Valorization of Hazardous Organic Solid Wastes towards Fuels and Chemicals via Fast (Catalytic) Pyrolysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials—Feeds
2.2. Raw Materials Physicochemical Properties
2.3. Fast Pyrolysis Experiments on the Py/GC-MS System
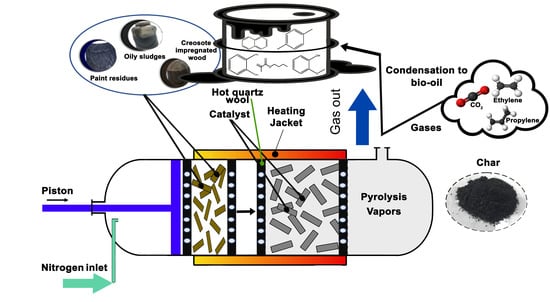
2.4. Fast Pyrolysis Experiments on Bench-Scale Fixed-Bed Reactor
3. Results & Discussion
3.1. Waste Materials Physicochemical Properties
3.2. Fast Pyrolysis of NAS Wastes on Py/GC-MS System
3.3. Fast Pyrolysis of NAS Wastes on Bench-Scale Fixed-Bed Reactor
3.3.1. Petroleum Containing Sludge (NAS-1) Pyrolysis Results
3.3.2. Residual Paint on Metal Containers (NAS-2) Pyrolysis Results
3.3.3. Creosote-Treated Wood Waste (NAS-3) Pyrolysis Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- European Parliament Council. Council Directive 2008/98/EC of 19 November 2008 on Waste and Repealing Certain Directives; European Union: Luxembourg, 2008; p. 02008L0098. [Google Scholar]
- Lombardi, L.; Carnevale, E.; Corti, A. A review of technologies and performances of thermal treatment systems for energy recovery from waste. Waste Manag. 2015, 37, 26–44. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.T.; Mohaddespour, A.; Nasr, A.T.; Carter, S. Municipal solid waste-to-energy processing for a circular economy in New Zealand. Renew. Sustain. Energy Rev. 2021, 145, 111080. [Google Scholar] [CrossRef]
- Block, C.; Van Caneghem, J.; Van Brecht, A.; Wauters, G.; Vandecasteele, C. Incineration of Hazardous Waste: A Sustainable Process. Waste Biomass Valorization 2015, 6, 137–145. [Google Scholar] [CrossRef]
- National Research Council. Opportunities and Obstacles in Large-Scale Biomass Utilization: The Role of the Chemical Sciences and Engineering Communities: A Workshop Summary; The National Academies Press: Washington, DC, USA, 2012; p. 60. [Google Scholar]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Sharifzadeh, M.; Sadeqzadeh, M.; Guo, M.; Borhani, T.N.; Murthy Konda, N.V.S.N.; Garcia, M.C.; Wang, L.; Hallett, J.; Shah, N. The multi-scale challenges of biomass fast pyrolysis and bio-oil upgrading: Review of the state of art and future research directions. Prog. Energy Combust. Sci. 2019, 71, 1–80. [Google Scholar] [CrossRef]
- Oasmaa, A.; Lehto, J.; Solantausta, Y.; Kallio, S. Historical Review on VTT Fast Pyrolysis Bio-oil Production and Upgrading. Energy Fuels 2021, 35, 5683–5695. [Google Scholar] [CrossRef]
- Sorunmu, Y.; Billen, P.; Spatari, S. A review of thermochemical upgrading of pyrolysis bio-oil: Techno-economic analysis, life cycle assessment, and technology readiness. GCB Bioenergy 2020, 12, 4–18. [Google Scholar] [CrossRef] [Green Version]
- Charisteidis, I.; Lazaridis, P.; Fotopoulos, A.; Pachatouridou, E.; Matsakas, L.; Rova, U.; Christakopoulos, P.; Triantafyllidis, K. Catalytic Fast Pyrolysis of Lignin Isolated by Hybrid Organosolv—Steam Explosion Pretreatment of Hardwood and Softwood Biomass for the Production of Phenolics and Aromatics. Catalysts 2019, 9, 935. [Google Scholar] [CrossRef] [Green Version]
- Lazaridis, P.A.; Fotopoulos, A.P.; Karakoulia, S.A.; Triantafyllidis, K.S. Catalytic Fast Pyrolysis of Kraft Lignin With Conventional, Mesoporous and Nanosized ZSM-5 Zeolite for the Production of Alkyl-Phenols and Aromatics. Front. Chem. 2018, 6, 295. [Google Scholar] [CrossRef] [Green Version]
- Kalogiannis, K.G.; Matsakas, L.; Lappas, A.A.; Rova, U.; Christakopoulos, P. Aromatics from Beechwood Organosolv Lignin through Thermal and Catalytic Pyrolysis. Energies 2019, 12, 1606. [Google Scholar] [CrossRef] [Green Version]
- Kalogiannis, K.G.; Stefanidis, S.D.; Michailof, C.M.; Lappas, A.A.; Sjöholm, E. Pyrolysis of lignin with 2DGC quantification of lignin oil: Effect of lignin type, process temperature and ZSM-5 in situ upgrading. J. Anal. Appl. Pyrolysis 2015, 115, 410–418. [Google Scholar] [CrossRef]
- Mondal, A.K.; Qin, C.; Ragauskas, A.J.; Ni, Y.; Huang, F. Preparation and Characterization of Various Kraft Lignins and Impact on Their Pyrolysis Behaviors. Ind. Eng. Chem. Res. 2020, 59, 3310–3320. [Google Scholar] [CrossRef]
- Margellou, A.G.; Lazaridis, P.A.; Charisteidis, I.D.; Nitsos, C.K.; Pappa, C.P.; Fotopoulos, A.P.; Van den Bosch, S.; Sels, B.F.; Triantafyllidis, K.S. Catalytic fast pyrolysis of beech wood lignin isolated by different biomass (pre)treatment processes: Organosolv, hydrothermal and enzymatic hydrolysis. Appl. Catal. A Gen. 2021, 623, 118298. [Google Scholar] [CrossRef]
- Liu, C.; Wang, H.; Karim, A.M.; Sun, J.; Wang, Y. Catalytic fast pyrolysis of lignocellulosic biomass. Chem. Soc. Rev. 2014, 43, 7594–7623. [Google Scholar] [CrossRef] [PubMed]
- Imran, A.; Bramer, E.A.; Seshan, K.; Brem, G. An overview of catalysts in biomass pyrolysis for production of biofuels. J. Biofuel Res. J. 2018, 5, 872–885. [Google Scholar] [CrossRef] [Green Version]
- Iliopoulou, E.F.; Lazaridis, P.A.; Triantafyllidis, K.S. Nanocatalysis in the Fast Pyrolysis of Lignocellulosic Biomass. In Nanotechnology in Catalysis; Springer: Berlin/Heidelberg, Germany, 2017; pp. 655–714. [Google Scholar]
- Iliopoulou, E.F.; Triantafyllidis, K.S.; Lappas, A.A. Overview of catalytic upgrading of biomass pyrolysis vapors toward the production of fuels and high-value chemicals. WIREs Energy Environ. 2019, 8, e322. [Google Scholar] [CrossRef] [Green Version]
- Triantafyllidis, K.S.; Stefanidis, S.D.; Karakoulia, S.A.; Pineda, A.; Margellou, A.; Kalogiannis, K.G.; Iliopoulou, E.F.; Lappas, A.A. Lappas. 5. State-of-the-art in biomass fast pyrolysis using acidic catalysts: Direct comparison between microporous zeolites, mesoporous aluminosilicates and hierarchical zeolites. In Biomass and Biowaste; Balu, A.M., Nuñez, A.G., Eds.; De Gruyter: Berlin, Germany, 2020; pp. 113–144. [Google Scholar]
- Rahman, M.M.; Liu, R.; Cai, J. Catalytic fast pyrolysis of biomass over zeolites for high quality bio-oil—A review. Fuel Processing Technol. 2018, 180, 32–46. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Moon, J.; Lee, J.H.; Jin, X.; Choi, J.W. Conversion of phenol intermediates into aromatic hydrocarbons over various zeolites during lignin pyrolysis. Fuel 2020, 279, 118484. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Zhou, G.; Wang, W.; Wang, C.; Komarneni, S.; Wang, Y. Catalytic fast pyrolysis of biomass with mesoporous ZSM-5 zeolites prepared by desilication with NaOH solutions. Appl. Catal. A Gen. 2014, 470, 115–122. [Google Scholar] [CrossRef]
- Carlson, T.R.; Cheng, Y.-T.; Jae, J.; Huber, G.W. Production of green aromatics and olefins by catalytic fast pyrolysis of wood sawdust. Energy Environ. Sci. 2011, 4, 145–161. [Google Scholar] [CrossRef] [Green Version]
- Foster, A.J.; Jae, J.; Cheng, Y.-T.; Huber, G.W.; Lobo, R.F. Optimizing the aromatic yield and distribution from catalytic fast pyrolysis of biomass over ZSM-5. Appl. Catal. A Gen. 2012, 423–424, 154–161. [Google Scholar] [CrossRef]
- Carlson, T.R.; Vispute, T.P.; Huber, G.W. Green Gasoline by Catalytic Fast Pyrolysis of Solid Biomass Derived Compounds. ChemSusChem 2008, 1, 397–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gayubo, A.G.; Aguayo, A.T.; Atutxa, A.; Aguado, R.; Olazar, M.; Bilbao, J. Transformation of Oxygenate Components of Biomass Pyrolysis Oil on a HZSM-5 Zeolite. II. Aldehydes, Ketones, and Acids. Ind. Eng. Chem. Res. 2004, 43, 2619–2626. [Google Scholar] [CrossRef]
- Mihalcik, D.J.; Mullen, C.A.; Boateng, A.A. Screening acidic zeolites for catalytic fast pyrolysis of biomass and its components. J. Anal. Appl. Pyrolysis 2011, 92, 224–232. [Google Scholar] [CrossRef]
- Gamliel, D.P.; Cho, H.J.; Fan, W.; Valla, J.A. On the effectiveness of tailored mesoporous MFI zeolites for biomass catalytic fast pyrolysis. Appl. Catal. A Gen. 2016, 522, 109–119. [Google Scholar] [CrossRef] [Green Version]
- Zheng, A.; Zhao, Z.; Chang, S.; Huang, Z.; Wu, H.; Wang, X.; He, F.; Li, H. Effect of crystal size of ZSM-5 on the aromatic yield and selectivity from catalytic fast pyrolysis of biomass. J. Mol. Catal. A Chem. 2014, 383–384, 23–30. [Google Scholar] [CrossRef]
- Wang, K.; Kim, K.H.; Brown, R.C. Catalytic pyrolysis of individual components of lignocellulosic biomass. Green Chem. 2014, 16, 727–735. [Google Scholar] [CrossRef]
- Hu, G.J.; Li, J.B.; Zeng, G.M. Recent development in the treatment of oily sludge from petroleum industry: A review. J. Hazard. Mater. 2013, 261, 470–490. [Google Scholar] [CrossRef]
- Chen, H.S.; Zhang, Q.M.; Yang, Z.J.; Liu, Y.S. Research on Treatment of Oily Sludge from the Tank Bottom by Ball Milling Combined with Ozone-Catalyzed Oxidation. ACS Omega 2020, 5, 12259–12269. [Google Scholar] [CrossRef]
- Badrul, I. Petroleum Sludge, its Treatment and Disposal: A Review. Int. J. Chem. Sci. 2015, 13, 1584–1602. [Google Scholar]
- Al-Futaisi, A.; Jamrah, A.; Yaghi, B.; Taha, R. Assessment of alternative management techniques of tank bottom petroleum sludge in Oman. J. Hazard. Mater. 2007, 141, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-T.; Lee, W.-J.; Mi, H.-H.; Su, C.-C. PAH emission from the incineration of waste oily sludge and PE plastic mixtures. Sci. Total Environ. 1995, 170, 171–183. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, X.; Zhou, L.; Han, X.; Cui, Z. Pyrolysis treatment of oil sludge and model-free kinetics analysis. J. Hazard. Mater. 2009, 161, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, X.; Han, X. Devolatilization of oil sludge in a lab-scale bubbling fluidized bed. J. Hazard. Mater. 2011, 185, 1205–1213. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Shie, J.-L.; Lin, J.-P.; Wu, C.-H.; Lee, D.-J.; Chang, C.-F. Major Products Obtained from the Pyrolysis of Oil Sludge. Energy Fuels 2000, 14, 1176–1183. [Google Scholar] [CrossRef]
- Wang, Z.; Gong, Z.; Wang, Z.; Li, X.; Chu, Z. Application and development of pyrolysis technology in petroleum oily sludge treatment. Environ. Eng. Res. 2021, 26, 190460. [Google Scholar] [CrossRef]
- Lin, B.; Huang, Q.; Ali, M.; Wang, F.; Chi, Y.; Yan, J. Continuous catalytic pyrolysis of oily sludge using U-shape reactor for producing saturates-enriched light oil. Proc. Combust. Inst. 2019, 37, 3101–3108. [Google Scholar] [CrossRef]
- Lenka, M.; Peter, E. End-of-Waste Criteria for Iron and Steel Scrap: Technical Proposals; European Commission. Joint Research Centre. Institute for Prospective Technological Studies; Publications Office: Luxembourg, 2010. [Google Scholar]
- Nitsos, C.K.; Choli-Papadopoulou, T.; Matis, K.A.; Triantafyllidis, K.S. Optimization of Hydrothermal Pretreatment of Hardwood and Softwood Lignocellulosic Residues for Selective Hemicellulose Recovery and Improved Cellulose Enzymatic Hydrolysis. ACS Sustain. Chem. Eng. 2016, 4, 4529–4544. [Google Scholar] [CrossRef]
- Tian, J.; Ni, F.J.; Chen, M. Application of pyrolysis in dealing with end-of-life vehicular products: A case study on car bumpers. J. Clean. Prod. 2015, 108, 1177–1183. [Google Scholar] [CrossRef]
- Santos, J.I.; Martín-Sampedro, R.; Fillat, Ú.; Oliva, J.M.; Negro, M.J.; Ballesteros, M.; Eugenio, M.E.; Ibarra, D. Evaluating Lignin-Rich Residues from Biochemical Ethanol Production of Wheat Straw and Olive Tree Pruning by FTIR and 2D-NMR. Int. J. Polym. Sci. 2015, 2015, 314891. [Google Scholar] [CrossRef] [Green Version]
- Thierfelder, T.; Sandstrom, E. The creosote content of used railway crossties as compared with European stipulations for hazardous waste. Sci. Total Environ. 2008, 402, 106–112. [Google Scholar] [CrossRef]
- Hutzinger, O. The Handbook of Environmental Chemistry. Volume 3 Pt. D: Anthropogenic Compounds; Addison, R.F., Hutzinger, O., Eds.; Springer: Berlin, Germany, 1986. [Google Scholar]
- Jung, S.H.; Koo, W.M.; Kim, J.S. Fast pyrolysis of creosote treated wood ties in a fluidized bed reactor and analytical characterization of product fractions. Energy 2013, 53, 33–39. [Google Scholar] [CrossRef]
- Hoang Pham, L.K.; Vi Tran, T.T.; Kongparakul, S.; Reubroycharoen, P.; Ding, M.; Guan, G.; Vo, D.-V.N.; Jaiyong, P.; Youngvises, N.; Samart, C. Data-driven prediction of biomass pyrolysis pathways toward phenolic and aromatic products. J. Environ. Chem. Eng. 2021, 9, 104836. [Google Scholar] [CrossRef]
- Maksimuk, Y.; Antonava, Z.; Krouk, V.; Korsakova, A.; Kursevich, V. Prediction of higher heating value based on elemental composition for lignin and other fuels. Fuel 2020, 263, 116727. [Google Scholar] [CrossRef]
- Annamalai, K.; Sweeten, J.M.; Ramalingam, S.C. Technical Notes: Estimation of Gross Heating Values of Biomass Fuels. Trans. ASAE 1987, 30, 1205–1208. [Google Scholar] [CrossRef]
- Bridle, T.; Unkovich, I. Critical Factors for Sludge Pyrolysis in Australia. Water 2002, 29, 43–48. [Google Scholar]
- Scholz, W. Paint Additives. In Surface Coatings: Volume 1 Raw Materials and Their Usage; Springer: Dordrecht, The Netherlands, 1993; pp. 539–580. [Google Scholar]
- Zandersons, J.; Zhurinsh, A.; Someus, E. Prospects for co-firing of clean coal and creosote-treated waste wood at small-scale power stations. Therm. Sci. 2006, 10, 109–118. [Google Scholar] [CrossRef]
- Nitsos, C.K.; Matis, K.A.; Triantafyllidis, K.S. Optimization of Hydrothermal Pretreatment of Lignocellulosic Biomass in the Bioethanol Production Process. ChemSusChem 2013, 6, 110–122. [Google Scholar] [CrossRef]
- Lee, K.-G.; Lee, S.-E.; Takeoka, G.R.; Kim, J.-H.; Park, B.-S. Antioxidant activity and characterization of volatile constituents of beechwood creosote. J. Sci. Food Agric. 2005, 85, 1580–1586. [Google Scholar] [CrossRef]
- Speight, J.G. The Chemistry and Technology of Coal, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Melber, C.; Kielhorn, J.; Mangelsdorf, I.; World Health Organization; The International Programme on Chemical Safety. Coal Tar Creosote. Available online: https://apps.who.int/iris/handle/10665/42943 (accessed on 15 January 2022).
- Davis, S.; Boundy, R.G. Transportation Energy Data Book: Edition 39; US Department of Energy: Oak Ridge, TN, USA, 2021.
- Demirbas, A. Higher heating values of lignin types from wood and non-wood lignocellulosic biomasses. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 39, 592–598. [Google Scholar] [CrossRef]
- Christoforou, E.A.; Fokaides, P.A.; Banks, S.W.; Nowakowski, D.; Bridgwater, A.V.; Stefanidis, S.; Kalogiannis, K.G.; Iliopoulou, E.F.; Lappas, A.A. Comparative Study on Catalytic and Non-Catalytic Pyrolysis of Olive Mill Solid Wastes. Waste Biomass Valorization 2017, 9, 301–313. [Google Scholar] [CrossRef] [Green Version]
- Yusuff, A.S.; Adeniyi, O.D.; Olutoye, M.A.; Akpan, U.G. Performance and Emission Characteristics of Diesel Engine Fuelled with Waste Frying Oil Derived Biodiesel-Petroleum Diesel Blend. Int. J. Eng. Res. Afr. 2017, 32, 100–111. [Google Scholar] [CrossRef]
- Jotun, P.E.L. Pioner Topcoat AV SAFETY DATA SHEET. Available online: https://www.jotun.com/Datasheets/Download?url=%2FSDS%2FSDS__1117__Pioner%20Topcoat%20AV__Eng__US.pdf (accessed on 19 February 2022).
- Jotun, P.E.L. Pioner Topcoat AV Technical Data Sheet. Available online: https://www.jotun.com/Datasheets/Download?url=%2FTDS%2FTDS__1117__Pioner%20Topcoat%20AV__Eng__US.pdf (accessed on 15 January 2022).
- Ocampo, C.; Armelin, E.; Liesa, F.; Alemán, C.; Ramis, X.; Iribarren, J.I. Application of a polythiophene derivative as anticorrosive additive for paints. Prog. Org. Coat. 2005, 53, 217–224. [Google Scholar] [CrossRef]
- Meilunas, R.J.; Bentsen, J.G.; Steinberg, A. Analysis of Aged Paint Binders by FTIR Spectroscopy. Stud. Conserv. 1990, 35, 33–51. [Google Scholar] [CrossRef]
- Stringari, C.; Pratt, E. The Identification and Characterization of Acrylic Emulsion Paint Media; Saving the Twentieth Century: The Conservation of Modern Materials; Grattan, D., Ed.; Canadian Conservation Institute (CCI): Ottawa, ON, Canada, 1993.
- Plav, B.; Kobe, S.; Orel, B. Identification of crystallization forms of CaCO3 with FTIR spectroscopy. Kovine Zlitine Teh. 1999, 33, 517–521. [Google Scholar]
- Nestler, F.H.M. The Characterization of Wood-Preserving Creosote by Physical and Chemical Methods of Analysis; U.S. Department of Agriculture, Forest Service, Forest Products Laboratory: Madison, WI, USA, 1974.
- Iliopoulou, E.F.; Stefanidis, S.D.; Kalogiannis, K.G.; Delimitis, A.; Lappas, A.A.; Triantafyllidis, K.S. Catalytic upgrading of biomass pyrolysis vapors using transition metal-modified ZSM-5 zeolite. Appl. Catal. B Environ. 2012, 127, 281–290. [Google Scholar] [CrossRef]
- Gusev, A.A.; Psarras, A.C.; Triantafyllidis, K.S.; Lappas, A.A.; Diddams, P.A. Effect of Steam Deactivation Severity of ZSM-5 Additives on LPG Olefins Production in the FCC Process. Molecules 2017, 22, 1784. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Zhang, C.; Zhu, L.; Xu, Z.; Gu, M.; Chu, H. Experimental study on pyrolysis of camphor wood catalyzed by CaO-calcined phosphate mixture. Fuel 2021, 288, 119642. [Google Scholar] [CrossRef]
- Iliopoulou, E.F.; Stefanidis, S.; Kalogiannis, K.; Psarras, A.C.; Delimitis, A.; Triantafyllidis, K.S.; Lappas, A.A. Pilot-scale validation of Co-ZSM-5 catalyst performance in the catalytic upgrading of biomass pyrolysis vapours. Green Chem. 2014, 16, 662–674. [Google Scholar] [CrossRef]

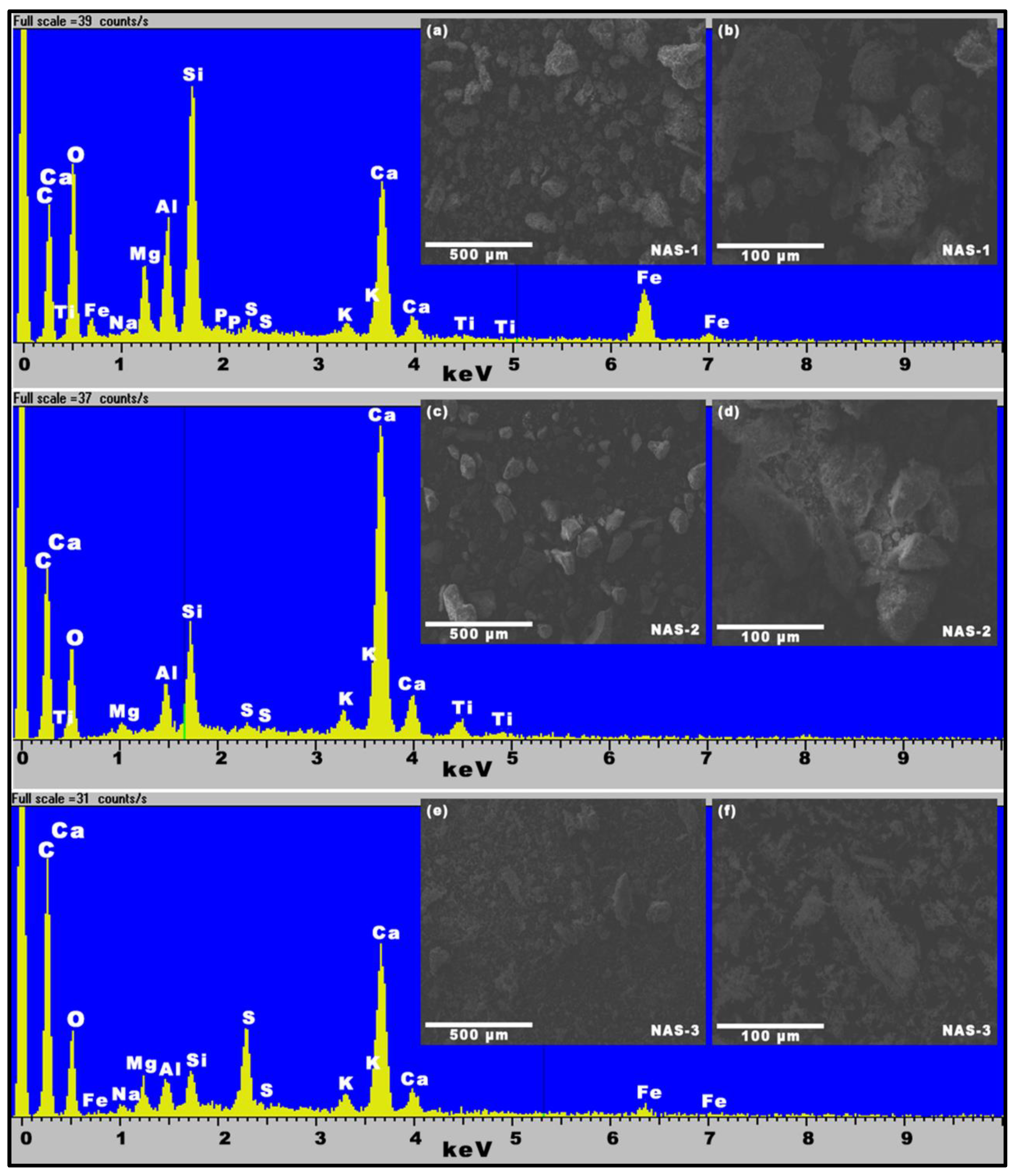
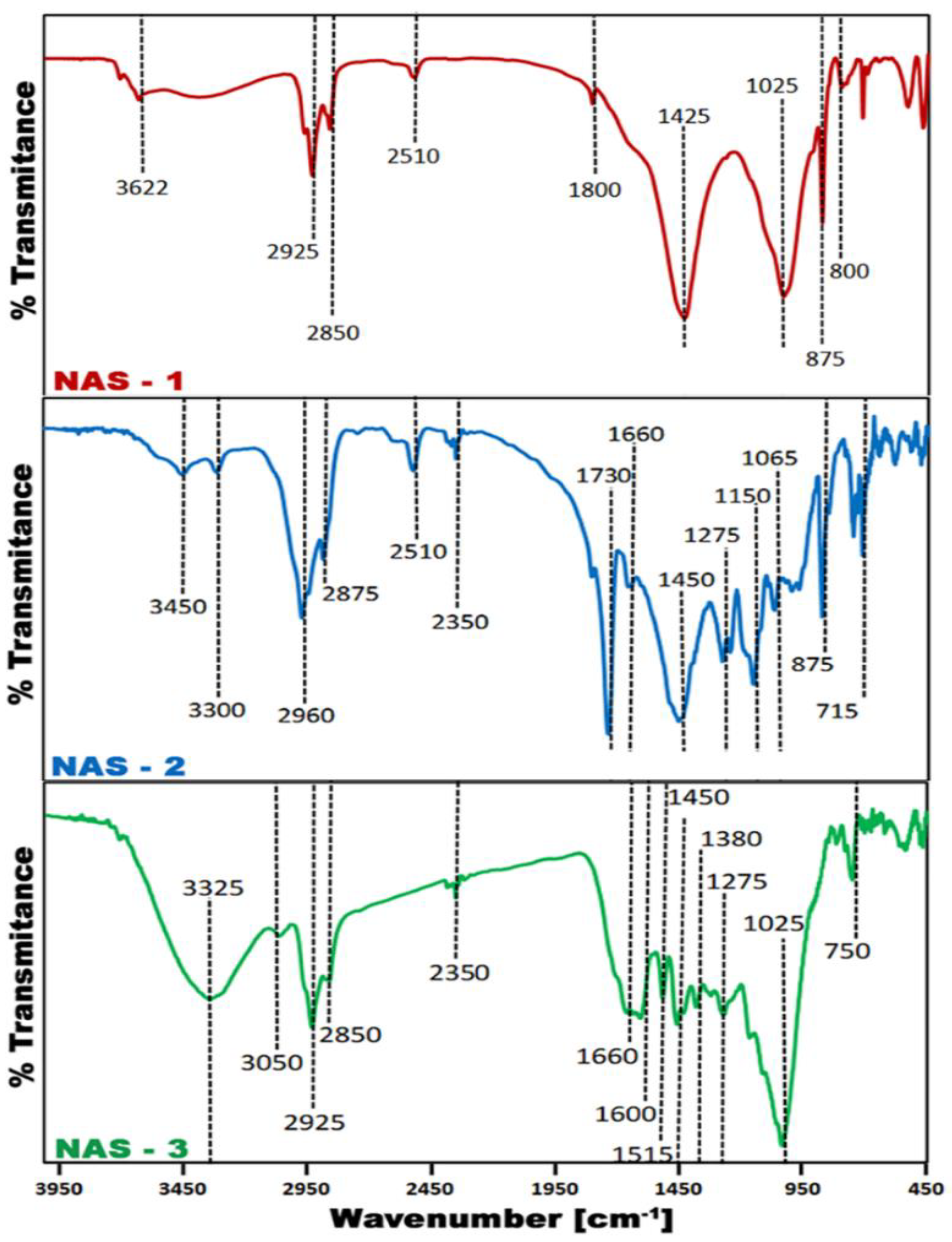
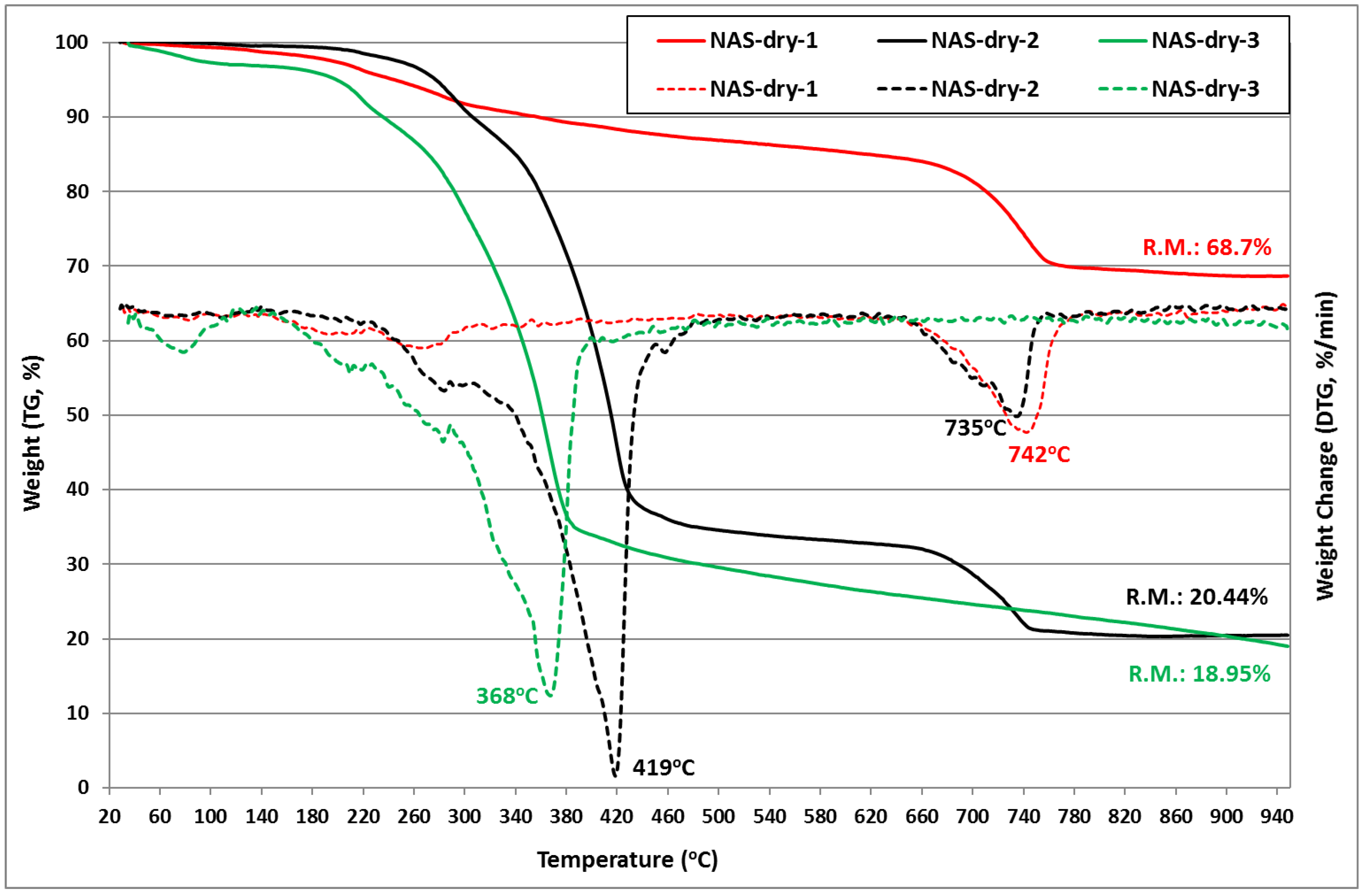


| Waste | C (% w.t.) | H (% w.t.) | Ν (% w.t.) | S (% w.t.) | O (% w.t.) | HHV (MJ/kg) | |
|---|---|---|---|---|---|---|---|
| Theoretical | Experimental | ||||||
| NAS-1 | 15.04 | 1.32 | 0.35 | 1.10 | 8.19 (a) | 6.05 | 5.90 |
| NAS-2 | 48.58 | 6.17 | 0.10 | 0.45 | 14.70 (b) | 22.67 | 21.97 |
| NAS-3 | 50.37 | 5.64 | 0.95 | 5.60 | 35.94 (c) | 20.92 | 20.95 |
| Compound | Group | % | Compound | Group | % |
|---|---|---|---|---|---|
| 2-Hexen-1-ol, (Z)- | AL | 0.15 | Methyl stearate | EST | 0.13 |
| 1,3,5-Hexatriene, (Z)- | ALI | 0.60 | Docosane | ALI | 0.48 |
| Propanoic acid, 2-methyl-, methyl ester | EST | 0.39 | Butyl 9-octadecenoate or 9-18:1 | ALI | 0.34 |
| 3-Pentanone | KET | 0.06 | Hexanedioic acid, bis(2-ethylhexyl) ester | EST | 0.28 |
| 1-Deoxy-2,4-methylene-3,5-anhydro-d-xylitol | AL | 21.76 | Dodecanoic acid, undecyl ester | EST | 0.23 |
| Propanoic acid, 2,2-dimethyl-, methyl ester | EST | 0.20 | Butanedioic acid, 2,3-bis(benzoyloxy)-, [S-(R*,R*)]- | OxyAR | 0.12 |
| Oxazole, 4,5-dimethyl- | NIT | 0.17 | 1,2-Benzenedicarboxylic acid, bis(8-methylnonyl) ester | EST | 2.74 |
| 1-Decene | ALI | 0.27 | 2(3H,4H)-Cyclopenta[b]furanone, 3a,6a-dihydro- | KET | 0.21 |
| 1-Heptanol, 3-methyl- | AL | 0.20 | Oxetane, 3,3-dimethyl- | ALI | 0.89 |
| 1,3-Hexanediol, 2-ethyl- | AL | 0.12 | 1,5-Hexadiene, 2,5-dimethyl- | ALI | 0.06 |
| Propanoic acid, 2-methyl-, butyl ester | EST | 0.45 | 1-Decene, 3,3,4-trimethyl- | ALI | 0.06 |
| Benzaldehyde | KET | 0.32 | 2-Propenoic acid, butyl ester | EST | 0.11 |
| n-Butyl methacrylate | EST | 54.25 | 2-Undecene, 2,5-dimethyl- | ALI | 0.09 |
| 1,1-Cyclobutanedicarboxamide, 2-phenyl-N,N’-bis(1-phenylethyl)- | NIT | 0.08 | Propanoic acid, 2-methylpropyl ester | EST | 0.57 |
| cis-2-Allylpyrrolidin-5-ol | AL | 0.13 | |||
| Benzene, 1,2,3-trimethyl- | AR | 0.11 | Acetic acid, octyl ester | EST | 0.27 |
| Butyl 2-methylbutanoate | ALI | 0.15 | 3-Undecene, 6-methyl-, (E)- | ALI | 0.11 |
| 3-Dodecanone | ALI | 0.09 | 2-Cyclopenten-1-one, 2,3-dimethyl- | KET | 0.06 |
| CH2=C(CH3)CH2COOH | AC | 0.20 | 4-Undecene, 3-methyl-, (Z)- | ALI | 0.16 |
| Acetophenone | KET | 0.23 | Benzonitrile | NIT | 0.29 |
| Benzoic acid, methyl ester | EST | 0.77 | 5-Amino-1-benzoyl-1H-pyrazole-3,4-dicarbonitrile | NIT | 0.14 |
| 1-Propanone, 1-phenyl- | KET | 0.18 | n-Butyl tiglate | ALI | 0.05 |
| 1-Dodecene | ALI | 3.14 | Butanoic acid, hexyl ester | EST | 0.11 |
| 1,3-Dioxocane, 2-pentadecyl- | ALI | 0.18 | (S)-(+)-5-Methyl-1-heptanol | AL | 0.13 |
| Phthalic anhydride | OxyAR | 4.61 | 2-Decene, 2,4-dimethyl- | ALI | 0.13 |
| 1,2-Benzenedicarbonitrile | NIT | 0.32 | Cyclohexane, 1,2,4-trimethyl- | ALI | 0.06 |
| 1(3H)-Isobenzofuranone | OxyAR | 0.23 | Dimethyl cyclohexane-1,4-dicarboxylate (trans isomer) | ALI | 0.04 |
| Butyl benzoate | OxyAR | 0.65 | 2,4-Pentadienoic acid, 3,4-dimethyl-, isopropyl ester | EST | 0.04 |
| .beta.-d-Lyxofuranoside, thio-nonyl- | SUL | 0.11 | Propanoic acid, 3-hydroxy-2,2-dimethyl-, 3-hydroxy-2,2-dimethylpropyl ester | AC | 0.08 |
| Benzophenone | KET | 0.16 | |||
| 2(1H)-Naphthalenone, 4a,5,6,7,8,8a-hexahydro-4a,8a-dimethyl-, cis- | KET | 0.26 | 1,10-Dimethyl-2-methylene-trans-decalin | ALI | 0.13 |
| n-Dodecylmethyl sulfide | SUL | 0.06 | |||
| 2-Hexadecene, 3,7,11,15-tetramethyl-, [R-[R*,R*-(E)]]- | ALI | 0.22 | 1,2-Cyclohexanedicarboxylic acid, butyl isobutyl ester | EST | 0.05 |
| Benzoic acid, hex-3-yl ester | AC | 0.05 | |||
| Cyclo-(glycyl-l-leucyl) | NIT | 0.25 | Hexadecanoic acid, methyl ester | AC | 0.09 |
| 1-Isobutylpiperidine-4-carboxylic acid, (tetrahydrofuran-2-ylmethyl)amide | NIT | 0.25 | Octadecanoic acid, 12-hydroxy-, methyl ester | EST | 0.13 |
| Butyl 9,12-octadecadienoate | ALI | 0.05 | |||
| 2-Cyclohexene-1,4-dione, 5,6-dibromo-2,6-dimethyl-, 1-oxime, o-benzoyl- | ALI | 0.09 | |||
| 9-Octadecenoic acid, methyl ester, (E)- | EST | 0.13 |
| Compound | Group | % | Compound | Group | % |
|---|---|---|---|---|---|
| Cyclopentene, 4-methyl- | ALI | 1.07 | Methyl methacrylate | EST | 0.18 |
| Benzene | AR | 6.90 | 3-Methylenecyclohexene | ALI | 0.17 |
| Cyclopentane, 1,3-dimethyl- | ALI | 0.67 | 2,4-Hexadiene, 3-methyl- | ALI | 0.30 |
| Cyclopentene, 4,4-dimethyl- | ALI | 1.11 | Cyclopentane, 1,2-dimethyl-3-methylene-, cis- | ALI | 0.07 |
| Cyclohexane, methyl- | ALI | 0.15 | .alpha.-Methylstyrene | AR | 0.05 |
| Cyclobutane, (1-methylethylidene)- | ALI | 0.49 | n-Butyl methacrylate | EST | 0.57 |
| Toluene | AR | 14.26 | Benzene, 1-ethenyl-3-methyl- | AR | 0.11 |
| Cyclopentene, 1,2,3-trimethyl- | ALI | 0.39 | Benzene, 2-propenyl- | AR | 0.06 |
| Ethylbenzene | AR | 3.46 | Benzene, 1,4-diethyl- | AR | 0.09 |
| Benzene, 1,3-dimethyl- | AR | 20.38 | Benzene, 1-methyl-4-propyl- | AR | 0.15 |
| o-Xylene | AR | 5.16 | 2-Tolyloxirane | OxyAR | 0.30 |
| Benzene, propyl- | AR | 0.31 | 5-Hexen-2-one, 5-methyl-3-methylene- | KET | 0.04 |
| Benzene, 1-ethyl-2-methyl- | AR | 5.49 | Benzene, 4-ethyl-1,2-dimethyl- | AR | 0.29 |
| Benzene, 1,2,4-trimethyl- | AR | 7.29 | Benzene, 1,2,3,4-tetramethyl- | AR | 0.23 |
| Benzonitrile | NIT | 1.13 | Benzene, 1,2,4,5-tetramethyl- | AR | 0.08 |
| Indane | AR | 1.29 | Benzene, 2-ethenyl-1,4-dimethyl- | AR | 0.05 |
| Indene | AR | 0.96 | Benzene, 1-methyl-4-(2-propenyl)- | AR | 0.05 |
| 1-Phenyl-1-butene | PH | 0.54 | 1H-Indene, 2,3-dihydro-5-methyl- | AR | 0.74 |
| 2,4-Dimethylstyrene | AR | 0.36 | 2-Methylindene | AR | 0.35 |
| 1H-Indene, 2,3-dihydro-4-methyl- | AR | 0.56 | 3-Phenylpropanoic anhydride | AR | 0.08 |
| 1H-Indene, 3-methyl- | AR | 1.41 | 1,4-Dihydronaphthalene | AR | 0.05 |
| 1H-Indene, 1-methyl- | AR | 0.37 | 2-Naphthalenol, 1,2-dihydro-, acetate | PAH | 0.78 |
| Naphthalene, 1,2,3,4-tetrahydro- | PAH | 0.15 | 1H-Indene, 2,3-dihydro-1,6-dimethyl- | AR | 0.08 |
| Naphthalene | PAH | 1.35 | Phenol, 2,3-dimethyl- | PH | 0.10 |
| 1H-Indene, 1,1-dimethyl- | AR | 0.18 | 2-Ethyl-2,3-dihydro-1H-indene | AR | 0.04 |
| Naphthalene, 2-methyl- | PAH | 3.22 | 1H-Indene, 1,3-dimethyl- | AR | 0.58 |
| Dodecane, 2,6,11-trimethyl- | ALI | 0.21 | Naphthalene, 1,2-dihydro-3-methyl- | PAH | 0.14 |
| Naphthalene, 1-ethyl- | PAH | 0.45 | Phenol, 2-ethyl-6-methyl- | PH | 0.04 |
| Naphthalene, 1,7-dimethyl- | PAH | 0.59 | Naphthalene, 6-ethyl-1,2,3,4-tetrahydro- | AR | 0.04 |
| Naphthalene, 2,3-dimethyl- | PAH | 0.47 | Naphthalene, 1-methyl- | PAH | 0.19 |
| Naphthalene, 1,5-dimethyl- | PAH | 0.23 | Phthalic anhydride | OxyAR | 3.25 |
| Hexadecane, 2,6,10,14-tetramethyl- | ALI | 0.95 | 1,3-Dicyanobenzene | NIT | 0.17 |
| Eicosanoic acid | AC | 0.18 | 1,2,3-Trimethylindene | AR | 0.05 |
| Pentadecane, 2,6,10-trimethyl- | ALI | 0.35 | 1H-Indene, 1,1,3-trimethyl- | AR | 0.06 |
| Pentadecane, 2,6,10,14-tetramethyl- | ALI | 0.63 | Naphthalene, 2,6-dimethyl- | PAH | 1.03 |
| Dotriacontyl heptafluorobutyrate | ALI | 0.49 | 1H-Isoindole-1,3(2H)-dione, 2-methyl- | AL | 0.16 |
| Tetrapentacontane, 1,54-dibromo- | ALI | 0.07 | 1H-Isoindole-1,3(2H)-dione, 2-(hydroxymethyl)- | AR | 0.22 |
| n-Tetracosanol-1 | AL | 0.15 | |||
| Oxalic acid, allyl octadecyl ester | EST | 0.16 | Naphthalene, 2-(1-methylethyl)- | PAH | 0.13 |
| Heptafluorobutyric acid, n-tetradecyl ester | EST | 0.41 | Naphthalene, 1,4,6-trimethyl- | PAH | 0.07 |
| 2-Octadecyl-propane-1,3-diol | AL | 1.48 | Naphthalene, 1,6,7-trimethyl- | PAH | 0.06 |
| Sulfurous acid, octadecyl 2-propyl ester | EST | 2.75 | Anthracene | PAH | 0.07 |
| 1,4-Hexadiene, (Z)- | ALI | 0.22 | Phenanthrene, 2-methyl- | PAH | 0.07 |
| Cyclopentene, 1,5-dimethyl- | ALI | 0.23 | Palmitoleic acid | AC | 0.04 |
| Petroleum Sludges Pyrolysis | Thermal | ZSM-5 (40) |
|---|---|---|
| Total Liquids (wt.%) | 15.8 | 12.6 |
| Organic oil (wt.%) | 10.3 | 2.3 |
| Water (wt.%) | 5.5 | 10.3 |
| Total Gases (wt.%) | 7.9 | 10.0 |
| H2 (wt.%) | 0.14 | 0.11 |
| CH4 (wt.%) | 0.90 | 0.55 |
| Ethylene (wt.%) | - | 1.67 |
| Propylene (wt.%) | - | 2.70 |
| C4+ (wt.%) | - | 1.23 |
| CO2 (wt.%) | 5.97 | 3.01 |
| CO (wt.%) | 0.86 | 0.74 |
| Total Solids (Ash + Char + Coke on catalyst) (wt.%) | 75.1 | 76.2 |
| Coke on catalyst (difference of Ash + char + coke minus Ash + char from the thermal pyrolysis experiment) (wt.%) | - | 1.1 |
| Total Mass Balance (wt.%) | 98.8 | 98.8 |
| Residual Paints Pyrolysis | Thermal | ZSM-5 (40) |
|---|---|---|
| Total Liquids (wt.%) | 35.0 | 32.4 |
| Organic oil (wt.%) | 24.0 | 16.5 |
| Water (wt.%) | 11.0 | 15.9 |
| Total Gases (wt.%) | 19.2 | 26.3 |
| H2 (wt.%) | 0.07 | 0.07 |
| CH4 (wt.%) | - | 0.21 |
| Ethane (wt.%) | 0.32 | 4.27 |
| Ethylene (wt.%) | - | 0.47 |
| Propane (wt.%) | 0.83 | 7.30 |
| Propylene (wt.%) | 8.39 | 3.55 |
| C4+ (wt.%) | 7.05 | 4.27 |
| CO2 (wt.%) | - | 0.21 |
| CO (wt.%) | 1.64 | 5.39 |
| Total Solids (Char + Coke on catalyst) (wt.%) | 36.9 | 38.3 |
| Coke on catalyst (difference of char + coke minus char from the thermal experiment) (wt.%) | - | 1.4 |
| Total Mass Balance (wt.%) | 91.1 | 97.1 |
| Creosote-Treated Wood Waste | Thermal | ZSM-5 (40) |
|---|---|---|
| Total Liquids (wt.%) | 46.9 | 41.9 |
| Organic oil (wt.%) | 31.6 | 17.3 |
| Water (wt.%) | 15.3 | 24.6 |
| Total Gases (wt.%) | 14.7 | 17.3 |
| H2 (wt.%) | 0.1 | 0.03 |
| CH4 (wt.%) | 1.44 | 0.79 |
| Ethane (wt.%) | - | - |
| Ethylene (wt.%) | - | 1.4 |
| Propane (wt.%) | - | - |
| Propylene (wt.%) | 0.14 | 1.07 |
| C4+ (wt.%) | - | 0.11 |
| CO2 (wt.%) | 8.29 | 6.18 |
| CO (wt.%) | 4.77 | 7.69 |
| Total Solids (Char + Coke on catalyst) (wt.%) | 36.0 | 38.4 |
| Coke on catalyst (difference of char + coke minus char from the thermal experiment) (wt.%) | - | 2.4 |
| Total Mass Balance (wt.%) | 97.6 | 97.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rekos, K.C.; Charisteidis, I.D.; Tzamos, E.; Palantzas, G.; Zouboulis, A.I.; Triantafyllidis, K.S. Valorization of Hazardous Organic Solid Wastes towards Fuels and Chemicals via Fast (Catalytic) Pyrolysis. Sustain. Chem. 2022, 3, 91-111. https://doi.org/10.3390/suschem3010007
Rekos KC, Charisteidis ID, Tzamos E, Palantzas G, Zouboulis AI, Triantafyllidis KS. Valorization of Hazardous Organic Solid Wastes towards Fuels and Chemicals via Fast (Catalytic) Pyrolysis. Sustainable Chemistry. 2022; 3(1):91-111. https://doi.org/10.3390/suschem3010007
Chicago/Turabian StyleRekos, Kyriazis C., Ioannis D. Charisteidis, Evangelos Tzamos, Georgios Palantzas, Anastasios I. Zouboulis, and Konstantinos S. Triantafyllidis. 2022. "Valorization of Hazardous Organic Solid Wastes towards Fuels and Chemicals via Fast (Catalytic) Pyrolysis" Sustainable Chemistry 3, no. 1: 91-111. https://doi.org/10.3390/suschem3010007
APA StyleRekos, K. C., Charisteidis, I. D., Tzamos, E., Palantzas, G., Zouboulis, A. I., & Triantafyllidis, K. S. (2022). Valorization of Hazardous Organic Solid Wastes towards Fuels and Chemicals via Fast (Catalytic) Pyrolysis. Sustainable Chemistry, 3(1), 91-111. https://doi.org/10.3390/suschem3010007