Imaging, Dynamic Histomorphometry, and Mechanical Testing in Preclinical Bone Research
Abstract
1. Introduction
2. Dual-Energy X-ray Absorptiometry (DXA)

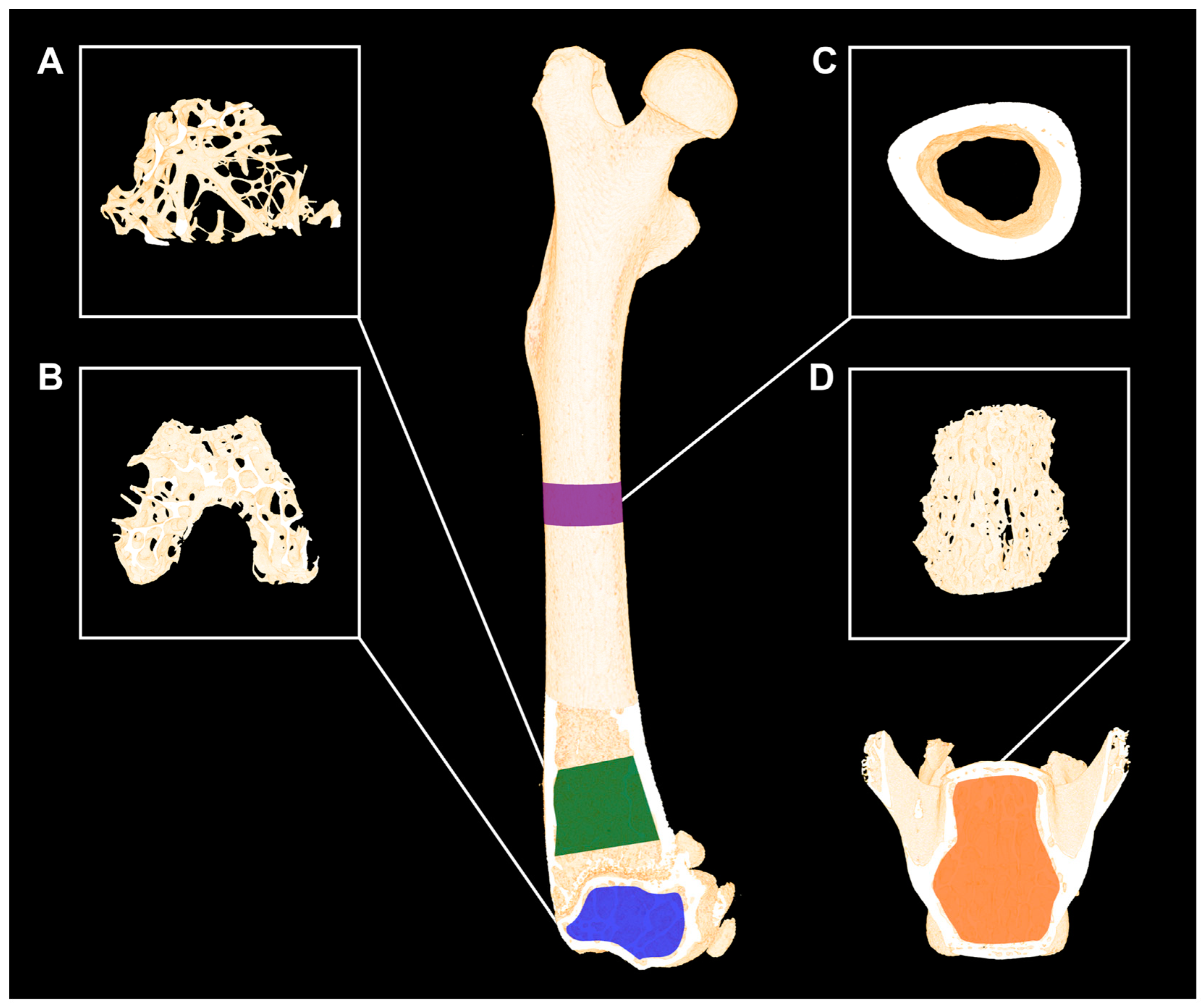
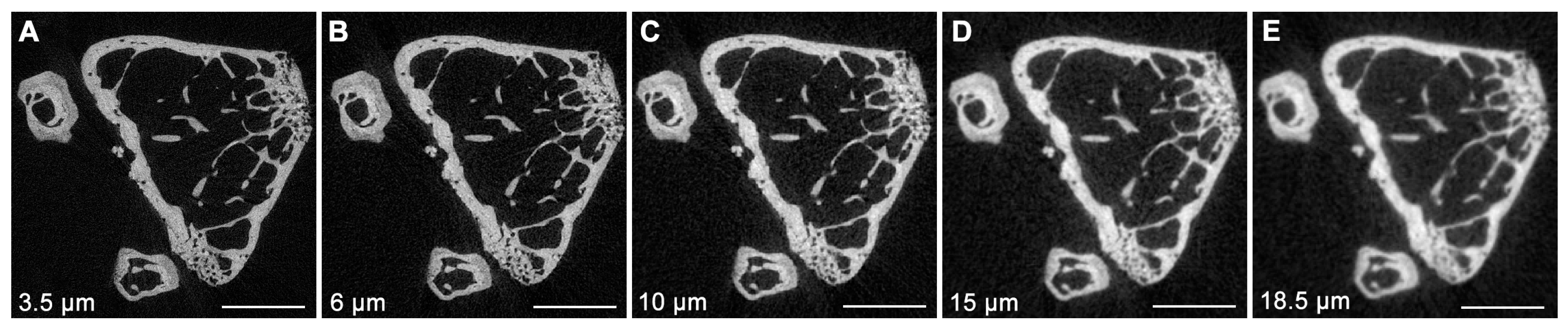
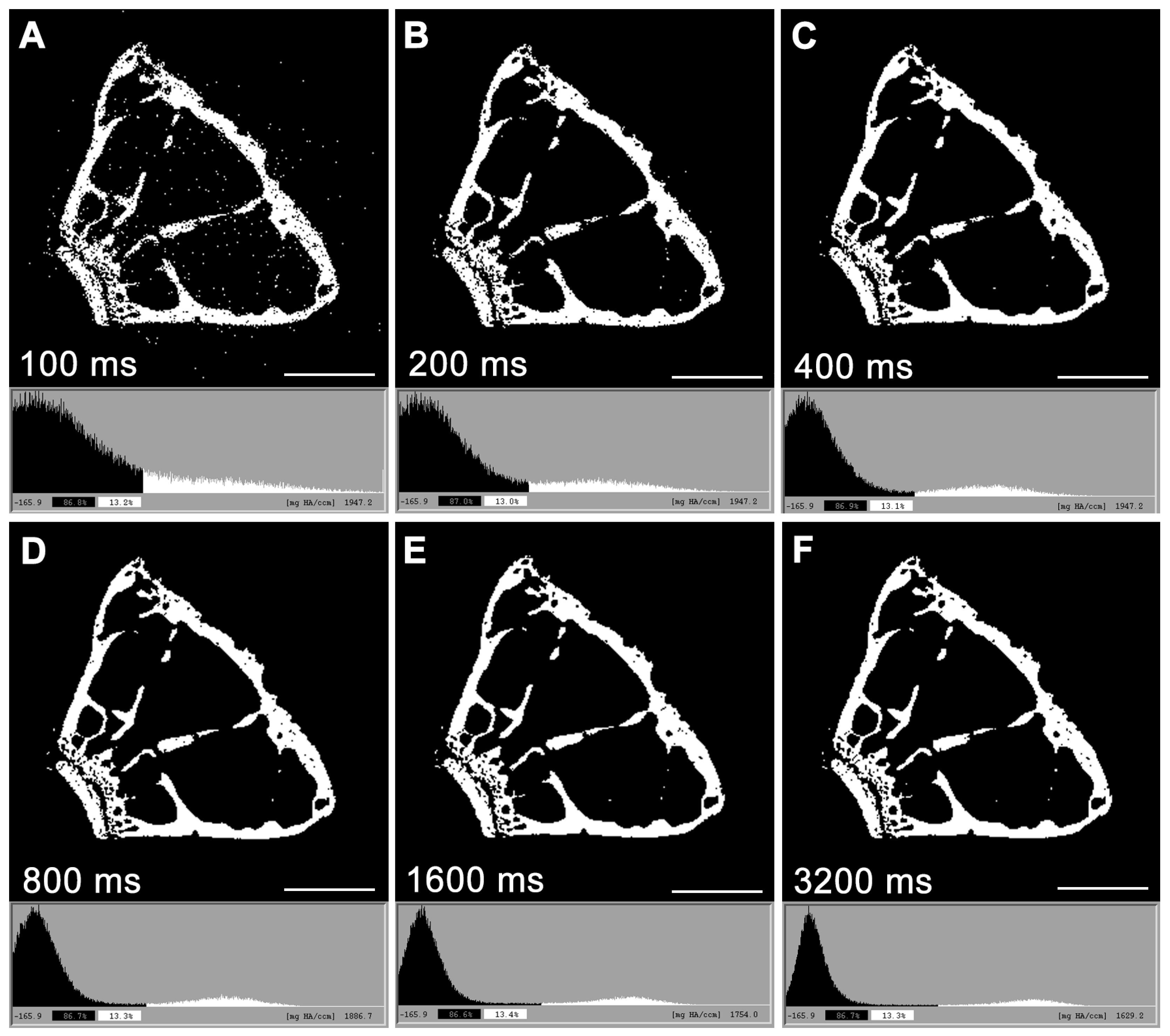
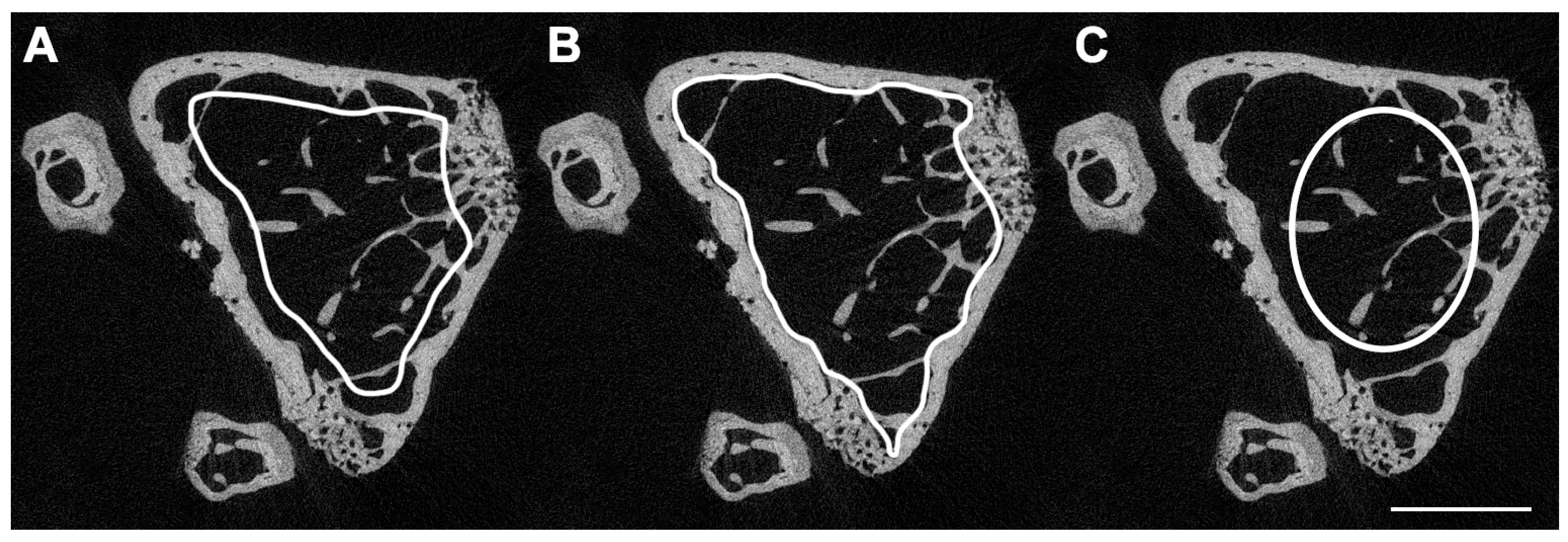
3. Micro-Computed Tomography (μCT)
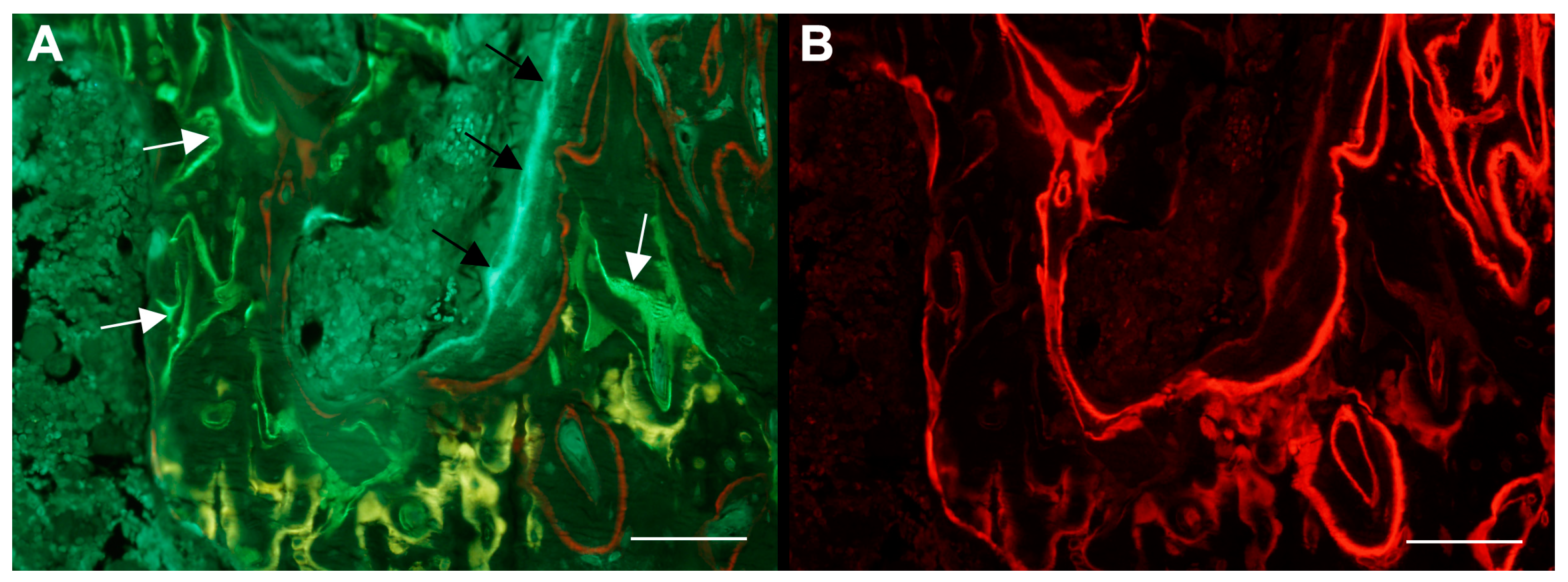
4. Dynamic Bone Histomorphometry
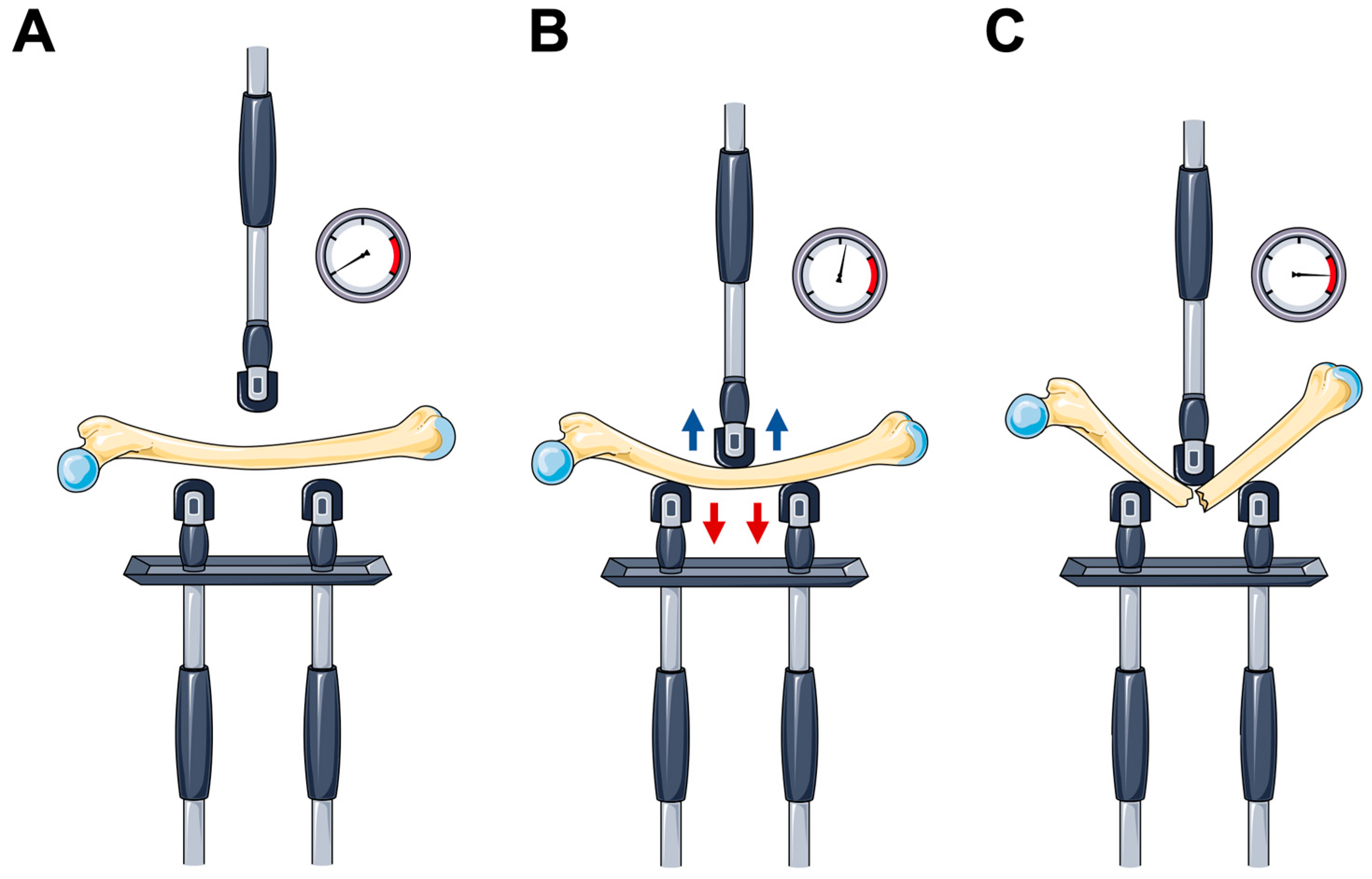
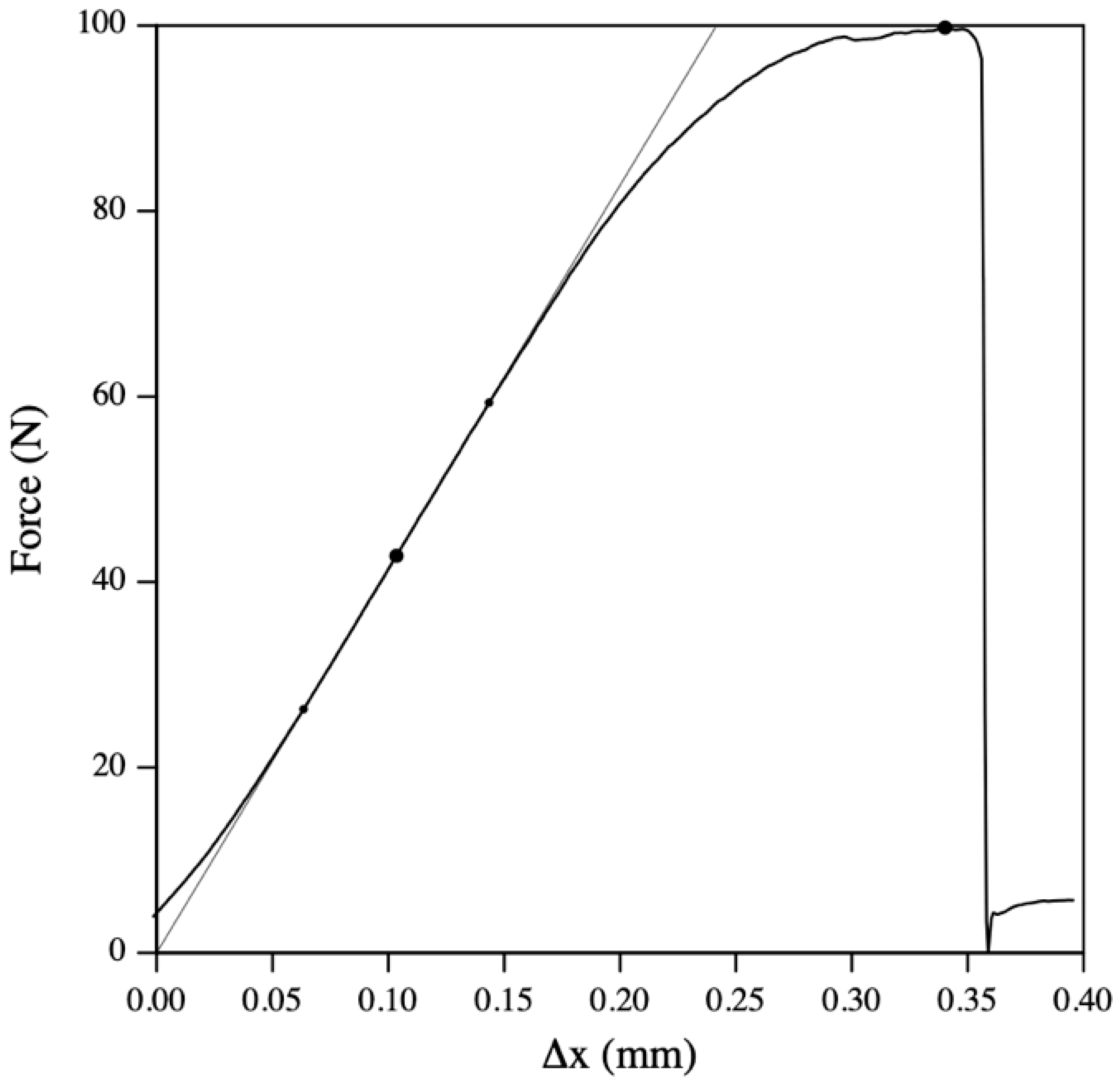
5. Mechanical Testing
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Kanis, J.A.; Melton, L.J.; Christiansen, C.; Johnston, C.C.; Khaltaev, N. The Diagnosis of Osteoporosis. J. Bone Miner. Res. 1994, 9, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; McCloskey, E.V.; Johansson, H.; Oden, A.; Melton, L.J.; Khaltaev, N. A Reference Standard for the Description of Osteoporosis. Bone 2008, 42, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Brent, M.B.; Brüel, A.; Thomsen, J.S. A Systematic Review of Animal Models of Disuse-Induced Bone Loss. Calcif. Tissue Int. 2021, 108, 561–575. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, E. Methods for Assessing Bone Quality: A Review. Clin. Orthop. Relat. Res. 2011, 469, 2128. [Google Scholar] [CrossRef] [PubMed]
- Blake, G.M.; Fogelman, I. Technical Principles of Dual Energy X-Ray Absorptiometry. Semin. Nucl. Med. 1997, 27, 210–228. [Google Scholar] [CrossRef] [PubMed]
- Cullum, I.D.; Ell, P.J.; Ryder, J.P. X-Ray Dual-Photon Absorptiometry: A New Method for the Measurement of Bone Density. Br. J. Radiol. 1989, 62, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Cherian, K.E.; Kapoor, N.; Meeta, M.; Paul, T.V. Dual-Energy X-Ray Absorptiometry Scanning in Practice, Technical Aspects, and Precision Testing. J. Midlife Health 2021, 12, 252. [Google Scholar] [CrossRef] [PubMed]
- Messina, C.; Maffi, G.; Vitale, J.A.; Ulivieri, F.M.; Guglielmi, G.; Sconfenza, L.M. Diagnostic Imaging of Osteoporosis and Sarcopenia: A Narrative Review. Quant. Imaging Med. Surg. 2018, 8, 86–99. [Google Scholar] [CrossRef]
- Brent, M.B.; Lodberg, A.; Thomsen, J.S.; Brüel, A. Rodent Model of Disuse-Induced Bone Loss by Hind Limb Injection with Botulinum Toxin A. MethodsX 2020, 7, 101079. [Google Scholar] [CrossRef]
- Martineau, P.; Leslie, W.D. Trabecular Bone Score (TBS): Method and Applications. Bone 2017, 104, 66–72. [Google Scholar] [CrossRef]
- Hans, D.; Barthe, N.; Boutroy, S.; Pothuaud, L.; Winzenrieth, R.; Krieg, M.A. Correlations Between Trabecular Bone Score, Measured Using Anteroposterior Dual-Energy X-Ray Absorptiometry Acquisition, and 3-Dimensional Parameters of Bone Microarchitecture: An Experimental Study on Human Cadaver Vertebrae. J. Clin. Densitom. 2011, 14, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Cherif, R.; Vico, L.; Laroche, N.; Sakly, M.; Attia, N.; Lavet, C. Dual-Energy X-Ray Absorptiometry Underestimates in Vivo Lumbar Spine Bone Mineral Density in Overweight Rats. J. Bone Miner. Metab. 2018, 36, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Lee, S.; Uyeda, M.; Tanjaya, J.; Kim, J.K.; Pan, H.C.; Reese, P.; Stodieck, L.; Lin, A.; Ting, K.; et al. Guidelines for Dual Energy X-Ray Absorptiometry Analysis of Trabecular Bone-Rich Regions in Mice: Improved Precision, Accuracy, and Sensitivity for Assessing Longitudinal Bone Changes. Tissue Eng. Part C Methods 2016, 22, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Lewiecki, E.M.; Binkley, N.; Morgan, S.L.; Shuhart, C.R.; Camargos, B.M.; Carey, J.J.; Gordon, C.M.; Jankowski, L.G.; Lee, J.K.; Leslie, W.D. Best Practices for Dual-Energy X-Ray Absorptiometry Measurement and Reporting: International Society for Clinical Densitometry Guidance. J. Clin. Densitom. 2016, 19, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.C.; Dover, S.D. X-ray Microtomography. J. Microsc. 1982, 126, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Feldkamp, L.A.; Goldstein, S.A.; Parfitt, M.A.; Jesion, G.; Kleerekoper, M. The Direct Examination of Three-dimensional Bone Architecture in Vitro by Computed Tomography. J. Bone Miner. Res. 1989, 4, 3–11. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for Assessment of Bone Microstructure in Rodents Using Micro-Computed Tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
- Brent, M.B.; Thomsen, J.S.; Brüel, A. Short-Term Glucocorticoid Excess Blunts Abaloparatide-Induced Increase in Femoral Bone Mass and Strength in Mice. Sci. Rep. 2021, 11, 12258. [Google Scholar] [CrossRef] [PubMed]
- Shivaramu Effective Atomic Numbers for Photon Energy Absorption and Photon Attenuation of Tissues from Human Organs. Med. Dosim. 2002, 27, 1–9. [CrossRef]
- Christiansen, B.A. Effect of Micro-Computed Tomography Voxel Size and Segmentation Method on Trabecular Bone Microstructure Measures in Mice. Bone Rep. 2016, 5, 136–140. [Google Scholar] [CrossRef]
- Brent, M.B.; Simonsen, U.; Thomsen, J.S.; Brüel, A. Effect of Acetazolamide and Zoledronate on Simulated High Altitude-Induced Bone Loss. Front. Endocrinol. 2022, 13, 831369. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Gang, G.J.; Siewerdsen, J.H. Noise, Sampling, and the Number of Projections in Cone-Beam CT with a Flat-Panel Detector. Med. Phys. 2014, 41, 061909. [Google Scholar] [CrossRef]
- du Plessis, A.; Broeckhoven, C.; Guelpa, A.; le Roux, S.G. Laboratory X-Ray Micro-Computed Tomography: A User Guideline for Biological Samples. Gigascience 2017, 6, gix027. [Google Scholar] [CrossRef] [PubMed]
- Chavez, M.B.; Chu, E.Y.; Kram, V.; de Castro, L.F.; Somerman, M.J.; Foster, B.L. Guidelines for Micro–Computed Tomography Analysis of Rodent Dentoalveolar Tissues. JBMR Plus 2021, 5, e10474. [Google Scholar] [CrossRef]
- Milch, R.A.; Rall, D.P.; Tobie, J.E. Bone Localization of the Tetracyclines. J. Natl. Cancer Inst. 1957, 19, 87–93. [Google Scholar] [CrossRef]
- Frost, H.M. Tetracycline-Based Histological Analysis of Bone Remodeling. Calcif. Tissue Res. 1969, 3, 211–237. [Google Scholar] [CrossRef] [PubMed]
- Frost, H.M. Measurement of Human Bone Formation By Means of Tetracycline Labelling. Can. J. Biochem. Physiol. 1963, 41, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Marotti, G.; Marotti, F. Topographic-Quantitative Study of Bone Tissue Formation and Reconstruction in Inert Bones. In Calcified Tissues 1965; Springer: Berlin/Heidelberg, Germany, 1966; pp. 89–93. [Google Scholar]
- Haas, H.G.; Müller, J.; Schenk, R.K. Osteomalacia: Metabolic and Quantitative Histologic Studies. Clin. Orthop. Relat. Res. 1967, 53, 213–222. [Google Scholar] [CrossRef]
- van Gaalen, S.M.; Kruyt, M.C.; Geuze, R.E.; de Bruijn, J.D.; Alblas, J.; Dhert, W.J.A. Use of Fluorochrome Labels in in Vivo Bone Tissue Engineering Research. Tissue Eng. Part B Rev. 2010, 16, 209–217. [Google Scholar] [CrossRef]
- Pautke, C.; Vogt, S.; Tischer, T.; Wexel, G.; Deppe, H.; Milz, S.; Schieker, M.; Kolk, A. Polychrome Labeling of Bone with Seven Different Fluorochromes: Enhancing Fluorochrome Discrimination by Spectral Image Analysis. Bone 2005, 37, 441–445. [Google Scholar] [CrossRef]
- Vegger, J.B.; Brüel, A.; Dahlgaard, A.F.; Thomsen, J.S. Alterations in Gene Expression Precede Sarcopenia and Osteopenia in Botulinum Toxin Immobilized Mice. J. Musculoskelet. Neuronal Interact. 2016, 16, 355–368. [Google Scholar] [PubMed]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized Nomenclature, Symbols, and Units for Bone Histomorphometry: A 2012 Update of the Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2013, 28, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.P.; Recker, R.R. The Label Escape Error: Determination of the Active Bone-Forming Surface in Histologic Sections of Bone Measured by Tetracycline Double Labels. Metab. Bone Dis. Relat. Res. 1982, 4, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Brent, M.B.; Brüel, A.; Thomsen, J.S. PTH (1–34) and Growth Hormone in Prevention of Disuse Osteopenia and Sarcopenia in Rats. Bone 2018, 110, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Brent, M.B.; Emmanuel, T. Contemporary Advances in Computer-Assisted Bone Histomorphometry and Identification of Bone Cells in Culture. Calcif. Tissue Int. 2023, 112, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sharir, A.; Barak, M.M.; Shahar, R. Whole Bone Mechanics and Mechanical Testing. Vet. J. 2008, 177, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B. Normal Bone Anatomy and Physiology. Clin. J. Am. Soc. Nephrol. 2008, 3 (Suppl. S3), S131. [Google Scholar] [CrossRef] [PubMed]
- Von Euw, S.; Wang, Y.; Laurent, G.; Drouet, C.; Babonneau, F.; Nassif, N.; Azaïs, T. Bone Mineral: New Insights into Its Chemical Composition. Sci. Rep. 2019, 9, 8456. [Google Scholar] [CrossRef]
- Simkin, A.; Robin, G. The Mechanical Testing of Bone in Bending. J. Biomech. 1973, 6, 31–39. [Google Scholar] [CrossRef]
- Turner, C.H. Bone Strength: Current Concepts. In Proceedings of the Annals of the New York Academy of Sciences; Blackwell Publishing Inc.: Hoboken, NJ, USA, 2006; Volume 1068, pp. 429–446. [Google Scholar]
- Cole, J.H.; Van Der Meulen, M.C.H. Whole Bone Mechanics and Bone Quality. Clin. Orthop. Relat. Res. 2011, 469, 2139–2149. [Google Scholar] [CrossRef]
- Brent, M.B.; Thomsen, J.S.; Brüel, A. The Efficacy of PTH and Abaloparatide to Counteract Immobilization-Induced Osteopenia Is in General Similar. Front. Endocrinol. 2020, 11, 588773. [Google Scholar] [CrossRef] [PubMed]
- Miller, A. Mechanical Testing of Sliding on Pivot-Locking Clamp (SOP-LC) Fracture Repair System in Four-Point Bending and Torsion. Vet. Comp. Orthop. Traumatol. 2024, 37, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Grote, S.; Noeldeke, T.; Blauth, M.; Mutschler, W.; Bürklein, D. Mechanical Torque Measurement in the Proximal Femur Correlates to Failure Load and Bone Mineral Density Ex Vivo. Orthop. Rev. 2013, 5, 77–81. [Google Scholar] [CrossRef]
- Nazarian, A.; Bauernschmitt, M.; Eberle, C.; Meier, D.; Müller, R.; Snyder, B.D. Design and Validation of a Testing System to Assess Torsional Cancellous Bone Failure in Conjunction with Time-Lapsed Micro-Computed Tomographic Imaging. J. Biomech. 2008, 41, 3496–3501. [Google Scholar] [CrossRef] [PubMed]
- Quenneville, C.E.; Fraser, G.S.; Dunning, C.E. Development of an Apparatus to Produce Fractures from Short-Duration High-Impulse Loading with an Application in the Lower Leg. J. Biomech. Eng. 2010, 132, 014502. [Google Scholar] [CrossRef] [PubMed]
- Zysset, P.K.; Dall’Ara, E.; Varga, P.; Pahr, D.H. Finite Element Analysis for Prediction of Bone Strength. Bonekey Rep. 2013, 2, 386. [Google Scholar] [CrossRef] [PubMed]
- Tiefenboeck, T.M.; Payr, S.; Bajenov, O.; Dangl, T.; Koch, T.; Komjati, M.; Sarahrudi, K. Different Storage Times and Their Effect on the Bending Load to Failure Testing of Murine Bone Tissue. Sci. Rep. 2020, 10, 17412. [Google Scholar] [CrossRef]
- Bailey, S.; Vashishth, D. Mechanical Characterization of Bone: State of the Art in Experimental Approaches—What Types of Experiments Do People Do and How Does One Interpret the Results? Curr. Osteoporos. Rep. 2018, 16, 423–433. [Google Scholar] [CrossRef]
| Minimum Reporting Requirements for Micro-Computed Tomography (μCT) | ||
|---|---|---|
| μCT scan acquisition | ||
| Variable | Description: | Standard unit |
| Voxel size | Basic discrete 3D unit of μCT image. The 3D volume represents two dimensions within the slice and slice thickness. | μm3 |
| X-ray tube potential (peak) | Applied peak electric potential of X-ray tube that accelerates electrons for generating X-ray photons. | kVp |
| Integration time | Duration of each tomographic projection. | ms |
| Trabecular bone microarchitecture | ||
| Bone volume fraction (BV/TV) | Ratio of the segmented bone volume to the total volume of the region of interest. | % |
| Trabecular number (Tb.N) | Measure of the average number of trabeculae per unit length. | 1/mm |
| Trabecular thickness (Tb.Th) | Mean thickness of trabeculae, assessed using direct 3D methods. | mm |
| Trabecular separation (Tb.Sp) | Mean distance between trabeculae, assessed using direct 3D methods. | mm |
| Cortical morphology | ||
| Total cross-sectional area (Tt.Ar) | Total cross-sectional area inside the periosteal envelope. | mm2 |
| Cortical area (Ct.Ar) | Cortical bone area = cortical volume (Ct.V) ÷ (number of slices × slice thickness). | mm2 |
| Relative cortical bone area to tissue area (Ct.Ar/Tt.Ar) | Cortical area fraction. | % |
| Cortical thickness (Ct.Th) | Average cortical thickness. | mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brent, M.B. Imaging, Dynamic Histomorphometry, and Mechanical Testing in Preclinical Bone Research. Osteology 2024, 4, 120-131. https://doi.org/10.3390/osteology4030010
Brent MB. Imaging, Dynamic Histomorphometry, and Mechanical Testing in Preclinical Bone Research. Osteology. 2024; 4(3):120-131. https://doi.org/10.3390/osteology4030010
Chicago/Turabian StyleBrent, Mikkel Bo. 2024. "Imaging, Dynamic Histomorphometry, and Mechanical Testing in Preclinical Bone Research" Osteology 4, no. 3: 120-131. https://doi.org/10.3390/osteology4030010
APA StyleBrent, M. B. (2024). Imaging, Dynamic Histomorphometry, and Mechanical Testing in Preclinical Bone Research. Osteology, 4(3), 120-131. https://doi.org/10.3390/osteology4030010





