Sulfur-Containing Polymers Prepared from Fatty Acid-Derived Monomers: Application of Atom-Economical Thiol-ene/Thiol-yne Click Reactions and Inverse Vulcanization Strategies
Abstract
:1. Introduction
1.1. Motivation for this Review
1.2. Sources and Classification of Fatty Acids
2. Applications of Thiol-ene and Thiol-yne Reactions
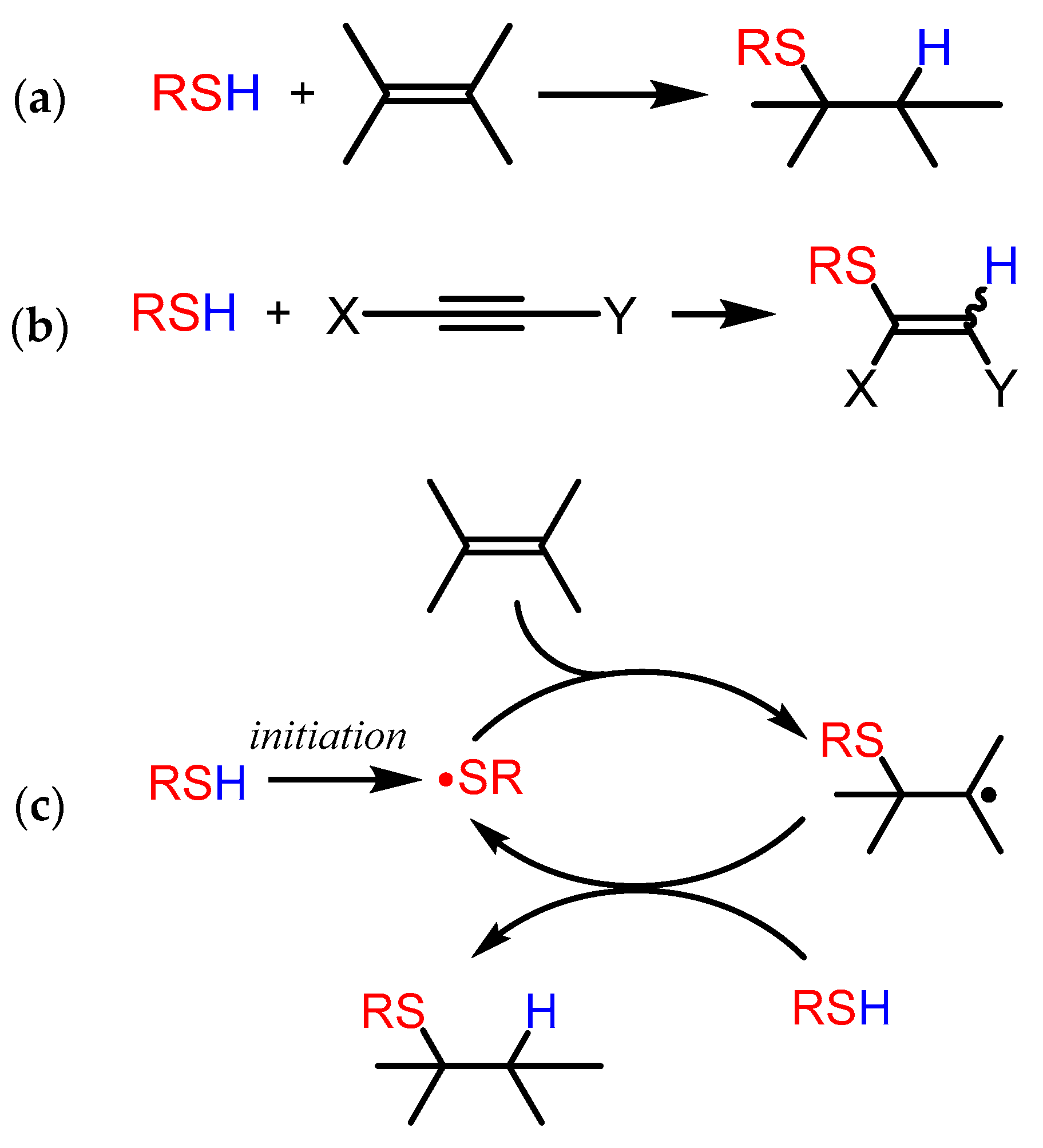
2.1. General Overview of Thiol-ene and Thiol-yne Reactions
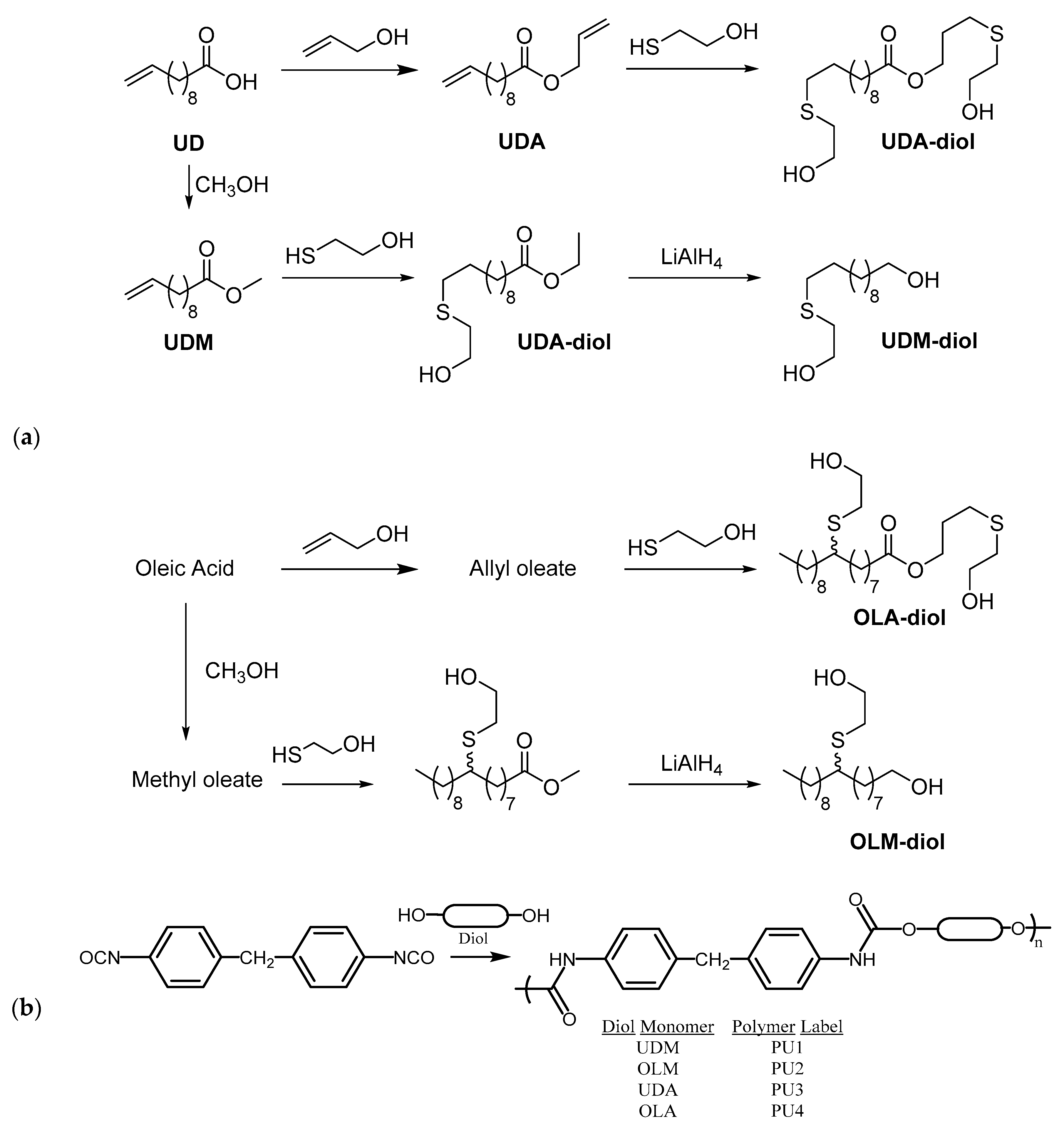
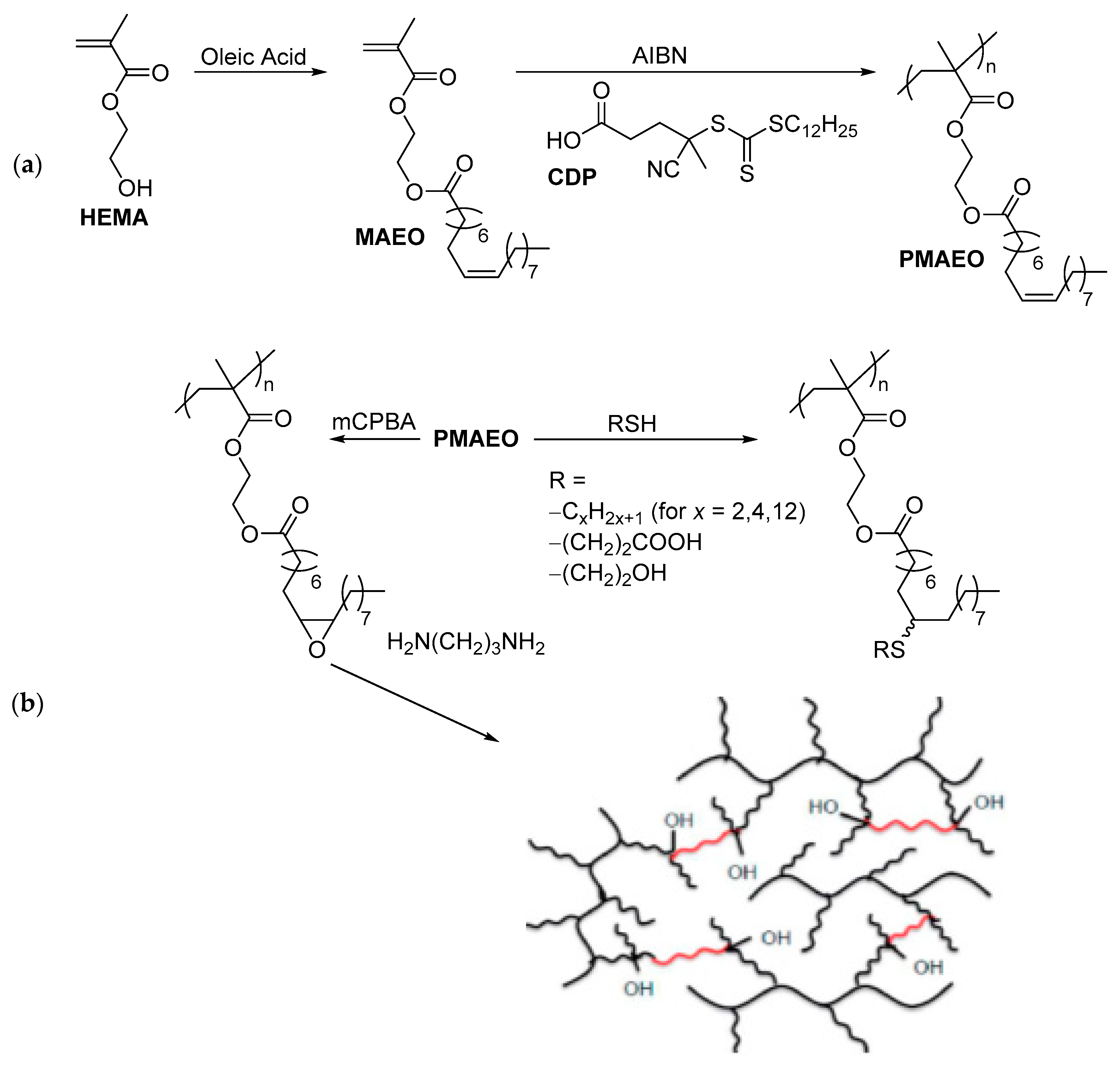
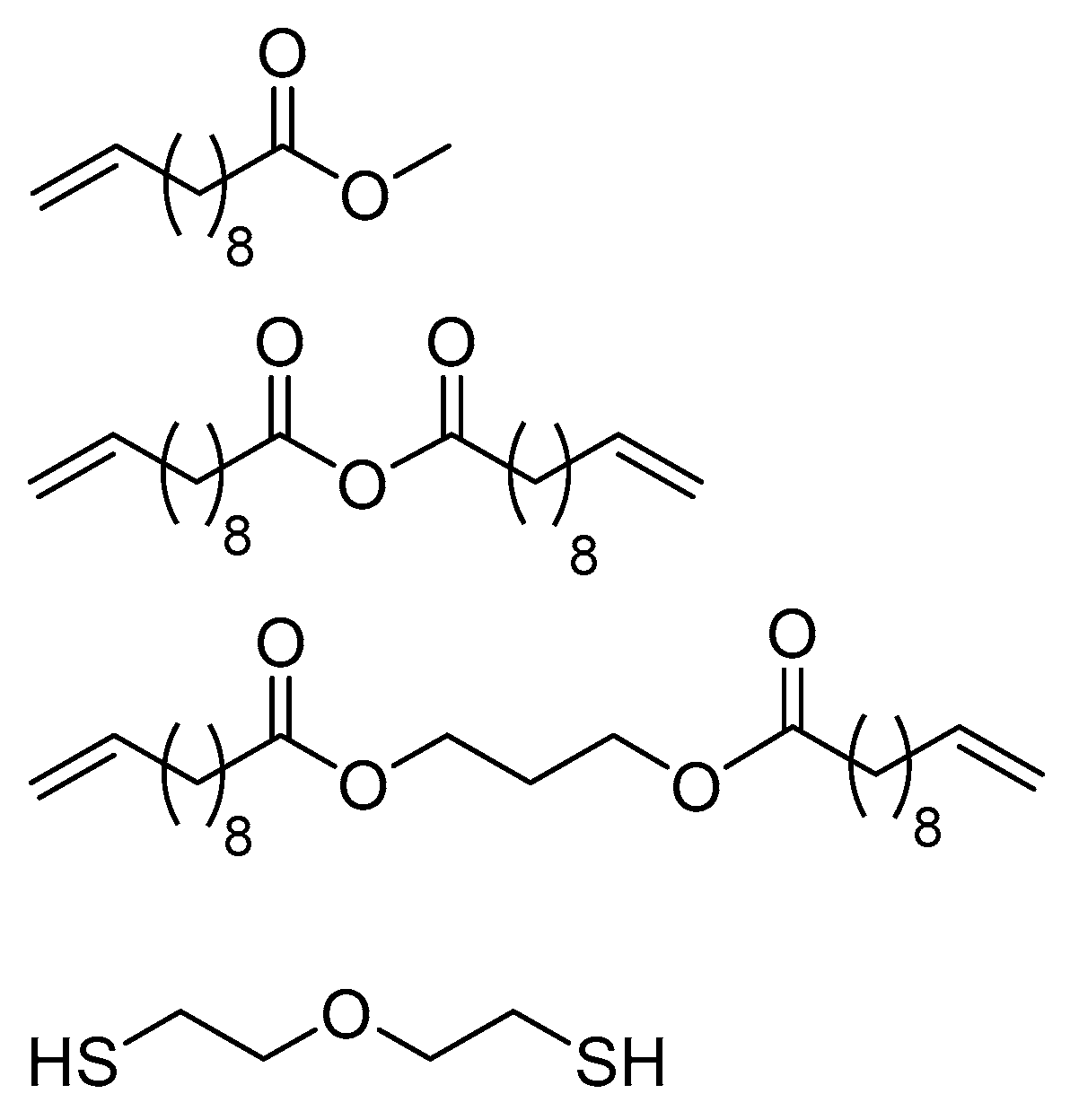
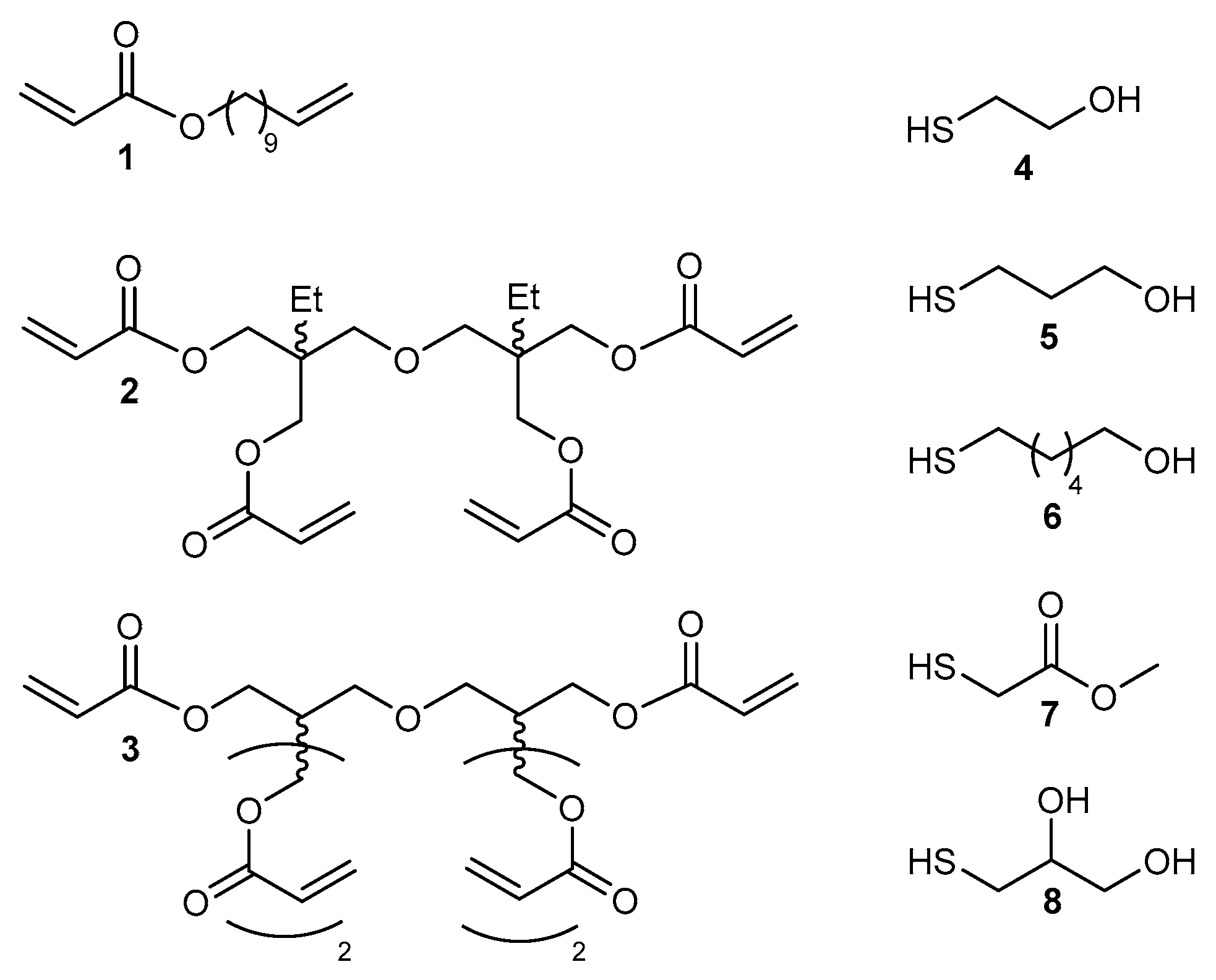
2.2. Applications of Thiol-ene Reactions to Fatty Acid-Derived Polymers
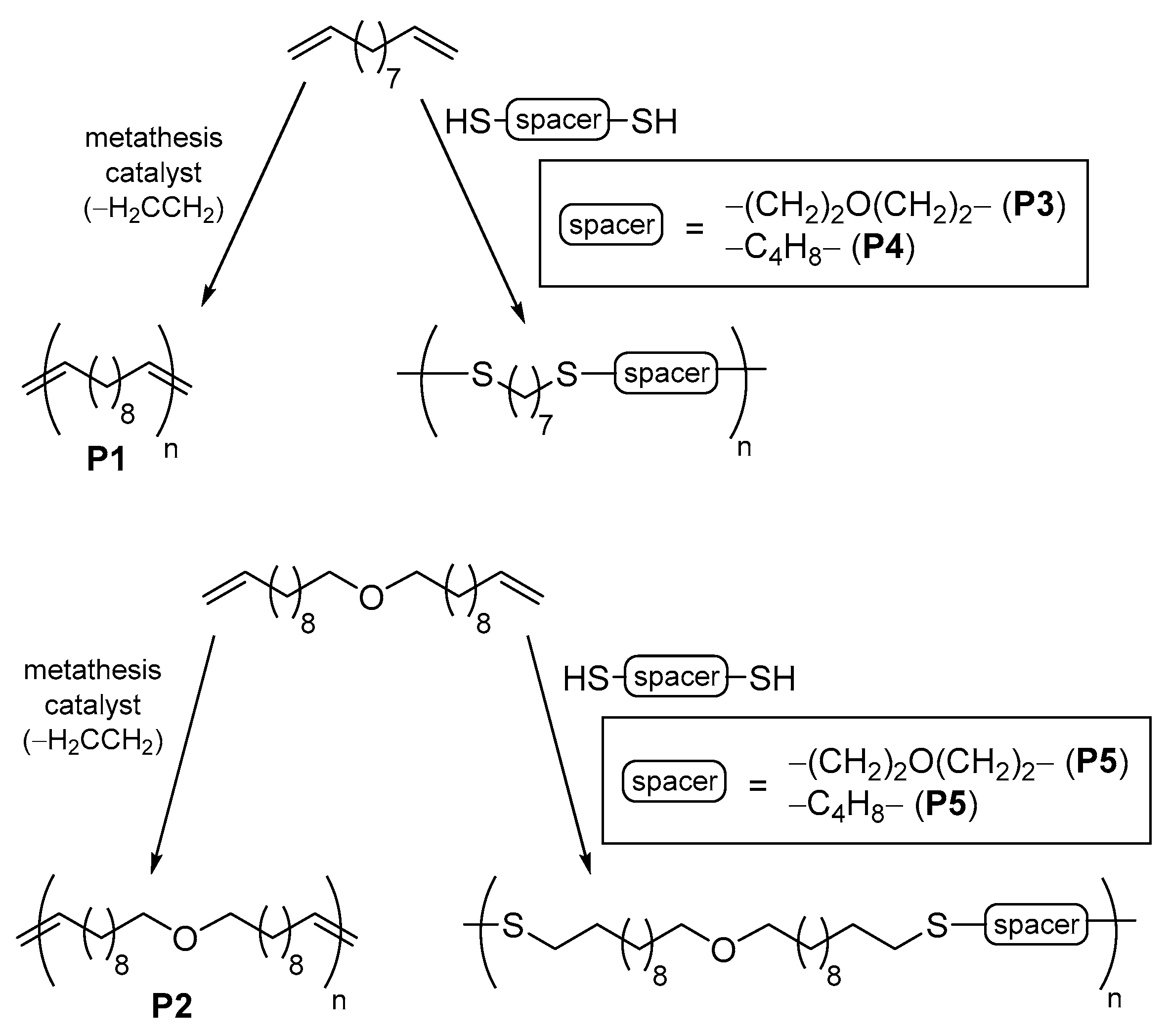
2.3. Comparison or Combination of Thiol-ene and Olefin Metathesis Reactions
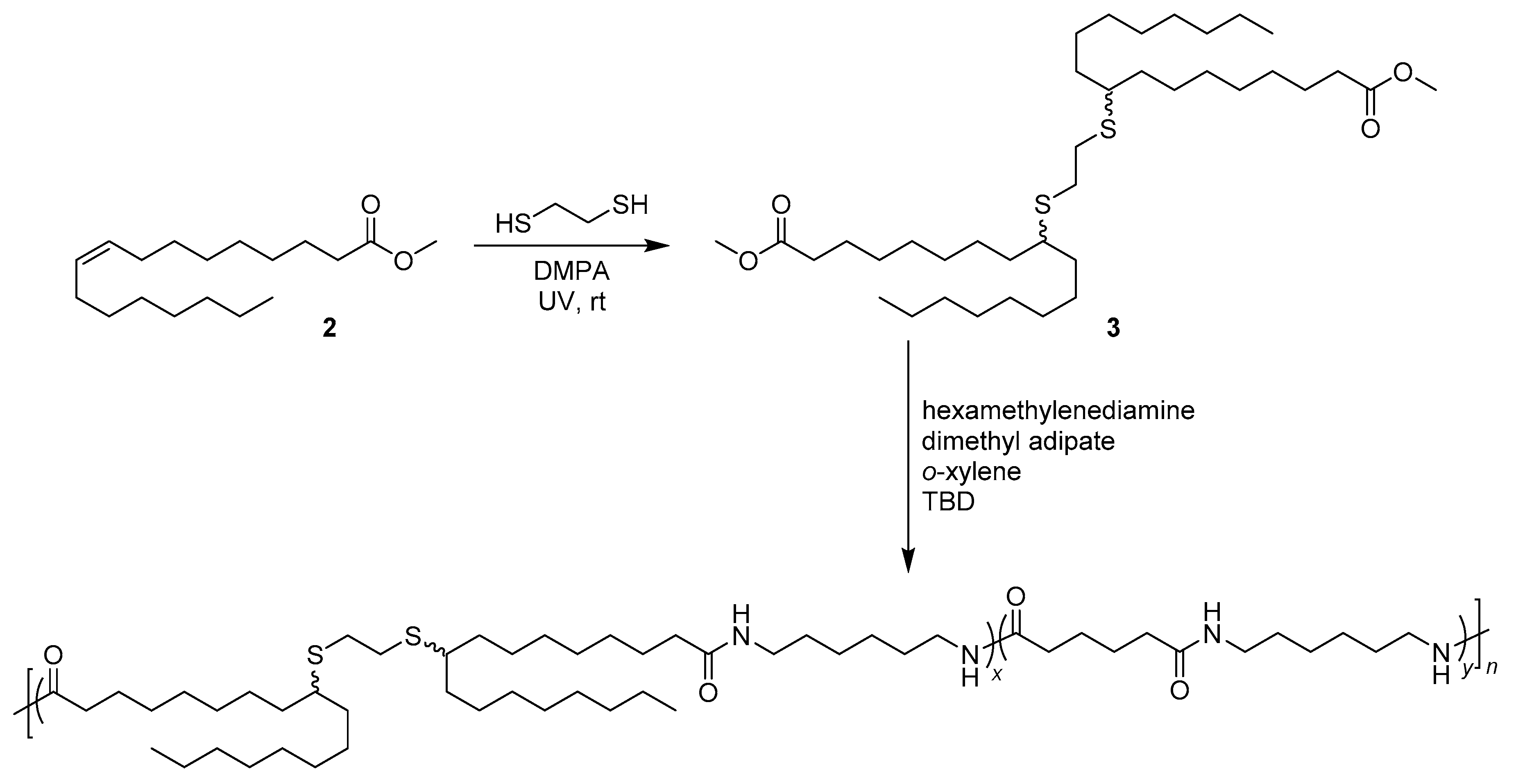
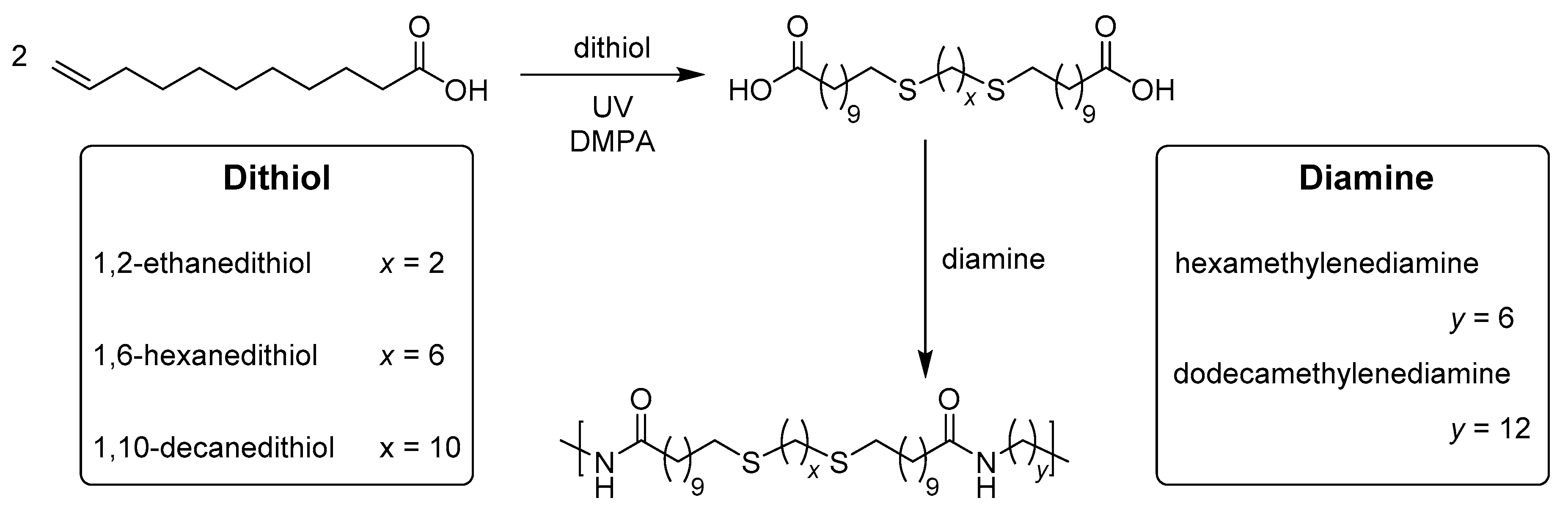
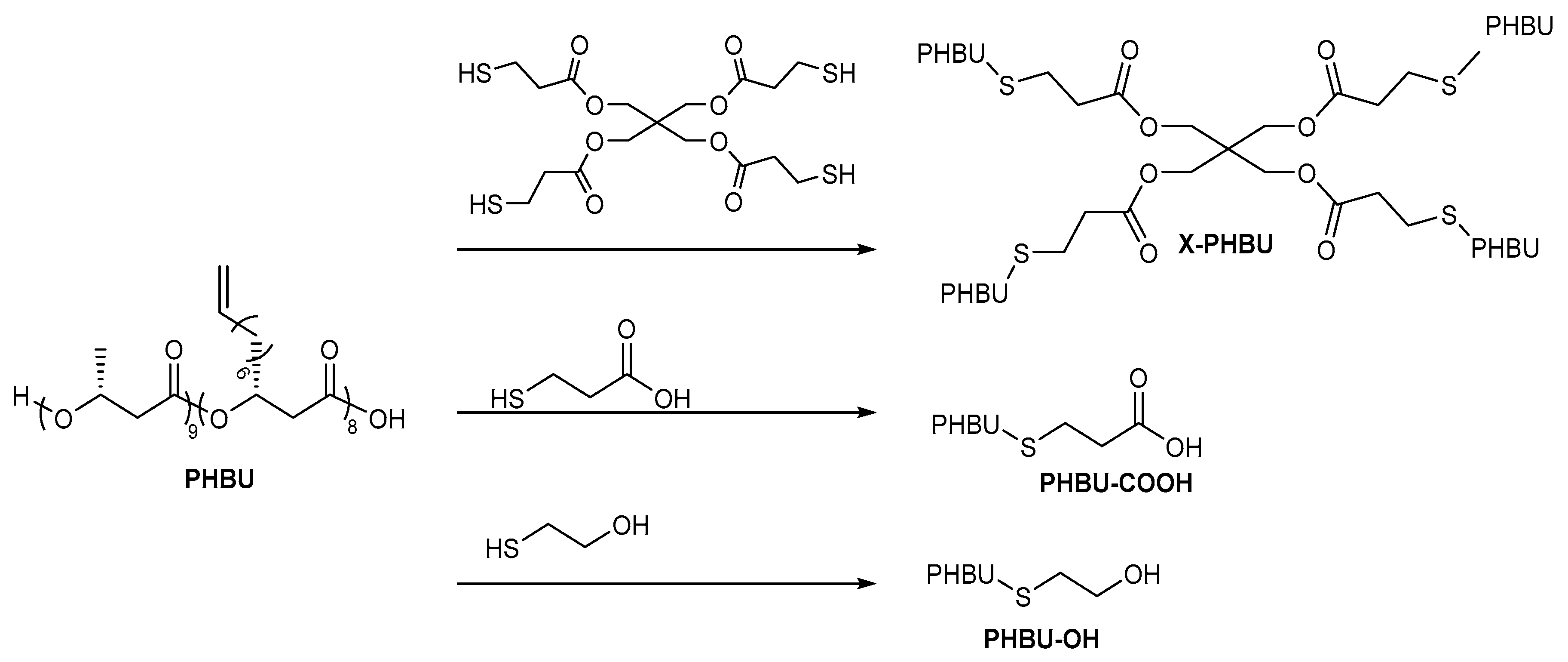
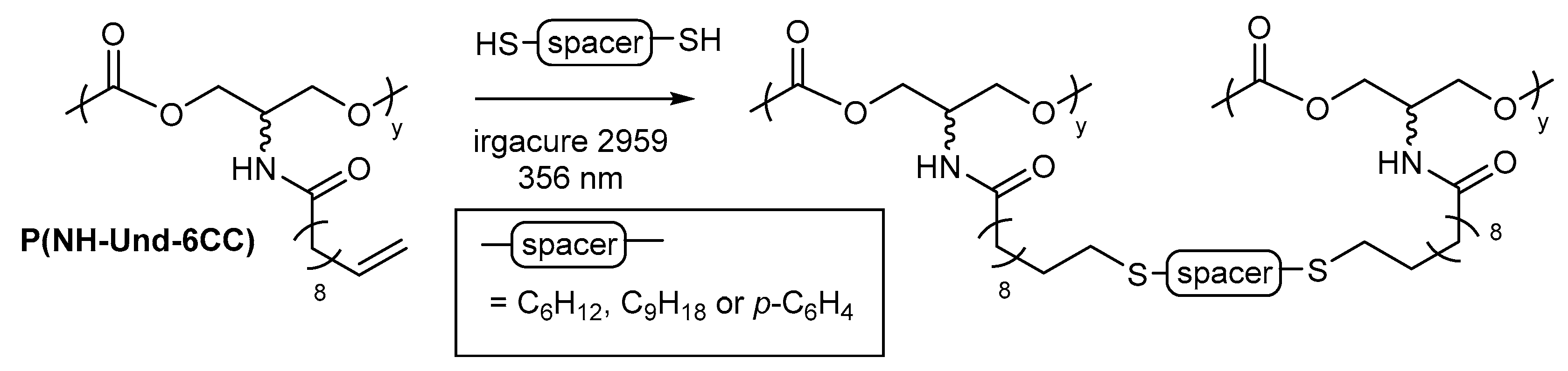
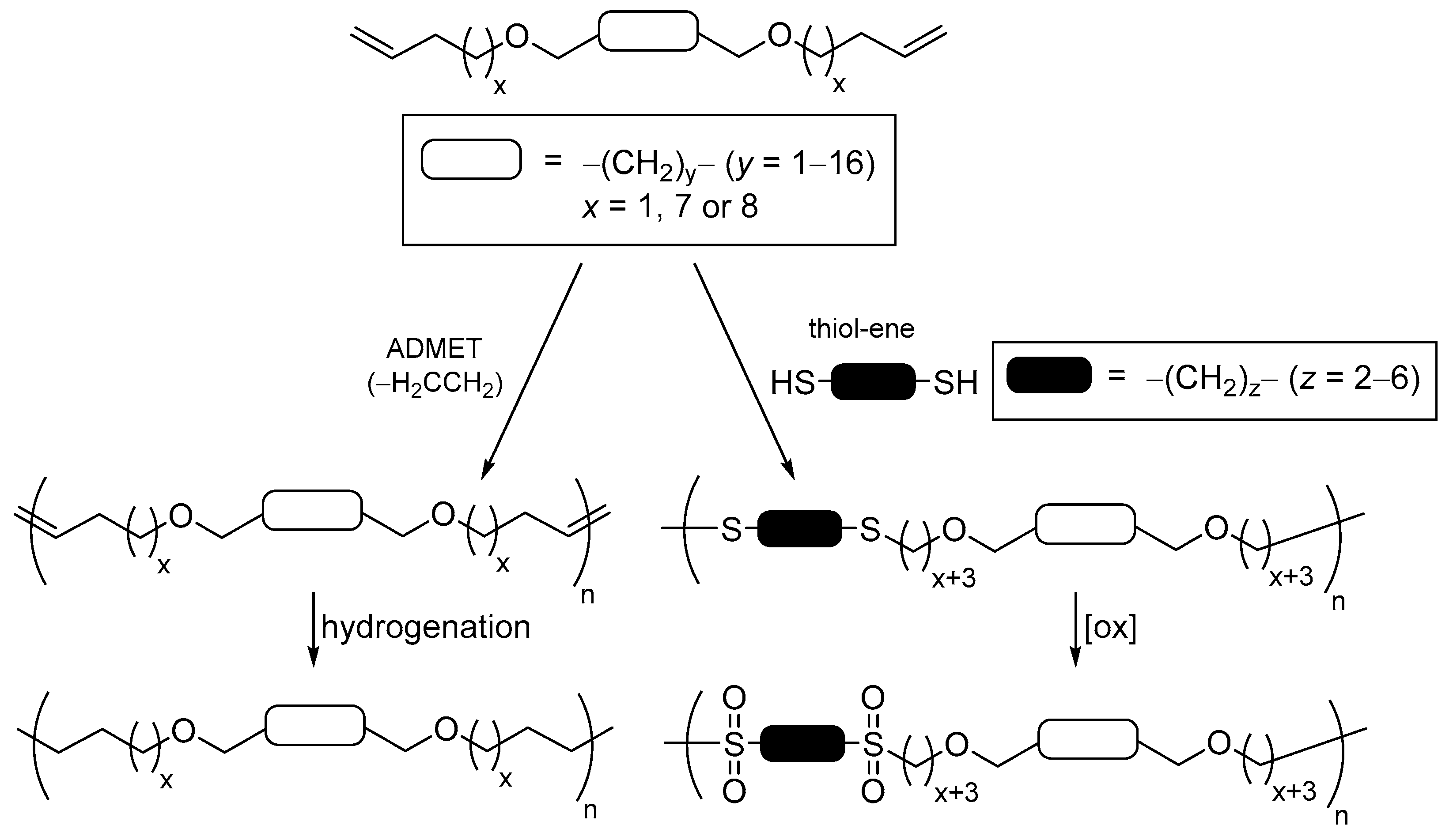
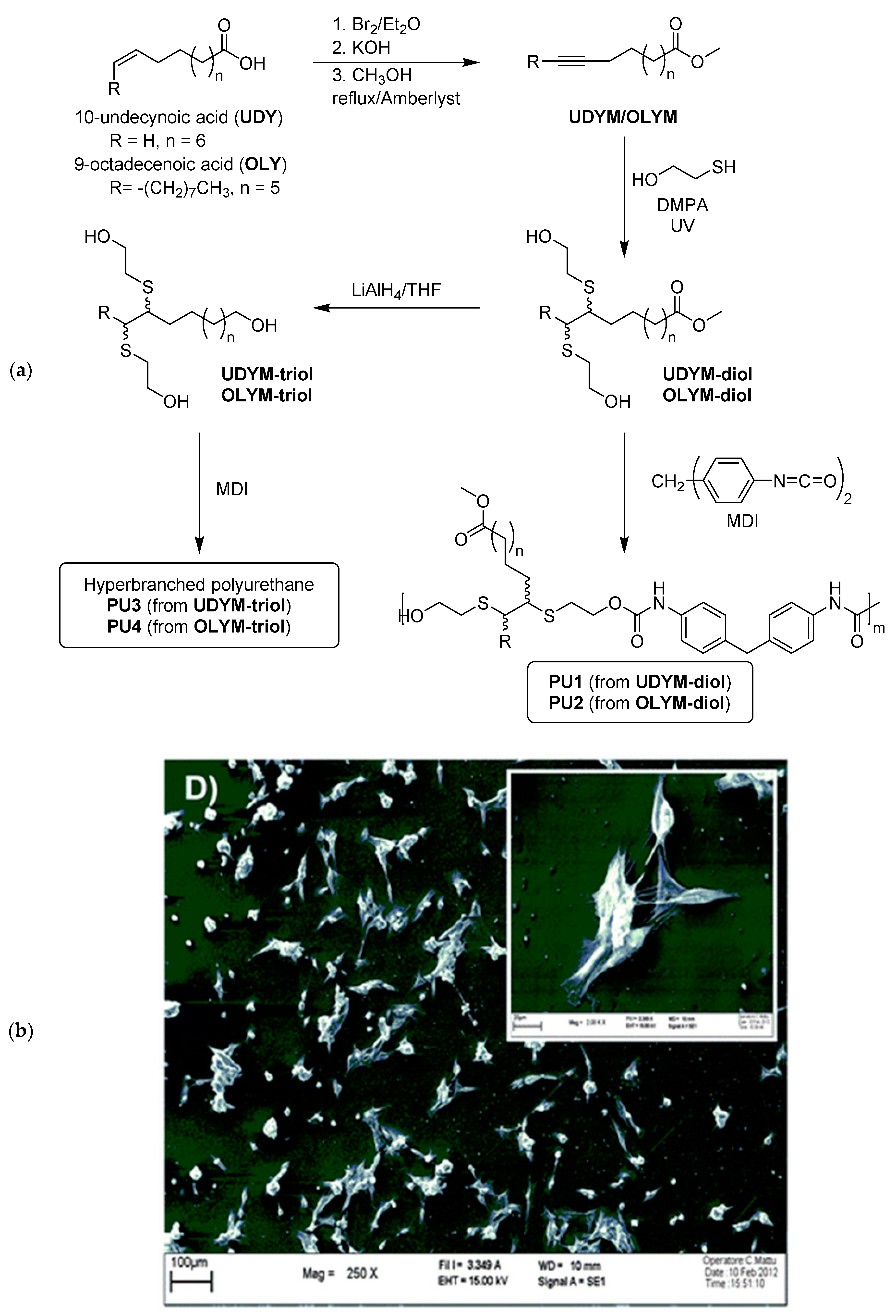
2.4. General Applications of Thiol-yne Reactions to Fatty Acid-Derived Polymers
3. Applications of Vulcanization and Inverse Vulcanization
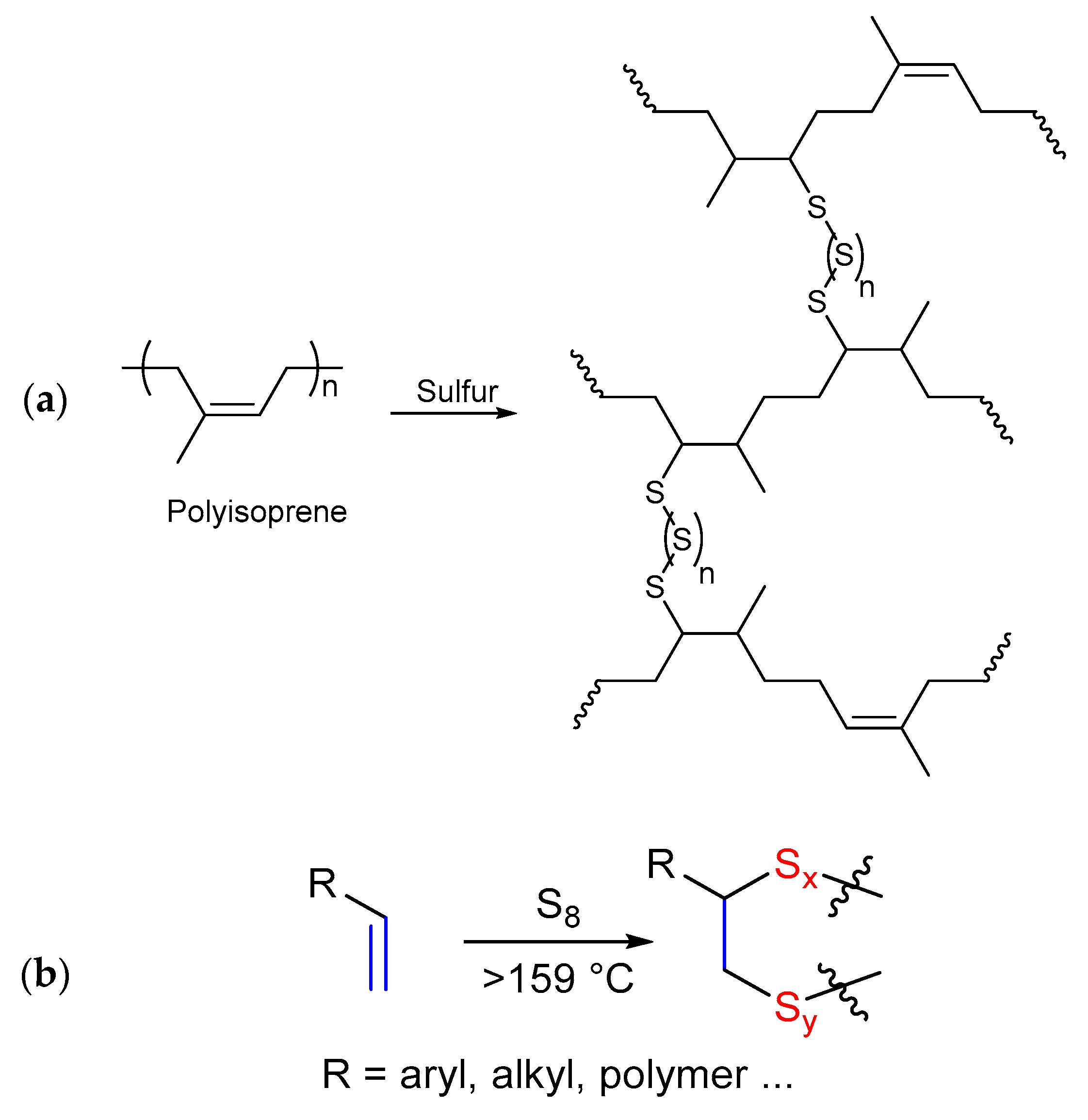
3.1. Inverse Vulcanization versus Classical Vulcanization
3.2. Applications of Vulcanization/Inverse Vulcanization to Fatty Acid-Derived Polymers
4. Recycling and Environmental Stability of Organosulfur Polymers
5. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Per-Capita Consumption of PE, PP & PVC Resins. 2014. Available online: https://www.plasticsinsight.com/world-per-capita-consumption-pe-pp-pvc-resins-2014/ (accessed on 7 April 2019).
- Malik, N.; Kumar, P.; Shrivastava, S.; Ghosh, S.B. An overview on PET waste recycling for application in packaging. Int. J. Plast. Technol. 2016, 21, 1–24. [Google Scholar] [CrossRef]
- Kaufmann, J. New materials for sports equipment made of anisotropic fiber-reinforced plastics with stiffness related coupling effect. Procedia Eng. 2015, 112, 140–145. [Google Scholar] [CrossRef] [Green Version]
- McKeen, L.W. Plastics Used in Medical Devices. In Handbook of Polymer Applications in Medicine and Medical Devices; Elsevier: Amsterdam, The Netherlands, 2014; pp. 21–53. [Google Scholar]
- Day, M.; Cooney, J.; Touchette-Barrette, C.; Sheehan, S. Pyrolysis of mixed plastics used in the electronics industry. J. Anal. Appl. Pyrolysis 1999, 52, 199–224. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.; Choi, J. Adverse outcome pathways potentially related to hazard identification of microplastics based on toxicity mechanisms. Chemosphere 2019, 231, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef] [PubMed]
- Gestoso, I.; Cacabelos, E.; Ramalhosa, P.; Canning-Clode, J. Plasticrusts: A new potential threat in the Anthropocene’s rocky shores. Sci. Total Environ. 2019, 687, 413–415. [Google Scholar] [CrossRef]
- Thiounn, T.; Smith, R.C. Advances and approaches for chemical recycling of plastic waste. J. Appl. Polym. Sci. 2020, 58, 1347–1364. [Google Scholar] [CrossRef] [Green Version]
- Karunarathna, M.S.; Smith, R.C. Valorization of lignin as a sustainable component of structural materials and composites: Advances from 2011 to 2019. Sustainability 2020, 12, 734. [Google Scholar] [CrossRef] [Green Version]
- Kristufek, S.L.; Wacker, K.T.; Tsao, Y.-Y.T.; Su, L.; Wooley, K.L. Monomer design strategies to create natural product-based polymer materials. Nat. Prod. Rep. 2017, 34, 433–459. [Google Scholar] [CrossRef]
- Yao, K.; Tang, C. Controlled polymerization of next-generation renewable monomers and beyond. Macromolecules 2013, 46, 1689–1712. [Google Scholar] [CrossRef]
- Leung, D.Y.; Wu, X.; Leung, M. A review on biodiesel production using catalyzed transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Ma, F.; Hanna, M.A. Biodiesel production: A review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Demirbaş, A. Biodiesel fuels from vegetable oils via catalytic and non-catalytic supercritical alcohol transesterifications and other methods: A survey. Energy Convers. Manag. 2003, 44, 2093–2109. [Google Scholar] [CrossRef]
- Ramadhas, A.S.; Jayaraj, S.; Muraleedharan, C. Biodiesel production from high FFA rubber seed oil. Fuel 2005, 84, 335–340. [Google Scholar] [CrossRef]
- Canakci, M.; Van Gerpen, J. Biodiesel production from oils and fats with high free fatty acids. Trans. ASAE 2001, 44, 1429–1436. [Google Scholar] [CrossRef]
- Mekonnen, T.; Mussone, P.; Bressler, D. Valorization of rendering industry wastes and co-products for industrial chemicals, materials and energy: Review. Crit. Rev. Biotechnol. 2016, 36, 120–131. [Google Scholar] [CrossRef]
- Pastore, C.; Lopez, A.; Mascolo, G. Efficient conversion of brown grease produced by municipal wastewater treatment plant into biofuel using aluminium chloride hexahydrate under very mild conditions. Bioresour. Technol. 2014, 155, 91–97. [Google Scholar] [CrossRef]
- Sim, Y.-L.; Meyappan, N.; Yen, N.S.; A/p, S.S.K.; Khoo, C.H.; Cheah, W.L.; Hilaire, D.S.; Pinnock, T.; Bacolod, B.; Cai, Z.B.; et al. Chemical reactions in the pyrolysis of brown grease. Fuel 2017, 207, 274–282. [Google Scholar] [CrossRef]
- Ward, P.M.L. Brown and black grease suitability for incorporation into feeds and suitability for biofuels. J. Food Prot. 2012, 75, 731–737. [Google Scholar] [CrossRef]
- Mol, J. Metathesis of unsaturated fatty acid esters and fatty oils. J. Mol. Catal. 1994, 90, 185–199. [Google Scholar] [CrossRef]
- Biermann, U.; Friedt, W.; Lang, S.; Lühs, W.; Machmüller, G.; Metzger, J.O.; Klaas, M.R.G.; Schäfer, H.J.; Schneider, M.P. New syntheses with oils and fats as renewable raw materials for the chemical industry. Angew. Chem. Int. Ed. 2000, 39, 2206–2224. [Google Scholar] [CrossRef]
- Kaminsky, W.; Fernandez, M. New polymers by copolymerization of olefins with bio oil components. Eur. J. Lipid Sci. Technol. 2008, 110, 841–845. [Google Scholar] [CrossRef]
- Ronda, J.C.; Lligadas, G.; Galià, M.; Cádiz, V. Vegetable oils as platform chemicals for polymer synthesis. Eur. J. Lipid Sci. Technol. 2011, 113, 46–58. [Google Scholar] [CrossRef]
- De Espinosa, L.M.; Meier, M.A.R. Plant oils: The perfect renewable resource for polymer science?! Eur. Polym. J. 2011, 47, 837–852. [Google Scholar] [CrossRef] [Green Version]
- Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. Monomers and polymers from plant oils via click chemistry reactions. J. Polym. Sci. Part A: Polym. Chem. 2013, 51, 2111–2124. [Google Scholar] [CrossRef]
- Adekunle, K.F. A review of vegetable oil-based polymers: Synthesis and applications. Open J. Polym. Chem. 2015, 5, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Kunduru, K.R.; Basu, A.; Zada, M.H.; Domb, A. Castor oil-based biodegradable polyesters. Biomacromolecules 2015, 16, 2572–2587. [Google Scholar] [CrossRef]
- Doll, K.M.; Moser, B.R.; Liu, Z.; Murray, R.E.; Sharma, B.K.; Biresaw, G. Producing monomers and polymers from plant oils. Environ. Frindly Biobased Lubr. 2016, 79–98. [Google Scholar] [CrossRef]
- Jain, J.P.; Sokolsky-Papkov, M.; Kumar, N.; Domb, A. Fatty acid based biodegradable polymer. Polym. Rev. 2008, 48, 156–191. [Google Scholar] [CrossRef]
- Maisonneuve, L.; Lebarbé, T.; Grau, E.; Cramail, H. Structure–properties relationship of fatty acid-based thermoplastics as synthetic polymer mimics. Polym. Chem. 2013, 4, 5472–5517. [Google Scholar] [CrossRef] [Green Version]
- Yelchuri, V.; Srikanth, K.; Prasad, R.B.N.; Lakshmi, K.M.S. Olefin metathesis of fatty acids and vegetable oils. J. Chem. Sci. 2019, 131, 39. [Google Scholar] [CrossRef] [Green Version]
- Lomege, J.; Lapinte, V.; Negrell, C.; Robin, J.-J.; Caillol, S. Fatty acid-based radically polymerizable monomers: From novel poly(meth)acrylates to cutting-edge properties. Biomacromolecules 2018, 20, 4–26. [Google Scholar] [CrossRef] [PubMed]
- Hoyle, C.E.; Bowman, C.N. Thiol-ene click chemistry. Angew. Chem. Int. Ed. 2010, 49, 1540–1573. [Google Scholar] [CrossRef]
- Cramer, N.B.; Bowman, C.N. Thiol-ene chemistry. Chemosel. Bioorthogonal Lig. React. 2017, 1, 117–145. [Google Scholar]
- Cramer, N.B.; Bowman, C.N. Thiol-ene and thiol-yne chemistry in ideal network synthesis. RSC Polym. Chem. Ser. 2013, 6, 1–27. [Google Scholar]
- Türünç, O.; Meier, M.A.R. Fatty acid derived monomers and related polymers via thiol-ene (Click) additions. Macromol. Rapid Commun. 2010, 31, 1822–1826. [Google Scholar] [CrossRef]
- González-Paz, R.J.; Lluch, C.; Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. A green approach toward oleic- and undecylenic acid-derived polyurethanes. J. Polym. Sci. Part A: Polym. Chem. 2011, 49, 2407–2416. [Google Scholar] [CrossRef]
- Desroches, M.; Caillol, S.; Auvergne, R.; Boutevin, B. Synthesis of pseudo-telechelic diols by transesterification and thiol-ene coupling. Eur. J. Lipid Sci. Technol. 2011, 114, 84–91. [Google Scholar] [CrossRef]
- Unverferth, M.; Meier, M.A.R. Selective formation of C36-dimer fatty acids via thiol-ene addition for copolyamide synthesis. Eur. J. Lipid Sci. Technol. 2016, 118, 1470–1474. [Google Scholar] [CrossRef]
- Pai, C.-C.; Jeng, R.-J.; Grossman, S.J.; Huang, J.-C. Effects of moisture on thermal and mechanical properties of nylon-6,6. Adv. Polym. Technol. 1989, 9, 157–163. [Google Scholar] [CrossRef]
- Nguyen, P.H.; Spoljaric, S.; Seppälä, J. Renewable polyamides via thiol-ene ‘click’ chemistry and long-chain aliphatic segments. Polymer 2018, 153, 183–192. [Google Scholar] [CrossRef]
- Kempe, K.; Hoogenboom, R.; Schubert, U.S. A green approach for the synthesis and thiol-ene modification of alkene functio1489lized poly(2-oxazoline)s. Macromol. Rapid Commun. 2011, 32, 1484–1489. [Google Scholar] [CrossRef] [PubMed]
- Maiti, B.; Kumar, S.; De, P. Controlled RAFT synthesis of side-chain oleic acid containing polymers and their post-polymerization functionalization. RSC Adv. 2014, 4, 56415–56423. [Google Scholar] [CrossRef]
- Levine, A.C.; Heberlig, G.W.; Nomura, C.T. Use of thiol-ene click chemistry to modify mechanical and thermal properties of polyhydroxyalkanoates (PHAs). Int. J. Boil. Macromol. 2016, 83, 358–365. [Google Scholar] [CrossRef]
- Durand, P.-L.; Brège, A.; Chollet, G.; Grau, E.; Cramail, H. Simple and efficient approach toward photosensitive biobased aliphatic polycarbonate materials. ACS Macro Lett. 2018, 7, 250–254. [Google Scholar] [CrossRef] [Green Version]
- Durand, P.-L.; Chollet, G.; Grau, E.; Cramail, H. Versatile cross-linked fatty acid-based polycarbonate networks obtained by thiol–ene coupling reaction. RSC Adv. 2019, 9, 145–150. [Google Scholar] [CrossRef] [Green Version]
- Moser, B.R.; Doll, K.M.; Peterson, S.C. Renewable poly(Thioether-Ester)s from fatty acid derivatives via thiol-ene photopolymerization. J. Am. Oil Chem. Soc. 2019, 96, 825–837. [Google Scholar] [CrossRef]
- Moser, B.R. Preparation and evaluation of multifunctional branched diesters as fuel property enhancers for biodiesel and petroleum diesel fuels. Energy Fuels 2014, 28, 3262–3270. [Google Scholar] [CrossRef]
- Türünç, O.; Meier, M.A.R. Thiol-ene vs. ADMET: A complementary approach to fatty acid-based biodegradable polymers. Green Chem. 2011, 13, 314–320. [Google Scholar] [CrossRef]
- Türünç, O.; De Espinosa, L.M.; Meier, M.A.R. Renewable polyethylene mimics derived from castor oil. Macromol. Rapid Commun. 2011, 32, 1357–1361. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-P.; Zhou, X.-P.; Cui, W.; Xie, X.; Tong, S.-Y. Toughening effect of maleic anhydride grafted linear low density polyethylene on linear low density polyethylene. J. Mater. Sci. 2008, 43, 4290–4296. [Google Scholar] [CrossRef]
- Unverferth, M.; Meier, M.A.R. Tuning the polarity of ADMET derived star-shaped polymers via thiol-ene chemistry. Polymer 2014, 55, 5571–5575. [Google Scholar] [CrossRef]
- Dannecker, P.-K.; Biermann, U.; Sink, A.; Bloesser, F.R.; Metzger, J.O.; Meier, M.A.R. Fatty acid–derived aliphatic long chain polyethers by a combination of catalytic ester reduction and ADMET or thiol-ene polymerization. Macromol. Chem. Phys. 2018, 220, 1800440. [Google Scholar] [CrossRef]
- González-Paz, R.J.; Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. Thiol–yne reaction of alkyne-derivatized fatty acids: Biobased polyols and cytocompatibility of derived polyurethanes. Polym. Chem. 2012, 3, 2471–2478. [Google Scholar] [CrossRef]
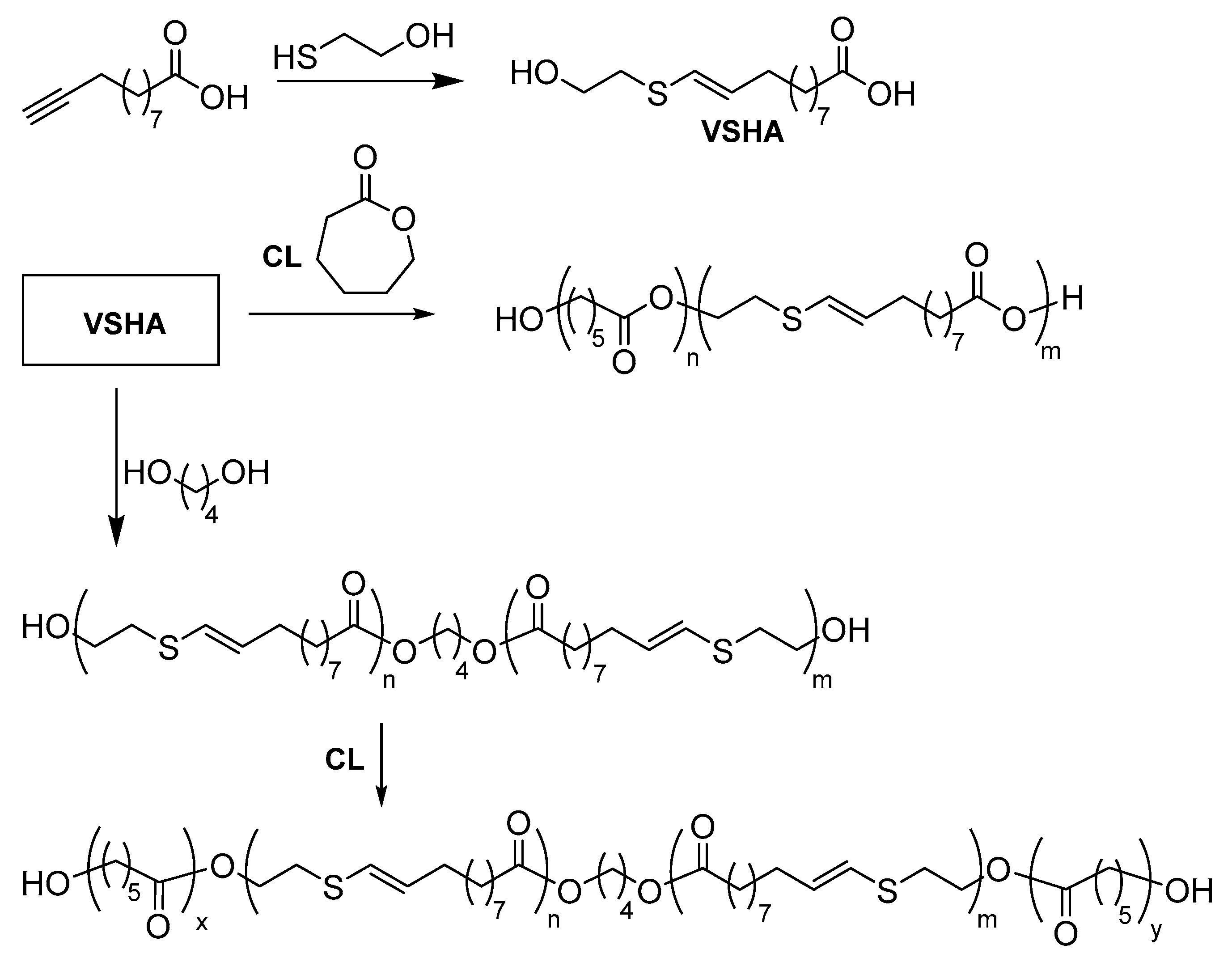
- Beyazkilic, Z.; Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. Vinylsulfide-containing polyesters and copolyesters from fatty acids: Thiol-yne monomer synthesis and thiol-ene functionalization. Macromol. Chem. Phys. 2014, 215, 2248–2259. [Google Scholar] [CrossRef]
- Goodyear, C. Improvement in India—Rubber Fabrics. US3633A, 9 March 1844. [Google Scholar]
- Chung, W.J.; Griebel, J.J.; Kim, E.T.; Yoon, H.; Simmonds, A.G.; Ji, H.J.; Dirlam, P.T.; Glass, R.S.; Wie, J.J.; Nguyen, N.A.; et al. The use of elemental sulfur as an alternative feedstock for polymeric materials. Nat. Chem. 2013, 5, 518–524. [Google Scholar] [CrossRef]
- Griebel, J.J.; Li, G.; Glass, R.S.; Char, K.; Pyun, J. Kilogram scale inverse vulcanization of elemental sulfur to prepare high capacity polymer electrodes for Li-S batteries. J. Polym. Sci. Part A Polym. Chem. 2014, 53, 173–177. [Google Scholar] [CrossRef] [Green Version]
- Karunarathna, M.S.; Lauer, M.K.; Tennyson, A.G.; Smith, R.C. Copolymerization of an aryl halide and elemental sulfur as a route to high sulfur content materials. Polym. Chem. 2020, 11, 1621–1628. [Google Scholar] [CrossRef]
- Thiounn, T.; Lauer, M.K.; Bedford, M.S.; Smith, R.C.; Tennyson, A.G. Thermally-healable network solids of sulfur-crosslinked poly(4-allyloxystyrene). RSC Adv. 2018, 8, 39074–39082. [Google Scholar] [CrossRef] [Green Version]
- Lopez, C.V.; Maladeniya, C.P.; Smith, R.C. Lithium-sulfur batteries: Advances and trends. Electrochem 2020, 1, 16. [Google Scholar] [CrossRef]
- Worthington, M.J.H.; Kucera, R.L.; Chalker, J.M. Green chemistry and polymers made from sulfur. Green Chem. 2017, 19, 2748–2761. [Google Scholar] [CrossRef] [Green Version]
- Chalker, J.M.; Worthington, M.J.H.; Lundquist, N.A.; Esdaile, L.J. Synthesis and applications of polymers made by inverse vulcanization. Top. Curr. Chem. 2019, 377, 16. [Google Scholar] [CrossRef] [PubMed]
- Wadi, V.S.; Jena, K.K.; Khawaja, S.Z.; Ranagraj, V.M.; Alhassan, S.M. Preparation and processing of porous sulfur foams having low thermal conductivity. RSC Adv. 2019, 9, 4397–4403. [Google Scholar] [CrossRef] [Green Version]
- Abraham, A.M.; Kumar, S.V.; Alhassan, S.M. Porous sulphur copolymer for gas-phase mercury removal and thermal insulation. Chem. Eng. J. 2018, 332, 1–7. [Google Scholar] [CrossRef]
- Zhang, B.; Petcher, S.; Hasell, T. A ternary system for delayed curing inverse vulcanisation. Chem. Commun. 2019, 55, 10681–10684. [Google Scholar] [CrossRef]
- Westerman, C.R.; Jenkins, C.L. Dynamic sulfur bonds initiate polymerization of vinyl and allyl ethers at mild temperatures. Macromolecules 2018, 51, 7233–7238. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, H.; Yan, P.; Petcher, S.; Hasell, T. Inverse vulcanization below the melting point of sulfur. Mater. Chem. Front. 2020, 4, 669–675. [Google Scholar] [CrossRef]
- Wu, X.; Smith, J.A.; Petcher, S.; Zhang, B.; Parker, D.J.; Griffin, J.M.; Hasell, T. Catalytic inverse vulcanization. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Tonkin, S.J.; Gibson, C.T.; Campbell, J.A.; Lewis, D.A.; Karton, A.; Hasell, T.; Chalker, J.M. Chemically induced repair, adhesion, and recycling of polymers made by inverse vulcanization. Chem. Sci. 2020, 11, 5537–5546. [Google Scholar] [CrossRef]
- Lundquist, N.; Tikoalu, A.; Worthington, M.; Shapter, R.; Tonkin, S.; Stojcevski, F.; Mann, M.; Gibson, C.; Gascooke, J.; Karton, A.; et al. Reactive compression molding post-inverse vulcanization: A method to assemble, recycle, and repurpose sulfur polymers and composites. Chem. Eur. J. 2020, 26, 10035–10044. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Green, S.J.; Petcher, S.; Parker, D.J.; Zhang, B.; Worthington, M.J.H.; Wu, X.; Kelly, C.A.; Baker, T.; Gibson, C.T.; et al. Crosslinker copolymerization for property control in inverse vulcanization. Chem. A Eur. J. 2019, 25, 10433–10440. [Google Scholar] [CrossRef] [PubMed]
- Thiounn, T.; Karunarathna, M.S.; Slann, L.M.; Lauer, M.K.; Smith, R.C. Sequential crosslinking for mechanical property development in high sulfur content composites. J. Poly. Sci. 2020. Ahead of print. [Google Scholar] [CrossRef]
- Oishi, S.; Oi, K.; Kuwabara, J.; Omoda, R.; Aihara, Y.; Fukuda, T.; Takahashi, T.; Choi, J.-C.; Watanabe, M.; Kanbara, T. Synthesis and characterization of sulfur-based polymers from elemental sulfur and algae oil. ACS Appl. Polym. Mater. 2019, 1, 1195–1202. [Google Scholar] [CrossRef]
- Parker, D.J.; Jones, H.A.; Petcher, S.; Cervini, L.; Griffin, J.M.; Akhtar, R.; Hasell, T. Low cost and renewable sulfur-polymers by inverse vulcanization, and their potential for mercury capture. J. Mater. Chem. A 2017, 5, 11682–11692. [Google Scholar] [CrossRef] [Green Version]
- Gomez, I.; Leonet, O.; Blazquez, J.A.; Mecerreyes, D.; Blazquez, A. Inverse vulcanization of sulfur using natural dienes as sustainable materials for lithium-sulfur batteries. ChemSusChem 2016, 9, 3419–3425. [Google Scholar] [CrossRef]
- Maladeniya, C.P.; Karunarathna, M.S.; Lauer, M.K.; Lopez, C.V.; Thiounn, T.; Smith, R.C. A role for terpenoid cyclization in the atom economical polymerization of terpenoids with sulfur to yield durable composites. Mater. Adv. 2020, 1, 1665–1674. [Google Scholar] [CrossRef]
- Tikoalu, A.D.; Lundquist, N.A.; Chalker, J.M. Mercury sorbents made by inverse vulcanization of sustainable triglycerides: The plant oil structure influences the rate of mercury removal from water. Adv. Sustain. Syst. 2020, 4, 1900111. [Google Scholar] [CrossRef]
- Mann, M.; Kruger, J.E.; Andari, F.; McErlean, J.; Gascooke, J.R.; Smith, J.A.; Worthington, M.J.H.; McKinley, C.C.C.; Campbell, J.A.; Lewis, D.A.; et al. Sulfur polymer composites as controlled-release fertilizers. Org. Biomol. Chem. 2019, 17, 1929–1936. [Google Scholar] [CrossRef]
- Worthington, M.J.H.; Shearer, C.J.; Esdaile, L.J.; Campbell, J.A.; Gibson, C.T.; Legg, S.K.; Yin, Y.; Lundquist, N.A.; Gascooke, J.R.; Albuquerque, I.S.; et al. Sustainable polysulfides for oil spill remediation: Repurposing industrial waste for environmental benefit. Adv. Sustain. Syst. 2018, 2, 1800024. [Google Scholar] [CrossRef] [Green Version]
- Lundquist, N.A.; Worthington, M.J.H.; Adamson, N.; Gibson, C.T.; Johnston, A.M.R.; Ellis, A.V.; Chalker, J.M. Polysulfides made from re-purposed waste are sustainable materials for removing iron from water. RSC Adv. 2018, 8, 1232–1236. [Google Scholar] [CrossRef] [Green Version]
- Esdaile, L.J.; Chalker, J.M. The mercury problem in artisanal and small-scale gold mining. Chem. A Eur. J. 2018, 24, 6905–6916. [Google Scholar] [CrossRef] [Green Version]
- Worthington, M.J.H.; Kucera, R.L.; Albuquerque, I.S.; Gibson, C.T.; Sibley, A.; Slattery, A.; Campbell, J.A.; Alboaiji, S.F.K.; Muller, K.A.; Young, J.; et al. Laying waste to mercury: Inexpensive sorbents made from sulfur and recycled cooking oils. Chem. A Eur. J. 2017, 23, 16219–16230. [Google Scholar] [CrossRef] [PubMed]
- Hoefling, A.; Lee, Y.J.; Theato, P. Sulfur-based polymer composites from vegetable oils and elemental sulfur: A sustainable active material for Li-S batteries. Macromol. Chem. Phys. 2016, 218, 1600303. [Google Scholar] [CrossRef]
- Lopez, C.V.; Karunarathna, M.S.; Lauer, M.K.; Maladeniya, C.P.; Thiounn, T.; Ackley, E.D.; Smith, R.C. High strength, acid-resistant composites from canola, sunflower, or linseed oils: Influence of triglyceride unsaturation on material properties. J. Appl. Polym. Sci. 2020, 58, 2259–2266. [Google Scholar] [CrossRef]
- Duarte, M.E.; Huber, B.; Theato, P.; Mutlu, H. The unrevealed potential of elemental sulfur for the synthesis of high sulfur content bio-based aliphatic polyesters. Polym. Chem. 2020, 11, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.D.; Smith, R.C.; Tennyson, A.G. Carbon-negative polymer cements by copolymerization of waste sulfur, oleic acid, and pozzolan cements. Sust. Chem. Pharm. 2020, 16, 100249. [Google Scholar]
- Smith, A.D.; McMillen, C.D.; Smith, R.C.; Tennyson, A.G. Copolymers by inverse vulcanization of sulfur with pure or technical-grade unsaturated fatty acids. J. Appl. Polym. Sci. 2020, 58, 438–445. [Google Scholar] [CrossRef]
- Smith, A.D.; Thiounn, T.; Lyles, E.W.; Kibler, E.K.; Smith, R.C.; Tennyson, A.G. Combining agriculture and energy industry waste products to yield recyclable, thermally healable copolymers of elemental sulfur and oleic acid. J. Polym. Sci. Part A: Polym. Chem. 2019, 57, 1704–1710. [Google Scholar] [CrossRef]
- Hasell, T.; Yan, P.; Zhao, W.; Zhang, B.; Petcher, S.; Smith Jessica, A.; Parker Douglas, J.; Cooper Andrew, I.; Jiang, L.; Lei, J. Inverse vulcanized polymers with shape memory, enhanced mechanical properties, and vitrimer behavior. Angew. Chem. 2020, 59, 2–10. [Google Scholar]
- Thiounn, T.; Tennyson, A.G.; Smith, R.C. Durable, acid-resistant copolymers from industrial by-product sulfur and microbially-produced tyrosine. RSC Adv. 2019, 9, 31460–31465. [Google Scholar] [CrossRef] [Green Version]
- Hoefling, A.; Nguyen, D.T.; Lee, Y.J.; Song, S.-W.; Theato, P. A sulfur–eugenol allyl ether copolymer: A material synthesized via inverse vulcanization from renewable resources and its application in Li–S batteries. Mater. Chem. Front. 2017, 1, 1818–1822. [Google Scholar] [CrossRef]
- Lauer, M.K.; Estrada-Mendoza, T.A.; McMillen, C.D.; Chumanov, G.; Tennyson, A.G.; Smith, R.C. Durable cellulose–sulfur composites derived from agricultural and petrochemical waste. Adv. Sustain. Syst. 2019, 3, 1900062. [Google Scholar] [CrossRef] [Green Version]
- Karunarathna, M.S.; Tennyson, A.G.; Smith, R.C. Facile new approach to high sulfur-content materials and preparation of sulfur–lignin copolymers. J. Mater. Chem. A 2020, 8, 548–553. [Google Scholar] [CrossRef]
- Karunarathna, M.S.; Lauer, M.K.; Thiounn, T.; Smith, R.C.; Tennyson, A.G. Valorization of waste to yield recyclable composites of elemental sulfur and lignin. J. Mater. Chem. A 2019, 7, 15683–15690. [Google Scholar] [CrossRef]
- Lauer, M.K.; Karunarathna, M.S.; Tennyson, A.G.; Smith, R.C. Recyclable, sustainable, and stronger than portland cement: A composite from unseparated biomass and fossil fuel waste. Mater. Adv. 2020, 1, 590–594. [Google Scholar] [CrossRef]
- Lauer, M.K.; Tennyson, A.G.; Smith, R.C. Green synthesis of thermoplastic composites from a terpenoid-cellulose ester. ACS Appl. Polym. Mater. 2020, 2, 3761–3765. [Google Scholar] [CrossRef]
- Dworakowska, S.; Le Coz, C.; Chollet, G.; Grau, E.; Cramail, H. Cross-linking of polyesters based on fatty acids. Eur. J. Lipid Sci. Technol. 2019, 121, 1900264. [Google Scholar] [CrossRef] [Green Version]
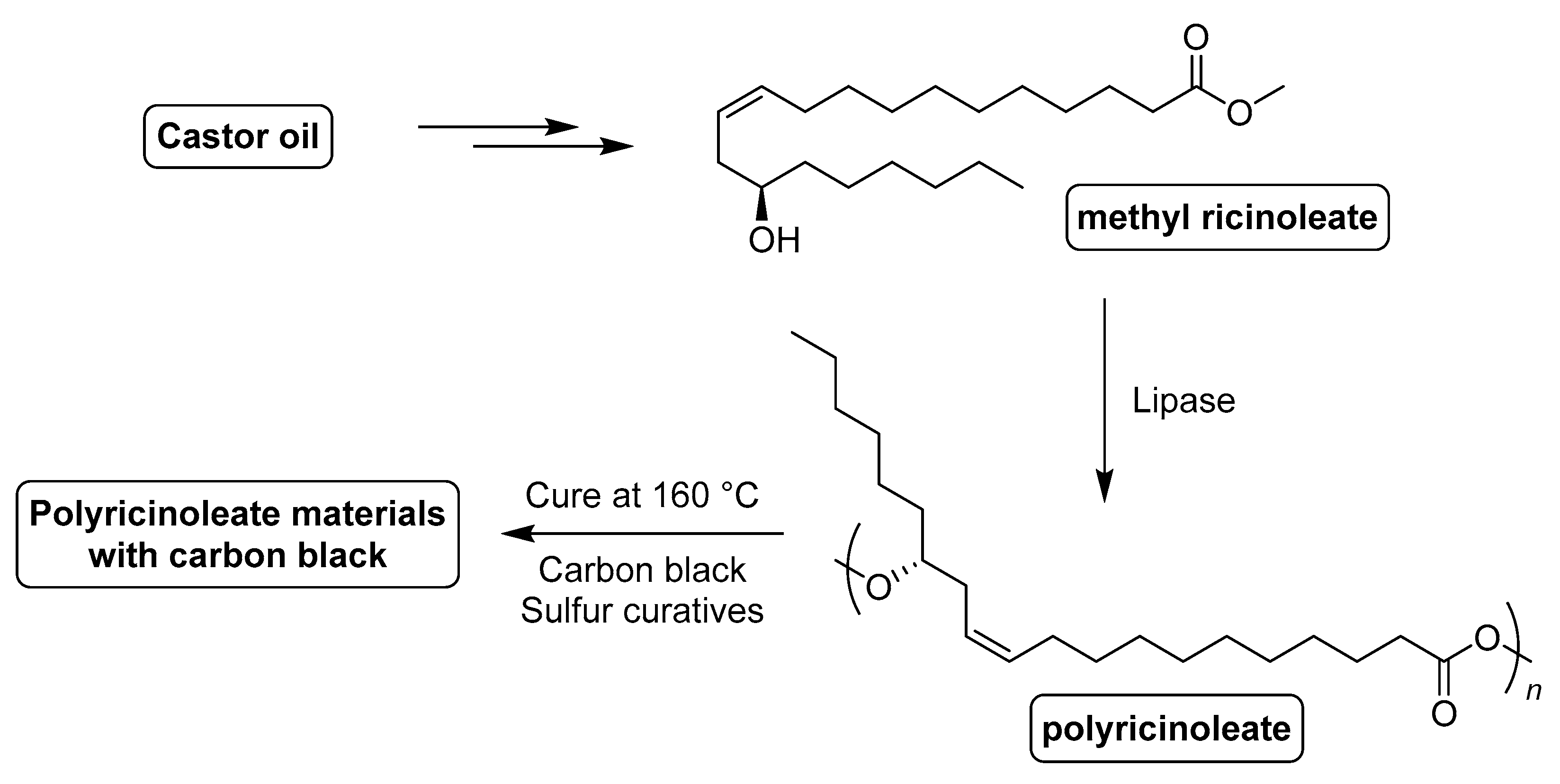
- Ebata, H.; Yasuda, M.; Toshima, K.; Matsumura, S. Poly (ricinoleic acid) based novel thermosetting elastomer. J. Oleo Sci. 2008, 57, 315–320. [Google Scholar] [CrossRef] [Green Version]
- Hernández, M.; Grande, A.M.; Dierkes, W.; Bijleveld, J.; Van Der Zwaag, S.; Garcia, S. Turning vulcanized natural rubber into a self-healing polymer: Effect of the disulfide/polysulfide ratio. ACS Sustain. Chem. Eng. 2016, 4, 5776–5784. [Google Scholar] [CrossRef]
- Xiang, H.P.; Rong, M.Z.; Zhang, M.Q. Self-healing, reshaping, and recycling of vulcanized chloroprene rubber: A case study of multitask cyclic utilization of cross-linked polymer. ACS Sustain. Chem. Eng. 2016, 4, 2715–2724. [Google Scholar] [CrossRef]
- Gordon, M.B.; French, J.M.; Wagner, N.J.; Kloxin, C.J. Dynamic bonds in covalently crosslinked polymer networks for photoactivated strengthening and healing. Adv. Mater. 2015, 27, 8007–8010. [Google Scholar] [CrossRef] [PubMed]
- Gwon, S.-W.; Ahn, E.; Shin, M. Self-healing of modified sulfur composites with calcium sulfoaluminate cement and superabsorbent polymer. Compos. Part B Eng. 2019, 162, 469–483. [Google Scholar] [CrossRef]
- Azcune, I.; Odriozola, I. Aromatic disulfide crosslinks in polymer systems: Self-healing, reprocessability, recyclability and more. Eur. Polym. J. 2016, 84, 147–160. [Google Scholar] [CrossRef]
- López, M.D.M.C.; Pernas, A.A.; Latorre, A.L.; López-Vilariño, J.; González-Rodríguez, M.V. Assessing changes on poly(ethylene terephthalate) properties after recycling: Mechanical recycling in laboratory versus postconsumer recycled material. Mater. Chem. Phys. 2014, 147, 884–894. [Google Scholar] [CrossRef]
- Rao, M.R.; Radhakrishnan, T.S. Thermal degradation of liquid polysulfide polymers: Pyrolysis–GC–MS and thermogravimetric studies. J. Appl. Polym. Sci. 1985, 30, 855–873. [Google Scholar] [CrossRef]
- Sundarrajan, S.; Surianarayanan, M.; Srinivasan, K.S.V.; Kishore, K. Thermal degradation processes in polysulfide copolymers investigated by direct pyrolysis mass spectrometry and flash pyrolysis-gas chromatography/mass spectrometry. Macromolecules 2002, 35, 3331–3337. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, Y.; Qu, S.; Da, J.; Hao, Z. H2S-selective catalytic oxidation: Catalysts and processes. ACS Catal. 2015, 5, 1053–1067. [Google Scholar] [CrossRef]
- Demirbas, A.; Alidrisi, H.; Balubaid, M.A. API gravity, sulfur content, and desulfurization of crude oil. Pet. Sci. Technol. 2014, 33, 93–101. [Google Scholar] [CrossRef]
- Yusupova, A.; Khatsrinov, A.I.; Akhmetova, R.T. Activating effect of aluminum chloride in the preparation of sulfur concrete from sulfur and silica. Inorg. Mater. 2018, 54, 809–814. [Google Scholar] [CrossRef]
- Mohamed, A.-M.O.; Gamal, M.E. Sulfur Concrete for the Construction Industry; J. Ross Publishing: Fort Lauderdale, FL, USA, 2010; p. 424. [Google Scholar]
- Abdel-Jawad, Y.; Al-Qudah, M. The combined effect of water and temperature on the strength of sulfur concrete. Cem. Concr. Res. 1994, 24, 165–175. [Google Scholar] [CrossRef]
- Pickard, S.S. Sulfur concrete for acid resistance. Chem. Eng. 1985, 92, 80. [Google Scholar]
- Platou, J.S. Corrosion resistant sulfur concrete—A new construction material for the chemical industry. CeerChem. Econ. Eng. Rev. 1984, 16, 19–22. [Google Scholar]
- Gregor, R.; Hackl, A. A New Approach to Sulfur Concrete. In New Uses of Sulfur—II; American Chemical Society: Washington, DC, USA, 1978; pp. 54–78. [Google Scholar] [CrossRef]
- Meltzer, R.; Lieb, K.; Horstman, R.; Moore, I.; Shrive, N.; Gillott, J.; Jordaan, I.; Loov, R. A study of durability in temperature cycles and water resistance of sulfur concretes and mortars. J. Test. Eval. 1977, 5, 484–493. [Google Scholar] [CrossRef]



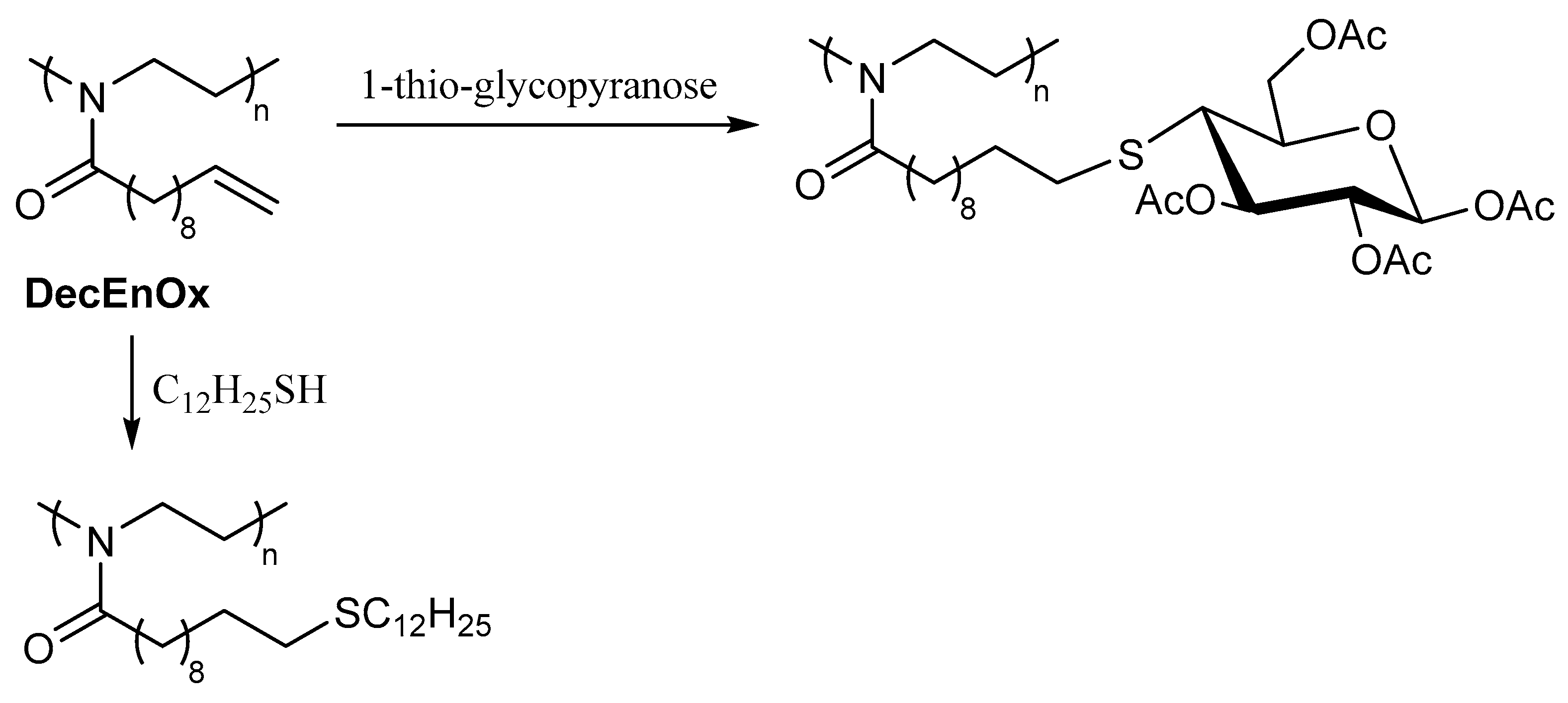



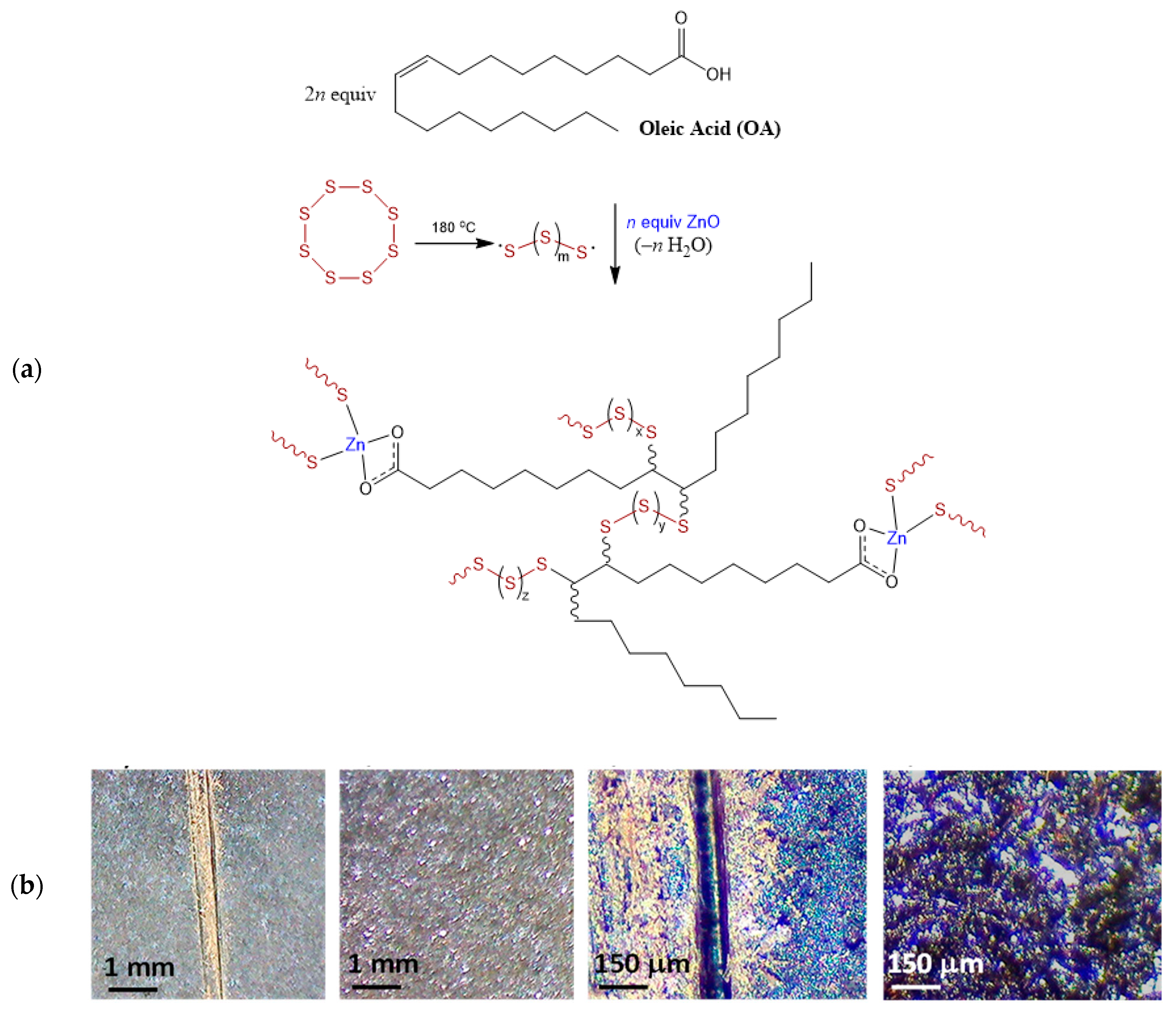

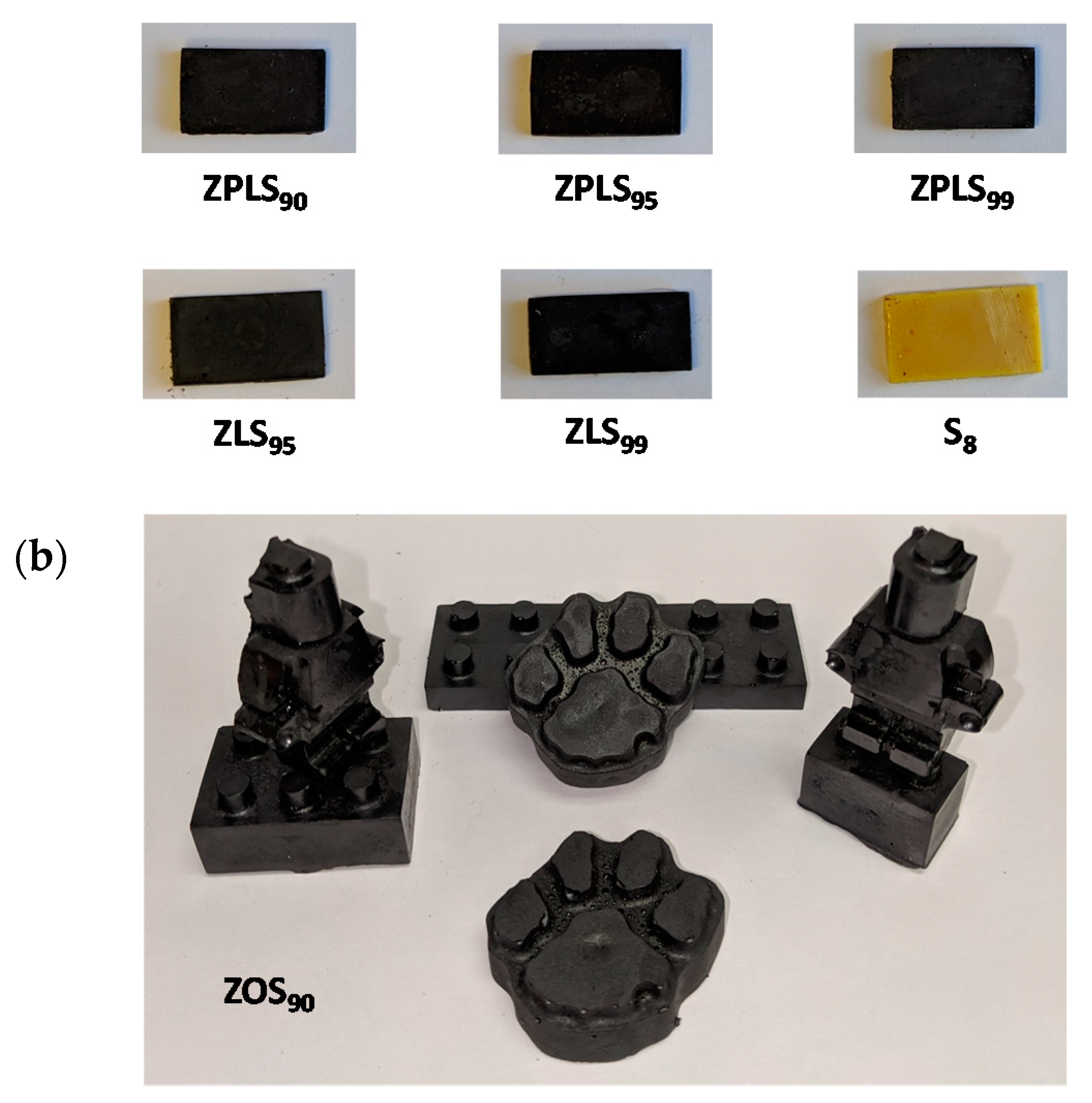
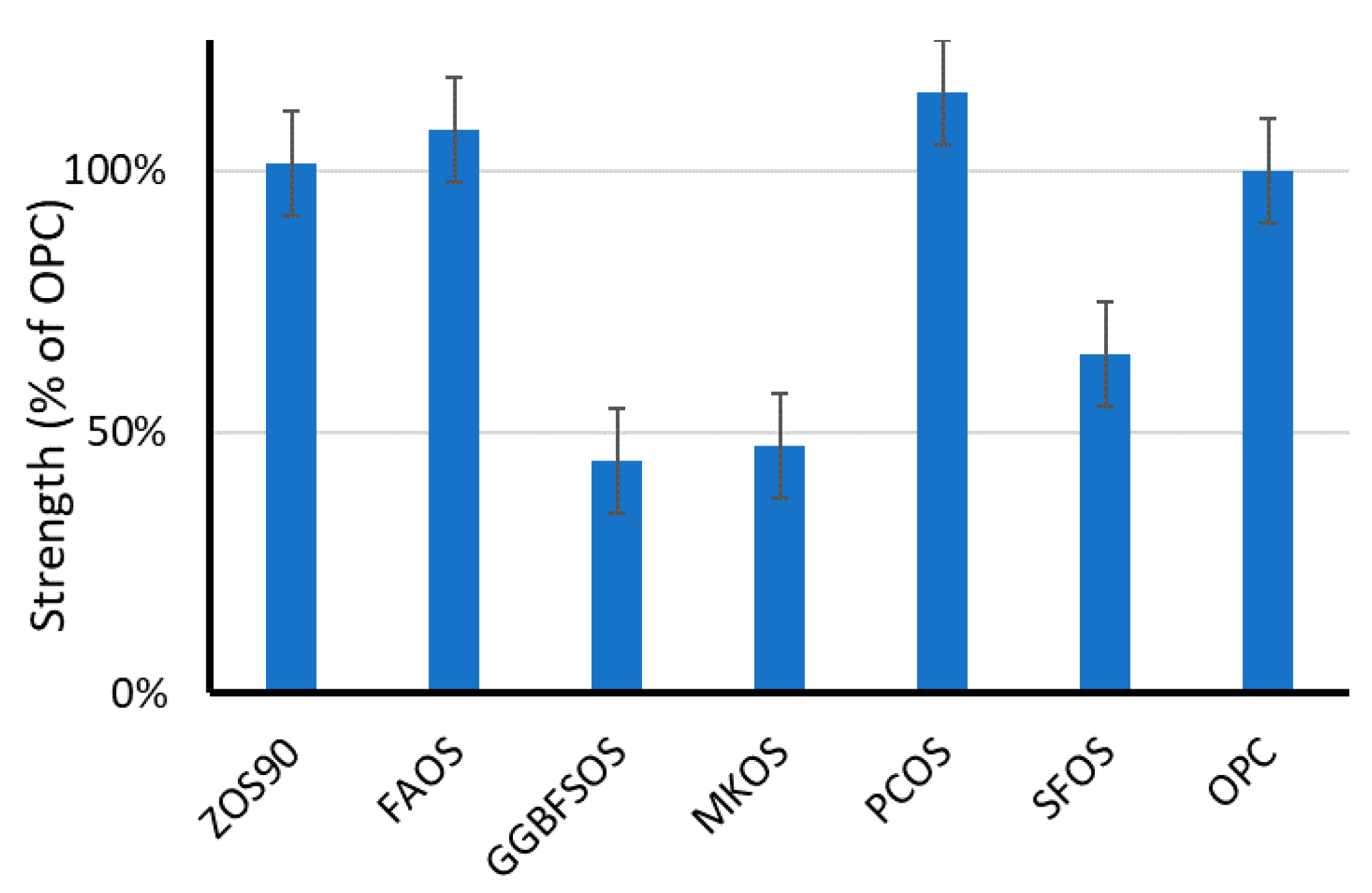
| Source | SFA Chains | MUFA Chains | PUFA Chains | |||
|---|---|---|---|---|---|---|
| Content (% of Total) a | Predominant Chain Length | Content (% of Total) | Predominant Chain Length | PUFA Content (% of Total) | Predominant Chain Length (Unsaturation) b | |
| Coconut | 92 | 12 | 6 | 18 | 2 | 18 (2) |
| Olive | 15 | 16 | 74 | 18 | 10 | 18 (2) |
| Canola | 8 | 16 | 62 | 18 | 32 | 18 (2) |
| Peanut | 18 | 16 | 50 | 18 | 32 | 18 (2) |
| Safflower | 9 | 16 | 14 | 18 | 77 | 18 (2) |
| Soybean | 16 | 16 | 24 | 18 | 60 | 18 (2) |
| Corn | 15 | 16 | 28 | 18 | 57 | 18 (2) |
| Sunflower | 13 | 16 | 22 | 18 | 66 | 18 (2) |
| Linseed | 10 | 16 | 19 | 18 | 72 | 18 (3) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, A.D.; Tennyson, A.G.; Smith, R.C. Sulfur-Containing Polymers Prepared from Fatty Acid-Derived Monomers: Application of Atom-Economical Thiol-ene/Thiol-yne Click Reactions and Inverse Vulcanization Strategies. Sustain. Chem. 2020, 1, 209-237. https://doi.org/10.3390/suschem1030015
Smith AD, Tennyson AG, Smith RC. Sulfur-Containing Polymers Prepared from Fatty Acid-Derived Monomers: Application of Atom-Economical Thiol-ene/Thiol-yne Click Reactions and Inverse Vulcanization Strategies. Sustainable Chemistry. 2020; 1(3):209-237. https://doi.org/10.3390/suschem1030015
Chicago/Turabian StyleSmith, Ashlyn D., Andrew G. Tennyson, and Rhett C. Smith. 2020. "Sulfur-Containing Polymers Prepared from Fatty Acid-Derived Monomers: Application of Atom-Economical Thiol-ene/Thiol-yne Click Reactions and Inverse Vulcanization Strategies" Sustainable Chemistry 1, no. 3: 209-237. https://doi.org/10.3390/suschem1030015
APA StyleSmith, A. D., Tennyson, A. G., & Smith, R. C. (2020). Sulfur-Containing Polymers Prepared from Fatty Acid-Derived Monomers: Application of Atom-Economical Thiol-ene/Thiol-yne Click Reactions and Inverse Vulcanization Strategies. Sustainable Chemistry, 1(3), 209-237. https://doi.org/10.3390/suschem1030015







