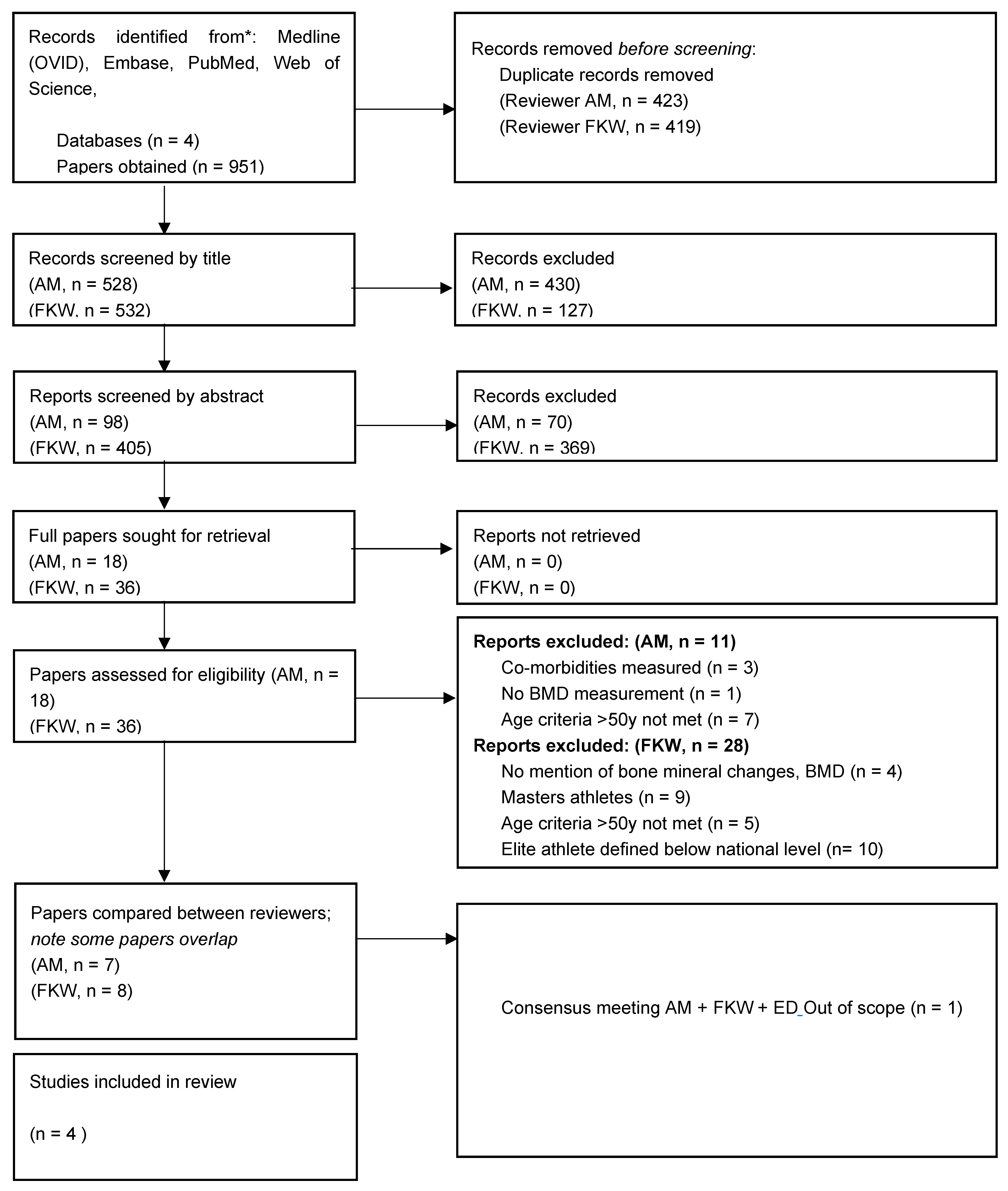
Participation in Elite Sport in Youth and Its Impact on Lifelong Bone Health
Abstract
1. Introduction
2. Methods
- 1.
- Study group selection
- Adequate case definition?
- Do participants represent the case hypothesis?
- Control selection process: from community?
- Control criteria: any limiting factors?
- 2.
- Comparability of participant groups
- Strong confounders
- Review of the outcome
- 3.
- Exposure
- Blinded/non-blinded?
- Same methods in all participant groups?
- Non-response rate (if applicable)?
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Drinkwater, L.; Goldsmith, R.S.; Conrad Johnston JrC, L.R.; Mack, T.M.; Meunier, P.J.; Christopher Nordin, B.E.; Michael Parfitt, A. Consensus development conference: Prophylaxis and treatment of osteoporosis. Br. Med. J. 1987, 295, 914–915. [Google Scholar]
- Johnell, O.; Kanis, J. Epidemiology of osteoporotic fractures. Osteoporos. Int. 2005, 16, S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Holroyd, C.; Cooper, C.; Dennison, E. Epidemiology of osteoporosis. Best Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.P.; Espada, M.C.; Massini, D.A.; Robalo, R.A.; Almeida, T.A.; Hernández-Beltrán, V.; Gamonales, J.M.; Castro, E.A.; Pessôa Filho, D.M. Effects of Exercise and Sports Intervention and the Involvement Level on the Mineral Health of Different Bone Sites in the Leg, Hip, and Spine: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 6537. [Google Scholar] [CrossRef]
- Cui, W.; Li, D.; Jiang, Y.; Gao, Y. Effects of exercise based on ACSM recommendations on bone mineral density in individuals with osteoporosis: A systematic review and meta-analyses of randomized controlled trials. Front. Physiol. 2023, 14, 1181327. [Google Scholar] [CrossRef] [PubMed]
- Andreoli, A.; Monteleone, M.; Van Loan, M.; Promenzio, L.; Tarantino, U.; De Lorenzo, A. Effects of different sports on bone density and muscle mass in highly trained athletes. Med. Sci. Sports Exerc. 2001, 33, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Hart, N.H.; Nimphius, S.; Rantalainen, T.; Ireland, A.; Siafarikas, A.; Newton, R.U. Mechanical basis of bone strength: Influence of bone material, bone structure and muscle action. J. Musculoskelet. Neuronal Interact. 2017, 17, 114. [Google Scholar] [PubMed]
- Patel, H.; Sammut, L.; Denison, H.; Teesdale-Spittle, P.; Dennison, E. The relationship between non-elite sporting activity and calcaneal bone density in adolescents and young adults: A narrative systematic review. Front. Physiol. 2020, 11, 505019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Parsons, C.; Fuggle, N.; Ward, K.A.; Cooper, C.; Dennison, E. Is regular weight-bearing physical activity throughout the lifecourse associated with better bone health in late adulthood? Calcif. Tissue Int. 2022, 111, 279–287. [Google Scholar]
- Khan, K.; McKay, H.A.; Haapasalo, H.; Bennell, K.L.; Forwood, M.R.; Kannus, P.; Wark, J.D. Does childhood and adolescence provide a unique opportunity for exercise to strengthen the skeleton? J. Sci. Med. Sport 2000, 3, 150–164. [Google Scholar]
- Ackerman, K.E.; Misra, M. Bone health and the female athlete triad in adolescent athletes. Physician Sportsmed. 2011, 39, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Kapczuk, K. Elite athletes and pubertal delay. Minerva Pediatr. 2017, 69, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. Strengthening the reporting of observational studies in epidemiology (STROBE). Epidemiology 2007, 18, 805–835. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Robertson, J.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ott. Hosp. Res. Inst. 2011, 2, 1–12. [Google Scholar]
- Kettunen, J.A.; Impivaara, O.; Kujala, U.M.; Linna, M.; Mäki, J.; Räty, H.; Alanen, E.; Kaprio, J.; Videman, T.; Sarna, S. Hip fractures and femoral bone mineral density in male former elite athletes. Bone 2010, 46, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Andreoli, A.; Celi, M.; Volpe, S.L.; Sorge, R.; Tarantino, U. Long-term effect of exercise on bone mineral density and body composition in post-menopausal ex-elite athletes: A retrospective study. Eur. J. Clin. Nutr. 2012, 66, 69–74. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tveit, M.; Rosengren, B.E.; Nilsson, J.Å.; Karlsson, M.K. Exercise in youth: High bone mass, large bone size, and low fracture risk in old age. Scand. J. Med. Sci. Sports 2015, 25, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Chen, W.; Yuwen, P.; Yang, N.; Yan, X.; Zhang, Y. Multivariate analysis of factors related to radiographic knee osteoarthritis based on the comparison between football players and matched nonsportsmen. Int. Orthop. 2018, 42, 519–527. [Google Scholar] [CrossRef]
- Heikura, I.A.; Uusitalo, A.L.; Stellingwerff, T.; Bergland, D.; Mero, A.A.; Burke, L.M. Low energy availability is difficult to assess but outcomes have large impact on bone injury rates in elite distance athletes. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 403–411. [Google Scholar] [CrossRef]
- Tam, N.; Santos-Concejero, J.; Tucker, R.; Lamberts, R.P.; Micklesfield, L.K. Bone health in elite Kenyan runners. J. Sports Sci. 2018, 36, 456–461. [Google Scholar] [CrossRef]
- Andersen, O.K.; Clarsen, B.; Garthe, I.; Mørland, M.; Stensrud, T. Bone Health in Elite Norwegian Endurance Cyclists and Runners: A Cross-Sectional Study. Med. Sci. Sports Exerc. 2019, 51, 679. [Google Scholar] [CrossRef]
- Tenforde, A.S.; Fredericson, M. Influence of sports participation on bone health in the young athlete: A review of the literature. PM&R 2011, 3, 861–867. [Google Scholar]
- Myburgh, K.H.; Hutchins, J.; Fataar, A.B.; Hough, S.F.; Noakes, T.D. Low bone density is an etiologic factor for stress fractures in athletes. Ann. Intern. Med. 1990, 113, 754–759. [Google Scholar] [CrossRef]
- Nordström, A.; Karlsson, C.; Nyquist, F.; Olsson, T.; Nordström, P.; Karlsson, M. Bone loss and fracture risk after reduced physical activity. J. Bone Miner. Res. 2005, 20, 202–207. [Google Scholar] [CrossRef]
- Gomez-Bruton, A.; Montero-Marín, J.; González-Agüero, A.; Gómez-Cabello, A.; García-Campayo, J.; Moreno, L.A.; Casajús, J.A. Vicente-Rodríguez G. Swimming and peak bone mineral density: A systematic review and meta-analysis. J. Sports Sci. 2018, 36, 365–377. [Google Scholar]
- Imeri, B.; Gheitasi, M.; Khaledi, A.; Mozafaripour, E. Bone Mineral Density and Content among Iranian Elite Male Athletes in Different Sports. Arch. Bone Jt. Surg. 2023, 11, 212–217. [Google Scholar]
- Hagman, M.; Helge, E.W.; Fristrup, B.; Jørgensen, N.R.; Helge, J.W.; Krustrup, P. High bone mineral density in lifelong trained female team handball players and young elite football players. Eur. J. Appl. Physiol. 2021, 121, 2825–2836. [Google Scholar] [CrossRef]
- Bellver, M.; Del Rio, L.; Jovell, E.; Drobnic, F.; Trilla, A. Bone mineral density and bone mineral content among female elite athletes. Bone 2019, 127, 393–400. [Google Scholar] [CrossRef]
| Medline (OVID): (athlete/ OR athlete* OR sports*) AND (elite OR professional) AND (bone adj3 (dens* OR content* OR measure*) AND (adult OR middle aged OR aged OR later life OR masters OR longevity/ OR “life span”) |
| Embase: (athlete/ OR athlete* OR sports*) AND (elite OR professional) AND (bone adj3 (dens* OR content* OR measure*) AND (adult OR middle aged OR aged OR later life OR masters OR longevity/ OR “life span”) |
| PubMed: (athlete/ OR athlete* OR sports*) AND (elite OR professional) AND (“bone mineral dens*” OR bone mineral content*” OR “bone mineral measure*” OR “bone dens*” OR “bone content*” OR “bone measure*”) AND (adult OR middle aged OR aged OR later life OR masters OR longevity/ OR “life span”) |
| Web of Science: (athlete/ OR athlete* OR sports*) AND (elite OR professional) AND (“bone mineral dens*” OR bone mineral content*” OR “bone mineral measure*” OR “bone dens*” OR “bone content*” OR “bone measure*”) AND (adult OR middle aged OR aged OR later life OR masters OR longevity/ OR “life span”) |
| Inclusion criteria: adults aged >50 years who have partaken in elite sport in young adulthood aged 15–30 years. This study includes both sexes (human only) and ‘elite’ is described as national level or above. |
| Exclusion criteria: individuals with chronic health conditions, including disabilities (physical and mental) which have impacted bone health or sport. Participation up to the regional level was excluded. Master athletes were excluded. Elite athletes who started the sport >30 years of age. | |
| Retired athletes who partook in elite sporting activity in young adulthood. |
| Controls, with no history of participation in elite sport. Level of activity was not limited. All permitted were matched with athletes in terms of age and sex. |
| Bone mineral density. |
| Newcastle–Ottawa Screening Tool | Kettunen J. et al. (2010) [16] | Andreoli A. et al. (2012) [17] | Tveit M. et al. (2015) [18] | Lv H. et al. (2018) [19] | |
|---|---|---|---|---|---|
| Study group comparison | Adequate case definition? | Y | Y | Y | Y |
| Do participants represent the case hypothesis? | Y | Y | Y | Y | |
| Control selection process: from the community? | Y | Y | Y | Y | |
| Control criteria: any limiting factors? | - | N | N | N | |
| Comparability of the participant groups | Strong confounders? | Y | Y | Y | Y |
| Review of the outcome? | Y | Y | Y | Y | |
| Exposure | Blinded? | N | N | N | N |
| Same methods for both participant groups? | Y | Y | Y | Y | |
| Sufficient response rate? | N | Y | Y | Y | |
| Scoring 0–9 | 6 | 8 | 8 | 8 | |
| Author (Year) | Kettunen J. et al. (2010) [16] | Andreoli A. et al. (2012) [17] | Tveit M. et al. (2015) [18] | Lv H. et al. (2018) [19] |
|---|---|---|---|---|
| Journal | Bone | European Journal of Clinical Nutrition | Scandinavian Journal of Medicine & Science in Sports | International Orthopaedics |
| Type of study | Case–control | Case–control | 24 Caucasian female ex-athletes | 12 runners, 12 swimmers |
| Athlete population size | 87 male ex-athletes (2147 contacted) | Cross-sectional cohort study | * 193 retired male elite soccer players | 193 footballers |
| Sport(s) | 31 football, 28 endurance running, 28 weight-lifting | Cross-sectional descriptive study | 86 retired male professional football players | 86 footballers |
| Controls | 194 male controls | 24 Caucasian female controls | 280 controls | 86 controls |
| Bone measurement sites | DEXA; FN, FT ** | DEXA; LA, RA, LS ** | DEXA; TB, A, L, LS, FN ** | DEXA; FN, FT, spine ** |
| Key results | Athletes (soccer, endurance, and weight-lifters) had a mean significantly higher BMD at both FN and FT than controls. Once adjusted for BMI and age, the p value was <0.0002 | BMD and BMC were higher in both groups (swimmers and runners) than controls, p < 0.01 | BMD is greater in both age groups > 50 years (p < 0.001). BMD becomes more similar between data groups at the 69 average age sub-group (p 0.02). | A greater BMD has a positive impact on reduced knee osteoarthritis risk + improved knee function (p < 0.001). |
| Author (Year) | Relevant Data Extracted | ||||
|---|---|---|---|---|---|
| Kettunen J. et al. (2010) [16] | Athletes n = 87 | Controls | Significance | ||
| Football (n = 31) | Endurance running (n = 28) | Weightlifting (n = 28) | n = 194 | p < 0.0002 | |
| Average age = 56.5 | Average age = 59.7 | Average age = 59.2 | Average age = 55.8 | ||
| FN = 1.032 (SD 0.163), FT 0.969 (SD 0.131) | FN = 0.977 (SD 0.145), FT = 0.885 (SD 0.128) | FN = 0.962 (SD 0.191), FT = 0.908 (SD 0.169) | FN = 0.905 (SD 0.131), FT = 0860 (SD 0.121) | ||
| Andreoli A. et al. (2012) [17] | Athletes | Controls | Significance | ||
| n = 12 runners | n = 12 swimmers | n = 24 | p < 0.01 | ||
| Average age = 57.8 | Average age = 58.4 | Average age = 60.8 | |||
| LA = 0.692 (SD 0.074), RA = 0.716 (SD 0.088), LS = 1.162 (SD 0.198), LL = 1.130 (SD 0.144), RL = 1.115 (SD 0.138) | LA = 0.703 (SD = 0.057), RA = 0.710 (SD 0.063), LS 0.938 (SD 0.0164), LL = 1.043 (SD 0.099), RL = 0.942 (SD 0.112) | LA = 0.640 (SD 0.068), RA = 0.647 (SD 0.068), LS = 0.938 (SD 0.164), LL 0.921 (SD = 0.107), RL = 0.942 (SD 0.112) | |||
| Tveit M. et al. (2015) [18] | Athletes | Controls | Significance | ||
| n = 48 Average age = 57.27 (retired for 20–29 years) | n = 157 Average age = 53.85 | p < 0.001 to 0.44 | |||
| TB = 1.23 (SD 0.07), A 0.97 (SD 0.06), L 1.40 (SD 0.10), LS 1.26 (SD 0.12), FN = 0.96 (SD 0.11) | TB = 1.18 (SD 0.08), A = 0.95 (SD 0.08), L 1.30 (SD 0.10), LS = 1.18 (SD 0.19), FN = 0.94 (SD 0.12) | ||||
| n = 83; Average age = 69.14 (retired for >30 years) | n = 141; Average age = 69.01 | p 0.002 to 0.66 | |||
| TB = 1.19 (SD 0.10), A = 0.92 (SD 0.08), L 1.32 (SD 0.13), LS = 1.25 (SD 0.22), FN = 0.91 (SD 0.16) | TB = 1.16 (SD 0.09), A = 0.92 (SD 0.10), L = 1.27 (SD 0.12), LS = 1.18 (0.22), FN = 0.90 (SD 0.14) | ||||
| Lv H. et al. (2018) [19] | Athletes | Controls | Significance | ||
| n = 86 | n = 86 | p < 0.001 | |||
| Average age = 53 | Average age = 52 | ||||
| FN = 0.913 (SD 0.254), FT = 0.860 (SD 0.177)), S = 0.921 (SD 0.098) | FN = 0.638 (SD 0.176), FT = 0.624 (SD 0.235), S = 0.720 (SD 0.099) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marriott, A.; Kirkham-Wilson, F.; Dennison, E. Participation in Elite Sport in Youth and Its Impact on Lifelong Bone Health. Osteology 2024, 4, 111-119. https://doi.org/10.3390/osteology4030009
Marriott A, Kirkham-Wilson F, Dennison E. Participation in Elite Sport in Youth and Its Impact on Lifelong Bone Health. Osteology. 2024; 4(3):111-119. https://doi.org/10.3390/osteology4030009
Chicago/Turabian StyleMarriott, Amelia, Fiona Kirkham-Wilson, and Elaine Dennison. 2024. "Participation in Elite Sport in Youth and Its Impact on Lifelong Bone Health" Osteology 4, no. 3: 111-119. https://doi.org/10.3390/osteology4030009
APA StyleMarriott, A., Kirkham-Wilson, F., & Dennison, E. (2024). Participation in Elite Sport in Youth and Its Impact on Lifelong Bone Health. Osteology, 4(3), 111-119. https://doi.org/10.3390/osteology4030009






