Understanding of Förster Resonance Energy Transfer (FRET) in Ionic Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Absorbance Studies
2.4. Photoluminescence and Quantum Yield Studies
2.5. FRET Calculations
2.6. Temperature Dependent Photophysical Characterization
2.7. Synthesis
3. Results and Discussion
3.1. Materials Characterization
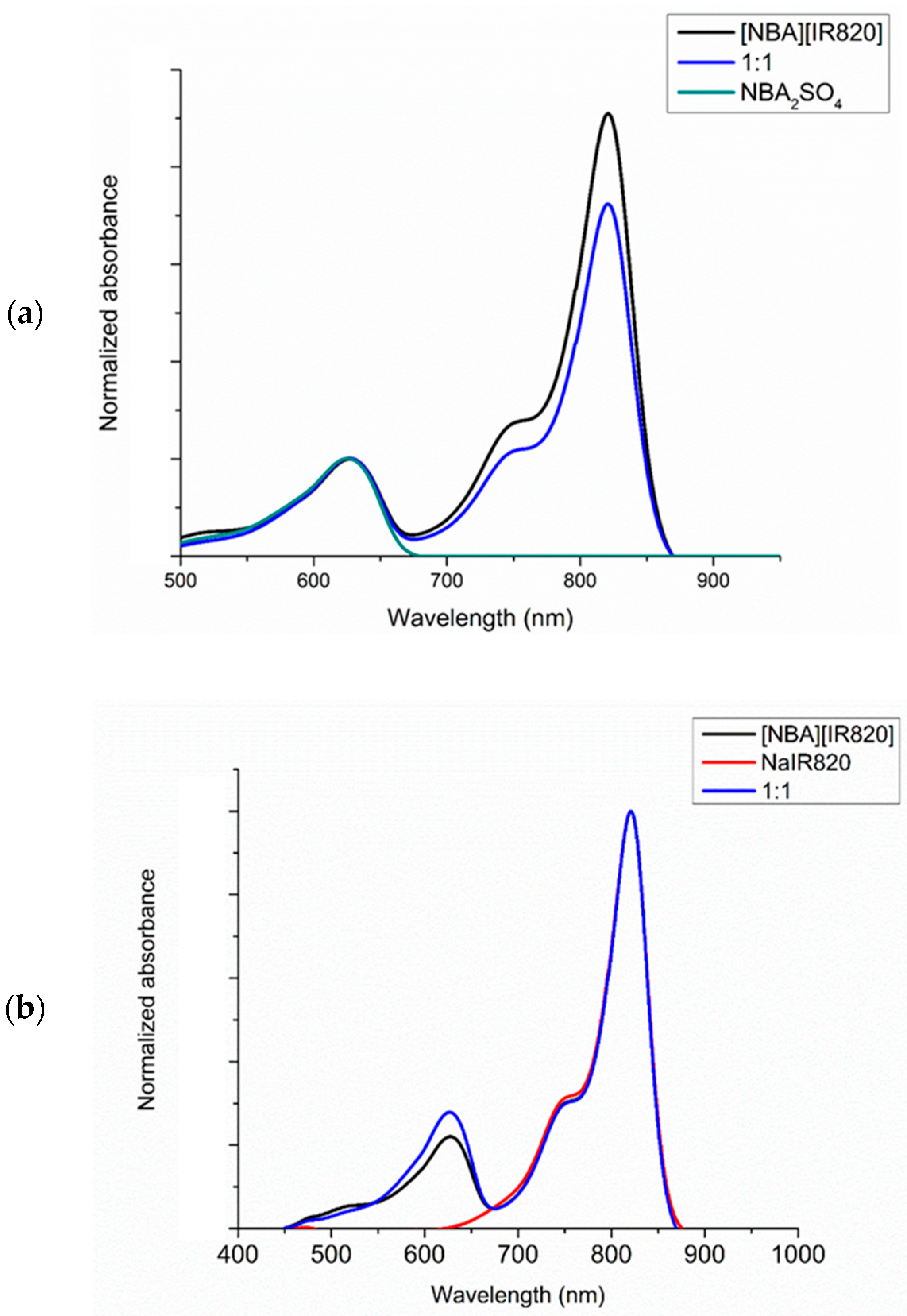
3.2. Photophysical Properties
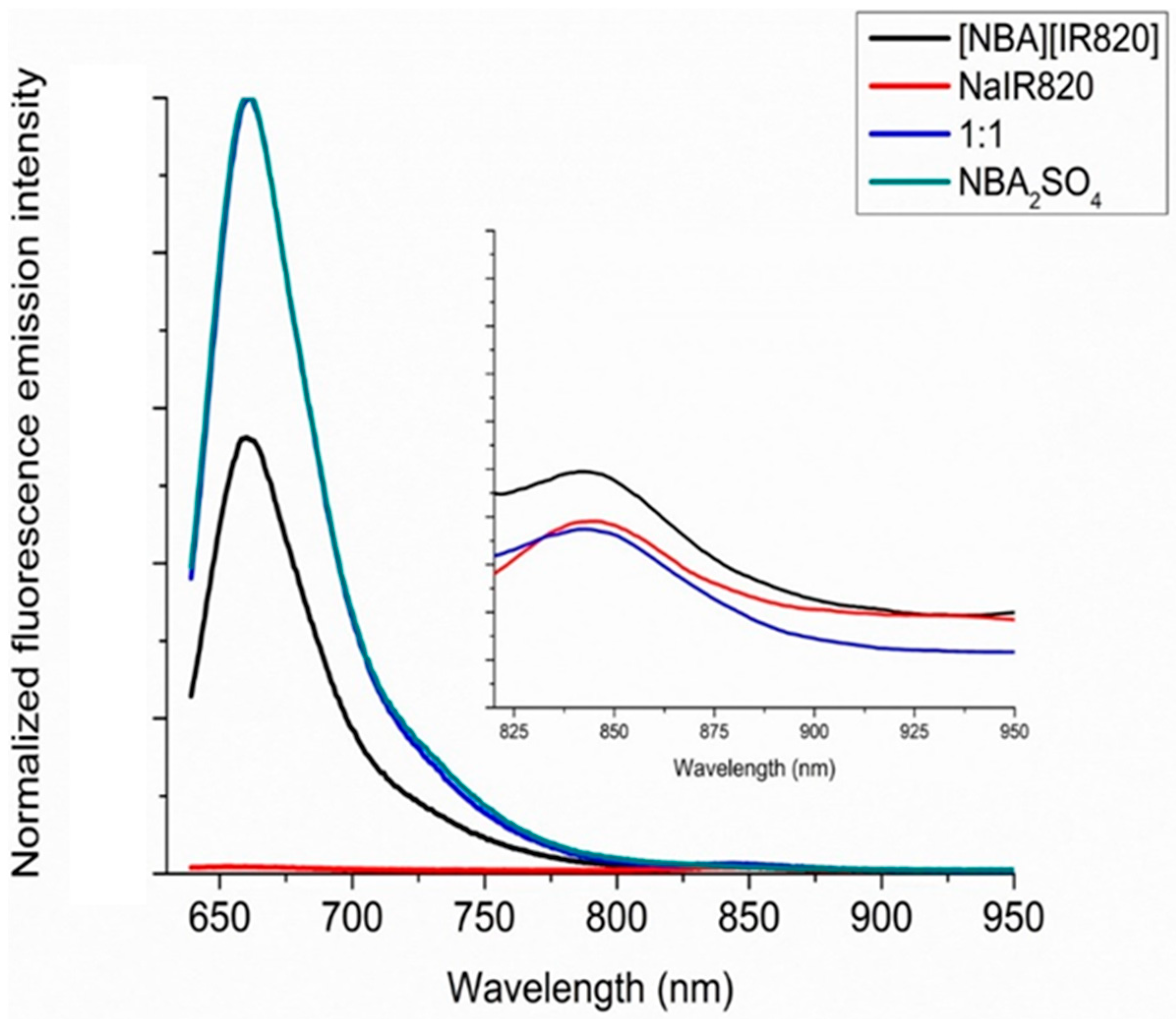
3.3. FRET Calculations
3.4. Temperature Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hug, H.; Bader, M.; Mair, P.; Glatzel, T. Biophotovoltaics: Natural Pigments in Dye-Sensitized Solar Cells. Appl. Energy 2014, 115, 216–225. [Google Scholar] [CrossRef] [Green Version]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef] [PubMed]
- Freitag, M.; Teuscher, J.; Saygili, Y.; Zhang, X.; Giordano, F.; Liska, P.; Hua, J.; Zakeeruddin, S.M.; Moser, J.E.; Grätzel, M.; et al. Dye-Sensitized Solar Cells for Efficient Power Generation under Ambient Lighting. Nat. Photonics 2017, 11, 372–378. [Google Scholar] [CrossRef]
- Kibria, M.T.; Ahammed, A.; Sony, S.M.; Hossain, F.; Islam, S.-U. A Review: Comparative Studies on Different Generation Solar Cells Technology. In Proceedings of the 5th International Conference on Environmental Aspects of Bangladesh, Dhaka, Bangladesh, 4–5 September 2014; pp. 51–53. [Google Scholar]
- Bisquert, J.; Cahen, D.; Hodes, G.; Rühle, S.; Zaban, A. Physical Chemical Principles of Photovoltaic Conversion with Nanoparticulate, Mesoporous Dye-Sensitized Solar Cells. J. Phys. Chem. B 2004, 108, 8106–8118. [Google Scholar] [CrossRef]
- Yin, D.; Liu, Y.; Tang, J.; Zhao, F.; Chen, Z.; Zhang, T.; Zhang, X.; Chang, N.; Wu, C.; Chen, D.; et al. Huge Enhancement of Upconversion Luminescence by Broadband Dye Sensitization of Core/Shell Nanocrystals. Dalton Trans. 2016, 45, 13392–13398. [Google Scholar] [CrossRef] [PubMed]
- Bella, F.; Vlachopoulos, N.; Nonomura, K.; Zakeeruddin, S.M.; Grätzel, M.; Gerbaldi, C.; Hagfeldt, A. Direct Light-Induced Polymerization of Cobalt-Based Redox Shuttles: An Ultrafast Way towards Stable Dye-Sensitized Solar Cells. Chem. Commun. 2015, 51, 16308–16311. [Google Scholar] [CrossRef] [Green Version]
- Das, T.K.; Ilaiyaraja, P.; Sudakar, C. Whispering Gallery Mode Assisted Enhancement in the Power Conversion Efficiency of DSSC and QDSSC Devices Using TiO 2 Microsphere Photoanodes. ACS Appl. Energy Mater. 2018, 1, 765–774. [Google Scholar] [CrossRef]
- Cell, S.; Jestin, Y. Learn More about Solar Spectra Photovoltaic Solar Energy an Introduction to Atmospheric Radia-Tion; Elservier Ltd.: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Kolic, P.E.; Siraj, N.; Cong, M.; Regmi, B.P.; Luan, X.; Wang, Y.; Warner, I.M. Improving Energy Relay Dyes for Dye-Sensitized Solar Cells by Use of a Group of Uniform Materials Based on Organic Salts (GUMBOS). RSC Adv. 2016, 6, 95273–95282. [Google Scholar] [CrossRef]
- Yen, S.K.; Jańczewski, D.; Lakshmi, J.L.; Dolmanan, S.B.; Tripathy, S.; Ho, V.H.B.; Vijayaragavan, V.; Hariharan, A.; Padmanabhan, P.; Bhakoo, K.K.; et al. Design and Synthesis of Polymer-Functionalized NIR Fluorescent Dyes-Magnetic Nanoparticles for Bioimaging. ACS Nano 2013, 7, 6796–6805. [Google Scholar] [CrossRef]
- Cheema, H.; Baumann, A.; Loya, E.K.; Brogdon, P.; McNamara, L.E.; Carpenter, C.A.; Hammer, N.I.; Mathew, S.; Risko, C.; Delcamp, J.H. Near-Infrared-Absorbing Indolizine-Porphyrin Push-Pull Dye for Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 16474–16489. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ko, M.J.; Lee, J.J. Novel Energy Relay Dyes for High Efficiency Dye-Sensitized Solar Cells. Nanoscale 2015, 7, 3526–3531. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.D.; Parkinson, P.; Torres, T.; Miura, H.; Herz, L.M.; Snaith, H.J. Surface Energy Relay between Cosensitized Molecules in Solid-State Dye-Sensitized Solar Cells. J. Phys. Chem. C 2011, 115, 23204–23208. [Google Scholar] [CrossRef]
- Margulis, G.Y.; Lim, B.; Hardin, B.E.; Unger, E.L.; Yum, J.H.; Feckl, J.M.; Fattakhova-Rohlfing, D.; Bein, T.; Grätzel, M.; Sellinger, A.; et al. Highly Soluble Energy Relay Dyes for Dye-Sensitized Solar Cells. Phys. Chem. Chem. Phys. 2013, 15, 11306–11312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, R.; Cui, Y.; Liu, X.; Wang, L. Multifunctional Interface Modification of Energy Relay Dye in Quasi-Solid Dye-Sensitized Solar Cells. Sci. Rep. 2014, 4, 1–6. [Google Scholar] [CrossRef]
- Ibrayev, N.; Seliverstova, E.; Nuraje, N. Materials Science in Semiconductor Processing FRET-Designed Dye-Sensitized Solar Cells to Enhance Light Harvesting. Mater. Sci. Semicond. Process. 2015, 31, 358–362. [Google Scholar] [CrossRef]
- Yuan, L.; Lin, W.; Zheng, K.; Zhu, S. FRET-Based Small-Molecule Fluorescent Probes: Rational Design and Bioimaging Applications. Acc. Chem. Res. 2013, 46, 1462–1473. [Google Scholar] [CrossRef]
- Piston, D.W.; Kremers, G.-J. Fluorescent Protein FRET: The Good, the Bad and the Ugly. Rev. TRENDS Biochem. Sci. 2007, 32, 407–414. [Google Scholar] [CrossRef]
- Eisenmenger, N.D.; Delaney, K.T.; Ganesan, V.; Fredrickson, G.H.; Chabinyc, M.L. Improving Energy Relay Dyes for Dye Sensitized Solar Cells by Increasing Donor Homotransfer. J. Phys. Chem. C 2014, 118, 14098–14106. [Google Scholar] [CrossRef]
- Warner, I.M.; El-Zahab, B.; Siraj, N. Perspectives on Moving Ionic Liquid Chemistry into the Solid Phase. Anal. Chem. 2014, 86, 7184–7191. [Google Scholar] [CrossRef]
- Jordan, A.N.; Siraj, N.; Das, S.; Warner, I.M. Tunable Near-Infrared Emission of Binary Nano- and Mesoscale GUMBOS. RSC Adv. 2014, 4, 28471–28480. [Google Scholar] [CrossRef]
- Pérez, R.L.; Cong, M.; Vaughan, S.R.; Ayala, C.E.; Galpothdeniya, W.I.S.; Mathaga, J.K.; Warner, I.M. Protein Discrimination Using a Fluorescence-Based Sensor Array of Thiacarbocyanine-GUMBOS. ACS Sens. 2020, 5, 2422–2429. [Google Scholar] [CrossRef]
- Jordan, A.N.; Das, S.; Siraj, N.; de Rooy, S.L.; Li, M.; El-Zahab, B.; Chandler, L.; Baker, G.A.; Warner, I.M. Anion-Controlled Morphologies and Spectral Features of Cyanine-Based NanoGUMBOS—An Improved Photosensitizer. Nanoscale 2012, 4, 5031–5038. [Google Scholar] [CrossRef]
- de Rooy, S.L.; Das, S.; Li, M.; El-Zahab, B.; Jordan, A.; Lodes, R.; Weber, A.; Chandler, L.; Baker, G.A.; Warner, I.M. Ionically Self-Assembled, Multi-Luminophore One-Dimensional Micro- and Nanoscale Aggregates of Thiacarbocyanine GUMBOS. J. Phys. Chem. C 2012, 116, 8251–8260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopi, A.; Lingamoorthy, S.; Soman, S.; Yoosaf, K.; Haridas, R.; Das, S. Modulating FRET in Organic-Inorganic Nanohybrids for Light Harvesting Applications. J. Phys. Chem. C 2016, 120, 26569–26578. [Google Scholar] [CrossRef]
- Dryza, V.; Bieske, E.J. Suppressing Förster Resonance Energy Transfer between Organic Dyes on a Cosensitized Metal Oxide Surface. J. Phys. Chem. C 2014, 118, 19646–19654. [Google Scholar] [CrossRef] [Green Version]
- Bajar, B.T.; Wang, E.S.; Zhang, S.; Lin, M.Z.; Chu, J. A Guide to Fluorescent Protein FRET Pairs. Sensors 2016, 16, 1488. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Das, S.; Magut, P.K.S.; Li, M.; El-Zahab, B.; Warner, I.M. Irradiation Induced Fluorescence Enhancement in PEGylated Cyanine-Based NIR Nano- and Mesoscale GUMBOS. Langmuir 2012, 28, 14415–14423. [Google Scholar] [CrossRef] [Green Version]
- Hazafy, D.; Salvia, M.V.; Mills, A.; Hutchings, M.G.; Evstigneev, M.P.; Parkinson, J.A. NMR Analysis of Nile Blue (C. I. Basic Blue 12) and Thionine (C. I. 52000) in Solution. Dye. Pigment. 2011, 88, 315–325. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, J.; Li, Q.; Tian, H.; Zhang, N.; Li, Z.; Luan, Y. PH-and Enzyme-Sensitive IR820−Paclitaxel Conjugate Self-Assembled Nanovehicles for Near-Infrared Fluorescence Imaging-Guided Chemo−Photothermal Therapy. ACS Appl. Mater. Interfaces 2018, 10, 30092–30102. [Google Scholar] [CrossRef]
- Johns, I.B.; Mcelhill, E.A.; Smith, J.O. Thermal Stability of Some Organic Compounds. J. Chem. Eng. Data 1962, 7, 277–281. [Google Scholar] [CrossRef]
- Leszczynska, A.; Njuguna, J.; Pielichowski, K.; Banerjee, J.R. Polymer/Montmorillonite Nanocomposites with Improved Thermal Properties. Part 1. Factors Influencing Thermal Stability and Mechanisms of Thermal Stability Improvement. Thermochim. Acta 2007, 453, 75–96. [Google Scholar] [CrossRef] [Green Version]
- Roy, A.; Kundu, N.; Banik, D.; Sarkar, N. Comparative Fluorescence Resonance Energy-Transfer Study in Pluronic Triblock Copolymer Micelle and Niosome Composed of Biological Component Cholesterol: An Investigation of Effect of Cholesterol and Sucrose on the FRET Parameters. J. Phys. Chem. B 2016, 120, 131–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porrès, L.; Holland, A.; Pålsson, L.O.; Monkman, A.P.; Kemp, C.; Beeby, A. Absolute Measurements of Photoluminescence Quantum Yields of Solutions Using an Integrating Sphere. J. Fluoresc. 2006, 16, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Jose, J.; Ueno, Y.; Burgess, K. Water-Soluble Nile Blue Derivatives: Syntheses and Photophysical Properties. Chem.—A Eur. J. 2009, 15, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Conceição, D.S.; Ferreira, D.P.; Vieira Ferreira, L.F. Photochemistry and Cytotoxicity Evaluation of Heptamethinecyanine near Infrared (NIR) Dyes. Int. J. Mol. Sci. 2013, 14, 18557–18571. [Google Scholar] [CrossRef]
- Zhao, W.; Leroy, F.; Heggen, B.; Zahn, S.; Kirchner, B.; Balasubramanian, S.; Müller-Plathe, F. Are There Stable Ion-Pairs in Room-Temperature Ionic Liquids? Molecular Dynamics Simulations of 1-n-Butyl-3-Methylimidazolium Hexafluorophosphate. J. Am. Chem. Soc. 2009, 131, 15825–15833. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gaylord, B.S.; Bazan, G.C. Fluorescein Provides a Resonance Gate for FRET from Conjugated Polymers to DNA Intercalated Dyes. J. Am. Chem. Soc. 2004, 126, 5446–5451. [Google Scholar] [CrossRef] [PubMed]



| % FRET efficiency | 31.20% |
| Förster distance (R0, nm) | 5.60 |
| Spectral Overlap Integral (J(λ)/M−1cm−1nm4) | 6.83 × 1015 |
| 25 °C | 40 °C | 50 °C | 60 °C | 70 °C | |
|---|---|---|---|---|---|
| % FRET efficiency | 28.82 | 26.19 | 36.16 | 24.04 | 20.58 |
| Förster distance (R0, nm) | 5.64 | 5.62 | 5.62 | 5.63 | 5.82 |
| Spectral Overlap Integral (J(λ)/M−1cm−1nm4) | 6.97 × 1015 | 6.78 × 1015 | 6.76 × 1015 | 6.83 × 1015 | 8.39 × 1015 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jalihal, A.; Le, T.; Macchi, S.; Krehbiel, H.; Bashiru, M.; Forson, M.; Siraj, N. Understanding of Förster Resonance Energy Transfer (FRET) in Ionic Materials. Sustain. Chem. 2021, 2, 564-575. https://doi.org/10.3390/suschem2040031
Jalihal A, Le T, Macchi S, Krehbiel H, Bashiru M, Forson M, Siraj N. Understanding of Förster Resonance Energy Transfer (FRET) in Ionic Materials. Sustainable Chemistry. 2021; 2(4):564-575. https://doi.org/10.3390/suschem2040031
Chicago/Turabian StyleJalihal, Amanda, Thuy Le, Samantha Macchi, Hannah Krehbiel, Mujeebat Bashiru, Mavis Forson, and Noureen Siraj. 2021. "Understanding of Förster Resonance Energy Transfer (FRET) in Ionic Materials" Sustainable Chemistry 2, no. 4: 564-575. https://doi.org/10.3390/suschem2040031
APA StyleJalihal, A., Le, T., Macchi, S., Krehbiel, H., Bashiru, M., Forson, M., & Siraj, N. (2021). Understanding of Förster Resonance Energy Transfer (FRET) in Ionic Materials. Sustainable Chemistry, 2(4), 564-575. https://doi.org/10.3390/suschem2040031







