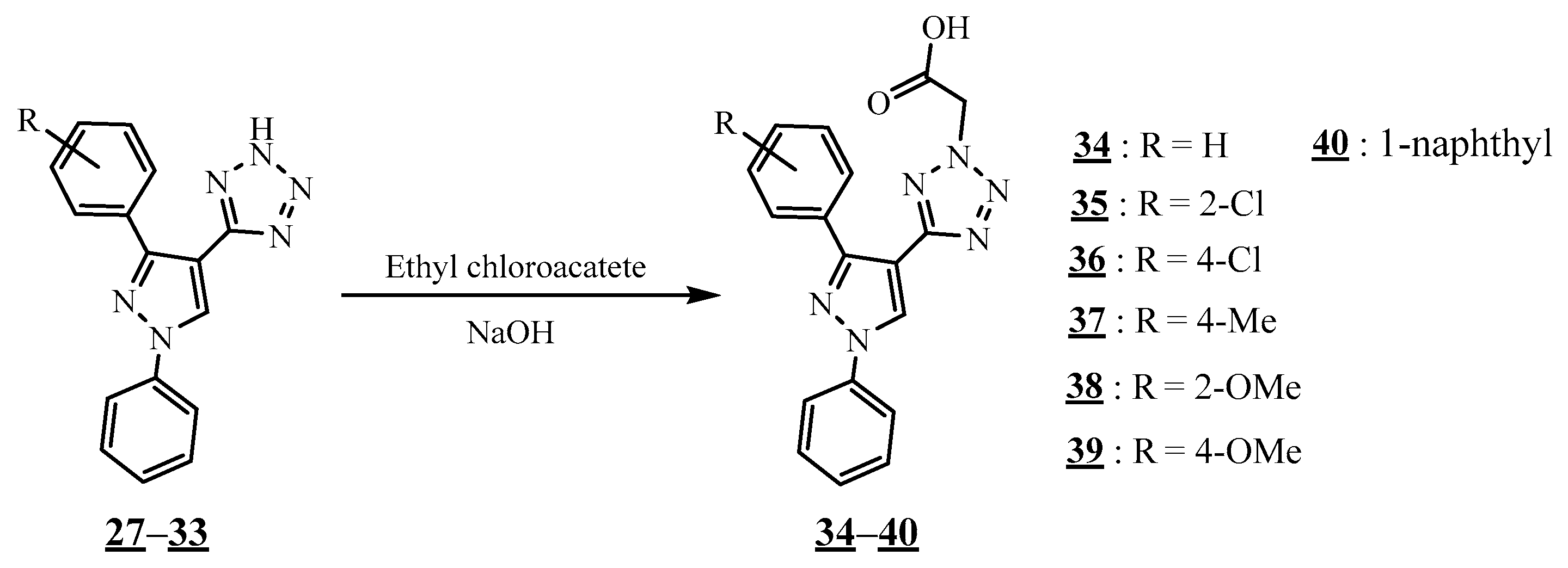
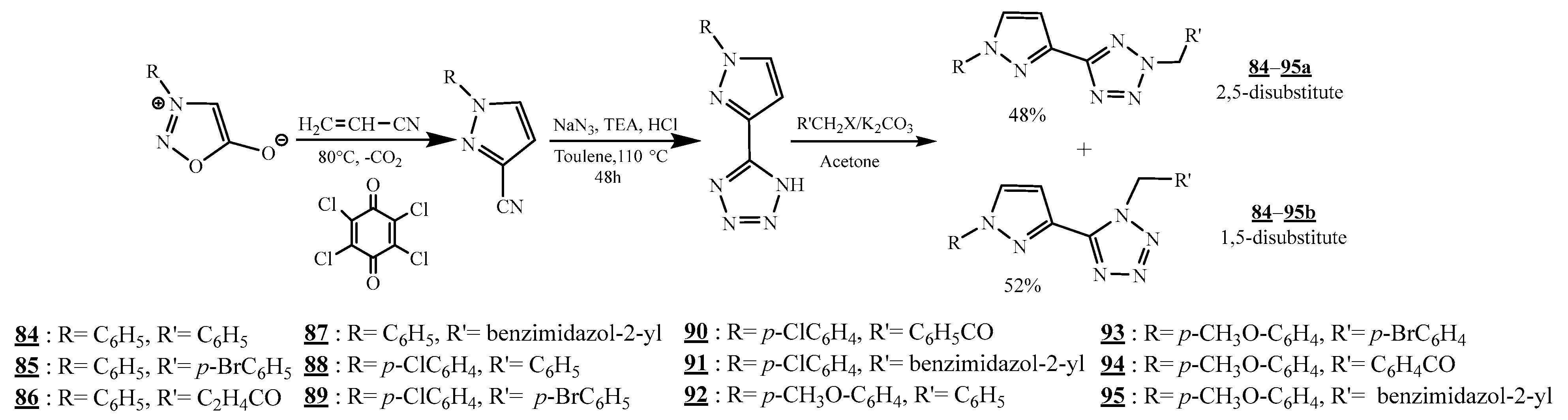
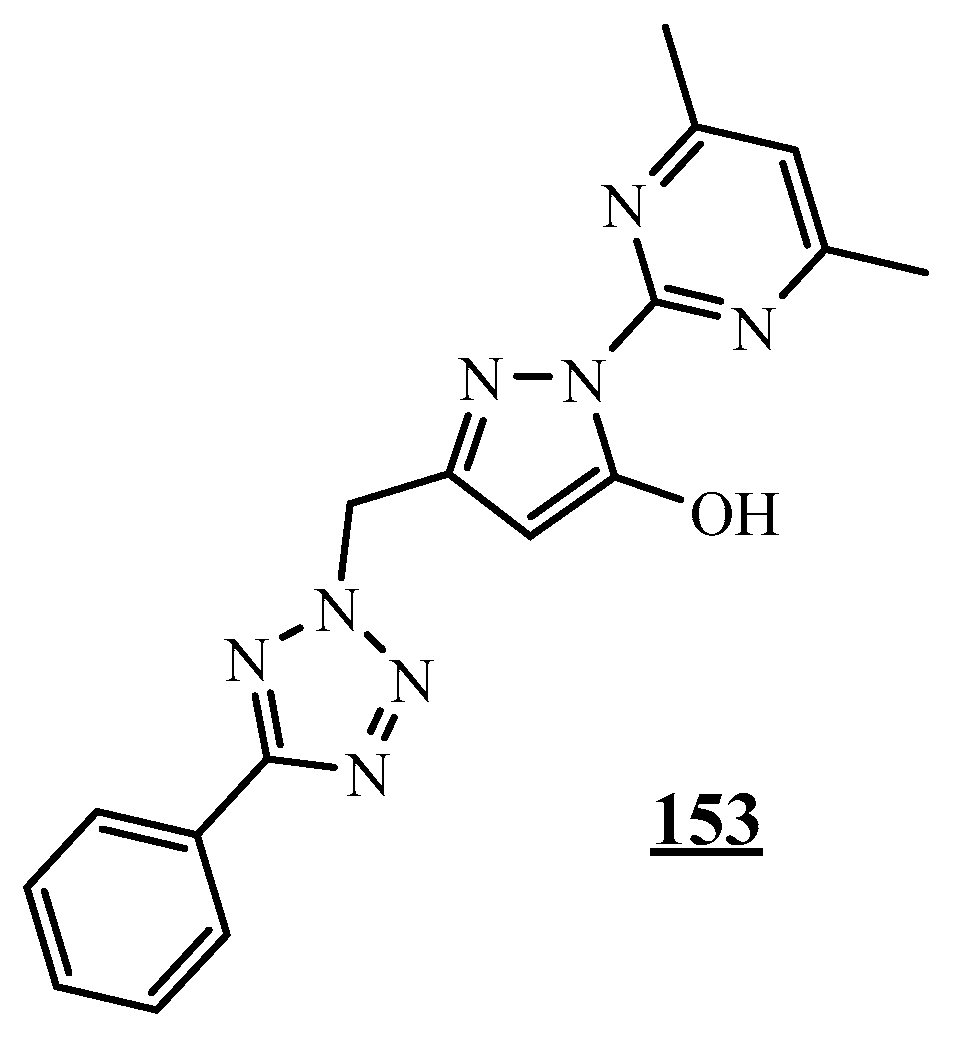
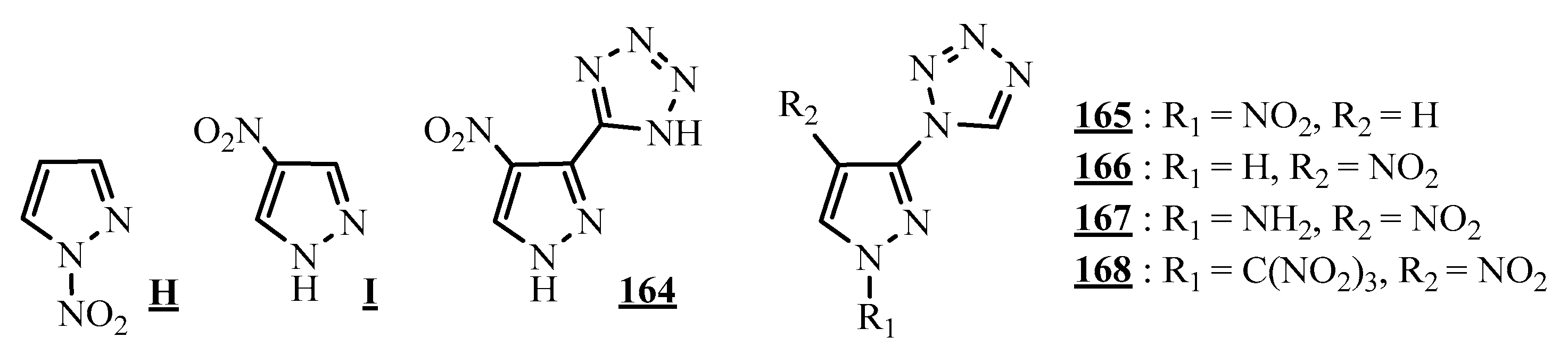
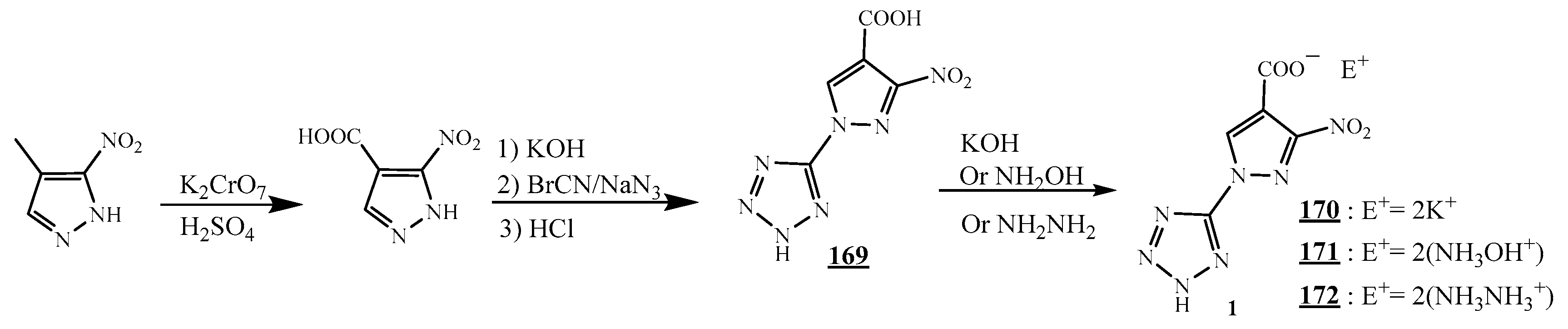
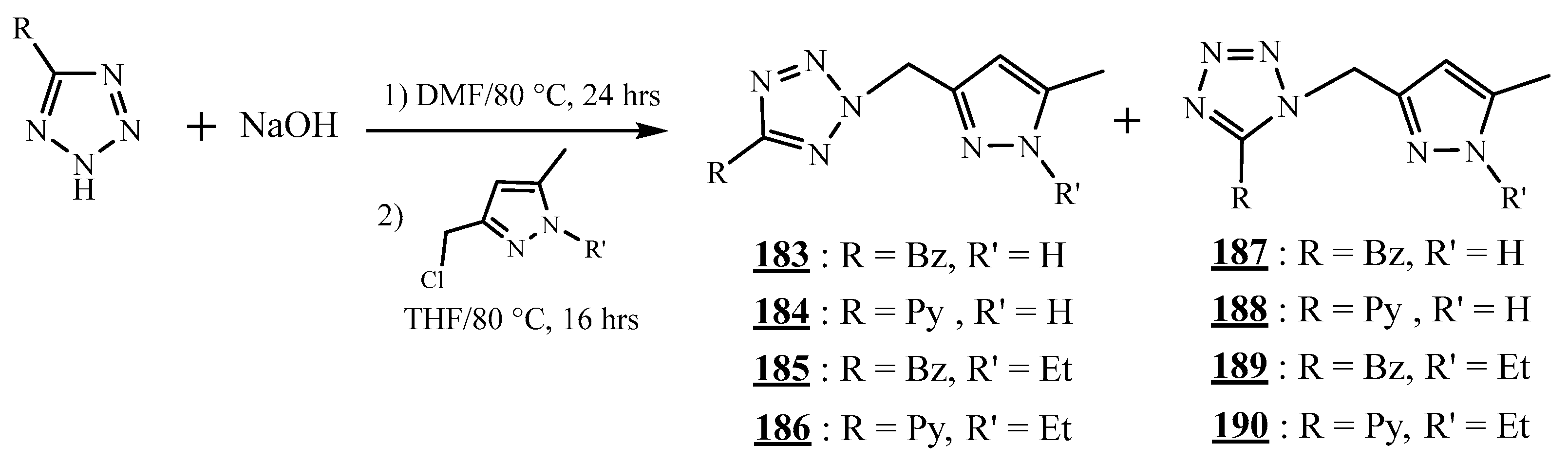
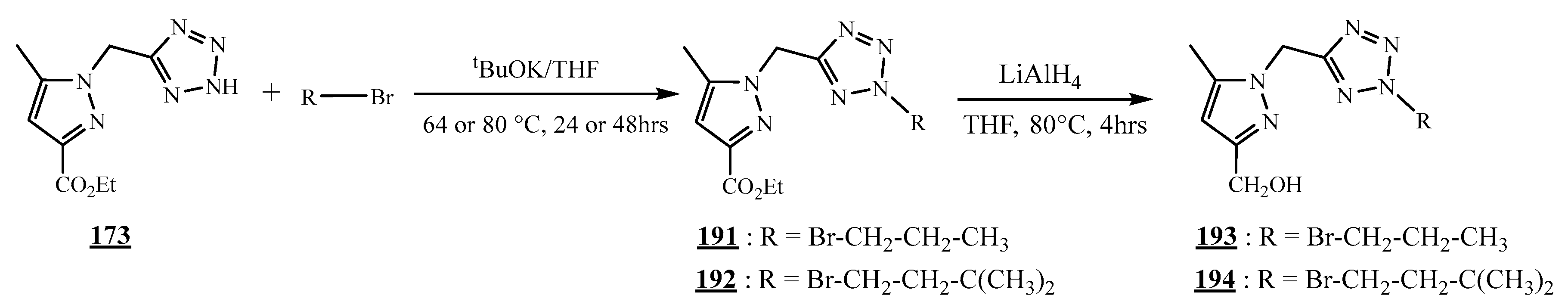
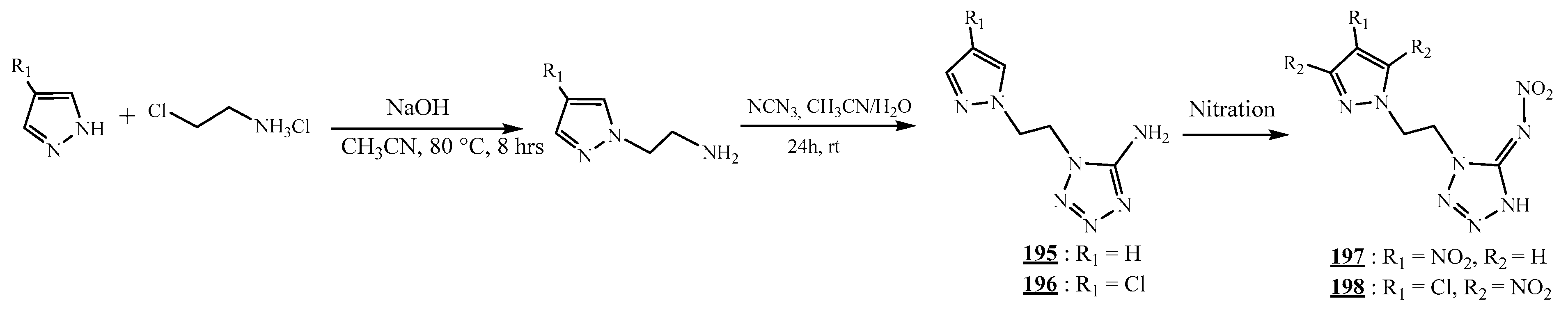
4.5. Junction Azote-Azote
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alivisatos, P.; Barbara, P.F.; Castleman, A.W.; Chang, J.; Dixon, D.A.; Klein, M.L.; McLendon, G.L.; Miller, J.S.; Ratner, M.A.; Rossky, P.J.; et al. From molecules to materials: Current trends and future directions. Adv. Mater. 1980, 10, 1297–1336. [Google Scholar] [CrossRef]
- Gupta, R.R.; Kumar, M.; Gupta, V. Heterocyclic Chemistry: Volume II: Five-Membered Heterocycles; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Dorababu, A. Pharmacology Profile of Recently Developed Multi-Functional Azoles; SAR-Based Predictive Structural Modification. ChemistrySelect 2020, 5, 6730–6758. [Google Scholar] [CrossRef]
- Umetsu, N.; Shirai, Y. Development of novel pesticides in the 21st century. J. Pestic. Sci. 2020, 45, 54–74. [Google Scholar] [CrossRef]
- Ghosh, D.; Ghosh, S.; Ghosh, A.; Pyne, P.; Majumder, S.; Hajra, A. Visible light-induced functionalization of indazole and pyrazole: A recent update. Chem. Commun. 2022, 58, 4435–4455. [Google Scholar] [CrossRef] [PubMed]
- Petrov, V.A. Fluorinated Heterocyclic Compounds: Synthesis, Chemistry, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Sadek, K.U.; Mekheimer, R.A.; Abd-Elmonem, M.; Abo-Elsoud, F.A.; Hayallah, A.M.; Mostafa, S.M.; Abdellattif, M.H.; Abourehab, M.A.S.; Farghaly, T.A.; Elkamhawy, A. Recent developments in the synthesis of hybrid heterocycles, a promising approach to develop multi-target antibacterial agents. J. Mol. Struct. 2023, 1286, 135616. [Google Scholar] [CrossRef]
- Mahmoud, E.; Hayallah, A.M.; Kovacic, S.; Abdelhamid, D.; Abdel-Aziz, M. Recent progress in biologically active indole hybrids: A mini review. Pharmacol. Rep. 2022, 74, 570–582. [Google Scholar] [CrossRef]
- Gattu, R.; Ramesh, S.S.; Nadigar, S.; Ramesh, S. Conjugation as a tool in therapeutics: Role of amino acids/peptides-bioactive (including Heterocycles) hybrid molecules in treating infectious diseases. Antibiotics 2023, 12, 532. [Google Scholar] [CrossRef]
- Araji, H.; Nakhoul, M.; Challita, E.; Barmo, N.; Wex, B. Cross-over from pyrene to acene optical and electronic properties: A theoretical investigation of a series of pyrene derivatives fused with N-, S, and O-containing heterocycles. Phys. Chem. Chem. Phys. 2024, 26, 18466–18475. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, Q.; Jean’ne, M.S. Fused heterocycle-based energetic materials (2012–2019). J. Mater. Chem. A 2020, 8, 4193–4216. [Google Scholar] [CrossRef]
- Cherfi, M.; Harit, T.; Malek, F. New macrocycles based on pyrazole-tetrazole subunit: Synthesis, characterization and their complexing properties toward heavy metal cations. J. Incl. Phenom. Macrocycl. Chem. 2023, 103, 63–70. [Google Scholar] [CrossRef]
- Sarkar, G.; Bandopadhyay, N.; Paramanik, K.; Saha, S.; Panda, S.J.; Purohit, C.S.; Biswas, B.; Das, H.S. An efficient 2-(2-Pyridyl) imidazole based copper catalyst for N-Arylation of N-heterocycles. Mol. Catal. 2023, 545, 113212. [Google Scholar] [CrossRef]
- Ebenezer, O.; Shapi, M.; Tuszynski, J.A. A review of the recent development in the synthesis and biological evaluations of pyrazole derivatives. Biomedicines 2022, 10, 1124. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, R.; Gadipelly, C.; Mannepalli, L.K. Advances in tetrazole synthesis–an overview. ChemistrySelect 2022, 7, e202200706. [Google Scholar] [CrossRef]
- Trofimenko, S. The coordination chemistry of pyrazole-derived ligands. Chem. Rev. 1972, 72, 497–509. [Google Scholar] [CrossRef]
- Bieller, S.; Haghiri, A.; Bolte, M.; Bats, J.W.; Wagner, M.; Lerner, H.-W. Transition metal complexes with pyrazole derivatives as ligands. Inorganica Chim. Acta 2006, 359, 1559–1572. [Google Scholar] [CrossRef]
- Kodadi, M.E.; Malek, F.; Touzani, R.; Ramdani, A. Synthesis of new tripodal ligand 5-(bis(3,5-dimethyl-1H-pyrazol-1-ylmethyl)amino)pentan-1-ol, catecholase activities studies of three functional tripodal pyrazolyl N-donor ligands, with different copper (II) salts. Catal. Commun. 2008, 9, 966–969. [Google Scholar] [CrossRef]
- Bouabdallah, I.; Touzani, R.; Zidane, I.; Ramdani, A. Effect of Two Isomeric Tetrapyrazolyl Ligands on the Catalytic Oxidation of 3,5-ditert-Butylcatechol. J. Iran. Chem. Soc. 2007, 3, 299–303. [Google Scholar] [CrossRef]
- Yan, W.; Wang, L.; Qi, H.; Zhan, G.; Fang, P.; Liu, Z.; Bian, Z. Highly Efficient Heteroleptic Cerium (III) Complexes with a Substituted Pyrazole Ancillary Ligand and Their Application in Blue Organic Light-Emitting Diodes. J. Inorg. Chem. 2021, 60, 18103–18111. [Google Scholar] [CrossRef]
- Harit, T.; Malek, F. New polymeric membrane incorporating a tetrapyrazolic macrocycle for the selective transport of cesium cation. Sep. Purif. Technol. 2017, 176, 8–14. [Google Scholar] [CrossRef]
- Harit, T.; Malek, F.; El Bali, B.; Dusek, M.; Kucerakova, M. Synthesis and characterization of two new tetrapyrazolic macrocycles for the selective extraction of cesium cation. Tetrahedron 2016, 72, 3966–3973. [Google Scholar] [CrossRef]
- Harit, T.; Ameduri, B.; Malek, F. Synthesis and characterization of new fluorinated copolymers based on azole groups for fuel cell membranes. Solid State Ion. 2018, 317, 108–114. [Google Scholar] [CrossRef]
- Harit, T.; Malek, F.; Ameduri, B. Fluorinated polymers based on pyrazole groups for fuel cell membranes. Eur. Polym. J. 2016, 79, 72–81. [Google Scholar] [CrossRef]
- Kumar, V.; Kaur, K.; Gupta, G.K.; Sharma, A.K. Pyrazole containing natural products: Synthetic preview and biological significance. Eur. J. Med. Chem. 2013, 69, 735–753. [Google Scholar] [CrossRef]
- Elguero, J.; Katritzky, A.R.; Denisko, O.V. Prototropic tautomerism of heterocycles: Heteroaromatic tautomerism-General overview and methodology. Adv. Heterocycl. Chem. 2000, 76, 1–84. [Google Scholar]
- Li, G.; Cheng, Y.; Han, C.; Song, C.; Huang, N.; Du, Y. Pyrazole-containing pharmaceuticals: Target, pharmacological activity, and their SAR studies. RSC Med. Chem. 2022, 13, 1300–1321. [Google Scholar] [CrossRef] [PubMed]
- Katritzky, A.R.; Jug, K.; Oniciu, D.C. Quantitative Measures of Aromaticity for Mono-, Bi-, and Tricyclic Penta- and Hexaatomic Heteroaromatic Ring Systems and Their Interrelationships. Chem. Rev. 2001, 101, 1421–1450. [Google Scholar] [CrossRef]
- Dhiman, N.; Kaur, K.; Jaitak, V. Tetrazoles as anticancer agents: A review on synthetic strategies, mechanism of action and SAR studies. Bioorg Med. Chem. 2020, 28, 115599. [Google Scholar] [CrossRef]
- Pandey, S.; Agarwal, P.; Srivastava, K.; Rajakumar, S.; Puri, S.K.; Verma, P.; Saxena, J.K.; Sharma, A.; Lal, J.; Chauhan, P.M. Synthesis and bioevaluation of novel 4-aminoquinoline-tetrazole derivatives as potent antimalarial agents. Eur. J. Med. Chem. 2013, 66, 69–81. [Google Scholar] [CrossRef]
- Gao, C.; Chang, L.; Xu, Z.; Yan, X.F.; Ding, C.; Zhao, F.; Wu, X.; Feng, L.S. Recent advances of tetrazole derivatives as potential anti-tubercular and anti-malarial agents. Eur. J. Med. Chem. 2019, 163, 404–412. [Google Scholar] [CrossRef]
- Wang, S.Q.; Wang, Y.F.; Xu, Z. Tetrazole hybrids and their antifungal activities. Eur. J. Med. Chem. 2019, 170, 225–234. [Google Scholar] [CrossRef]
- He, X.Y.; Zou, P.; Qiu, J.; Hou, L.; Jiang, S.; Liu, S.; Xie, L. Design, synthesis and biological evaluation of 3-substituted 2,5-dimethyl-N-(3-(1H-tetrazol-5-yl)phenyl)pyrroles as novel potential HIV-1 gp41 inhibitors. Bioorg Med. Chem. 2011, 19, 6726–6734. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Xiao, J.; Huang, G. Current scenario of tetrazole hybrids for antibacterial activity. Eur. J. Med. Chem. 2019, 184, 111744. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.-M.; Tang, M.-H.; Yang, G.-L.; Zhao, B. Cluster/cage-based coordination polymers with tetrazole derivatives. Coord. Chem. Rev. 2020, 422, 213424. [Google Scholar] [CrossRef]
- Li, Y.T.; Yao, W.Q.; Zhou, S.; Xu, J.X.; Lu, H.; Lin, J.; Hu, X.Y.; Zhang, S.K. Synthesis, fungicidal activity, and 3D-QSAR of tetrazole derivatives containing phenyloxadiazole moieties. Bioorg Med. Chem. Lett. 2021, 34, 127762. [Google Scholar] [CrossRef]
- Soylak, M.; Topalak, Z. Enrichment-separation and determinations of cadmium(II) and lead(II)-1-phenyl-1H-tetrazole-5-thiol chelates on Diaion SP-207 by solid phase extraction-flame atomic absorption spectrometry. Arab. J. Chem. 2015, 8, 720–725. [Google Scholar] [CrossRef]
- Wang, J.; Deng, S.Q.; Zhao, T.T.; Zheng, S.R.; Cai, S.L.; Fan, J.; Zhang, W.G. A Mn(II)-MOF with inherent missing metal-ion defects based on an imidazole-tetrazole tripodal ligand and its application in supercapacitors. Dalton Trans. 2020, 49, 12150–12155. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Nezafat, Z.; Bidgoli, N.S.S.; Shafiei, N. Use of tetrazoles in catalysis and energetic applications: Recent developments. Mol. Catal. 2021, 513, 111788. [Google Scholar] [CrossRef]
- Krishnan, N.N.; Duong, N.M.H.; Konovalova, A.; Jang, J.H.; Park, H.S.; Kim, H.J.; Roznowska, A.; Michalak, A.; Henkensmeier, D. Polybenzimidazole / tetrazole-modified poly(arylene ether) blend membranes for high temperature proton exchange membrane fuel cells. J. Membr. Sci. 2020, 614, 118494. [Google Scholar] [CrossRef]
- Ostrovskii, V.A.; Koldobskii, G.; Trifonov, R.E. Tetrazoles. In Comprehensive Heterocyclic Chemistry III; Elsevier Science: Amsterdam, The Netherlands, 2008; pp. 257–423. [Google Scholar]
- Trifonov, R.E.; Ostrovskii, V.A. Protolytic equilibria in tetrazoles. Russ. J. Org. Chem. 2006, 42, 1585–1605. [Google Scholar] [CrossRef]
- Yi, K.Y.; Yoo, S.E. Synthesis of 5-aryl and vinyl tetrazoles by the palladium-catalyzed cross-coupling reaction. Tetrahedron Lett. 1995, 36, 1679–1682. [Google Scholar] [CrossRef]
- Koldobskii, G.I.; Kharbash, R.B. 2-Substituted and 2,5-Disubstituted Tetrazoles. Russ. J. Org. Chem. 2003, 39, 453–470. [Google Scholar] [CrossRef]
- Faria, J.V.; Santos, M.S.D.; Bernardino, A.M.; Becker, K.M.; Machado, G.M.; Rodrigues, R.F.; Canto-Cavalheiro, M.M.; Leon, L.L. Synthesis and activity of novel tetrazole compounds and their pyrazole-4-carbonitrile precursors against Leishmania spp. Bioorg. Med. Chem. Lett. 2013, 23, 6310–6312. [Google Scholar] [CrossRef] [PubMed]
- Faioes, V.D.S.; Leon, L.L.; Canto-Cavalheiro, M.M.; Torres-Santos, E.C.; Bernardino, A.M.; Vegi, P.F.; Santos, M.S.D. Effectiveness of novel 5-(5-amino-1-aryl-1H-pyrazol-4-yl)-1H-tetrazole derivatives against promastigotes and amastigotes of Leishmania amazonensis. Chem. Biol. Drug Des. 2014, 83, 272–277. [Google Scholar] [CrossRef]
- De Oliveira, C.H.A.; Mairink, L.M.; Pazini, F.; Lião, L.M.; De Oliveira, A.L.; Viegas, C.; De Oliveira, V.; Cunha, L.C.; Oliveira, F.G.F.; Paz, J.L.; et al. Chemoselective and Regiospecific Formylation of 1-Phenyl-1H-pyrazoles Through the Duff Reaction. Synth. Commun. 2013, 43, 1633–1639. [Google Scholar] [CrossRef]
- Martins, D.R.; Pazini, F.; De Medeiros Alves, V.; De Moura, S.S.; Lião, L.M.; De Magalhães, M.T.Q.; Valadares, M.C.; Andrade, C.H.; Menegatti, R.; Rocha, M.L. Synthesis, docking studies, pharmacological activity and toxicity of a novel Pyrazole derivative (LQFM 021)—Possible effects on phosphodiesterase. Chem. Pharm. Bull. 2013, 61, 524–531. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, L.P.; Da Silva, D.P.; Florentino, I.F.; Fajemiroye, J.O.; De Oliveira, T.S.; Marcelino, R.I.; Pazini, F.; Liao, L.M.; Ghedini, P.C.; De Moura, S.S.; et al. New pyrazole derivative 5-[1-(4-fluorophenyl)-1H-pyrazol-4-yl]-2H-tetrazole: Synthesis and assessment of some biological activities. Chem. Biol. Drug Des. 2017, 89, 124–135. [Google Scholar] [CrossRef]
- Florentino, I.F.; Galdino, P.M.; De Oliveira, L.P.; Silva, D.P.; Pazini, F.; Vanderlinde, F.A.; Liao, L.M.; Menegatti, R.; Costa, E.A. Involvement of the NO/cGMP/KATP pathway in the antinociceptive effect of the new pyrazole 5-(1-(3-fluorophenyl)-1H-pyrazol-4-yl)-2H-tetrazole (LQFM-021). Nitric Oxide 2015, 47, 17–24. [Google Scholar] [CrossRef]
- Ravula, P.; Vamaraju, H.B.; Paturi, M.; Jn, N.S.C.; Kolli, S. Design, synthesis, in silico toxicity prediction, molecular docking, and evaluation of novel pyrazole derivatives as potential antiproliferative agents. EXCLI J. 2016, 15, 187–202. [Google Scholar]
- Mason, D.T.; Foerster, J.M. Side Effects and Intoxication of Cardiac Glycosides: Manifestations and Treatment. In Cardiac Glycosides Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1981; pp. 275–297. [Google Scholar]
- Hauptman, P.J.; Kelly, R.A. Digitalis. Circulation 1999, 99, 1265–1270. [Google Scholar] [CrossRef]
- Duan, L.M.; Yu, H.Y.; Li, Y.L.; Jia, C.J. Design and discovery of 2-(4-(1H-tetrazol-5-yl)-1H-pyrazol-1-yl)-4-(4-phenyl)thiazole derivatives as cardiotonic agents via inhibition of PDE3. Bioorg Med. Chem. 2015, 23, 6111–6117. [Google Scholar] [CrossRef]
- Kaushik, N.; Kumar, N.; Kumar, A. Synthesis, Antioxidant and Antidiabetic Activity of 1-[(5-Substituted phenyl)-4,5-dihydro-1H-pyrazol-3-yl]-5-phenyl-1H-tetrazole. Indian J. Pharm. Sci. 2016, 78, 352–359. [Google Scholar] [CrossRef]
- Mao, Z.W.; Li, Q.L.; Wang, P.; Sun, Y.L.; Liu, Y. Design and Development of Novel 4-(4-(1H-Tetrazol-5-yl)-1H-pyrazol-1-yl)-6-morpholino-N-(4-nitrophenyl)-1,3,5-triazin-2-amine as Cardiotonic Agent via Inhibition of PDE3. Arch Pharm 2016, 349, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Kumbar, M.N.; Sannaikar, M.S.; Shaikh, S.K.J.; Kamble, A.A.; Wari, M.N.; Inamdar, S.R.; Qiao, Q.; Revanna, B.N.; Madegowda, M.; Dasappa, J.P.; et al. Synthesis, Photophysical and Computational Study of Novel Coumarin-based Organic Dyes. Photochem. Photobiol. 2018, 94, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Kattimani, P.P.; Somagond, S.M.; Bayannavar, P.K.; Kamble, R.R.; Bijjaragi, S.C.; Hunnur, R.K.; Joshi, S.D. Novel 5-(1-aryl-1H-pyrazol-3-yl)-1H-tetrazoles as glycogen phosphorylase inhibitors: An in vivo antihyperglycemic activity study. Drug Dev. Res. 2019, 81, 70–84. [Google Scholar] [CrossRef]
- Kushwaha, P.; Fatima, S.; Upadhyay, A.; Gupta, S.; Bhagwati, S.; Baghel, T.; Siddiqi, M.I.; Nazir, A.; Sashidhara, K.V. Synthesis, biological evaluation and molecular dynamic simulations of novel Benzofuran-tetrazole derivatives as potential agents against Alzheimer’s disease. Bioorg Med. Chem. Lett. 2019, 29, 66–72. [Google Scholar] [CrossRef]
- Kumbar, M.N.; Kamble, R.R.; Dasappa, J.P.; Bayannavar, P.K.; Khamees, H.A.; Mahendra, M.; Joshi, S.D.; Dodamani, S.; Rasal, V.P.; Jalalpure, S. 5-(1-Aryl-3-(thiophen-2-yl)-1H-pyrazol-4-yl)-1H-tetrazoles: Synthesis, structural characterization, Hirshfeld analysis, anti-inflammatory and anti-bacterial studies. J. Mol. Struct. 2018, 1160, 63–72. [Google Scholar] [CrossRef]
- Lempert, D.; Kazakov, A.; Soglasnova, S.; Dalinger, I.; Sheremetev, A.B. Energetic abilities of nitro derivatives of isomeric (pyrazol-3-yl)tetrazoles as components of solid composite propellants. Russ. Chem. Bull. 2018, 67, 1580–1588. [Google Scholar] [CrossRef]
- Dhevaraj, J.; Gopalakrishnan, M.; Pazhamalai, S. Synthesis, characterization, molecular docking, ADME and biological evaluation of 3-(4-(tetrazol-1-yl)phenyl)-5-phenyl-1H-pyrazoles. J. Mol. Struct. 2019, 1193, 450–467. [Google Scholar] [CrossRef]
- Ashok, D.; Nagaraju, N.; Lakshmi, B.V.; Sarasija, M. Microwave Assisted Synthesis of 5-[4-(3-Phenyl-4,5-dihydro-1H-pyrazol-5-yl)phenyl]-1H-tetrazole Derivatives and Their Antimicrobial Activity. Russ. J. Gen. Chem. 2019, 89, 1905–1910. [Google Scholar] [CrossRef]
- Dofe, V.S.; Sarkate, A.P.; Shaikh, Z.M.; Gill, C.H. Ultrasound-assisted synthesis and antimicrobial activity of tetrazole-based pyrazole and pyrimidine derivatives. Heterocycl. Commun. 2018, 24, 59–65. [Google Scholar] [CrossRef]
- Elmonaem, H.S.A.; Abdel-Aziz, N.I.; El-Ashmawy, M.B.; Morsy, M.A.; Moustafa, M.A. Synthesis, In Vitro Antiproliferative Evaluation and Molecular Docking of New tetrazole-chalcone and tetrazole-pyrazoline Hybrids. J. Appl. Pharm. Sci. 2018, 8, 75–87. [Google Scholar]
- Nesterova, O.M.; Zarubina, O.S.; Tolstyakov, V.V.; Danagulyan, G.; Trifonov, R.; Smirnov, S.; Slepukhin, P.; Ignatenko, N.K.; Ostrovskii, V. Synthesis and structure of N-(4,6-dimethylpyrimidin-2-yl)-2-(5-phenyl-2H-tetrazol-2-yl)acetohydrazide and 1-(4,6-dimethylpyrimidin-2-yl)-3-[(5-phenyl-2H-tetrazol-2-yl)methyl]-1H-pyrazol-5-ol. Russ. J. Org. Chem. 2017, 53, 1766–1768. [Google Scholar] [CrossRef]
- Vatsadze, I.A.; Serushkina, O.V.; Dutov, M.D.; Shkineva, T.K.; Suponitsky, K.Y.; Ugrak, B.I.; Dalinger, I.L. Synthesis of 1-(N-nitropyrazolyl)-1H-tetrazoles—A new type of heteronuclear N-nitropyrazole derivatives. Chem. Heterocycl. Compd. 2015, 51, 695–703. [Google Scholar] [CrossRef]
- Kumar, D.; Imler, G.H.; Parrish, D.A.; Shreeve, J.M. N-Acetonitrile Functionalized Nitropyrazoles: Precursors to Insensitive Asymmetric N-Methylene-C Linked Azoles. Chemistry 2017, 23, 7876–7881. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; He, C.; Mitchell, L.A.; Parrish, D.A.; Shreeve, J.N.M. Connecting energetic nitropyrazole and aminotetrazole moieties with N,N′-ethylene bridges: A promising approach for fine tuning energetic properties. J. Mater. Chem. A 2016, 4, 9220–9228. [Google Scholar] [CrossRef]
- Kumar, D.; Mitchell, L.A.; Parrish, D.A.; Shreeve, J.N.M. Asymmetric N,N′-ethylene-bridged azole-based compounds: Two way control of the energetic properties of compounds. J. Mater. Chem. A 2016, 4, 9931–9940. [Google Scholar] [CrossRef]
- Kumar, D.; Imler, G.H.; Parrish, D.A.; Shreeve, J.N.M. Resolving synthetic challenges faced in the syntheses of asymmetric N,N′-ethylene-bridged energetic compounds. New J. Chem. 2017, 41, 4040–4047. [Google Scholar] [CrossRef]
- Kazakov, A.I.; Kurochkina, L.S.; Nabatova, A.V.; Lempert, D.B.; Dalinger, I.L.; Kormanov, A.V.; Serushkina, O.V.; Sheremetev, A.B. Pyrazolyltetrazoles—A High-Enthalpy Backbone for Designing High-Energy Compounds: An Experimental Study of the Enthalpy of Formation. Dokl. Phys. Chem. 2018, 478, 15–18. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, X.; Qi, X.; Wang, K.; Liu, T. Synthesis of 5-(1H-pyrazol-1-yl)-2H-tetrazole-derived energetic salts with high thermal stability and low sensitivity. Energ. Mater. Front. 2020, 1, 83–89. [Google Scholar] [CrossRef]
- Cherfi, M.; Dib, I.; Harit, T.; Ziyyat, A.; Malek, F. Synthesis and characterization of new pyrazole-tetrazole derivatives as new vasorelaxant agents. Drug Dev. Res. 2021, 82, 1055–1062. [Google Scholar] [CrossRef]
- Harit, T.; Cherfi, M.; Daoudi, N.E.; Isaad, J.; Bnouham, M.; Malek, F. Hybrid Pyrazole-Tetrazole Derivatives with High α-Amylase Inhibition Activity: Synthesis, Biological Evaluation and Docking Study. ChemistrySelect 2022, 7, e202203757. [Google Scholar] [CrossRef]
- Oulous, A.; Daoudi, N.E.; Harit, T.; Cherfi, M.; Bnouham, M.; Malek, F. New pyrazole-tetrazole hybrid compounds as potent alpha-amylase and non-enzymatic glycation inhibitors. Bioorg Med. Chem. Lett. 2022, 69, 128785–128789. [Google Scholar] [CrossRef] [PubMed]
- Cherfi, M.; Harit, T.; Dib, I.; Yahyaoui, M.I.; Asehraou, A.; Yahyi, A.; Ziyyat, A.; Malek, F. Pyrazole-tetrazole hybrid compounds: Synthesis, characterization and their biological activities. Chem. Data Collect. 2023, 45, 101026. [Google Scholar] [CrossRef]
- Yin, P.; Zhang, J.; Parrish, D.A.; Shreeve, J.M. Energetic N,N′-ethylene-bridged bis(nitropyrazoles): Diversified functionalities and properties. Chemistry 2014, 20, 16529–16536. [Google Scholar] [CrossRef] [PubMed]
- Joo, Y.H.; Shreeve, J.M. Energetic mono-, di-, and trisubstituted nitroiminotetrazoles. Angew. Chem. Int. Ed. Engl. 2009, 48, 564–567. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Sproll, S.M. Alkyl-Bridged Bis-5-azidotetrazoles: A Safe Way of Preparation. Eur. J. Org. Chem. 2009, 2009, 4284–4289. [Google Scholar] [CrossRef]
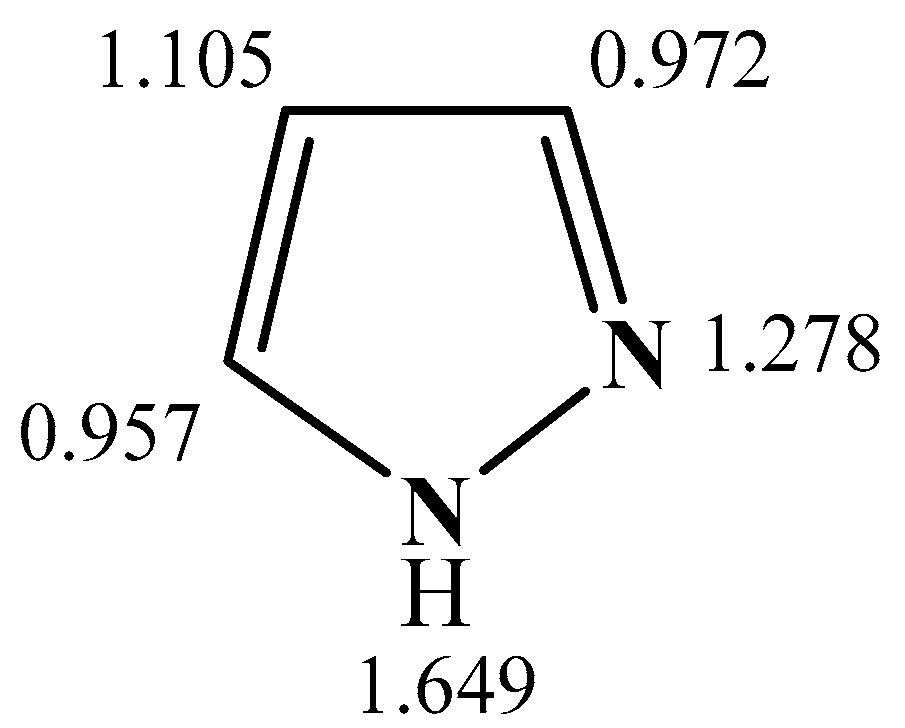


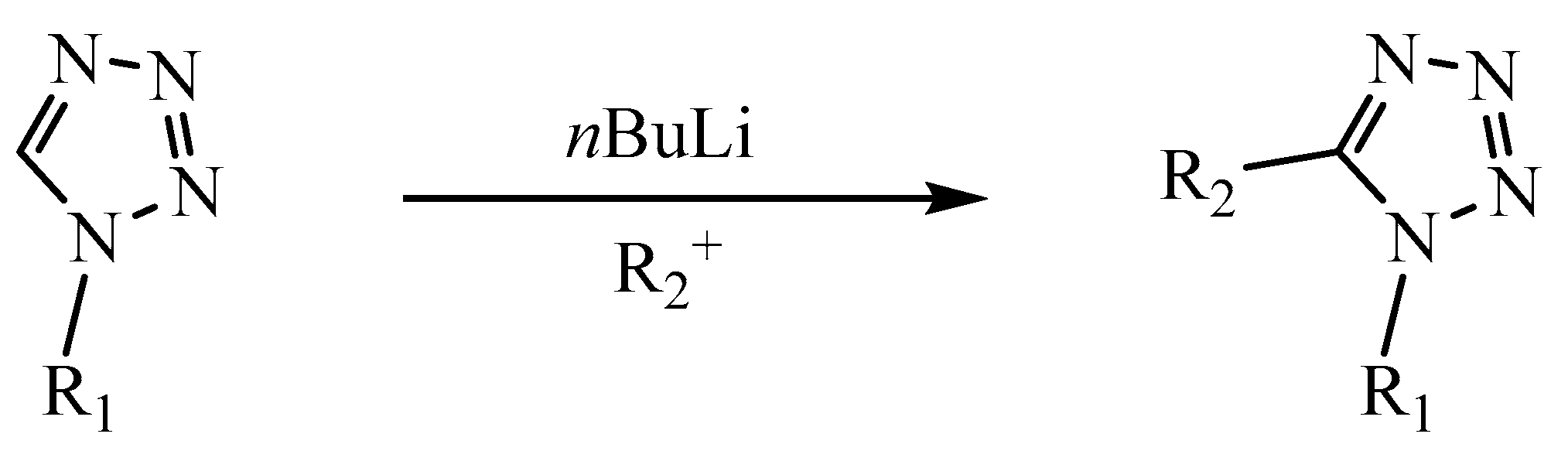






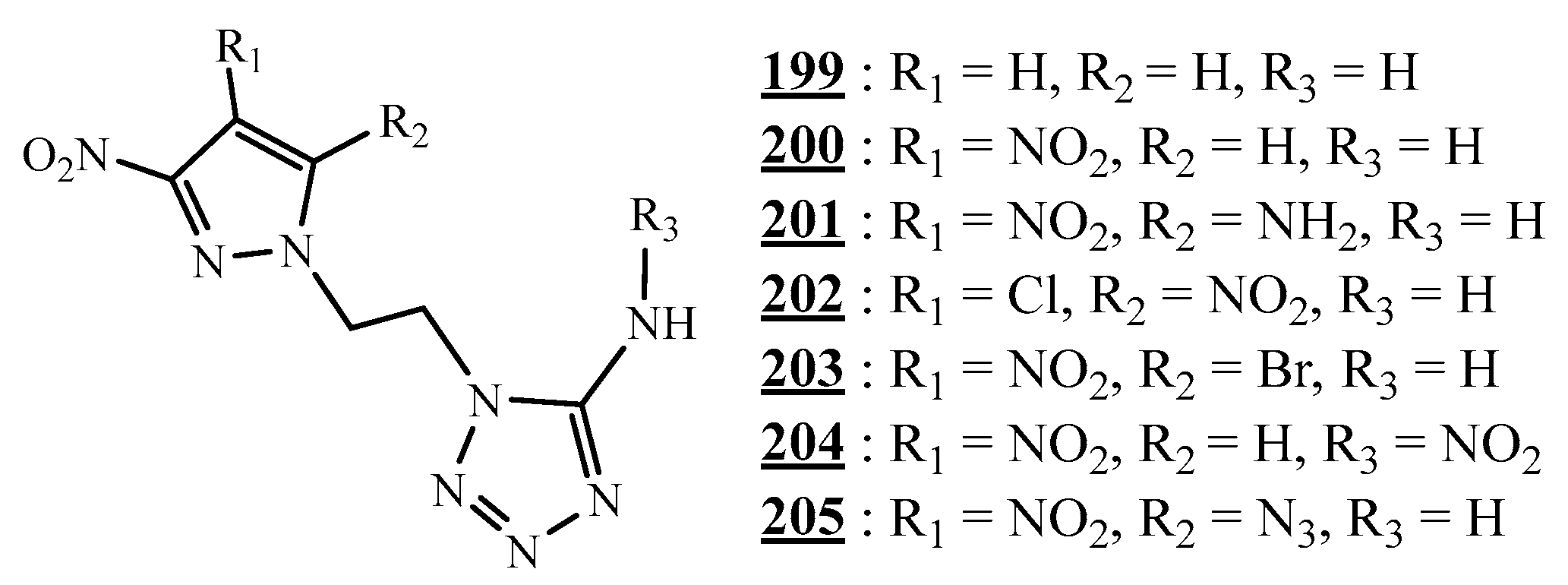
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cherfi, M.; Harit, T.; Amanchar, M.; Oulous, A.; Malek, F. An Overview of Pyrazole-Tetrazole-Based Hybrid Compounds: Synthesis Methods, Biological Activities and Energetic Properties. Organics 2024, 5, 575-597. https://doi.org/10.3390/org5040030
Cherfi M, Harit T, Amanchar M, Oulous A, Malek F. An Overview of Pyrazole-Tetrazole-Based Hybrid Compounds: Synthesis Methods, Biological Activities and Energetic Properties. Organics. 2024; 5(4):575-597. https://doi.org/10.3390/org5040030
Chicago/Turabian StyleCherfi, Mounir, Tarik Harit, Malika Amanchar, Ahlam Oulous, and Fouad Malek. 2024. "An Overview of Pyrazole-Tetrazole-Based Hybrid Compounds: Synthesis Methods, Biological Activities and Energetic Properties" Organics 5, no. 4: 575-597. https://doi.org/10.3390/org5040030
APA StyleCherfi, M., Harit, T., Amanchar, M., Oulous, A., & Malek, F. (2024). An Overview of Pyrazole-Tetrazole-Based Hybrid Compounds: Synthesis Methods, Biological Activities and Energetic Properties. Organics, 5(4), 575-597. https://doi.org/10.3390/org5040030





