Dual UV-Thermal Curing of Biobased Resorcinol Epoxy Resin-Diatomite Composites with Improved Acoustic Performance and Attractive Flame Retardancy Behavior
Abstract
1. Introduction
2. Experimental
Materials
3. Sample preparation
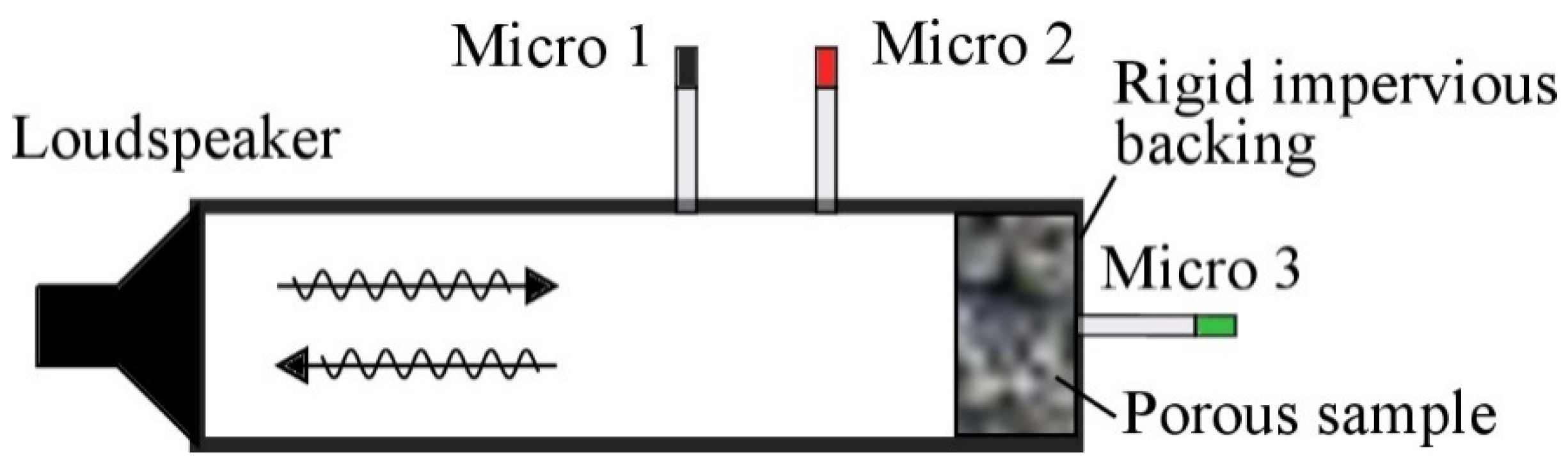
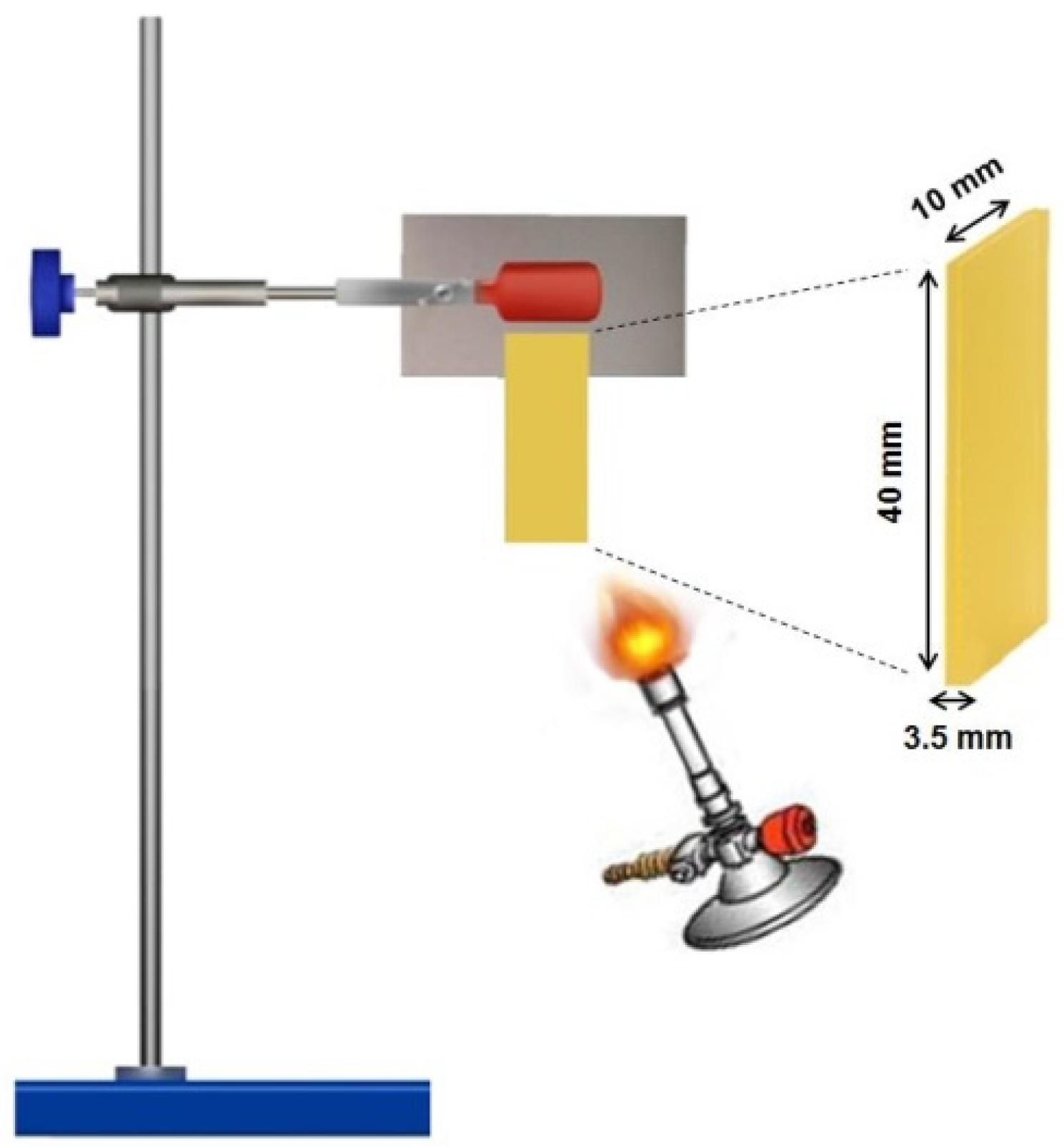
Experimental Techniques
4. Results and Discussion
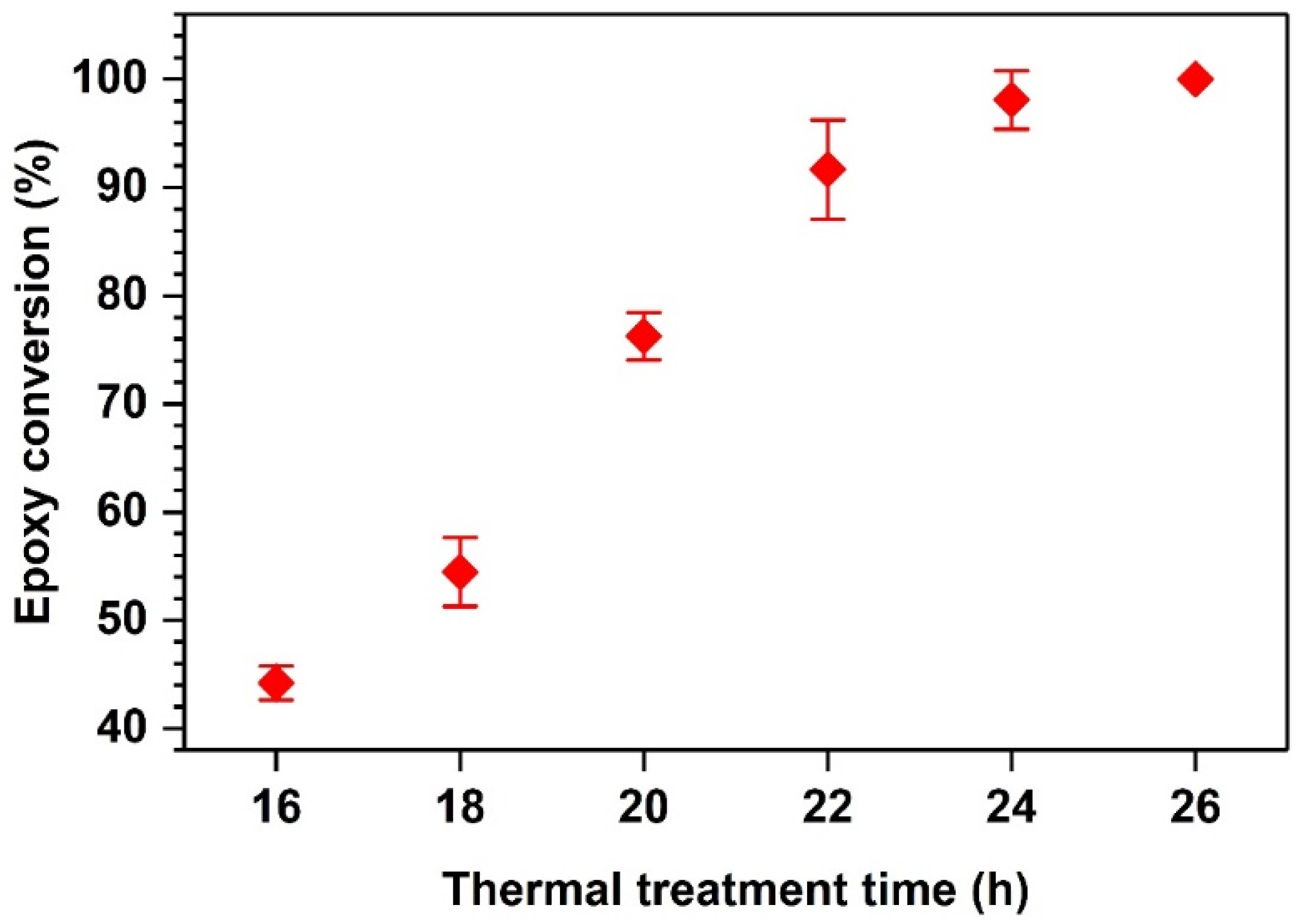
4.1. Photoinitiation and Thermal Dark Curing Process
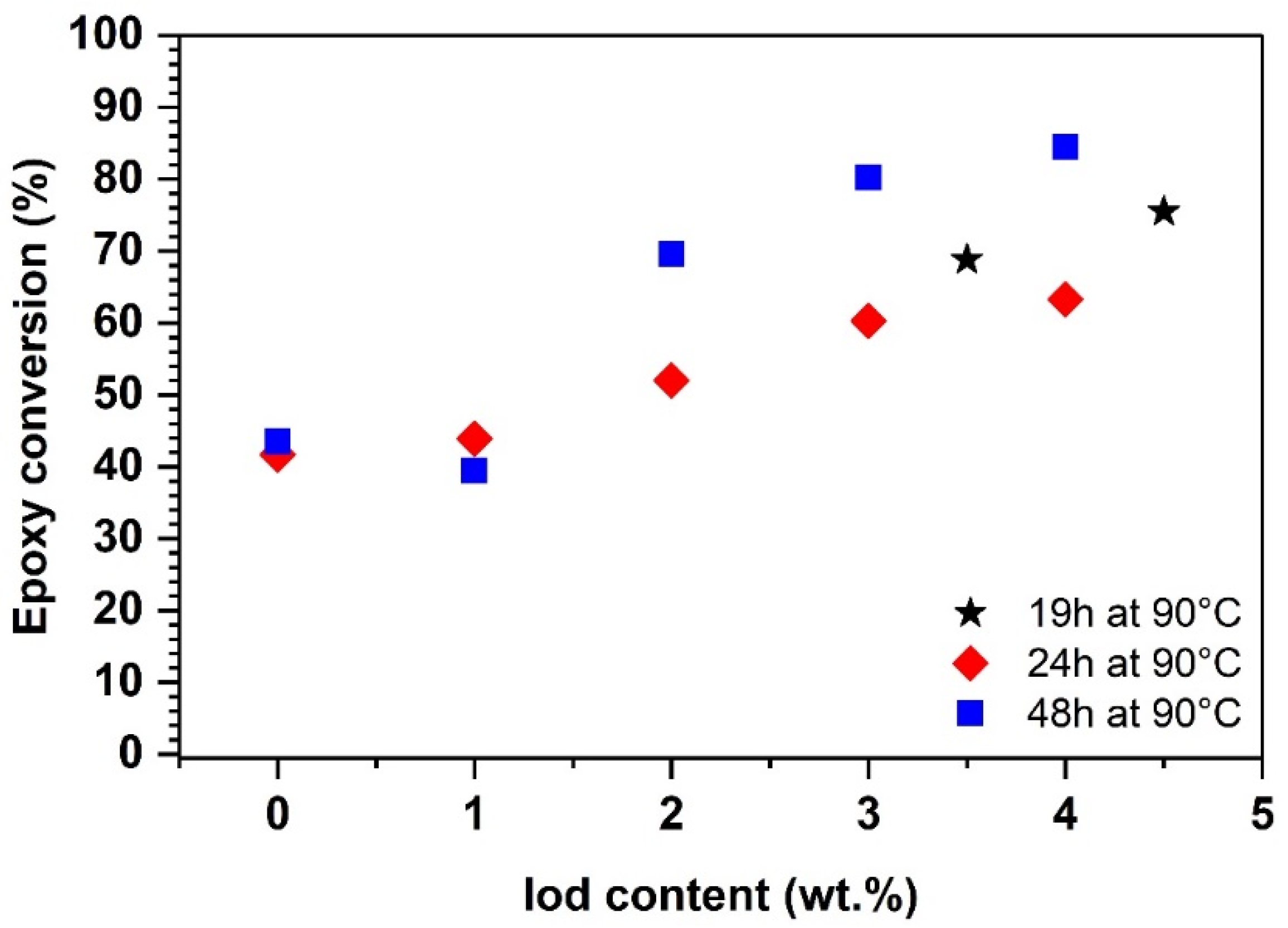
4.2. UV-Induced Epoxy-Diatomite Composites with Thermal Post-Curing
4.3. RDGE Composites with Diatomite Granules: Effects of Diatomite Content
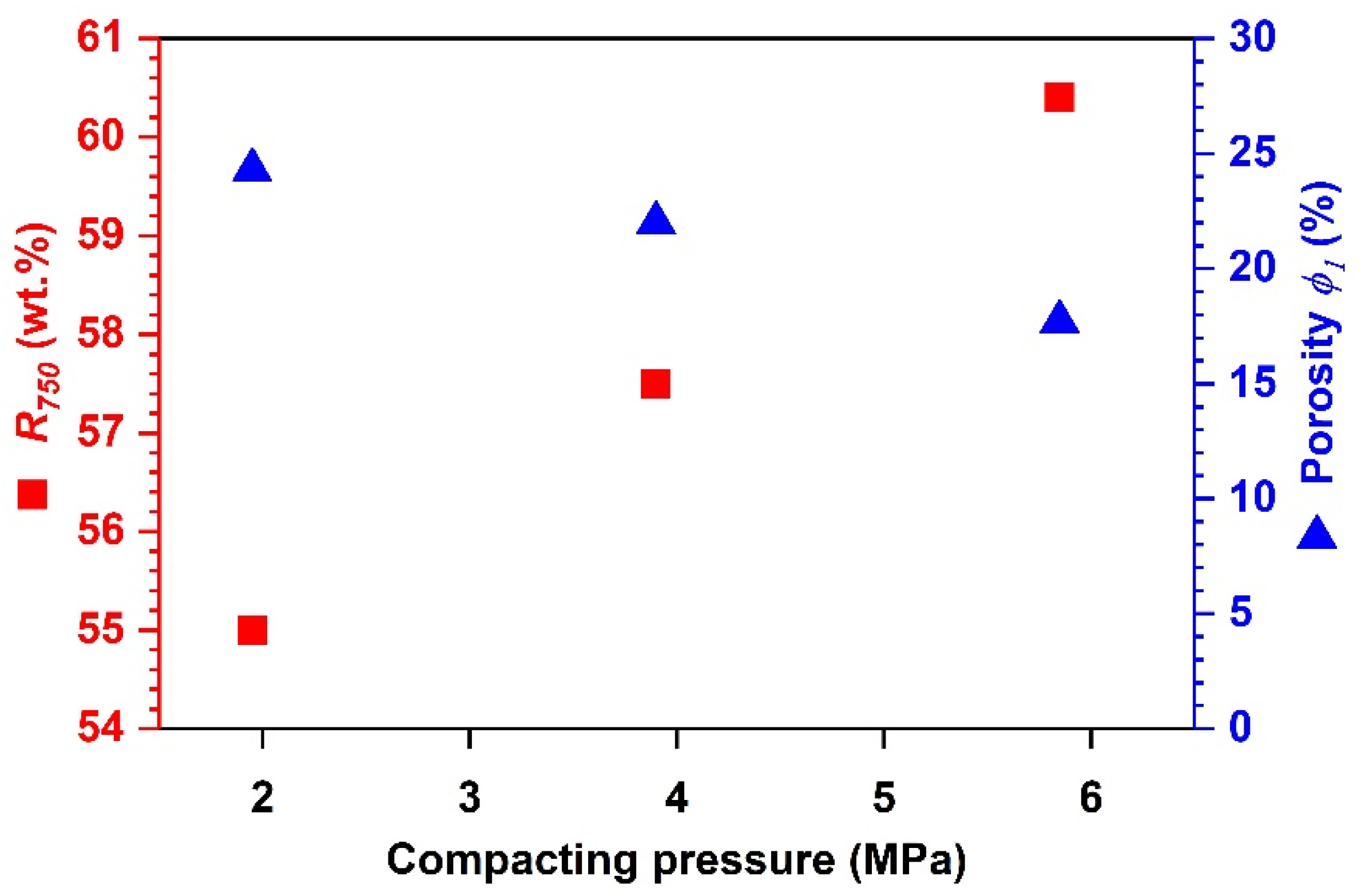
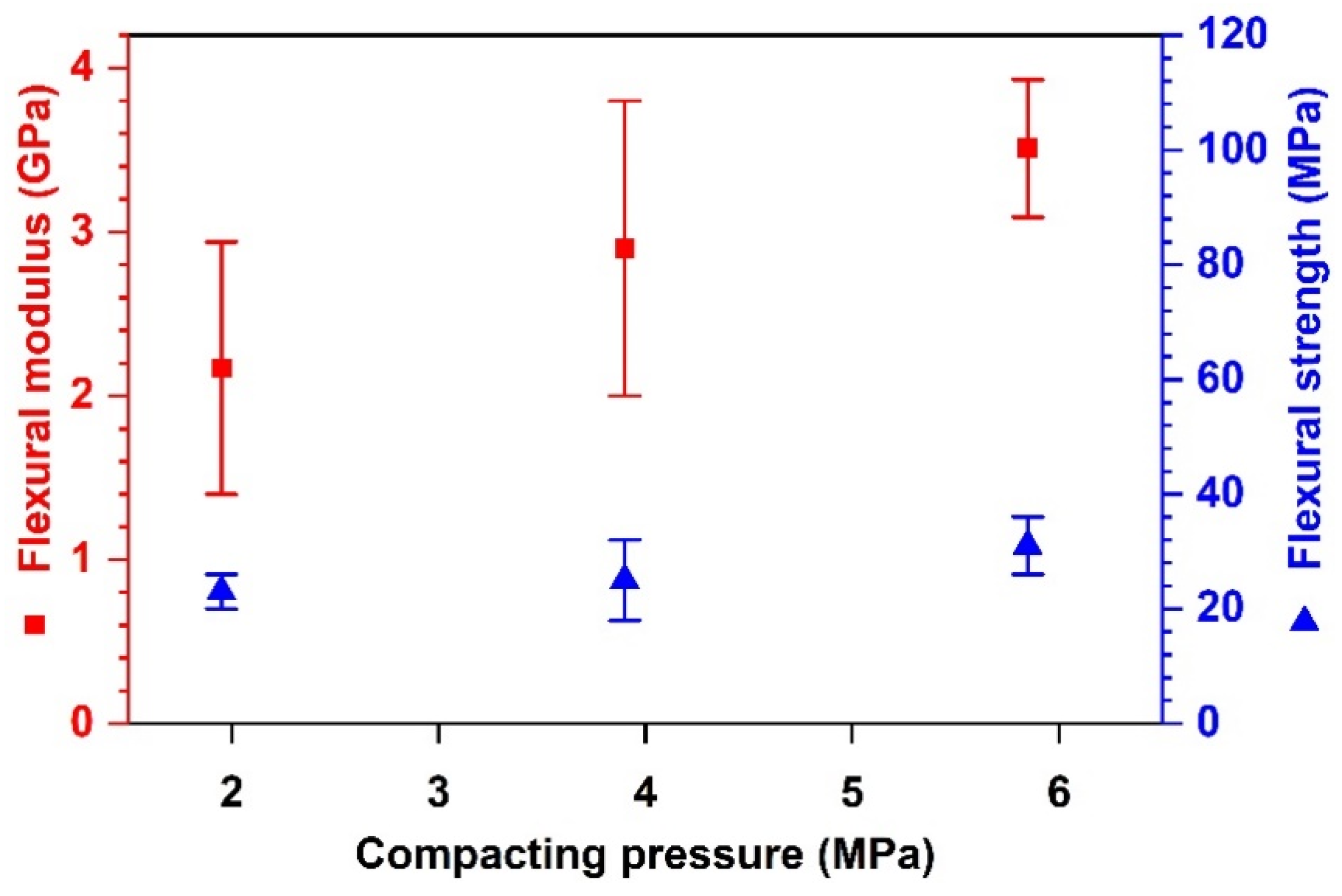
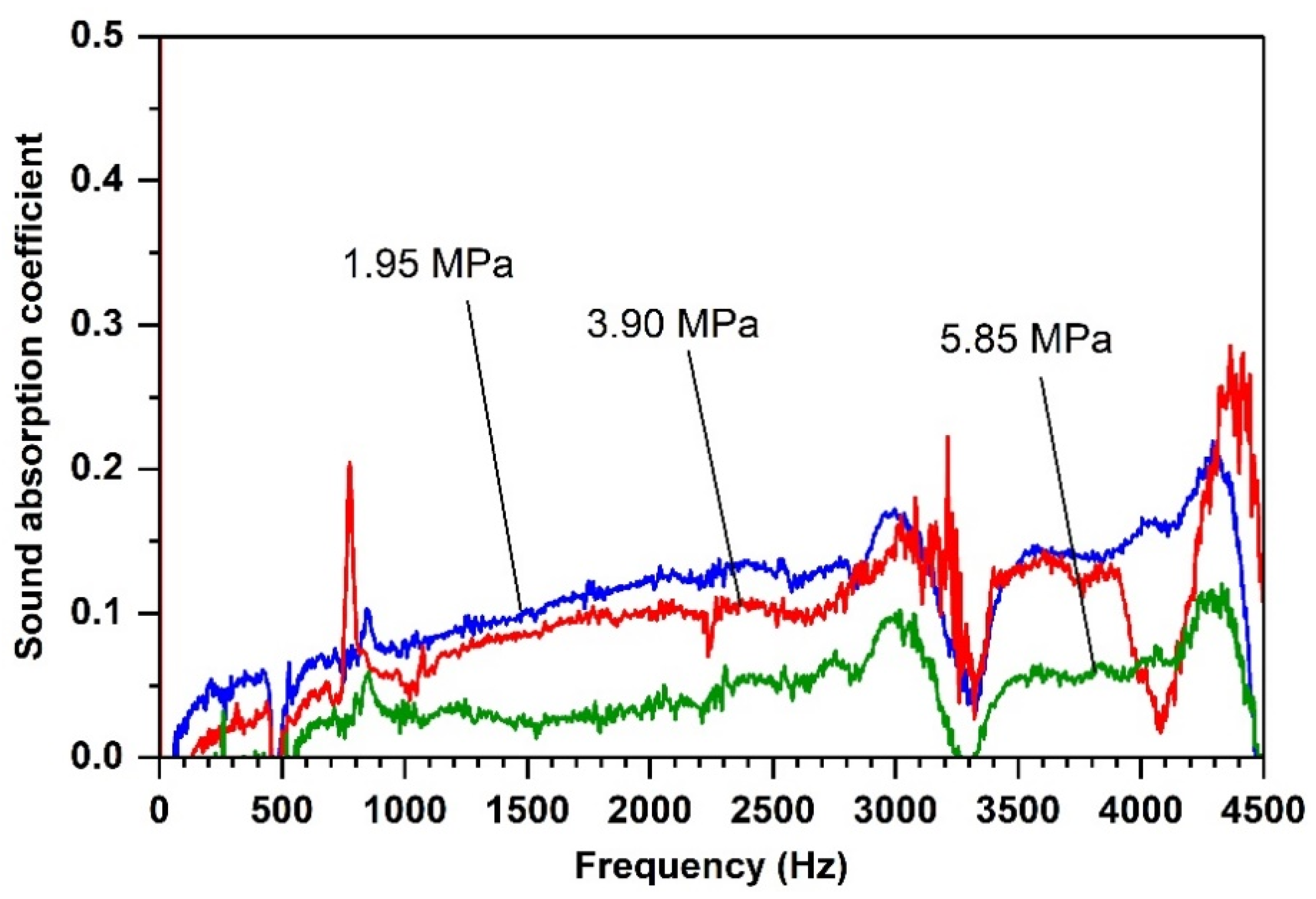
Effects of Compacting Pressure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Van Garderen, N.; Clemens, F.J.; Mezzomo, M.; Bergmann, C.P.; Graule, T. Investigation of Clay Content and Sintering Temperature on Attrition Resistance of Highly Porous Diatomite Based Material. Appl. Clay Sci. 2011, 52, 115–121. [Google Scholar] [CrossRef]
- Parkinson, J.; Gordon, R. Beyond Micromachining: The Potential of Diatoms. Trends Biotechnol. 1999, 17, 190–196. [Google Scholar] [CrossRef]
- Akin, S.; Schembre, J.M.; Bhat, S.K.; Kovscek, A.R. Spontaneous Imbibition Characteristics of Diatomite. J. Pet. Sci. Eng. 2000, 25, 149–165. [Google Scholar] [CrossRef]
- Lee, S.; Ha, J.-H.; Lee, J.; Song, I.-H.; Kwon, S.-H. Preparation and Characterization of a Low-Cost and Natural Material-Based Reticulated Porous Diatomite-Kaolin Composite. Appl. Sci. 2020, 10, 2125. [Google Scholar] [CrossRef]
- Mateo, S.; Cuevas, M.; La Rubia, M.D.; Eliche-Quesada, D. Preliminary Study of the Use of Spent Diatomaceous Earth from the Brewing Industry in Clay Matrix Bricks. Adv. Appl. Ceram. 2017, 116, 77–84. [Google Scholar] [CrossRef]
- Pimraksa, K.; Chindaprasirt, P. Lightweight Bricks Made of Diatomaceous Earth, Lime and Gypsum. Ceram. Int. 2009, 35, 471–478. [Google Scholar] [CrossRef]
- Escalera, E.; Garcia, G.; Terán, R.; Tegman, R.; Antti, M.-L.; Odén, M. The Production of Porous Brick Material from Diatomaceous Earth and Brazil Nut Shell Ash. Constr. Build. Mater. 2015, 98, 257–264. [Google Scholar] [CrossRef]
- Zheng, S.; Bai, C.; Gao, R. Preparation and Photocatalytic Property of TiO2 /Diatomite-Based Porous Ceramics Composite Materials. Int. J. Photoenergy 2012, 2012, 1–4. [Google Scholar] [CrossRef]
- Zeren, D.; Güden, M. The Increased Compression Strength of an Epoxy Resin with the Addition of Heat-Treated Natural Nano-Structured Diatom Frustules. J. Compos. Mater. 2017, 51, 1681–1691. [Google Scholar] [CrossRef]
- Leskovac, M.; Kovačević, V.; Lučić, S.; Perrott, H.R.; Šmit, I. Composites of Poly(Acrylate) Copolymer Filled with Diatomaceous Earth: Morphology and Mechanical Behaviour. Mater. Res. Innov. 2002, 6, 206–213. [Google Scholar] [CrossRef]
- Cacciotti, I.; Rinaldi, M.; Fabbrizi, J.; Nanni, F. Innovative Polyetherimide and Diatomite Based Composites: Influence of the Diatomite Kind and Treatment. J. Mater. Res. Technol. 2019, 8, 1737–1745. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, D.; Liu, Z.; Chen, H.; Zhou, Y.; Zhou, Y.; Zhu, B. Effects of Biomass Diatom Frustule on Structure and Properties of Polyurethane Elastomer. J. Appl. Polym. Sci. 2020, 137, 48452. [Google Scholar] [CrossRef]
- Dobrosielska, M.; Przekop, R.; Sztorch, B.; Brząkalski, D.; Zgłobicka, I.; Łępicka, M.; Dobosz, R.; Kurzydłowski, K. Biogenic Composite Filaments Based on Polylactide and Diatomaceous Earth for 3D Printing. Materials 2020, 13, 4632. [Google Scholar] [CrossRef]
- Fu, Y.; Xu, X.; Huang, Y.; Hu, J.; Chen, Q.; Wu, Y. Preparation of New Diatomite–Chitosan Composite Materials and Their Adsorption Properties and Mechanism of Hg(II). R. Soc. Open Sci. 2017, 4, 170829. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Cong, S. Modified Diatomite Forms in the Rubber Nanocomposites. J. Thermoplast. Compos. Mater. 2020, 33, 659–672. [Google Scholar] [CrossRef]
- Benayache, S.; Alleg, S.; Mebrek, A.; Suñol, J.J. Thermal and Microstructural Properties of Paraffin/Diatomite Composite. Vacuum 2018, 157, 136–144. [Google Scholar] [CrossRef]
- Xu, G.; Leng, G.; Yang, C.; Qin, Y.; Wu, Y.; Chen, H.; Cong, L.; Ding, Y. Sodium Nitrate – Diatomite Composite Materials for Thermal Energy Storage. Sol. Energy 2017, 146, 494–502. [Google Scholar] [CrossRef]
- Wang, R.-M.; Zheng, S.-R.; Zheng, Y.-P. Polymer Matrix Composites and Technology; Woodhead Publishing Limited: Cambridge, UK, 2011; ISBN 978-0-85709-221-2. [Google Scholar]
- Jin, F.-L.; Li, X.; Park, S.-J. Synthesis and Application of Epoxy Resins: A Review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Hsu, C.-Y.; Wei, W.-L.; Jeng, R.-J. Preparation and Thermal Properties of Epoxy-Silica Nanocomposites from Nanoscale Colloidal Silica. Polymer 2003, 44, 5159–5167. [Google Scholar] [CrossRef]
- Kosbar, L.L.; Gelorme, J.D.; Japp, R.M.; Fotorny, W.T. Introducing Biobased Materials into the Electronics Industry. J. Ind. Ecol. 2000, 4, 93–105. [Google Scholar] [CrossRef]
- Pan, H. Synthesis of Polymers from Organic Solvent Liquefied Biomass: A Review. Renew. Sustain. Energy Rev. 2011, 15, 3454–3463. [Google Scholar] [CrossRef]
- Nikafshar, S.; Zabihi, O.; Hamidi, S.; Moradi, Y.; Barzegar, S.; Ahmadi, M.; Naebe, M. A Renewable Bio-Based Epoxy Resin with Improved Mechanical Performance That Can Compete with DGEBA. RSC Adv. 2017, 7, 8694–8701. [Google Scholar] [CrossRef]
- Nguyen, Q.; Nguyen, N.; Rios de Anda, A.; Nguyen, V.; Versace, D.; Langlois, V.; Naili, S.; Renard, E. Photocurable Bulk Epoxy Resins Based on Resorcinol Derivative through Cationic Polymerization. J. Appl. Polym. Sci. 2020, 137, 10. [Google Scholar] [CrossRef]
- Bourne, L.B.; Milner, F.J.M.; Alberman, K.B. Health Problems of Epoxy Resins and Amine-Curing Agents. Occup. Environ. Med. 1959, 16, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Xiao-Xue, W.; Qian-ying, W.; Ying, Z. Sound Absorption Performance of Diatom Mud Coating and Its Influence on Indoor Acoustic Environment. Ferroelectrics 2019, 549, 241–253. [Google Scholar] [CrossRef]
- Jin, H.-Y.; Yang, Y.-Q.; Xu, L.; Hou, S.-E. Effects of Spherical Silica on the Properties of an Epoxy Resin System. J. Appl. Polym. Sci. 2011, 121, 648–653. [Google Scholar] [CrossRef]
- Yang, P.; Ren, M.; Chen, K.; Liang, Y.; Lü, Q.-F.; Zhang, T. Synthesis of a Novel Silicon-Containing Epoxy Resin and Its Effect on Flame Retardancy, Thermal, and Mechanical Properties of Thermosetting Resins. Mater. Today Commun. 2019, 19, 186–195. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Wu, C.-S.; Chiu, Y.-S.; Ho, W.-H. Preparation, Thermal Properties, and Flame Retardance of Epoxy-Silica Hybrid Resins. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 2354–2367. [Google Scholar] [CrossRef]
- Gu, H.; Guo, J.; He, Q.; Tadakamalla, S.; Zhang, X.; Yan, X.; Huang, Y.; Colorado, H.A.; Wei, S.; Guo, Z. Flame-Retardant Epoxy Resin Nanocomposites Reinforced with Polyaniline-Stabilized Silica Nanoparticles. Ind. Eng. Chem. Res. 2013, 52, 7718–7728. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Song, S. Preparation of a Novel Type of Flame Retardant Diatomite and Its Application in Silicone Rubber Composites. Adv. Powder Technol. 2019, 30, 1567–1575. [Google Scholar] [CrossRef]
- Bulut, U.; Crivello, J.V. Investigation of the Reactivity of Epoxide Monomers in Photoinitiated Cationic Polymerization. Macromolecules 2005, 38, 3584–3595. [Google Scholar] [CrossRef]
- Goethals, E.; Duprez, F. Carbocationic Polymerizations. Prog. Polym. Sci. 2007, 32, 220–246. [Google Scholar] [CrossRef]
- Washburn, E.W. The Dynamics of Capillary Flow. Phys. Rev. 1921, 17, 273–283. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Particle Technology Series; Springer: Dordrecht, The Netherlands, 2004; Volume 16, ISBN 978-90-481-6633-6. [Google Scholar]
- De Anda, A.R.; Fillot, L.A.; Rossi, S.; Long, D.; Sotta, P. Influence of the Sorption of Polar and Non-Polar Solvents on the Glass Transition Temperature of Polyamide 6,6 Amorphous Phase. Polym. Eng. Sci. 2011, 51, 2129–2135. [Google Scholar] [CrossRef]
- Rios De Anda, A.; Fillot, L.A.; Preda, F.M.; Rossi, S.; Long, D.R.; Sotta, P. Sorption and Plasticization Effects of Ethanol–Toluene–Isooctane Ternary Mixtures in Polyamide 6,6 and Induced Plasticization Effects. Eur. Polym. J. 2014, 55, 199–209. [Google Scholar] [CrossRef]
- Rios de Anda, A.; Fillot, L.-A.; Long, D.R.; Sotta, P. Influence of the Amorphous Phase Molecular Mobility on Impact and Tensile Properties of Polyamide 6,6. J. Appl. Polym. Sci. 2016, 133, 9. [Google Scholar] [CrossRef]
- ASTM D790-03: Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials; ASTM International: West Conshohocken, PA, USA, 2003.
- ISO 10534-2: Acoustics-Determination of Sound Absorption Coefficient and Impedance in Impedance Tubes. Part 2: Transfer-Function Method; International Organization for Standardization: Geneva, Switzerland, 1998.
- Salissou, Y.; Panneton, R. Wideband Characterization of the Complex Wave Number and Characteristic Impedance of Sound Absorbers. J. Acoust. Soc. Am. 2010, 128, 2868–2876. [Google Scholar] [CrossRef]
- ASTM C423: Standard Test Method for Sound Absorption and Sound Absorption Coefficients by the Reverberation Room Method; ASTM International: West Conshohocken, PA, USA, 2002.
- Huggett, C. Estimation of Rate of Heat Release by Means of Oxygen Consumption Measurements. Fire Mater. 1980, 4, 61–65. [Google Scholar] [CrossRef]
- Chaisena, A.; Rangsriwatananon, K. Effects of Thermal and Acid Treatments on Some Physico-Chemical Properties of Lampang Diatomite. Suranaree J. Sci. Technol. 2004, 11, 289–299. [Google Scholar]
- Kulpe, J.A.; Lee, C.-Y.; Leamy, M.J. Computation of Acoustic Absorption in Media Composed of Packed Microtubes Exhibiting Surface Irregularity. J. Acoust. Soc. Am. 2011, 130, 826–834. [Google Scholar] [CrossRef]
- Swift, M.J.; Bris, P.; Horoshenkov, K.V. Acoustic Absorption in Re-Cycled Rubber Granulate. Appl. Acoust. 1999, 57, 203–212. [Google Scholar] [CrossRef]
- Bifulco, A.; Parida, D.; Salmeia, K.A.; Nazir, R.; Lehner, S.; Stämpfli, R.; Markus, H.; Malucelli, G.; Branda, F.; Gaan, S. Fire and Mechanical Properties of DGEBA-Based Epoxy Resin Cured with a Cycloaliphatic Hardener: Combined Action of Silica, Melamine and DOPO-Derivative. Mater. Des. 2020, 193, 108862. [Google Scholar] [CrossRef]
- Butler, S.; Fotsing, E.R.; Ross, A. Acoustic Thermoset Open-Cell Foams Produced by Particulate Leaching Process. J. Mater. Sci. 2019, 54, 12553–12572. [Google Scholar] [CrossRef]
- Ali, M.S.; Mohamed Ariff, A.H.; Jaafar, C.N.A.; Tahir, S.M.; Mazlan, N.; Maori, K.A.; Naser, H. Factors Affecting the Porosity and Mechanical Properties of Porous Ceramic Composite Materials. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 978-0-12-803581-8. [Google Scholar]
- Patel, P.S.; Shepherd, D.E.; Hukins, D.W. Compressive Properties of Commercially Available Polyurethane Foams as Mechanical Models for Osteoporotic Human Cancellous Bone. BMC Musculoskelet. Disord. 2008, 9. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-Y. Advanced Polyimide Materials: Synthesis, Characterization, and Applications; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 978-0-12-812641-7. [Google Scholar]
- Sonnier, R.; Vahabi, H.; Ferry, L.; Lopez-Cuesta, J.-M. Pyrolysis-Combustion Flow Calorimetry: A Powerful Tool To Evaluate the Flame Retardancy of Polymers. In Fire and Polymers VI: New Advances in Flame Retardant Chemistry and Science; Morgan, A.B., Wilkie, C.A., Nelson, G.L., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2012; Volume 1118, pp. 361–390. ISBN 978-0-8412-2780-4. [Google Scholar]
- ASTM D7309-20: Standard Test Method for Determining Flammability Characteristics of Plastics and Other Solid Materials Using Microscale Combustion Calorimetry; ASTM International: West Conshohocken, PA, USA, 2020.
- Lyon, R.E.; Walters, R.N. A Microscale Combustion Calorimeter; Federal Aviation Administration, Office of Aviation Research: Washington, DC, USA, 2002. [Google Scholar]
- Wu, H.; Sulkis, M.; Driver, J.; Saade-Castillo, A.; Thompson, A.; Koo, J.H. Multi-Functional ULTEMTM1010 Composite Filaments for Additive Manufacturing Using Fused Filament Fabrication (FFF). Addit. Manuf. 2018, 24, 298–306. [Google Scholar] [CrossRef]
- Butnaru, I.; Bruma, M.; Gaan, S. Phosphine Oxide Based Polyimides: Structure–Property Relationships. RSC Adv. 2017, 7, 50508–50518. [Google Scholar] [CrossRef]
- Schartel, B.; Wilkie, C.A.; Camino, G. Recommendations on the Scientific Approach to Polymer Flame Retardancy: Part 1—Scientific Terms and Methods. J. Fire Sci. 2016, 34, 447–467. [Google Scholar] [CrossRef]
- Nguyen, Q.-B.; Nguyen, V.-H.; Perrot, C.; Rios de Anda, A.; Renard, E.; Naili, S. Multiscale Approach to Characterize Effective Mechanical, Hydraulic and Acoustic Properties of a New Bio-Based Porous Material. Mater. Today Commun. 2021, 26, 101938. [Google Scholar] [CrossRef]
- Yorov, K.E.; Kottsov, S.Y.; Baranchikov, А.Е.; Boytsova, O.V.; Kiskin, M.A.; Varaksina, E.A.; Kopitsa, G.P.; Lermontov, S.A.; Sidorov, A.A.; Pipich, V.; et al. Photoluminescent Porous Aerogel Monoliths Containing ZnEu-Complex: The First Example of Aerogel Modified with a Heteronuclear Metal Complex. J. Sol Gel Sci. Technol. 2019. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science-The Physics and Chemistry of Sol-Gel Processing; Academic Press Inc.: Cambridge, MA, USA, 1990. [Google Scholar]
- Al-Oweini, R.; El-Rassy, H. Synthesis and Characterization by FTIR Spectroscopy of Silica Aerogels Prepared Using Several Si(OR)4 and R′′Si(OR′)3 Precursors. J. Mol. Struct. 2009, 919, 140–145. [Google Scholar] [CrossRef]

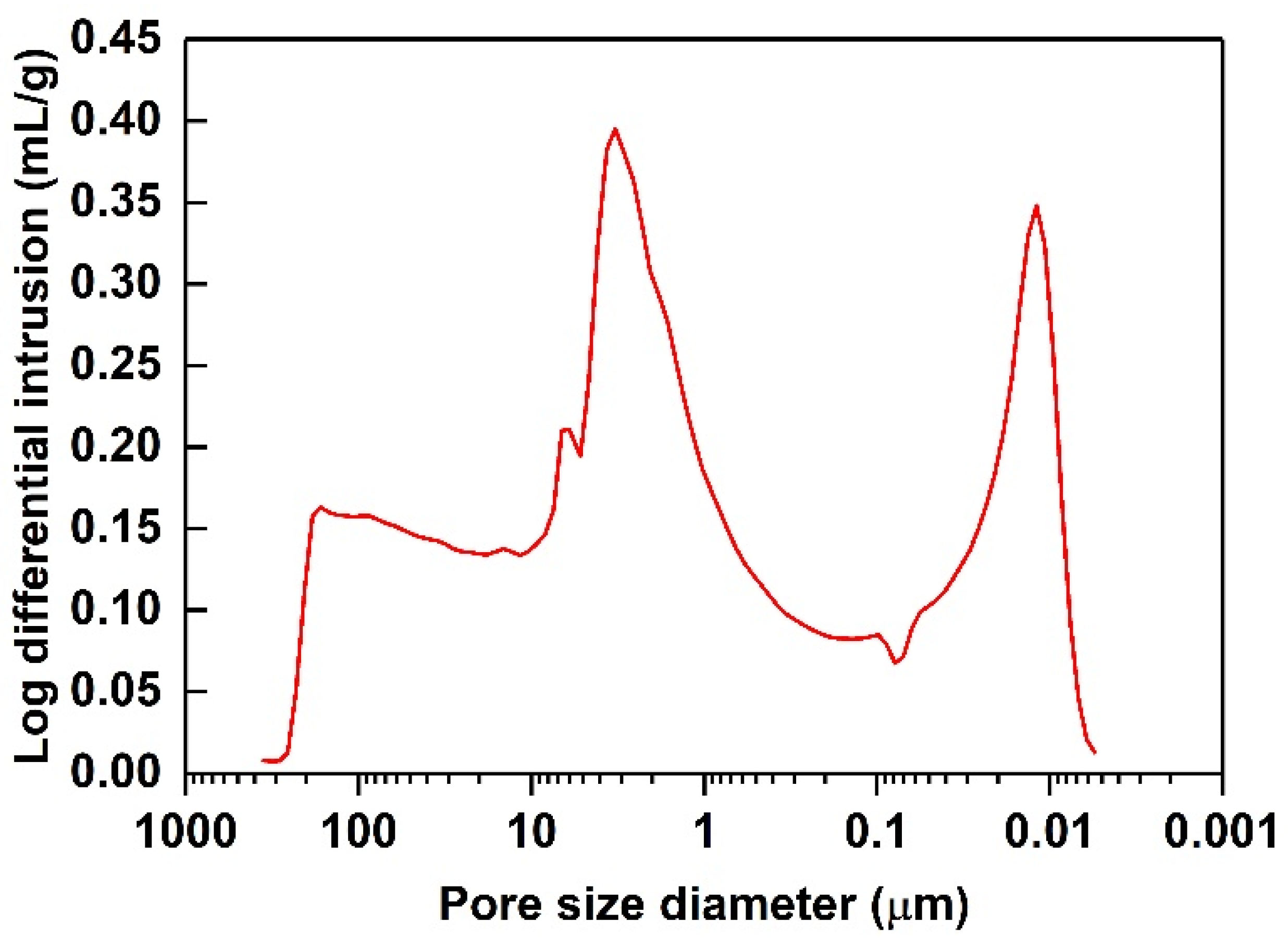
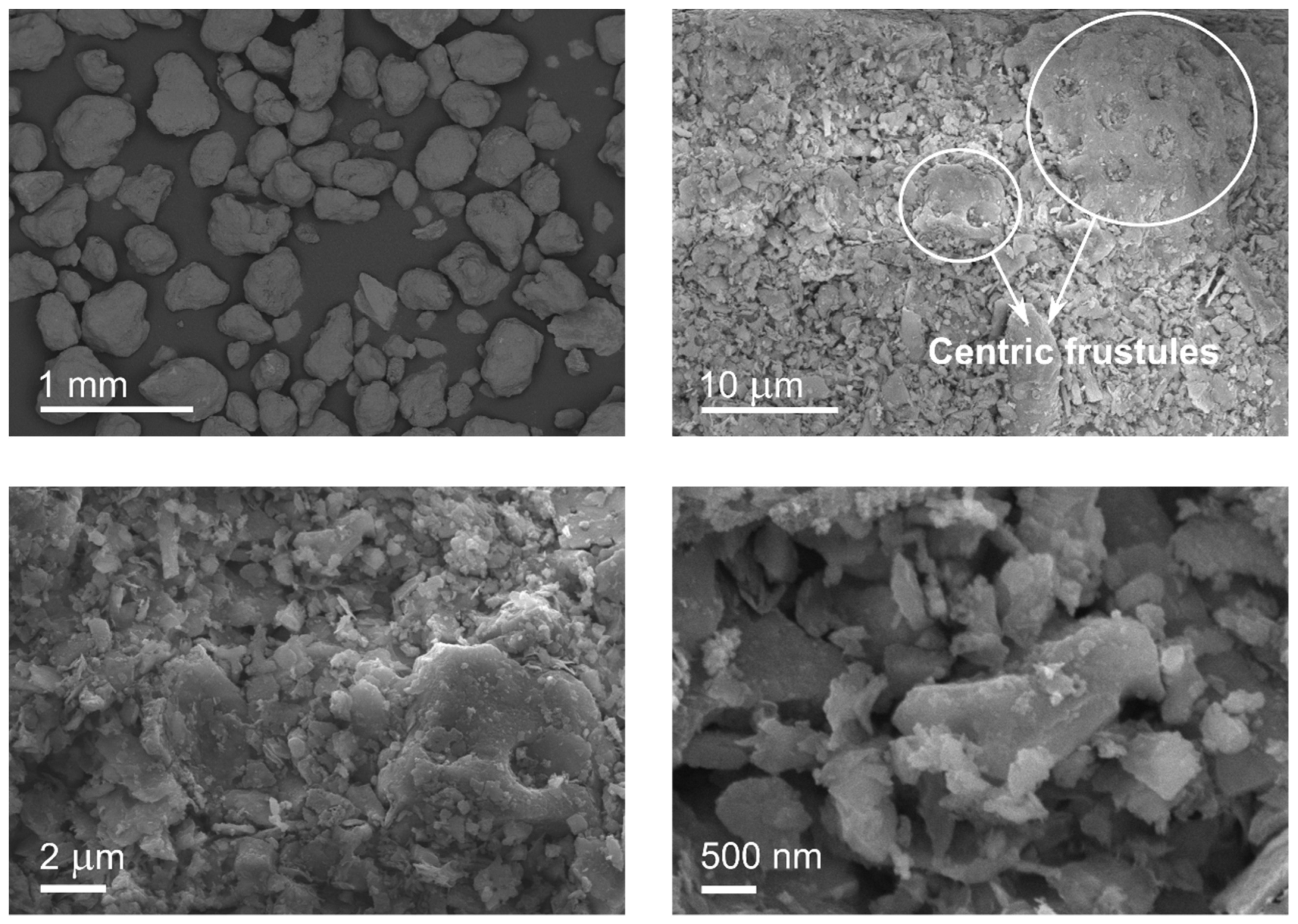
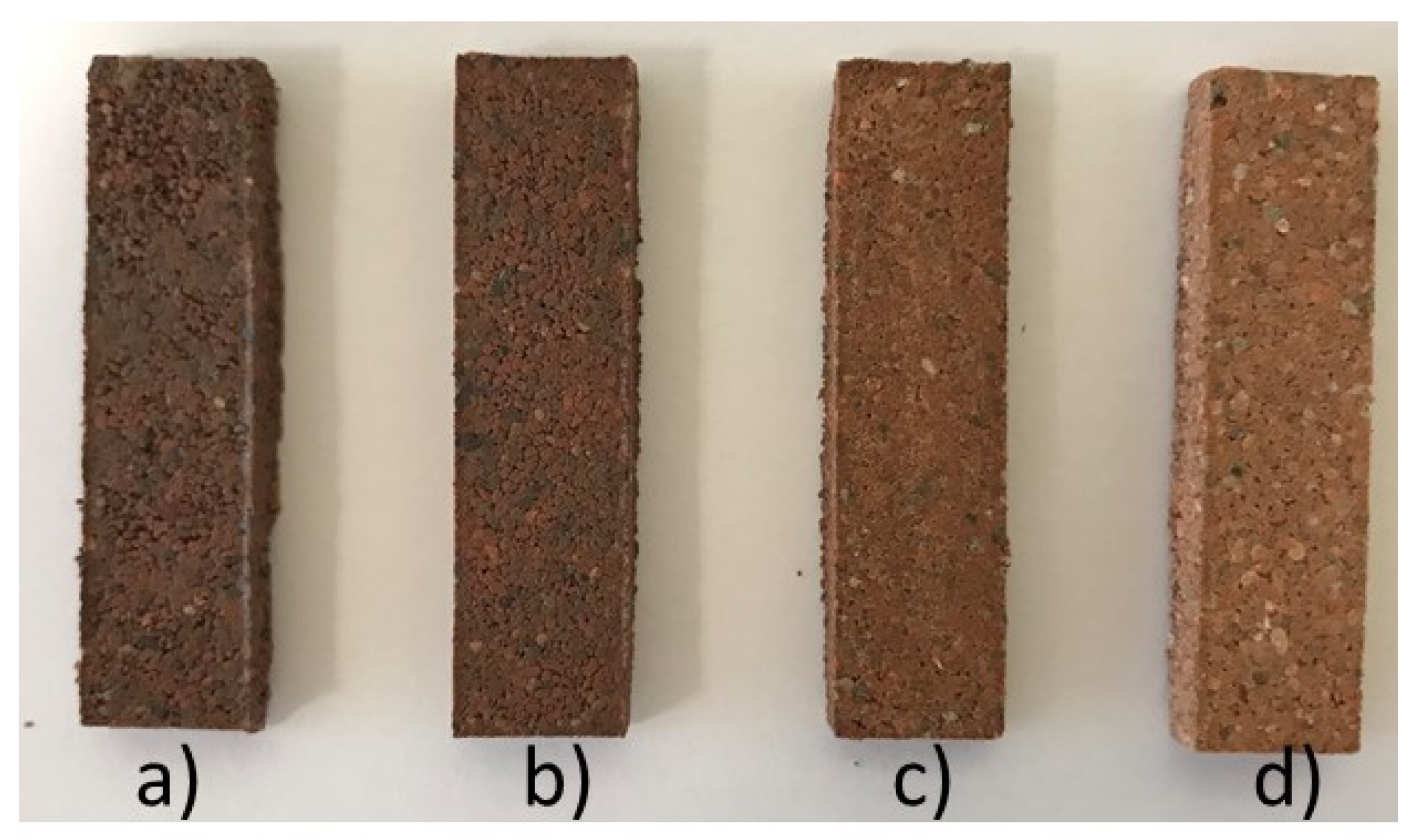
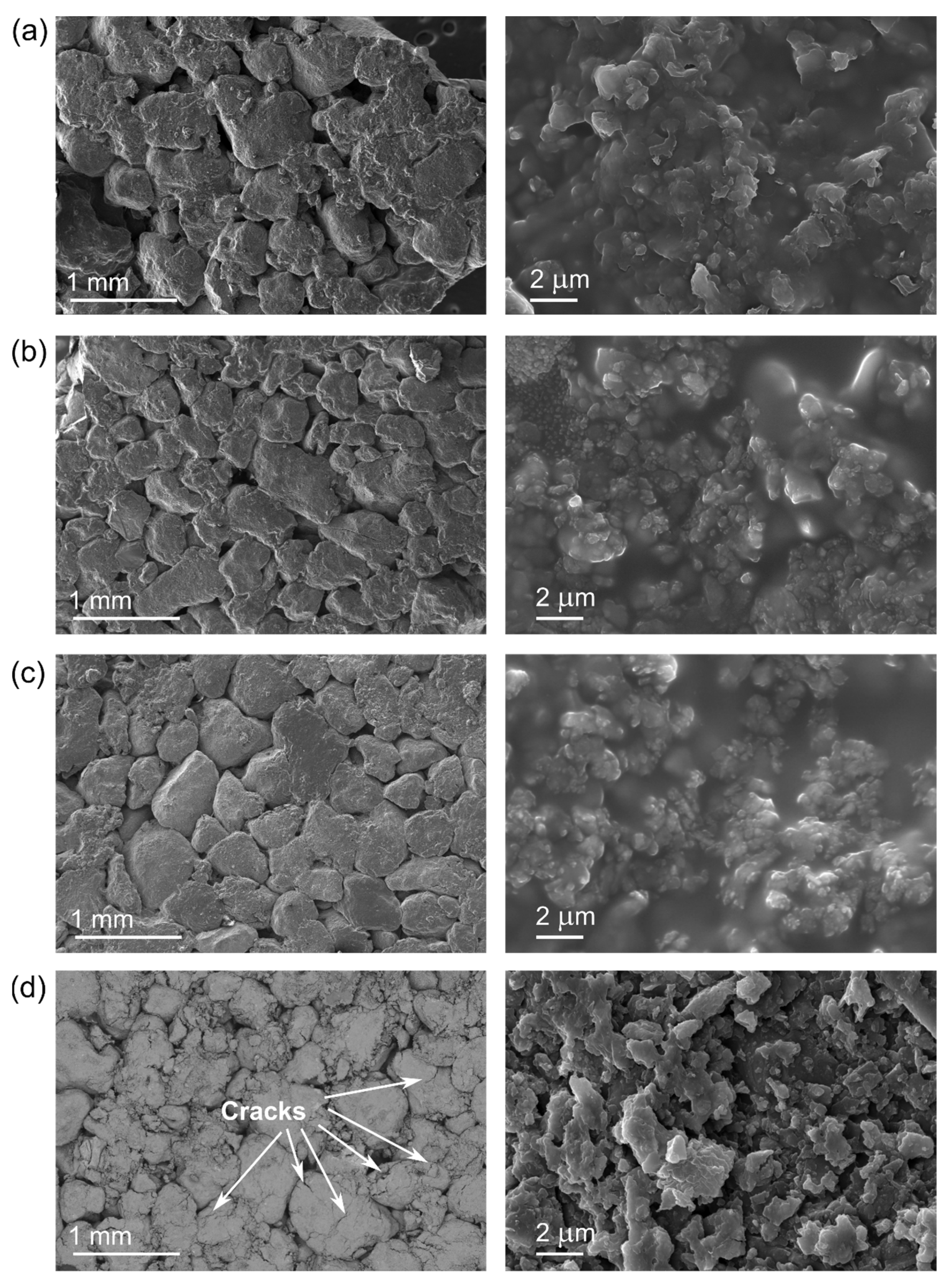
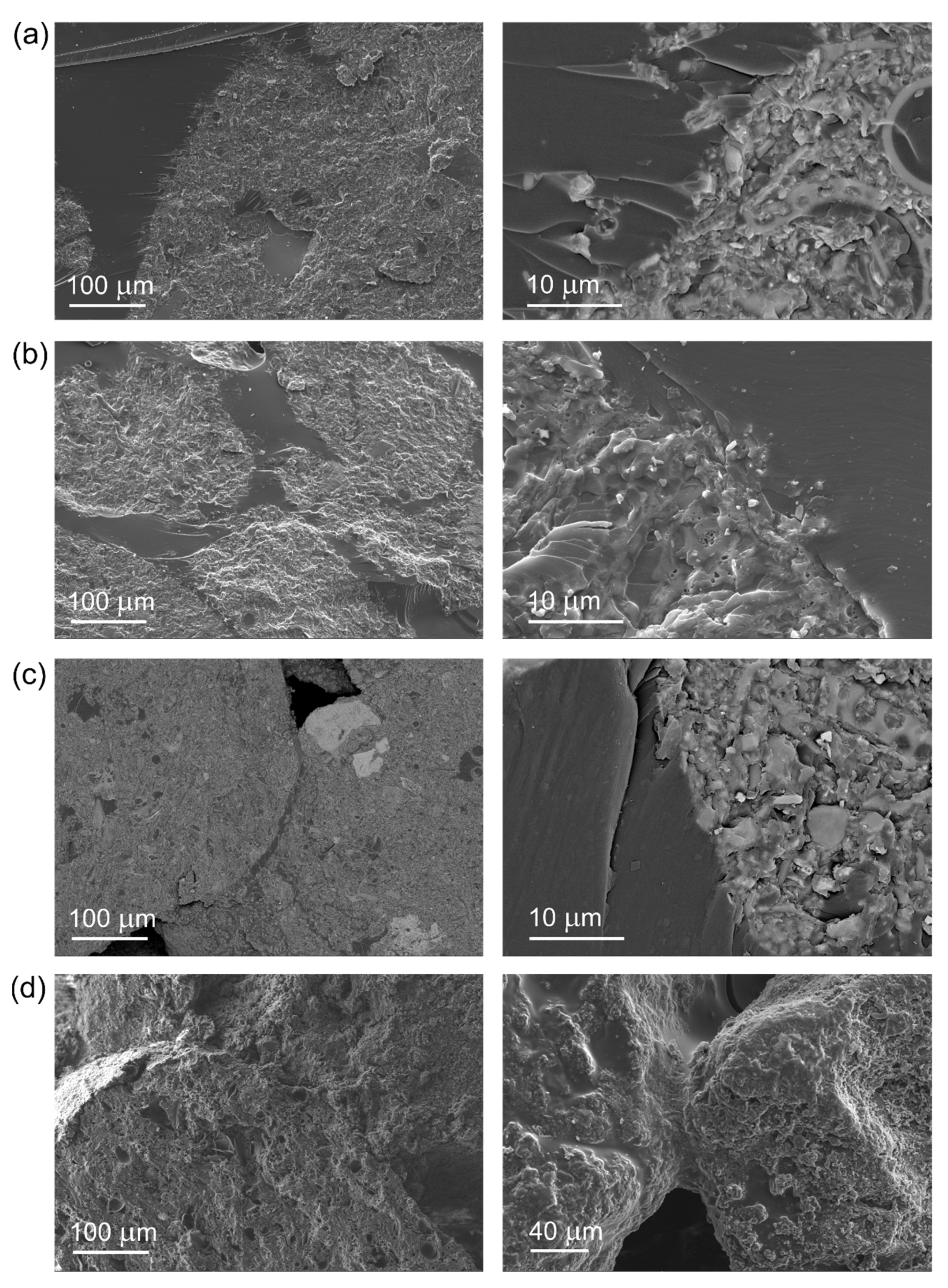

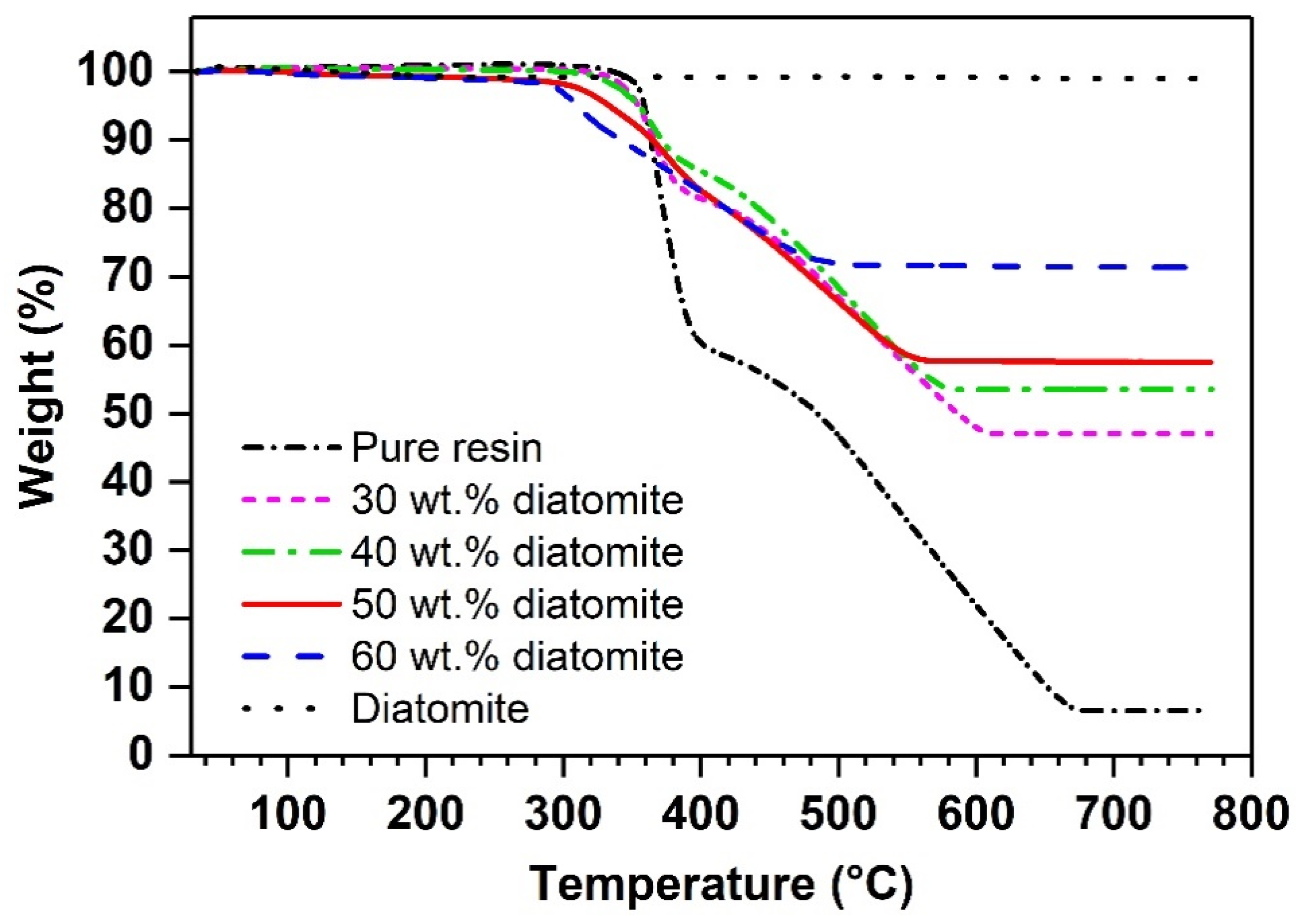

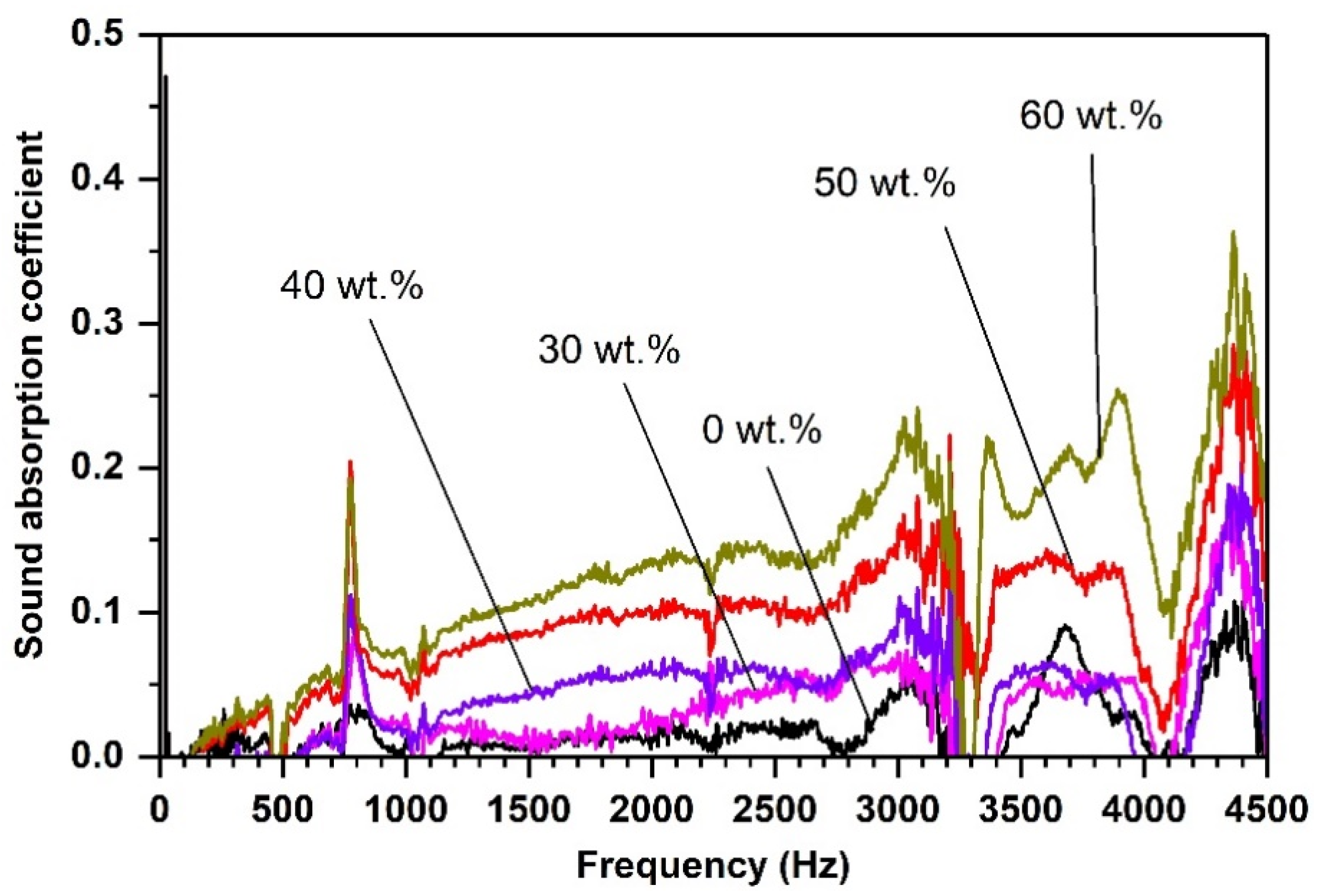
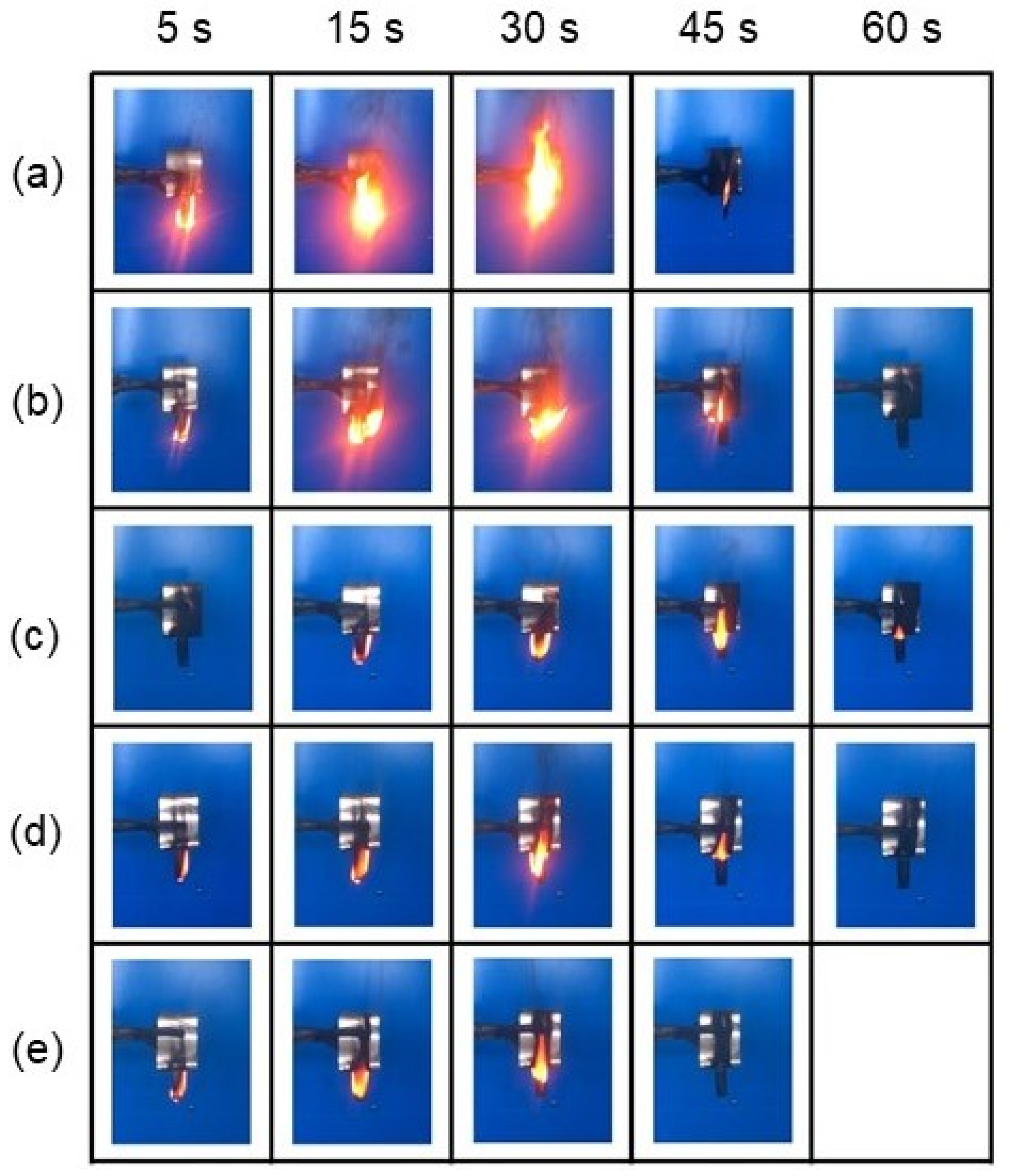

| Diatomite Content (wt.%) | 0 | 30 | 40 | 50 | 60 |
|---|---|---|---|---|---|
| (°C) | 153 ± 3 | 105 ± 1 | 100 ± 1 | 99 ± 1 | 96 ± 1 |
| (°C) | 359 | 355 | 355 | 334 | 310 |
| (wt.%) | 6.5 | 47.0 | 53.5 | 57.5 | 71.4 |
| Calculated diatomite cntent (wt.%) * | - | 43.8 | 50.8 | 55.1 | 70.2 |
| (%) * | - | 17.5 | 19.3 | 24.2 | 41.9 |
| (%) * | - | 19.6 | 25.6 | 31.5 | 37.2 |
| Density (g/cm3) | 1.318 | 1.437 | 1.427 | 1.380 | 1.181 |
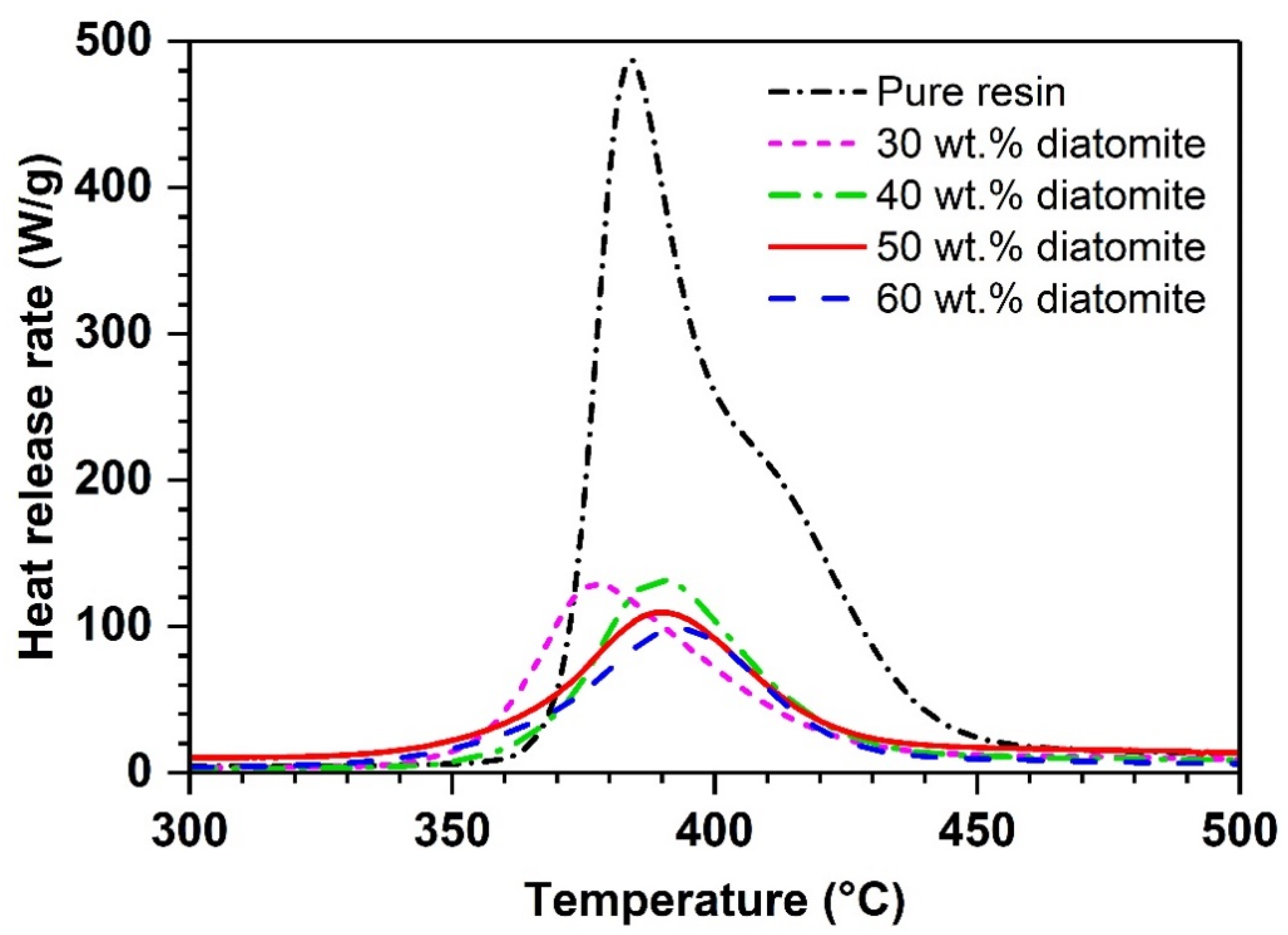
| Diatomite Content (wt.%) | pHRR (W/g) | TpHRR (°C) | THR (kJ/g) |
|---|---|---|---|
| 0 | 487 | 384 | 16.2 |
| 30 | 129 | 377 | 5.7 |
| 40 | 132 | 390 | 6 |
| 50 | 109 | 390 | 5 |
| 60 | 97 | 394 | 4.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, Q.-B.; Vahabi, H.; Rios de Anda, A.; Versace, D.-L.; Langlois, V.; Perrot, C.; Nguyen, V.-H.; Naili, S.; Renard, E. Dual UV-Thermal Curing of Biobased Resorcinol Epoxy Resin-Diatomite Composites with Improved Acoustic Performance and Attractive Flame Retardancy Behavior. Sustain. Chem. 2021, 2, 24-48. https://doi.org/10.3390/suschem2010003
Nguyen Q-B, Vahabi H, Rios de Anda A, Versace D-L, Langlois V, Perrot C, Nguyen V-H, Naili S, Renard E. Dual UV-Thermal Curing of Biobased Resorcinol Epoxy Resin-Diatomite Composites with Improved Acoustic Performance and Attractive Flame Retardancy Behavior. Sustainable Chemistry. 2021; 2(1):24-48. https://doi.org/10.3390/suschem2010003
Chicago/Turabian StyleNguyen, Quoc-Bao, Henri Vahabi, Agustín Rios de Anda, Davy-Louis Versace, Valérie Langlois, Camille Perrot, Vu-Hieu Nguyen, Salah Naili, and Estelle Renard. 2021. "Dual UV-Thermal Curing of Biobased Resorcinol Epoxy Resin-Diatomite Composites with Improved Acoustic Performance and Attractive Flame Retardancy Behavior" Sustainable Chemistry 2, no. 1: 24-48. https://doi.org/10.3390/suschem2010003
APA StyleNguyen, Q.-B., Vahabi, H., Rios de Anda, A., Versace, D.-L., Langlois, V., Perrot, C., Nguyen, V.-H., Naili, S., & Renard, E. (2021). Dual UV-Thermal Curing of Biobased Resorcinol Epoxy Resin-Diatomite Composites with Improved Acoustic Performance and Attractive Flame Retardancy Behavior. Sustainable Chemistry, 2(1), 24-48. https://doi.org/10.3390/suschem2010003







