Chlorophylls Extraction from Spinach Leaves Using Aqueous Solutions of Surface-Active Ionic Liquids
Abstract
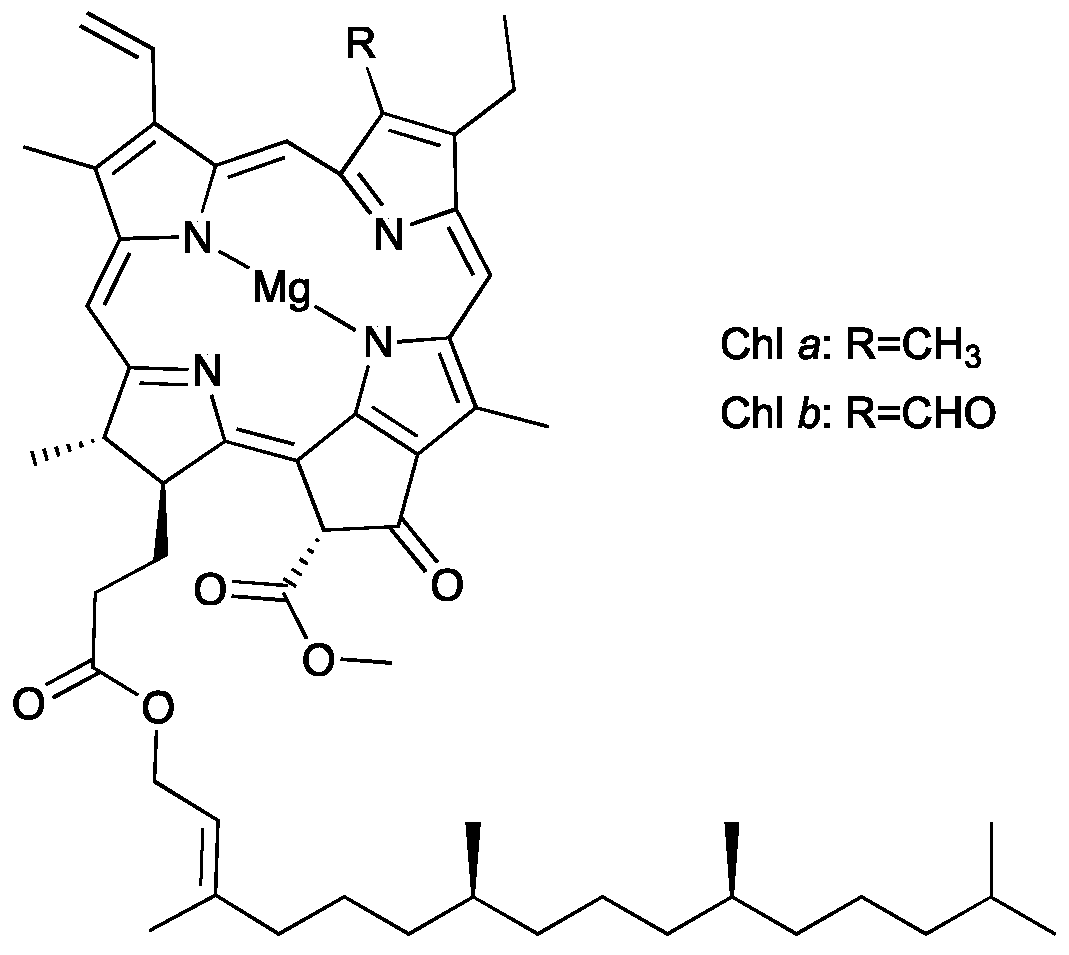
:1. Introduction
2. Materials and Methods
2.1. Materials
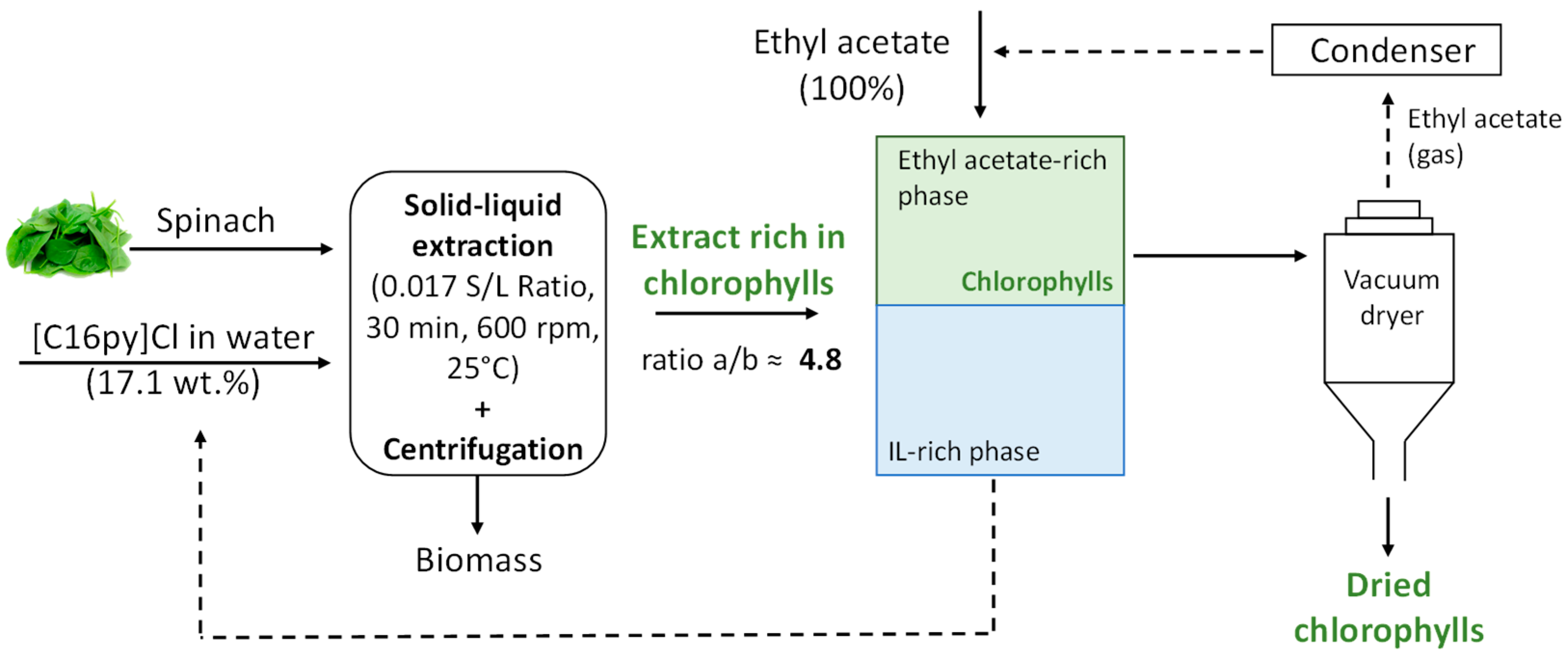
2.2. Chlorophylls Extraction
2.3. Surface Response Methodology
2.4. Chlorophylls Recovery and Solvent Reuse
3. Results and Discussion
3.1. Screening of the IL Chemical Structure to Efficiently Extract Chlorophylls from Spinach Leaves
3.2. Optimization of the Operating Conditions by Response Surface Methodology
3.3. Chlorophylls Recovery and Solvent Reuse
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hosikian, A.; Lim, S.-B.; Halim, R.; Danquah, M.K. Chlorophyll Extraction from Microalgae: A Review on the Process Engineering Aspects. Int. J. Chem. Eng. 2010, 2010, 391632. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.C.; Hung, C.F.; Wu, W.B.; Chen, B.H. Determination of chlorophylls and their derivatives in Gynostemma pentaphyllum Makino by liquid chromatography–mass spectrometry. J. Pharm. Biomed. Anal. 2008, 48, 105–112. [Google Scholar] [CrossRef]
- Makarska-Bialokoz, M.; Kaczor, A.A. Computational Analysis of Chlorophyll Structure and UV-Vis Spectra: A Student Research Project on the Spectroscopy of Natural Complexes. Spectrosc. Lett. 2014, 47, 147–152. [Google Scholar] [CrossRef]
- Cubas, C.; Gloria Lobo, M.; González, M. Optimization of the extraction of chlorophylls in green beans (Phaseolus vulgaris L.) by N,N-dimethylformamide using response surface methodology. J. Food Compos. Anal. 2008, 21, 125–133. [Google Scholar] [CrossRef]
- Schoefs, B. Determination of pigments in vegetables. J. Chromatogr. A 2004, 1054, 217–226. [Google Scholar] [CrossRef]
- Hojnik, M.; Škerget, M.; Knez, Ž. Isolation of chlorophylls from stinging nettle (Urtica dioica L.). Sep. Purif. Technol. 2007, 57, 37–46. [Google Scholar] [CrossRef]
- Kong, W.; Liu, N.; Zhang, J.; Yang, Q.; Hua, S.; Song, H.; Xia, C. Optimization of ultrasound-assisted extraction parameters of chlorophyll from Chlorella vulgaris residue after lipid separation using response surface methodology. J. Food Sci. Technol. 2014, 51, 2006–2013. [Google Scholar] [CrossRef] [Green Version]
- Damerum, A.; Selmes, S.L.; Biggi, G.F.; Clarkson, G.J.; Rothwell, S.D.; Truco, M.J.; Michelmore, R.W.; Hancock, R.D.; Shellcock, C.; Chapman, M.A.; et al. Elucidating the genetic basis of antioxidant status in lettuce (Lactuca sativa). Hortic. Res. 2015, 2, 15055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, A.A.; Senge, M.O. How green is green chemistry? Chlorophylls as a bioresource from biorefineries and their commercial potential in medicine and photovoltaics. Photochem. Photobiol. Sci. 2015, 14, 638–660. [Google Scholar] [CrossRef] [Green Version]
- Cha, K.H.; Lee, H.J.; Koo, S.Y.; Song, D.G.; Lee, D.U.; Pan, C.H. Optimization of pressurized liquid extraction of carotenoids and chlorophylls from Chlorella vulgaris. J. Agric. Food Chem. 2010, 58, 793–797. [Google Scholar] [CrossRef]
- Ballschmiter, K.; Cotton, T.M.; Strain, H.H.; Katz, J.J. Chlorophyll-water interactions Hydration, dehydration and hydrates of chlorophyll. Biochim. Biophys. Acta Bioenergy 1969, 180, 347–359. [Google Scholar] [CrossRef]
- Walden, P. Molecular weights and electrical conductivity of several fused salts. Bull. Acad. Imper. Sci. 1914, 8, 405–422. [Google Scholar]
- e Silva, F.A.; Pereira, J.F.B.; Kurnia, K.A.; Ventura, S.P.M.; Silva, A.M.S.; Rogers, R.D.; Coutinho, J.A.P.; Freire, M.G. Temperature dependency of aqueous biphasic systems: An alternative approach for exploring the differences between Coulombic-dominated salts and ionic liquids. Chem. Commun. 2017, 53, 7298–7301. [Google Scholar] [CrossRef]
- Mariani, A.; Bonomo, M.; Gao, X.; Centrella, B.; Nucara, A.; Buscaino, R.; Barge, A.; Barbero, N.; Gontrani, L.; Passerini, S. The unseen evidence of Reduced Ionicity: The elephant in (the) room temperature ionic liquids. J. Mol. Liq. 2021, 324, 115069. [Google Scholar] [CrossRef]
- Ghandi, K. A Review of Ionic Liquids, Their Limits and Applications. Green Sustain. Chem. 2014, 4, 10. [Google Scholar] [CrossRef] [Green Version]
- Gupta, H.; Chandrasekhar, M.; Krishna, T.S.; Sharma, V.K. Thermodynamic properties of mixtures containing 1-butyl-2,3-dimethylimidazolium tetrafluoroborate and cyclopentanone or cyclohexanone. J. Mol. Liq. 2017, 231, 225–237. [Google Scholar] [CrossRef]
- Philippi, F.; Welton, T. Targeted modifications in ionic liquids—from understanding to design. Phys. Chem. Chem. Phys. 2021, 23, 6993–7021. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, M. Ionic Liquids Toxicity—Benefits and Threats. Int. J. Mol. Sci. 2020, 21, 6267. [Google Scholar] [CrossRef] [PubMed]
- Ventura, S.P.M.; Marques, C.S.; Rosatella, A.A.; Afonso, C.A.M.; Gonçalves, F.; Coutinho, J.A.P. Toxicity assessment of various ionic liquid families towards Vibrio fischeri marine bacteria. Ecotoxicol. Environ. Saf. 2012, 76, 162–168. [Google Scholar] [CrossRef]
- Ventura, S.P.; Santos, L.D.; Saraiva, J.A.; Coutinho, J.A. Concentration effect of hydrophilic ionic liquids on the enzymatic activity of Candida antarctica lipase B. World J. Microbiol. Biotechnol. 2012, 28, 2303–2310. [Google Scholar] [CrossRef]
- Pernak, J.; Borucka, N.; Walkiewicz, F.; Markiewicz, B.; Fochtman, P.; Stolte, S.; Steudte, S.; Stepnowski, P. Synthesis, toxicity, biodegradability and physicochemical properties of 4-benzyl-4-methylmorpholinium-based ionic liquids. Green Chem. 2011, 13, 2901–2910. [Google Scholar] [CrossRef]
- Arning, J.; Stolte, S.; Böschen, A.; Stock, F.; Pitner, W.-R.; Welz-Biermann, U.; Jastorff, B.; Ranke, J. Qualitative and quantitative structure activity relationships for the inhibitory effects of cationic head groups, functionalised side chains and anions of ionic liquids on acetylcholinesterase. Green Chem. 2008, 10, 47–58. [Google Scholar] [CrossRef]
- Losada-Pérez, P.; Khorshid, M.; Renner, F.U. Interactions of Aqueous Imidazolium-Based Ionic Liquid Mixtures with Solid-Supported Phospholipid Vesicles. PLoS ONE 2016, 11, e0163518. [Google Scholar]
- Passos, H.; Freire, M.G.; Coutinho, J.A.P. Ionic liquid solutions as extractive solvents for value-added compounds from biomass. Green Chem. 2014, 16, 4786–4815. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, N.S.d.; Carlosa, A.L.d.S.; Mattedib, S.; Soaresa, C.M.F.; Souzaa, R.L.; Fricksa, A.T.; Lima, Á. Ionic Liquid-Based Ultrasonic-Assisted Extraction of Alkaloids from Cacao (Theobroma cacao). Chem. Eng. Trans. 2018, 64, 49–54. [Google Scholar]
- Syahmina, A.; Usuki, T. Ionic Liquid-Assisted Extraction of Essential Oils from Thujopsis dolobrata (Hiba). ACS Omega 2020, 5, 29618–29622. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Zhang, Q.; Liu, Q.; Tang, F.; Long, Y.; Yao, S. Ionic liquid surfactant-mediated ultrasonic-assisted extraction coupled to HPLC: Application to analysis of tanshinones in Salvia miltiorrhiza bunge. J. Sep. Sci. 2009, 32, 4220–4226. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Gomes, H.M.D.; Coutinho, J.A.P.; Freire, M.G. Valorization of Spent Coffee by Caffeine Extraction Using Aqueous Solutions of Cholinium-Based Ionic Liquids. Sustainability 2021, 13, 7509. [Google Scholar] [CrossRef]
- Cláudio, A.F.M.; Neves, M.C.; Shimizu, K.; Canongia Lopes, J.N.; Freire, M.G.; Coutinho, J.A.P. The magic of aqueous solutions of ionic liquids: Ionic liquids as a powerful class of catanionic hydrotropes. Green Chem. 2015, 17, 3948–3963. [Google Scholar] [CrossRef]
- de Faria, E.L.P.; Ferreira, A.M.; Cláudio, A.F.M.; Coutinho, J.A.P.; Silvestre, A.J.D.; Freire, M.G. Recovery of Syringic Acid from Industrial Food Waste with Aqueous Solutions of Ionic Liquids. ACS Sustain. Chem. Eng. 2019, 7, 14143–14152. [Google Scholar] [CrossRef]
- Abranches, D.O.; Benfica, J.; Soares, B.P.; Ferreira, A.M.; Sintra, T.E.; Shimizu, S.; Coutinho, J.A.P. The impact of the counterion in the performance of ionic hydrotropes. Chem. Commun. 2021, 57, 2951–2954. [Google Scholar] [CrossRef]
- Sales, I.; Abranches, D.O.; Costa, P.; Sintra, T.E.; Ventura, S.P.M.; Mattedi, S.; Coutinho, J.A.P.; Freire, M.G.; Pinho, S.P. Enhancing Artemisinin Solubility in Aqueous Solutions: Searching for Hydrotropes based on Ionic Liquids. Fluid Ph. Equilibria 2021, 534, 112961. [Google Scholar] [CrossRef]
- Ressmann, A.K.; Zirbs, R.; Pressler, M.; Gaertner, P.; Bica, K. Surface-active Ionic Liquids for Micellar Extraction of Piperine from Black Pepper. Z. Naturforsch. B 2013, 68, 1129–1137. [Google Scholar] [CrossRef]
- Jin, W.; Yang, Q.; Huang, B.; Bao, Z.; Su, B.; Ren, Q.; Yang, Y.; Xing, H. Enhanced solubilization and extraction of hydrophobic bioactive compounds using water/ionic liquid mixtures. Green Chem. 2016, 18, 3549–3557. [Google Scholar] [CrossRef]
- de Faria, E.L.P.; Shabudin, S.V.; Claúdio, A.F.M.; Válega, M.; Domingues, F.M.J.; Freire, C.S.R.; Silvestre, A.J.D.; Freire, M.G. Aqueous Solutions of Surface-Active Ionic Liquids: Remarkable Alternative Solvents To Improve the Solubility of Triterpenic Acids and Their Extraction from Biomass. ACS Sustain. Chem. Eng. 2017, 5, 7344–7351. [Google Scholar] [CrossRef]
- De Faria, E.L.P.; Do Carmo, R.S.; Cláudio, A.F.M.; Freire, C.S.R.; Freire, M.G.; Silvestre, A.J.D. Deep Eutectic Solvents as Efficient Media for the Extraction and Recovery of Cynaropicrin from Cynara cardunculus L. Leaves. Int. J. Mol. Sci. 2017, 18, 2276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, M.; Vieira, F.A.; Correia, I.; Ferreira, R.A.S.; Abreu, H.; Coutinho, J.A.P.; Ventura, S.P.M. Recovery of phycobiliproteins from the red macroalga Gracilaria sp. using ionic liquid aqueous solutions. Green Chem. 2016, 18, 4287–4296. [Google Scholar] [CrossRef]
- Martins, M.; Albuquerque, C.M.; Pereira, C.F.; Coutinho, J.A.P.; Neves, M.G.P.M.S.; GA Pinto, D.C.; Faustino, M.A.F.; Ventura, S.P.M. Recovery of Chlorophyll a Derivative from Spirulina maxima: Its Purification and Photosensitizing Potential. ACS Sustain. Chem. Eng. 2021, 9, 1772–1780. [Google Scholar] [CrossRef]
- Martins, M.; Fernandes, A.P.M.; Torres-Acosta, M.A.; Collén, P.N.; Abreu, M.H.; Ventura, S.P.M. Extraction of chlorophyll from wild and farmed Ulva spp. using aqueous solutions of ionic liquids. Sep. Purif. Technol. 2021, 254, 117589. [Google Scholar] [CrossRef]
- Goodchild, I.; Collier, L.; Millar, S.L.; Prokeš, I.; Lord, J.C.D.; Butts, C.P.; Bowers, J.; Webster, J.R.P.; Heenan, R.K. Structural studies of the phase, aggregation and surface behaviour of 1-alkyl-3-methylimidazolium halide + water mixtures. J. Colloid Interface Sci. 2007, 307, 455–468. [Google Scholar] [CrossRef]
- Leite, A.C.; Ferreira, A.M.; Morais, E.S.; Khan, I.; Freire, M.G.; Coutinho, J.A.P. Cloud Point Extraction of Chlorophylls from Spinach Leaves Using Aqueous Solutions of Nonionic Surfactants. ACS Sustain. Chem. Eng. 2018, 6, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Zhou, Y.; Qin, J.G.; Yao, W.; Ma, Z. Optimization of the Method for Chlorophyll Extraction in Aquatic Plants. J. Freshw. Ecol. 2010, 25, 531–538. [Google Scholar] [CrossRef] [Green Version]
- Tsuji, T.; Mori, T.; Taniguchi, M.; Shimizu, K.; Kobayashi, T. Solvent extraction of plant pigments from leaf protein concentrate. J. Chem. Eng. Jpn. 1985, 18, 539–544. [Google Scholar] [CrossRef] [Green Version]
- Derrien, M.; Badr, A.; Gosselin, A.; Desjardins, Y.; Angers, P. Optimization of a green process for the extraction of lutein and chlorophyll from spinach by-products using response surface methodology (RSM). LWT—Food Sci. Technol. 2017, 79, 170–177. [Google Scholar] [CrossRef]
- Scientific Committee on Consumer Safety. Opinion on Cetylpyridinium Chloride—Submission II ( COLIPA n° P97); European Union. 2015. Available online: https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_171.pdf (accessed on 22 November 2021).




| Extraction Conditions | Yield of Chlorophyll a (wt.%) | Yield of Total Chlorophylls (wt.%) | Chlorophyll a/b Ratio | Ref. |
|---|---|---|---|---|
| C11-C139EO’s at 12.4 mM, solid–liquid ratio of 0.07, 30 min at 41 °C | --- | 0.094 | --- | [41] |
| Brij 98 at 3.3 mM, solid–liquid ratio of 0.02, 30 min at 25 °C (not optimized) | 0.007 | 0.010 | 4.71 | [31] |
| Ethanol at 100%, solid–liquid ratio of 0.01, 30 min at 25 °C (not optimized) | 0.099 | 0.124 | 3.92 | This study |
| Ethanol–water mixtures at 93%, 258 min at 43 °C | --- | 0.104 | ---- | [44] |
| [C16py]Cl at 17.1 wt.%, solid–liquid ratio of 0.017, 30 min at 25 °C | 0.104 | 0.126 | 4.79 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, A.M.; Leite, A.C.; Coutinho, J.A.P.; Freire, M.G. Chlorophylls Extraction from Spinach Leaves Using Aqueous Solutions of Surface-Active Ionic Liquids. Sustain. Chem. 2021, 2, 764-777. https://doi.org/10.3390/suschem2040040
Ferreira AM, Leite AC, Coutinho JAP, Freire MG. Chlorophylls Extraction from Spinach Leaves Using Aqueous Solutions of Surface-Active Ionic Liquids. Sustainable Chemistry. 2021; 2(4):764-777. https://doi.org/10.3390/suschem2040040
Chicago/Turabian StyleFerreira, Ana M., Ana Cláudia Leite, João A. P. Coutinho, and Mara G. Freire. 2021. "Chlorophylls Extraction from Spinach Leaves Using Aqueous Solutions of Surface-Active Ionic Liquids" Sustainable Chemistry 2, no. 4: 764-777. https://doi.org/10.3390/suschem2040040
APA StyleFerreira, A. M., Leite, A. C., Coutinho, J. A. P., & Freire, M. G. (2021). Chlorophylls Extraction from Spinach Leaves Using Aqueous Solutions of Surface-Active Ionic Liquids. Sustainable Chemistry, 2(4), 764-777. https://doi.org/10.3390/suschem2040040








