Recent Advances in RO(CO)P of Bio-Based Monomers
Abstract
:1. Introduction
2. Polyesters
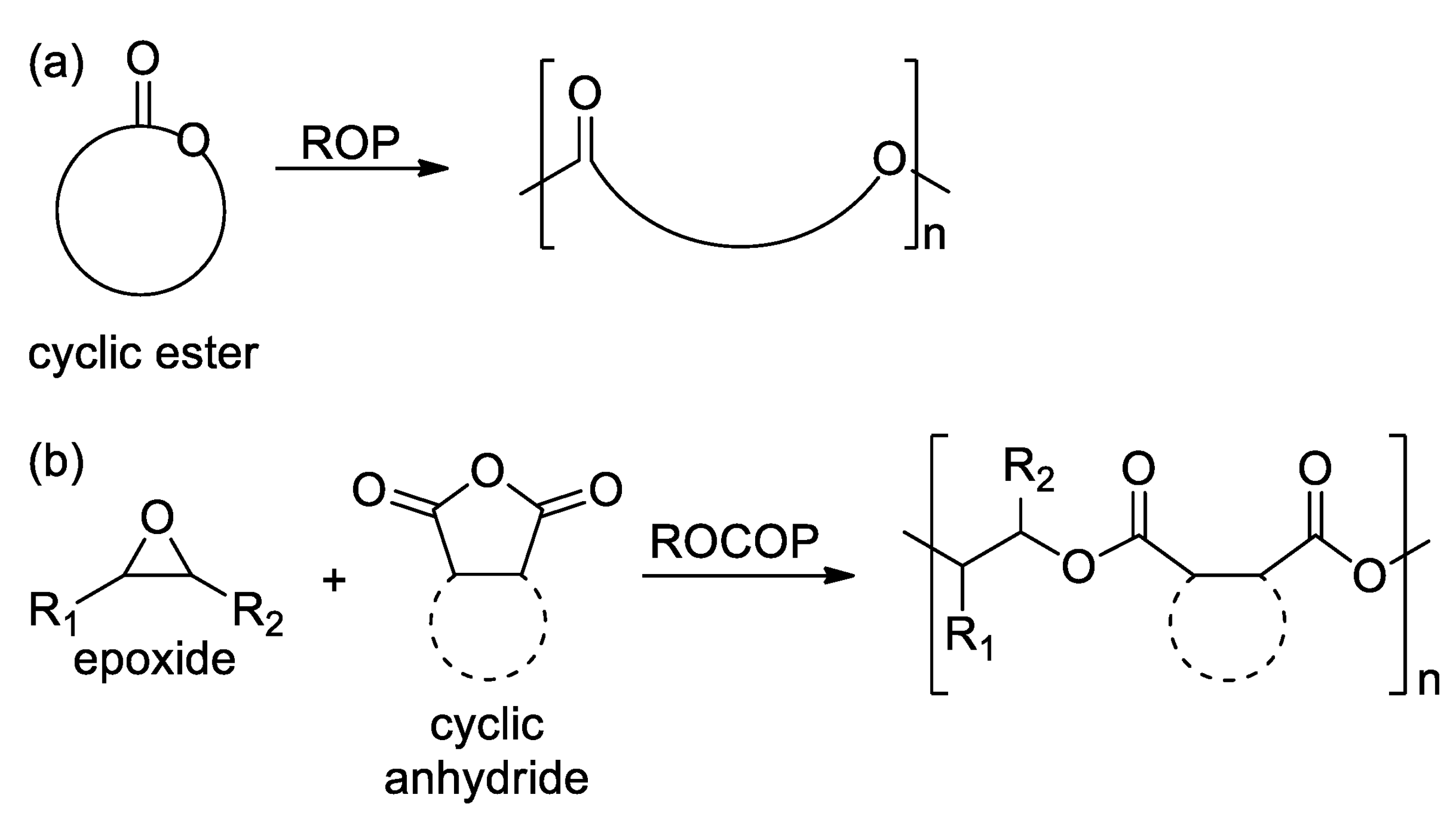
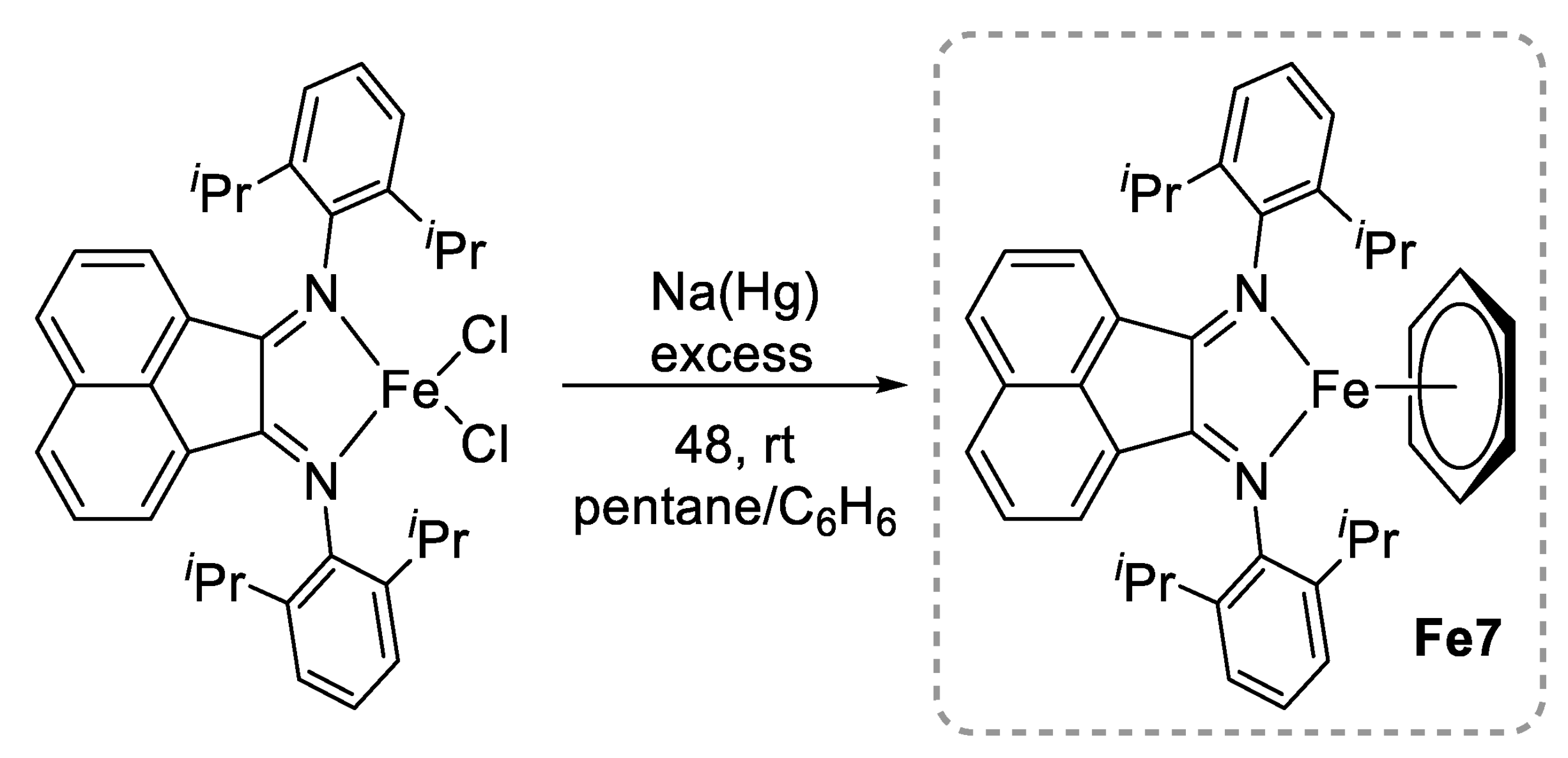
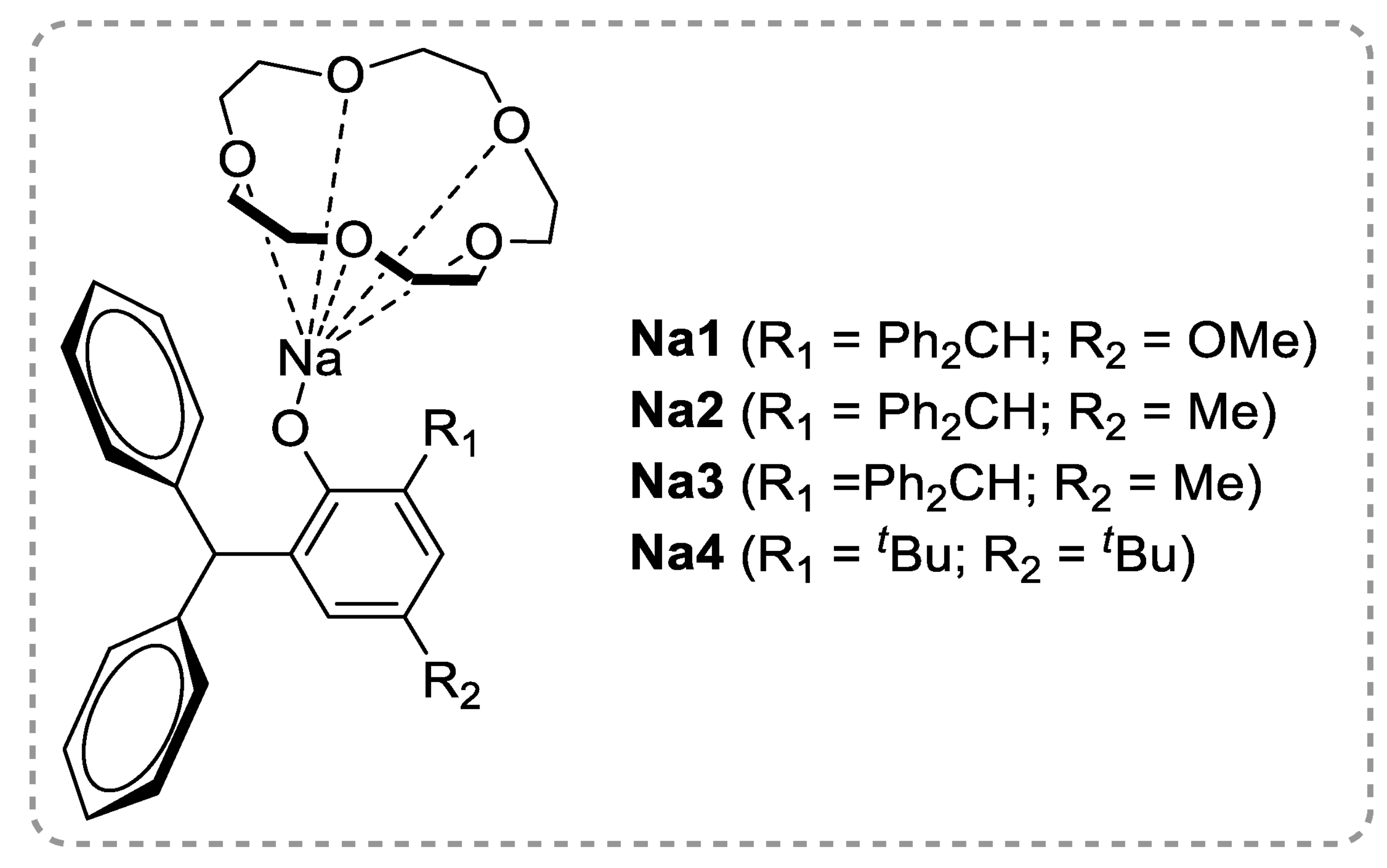
2.1. Polyesters from ROP of Lactones
2.2. Polyesters from ROCOP of Epoxides and Cyclic Anhydrides
3. Polycarbonates
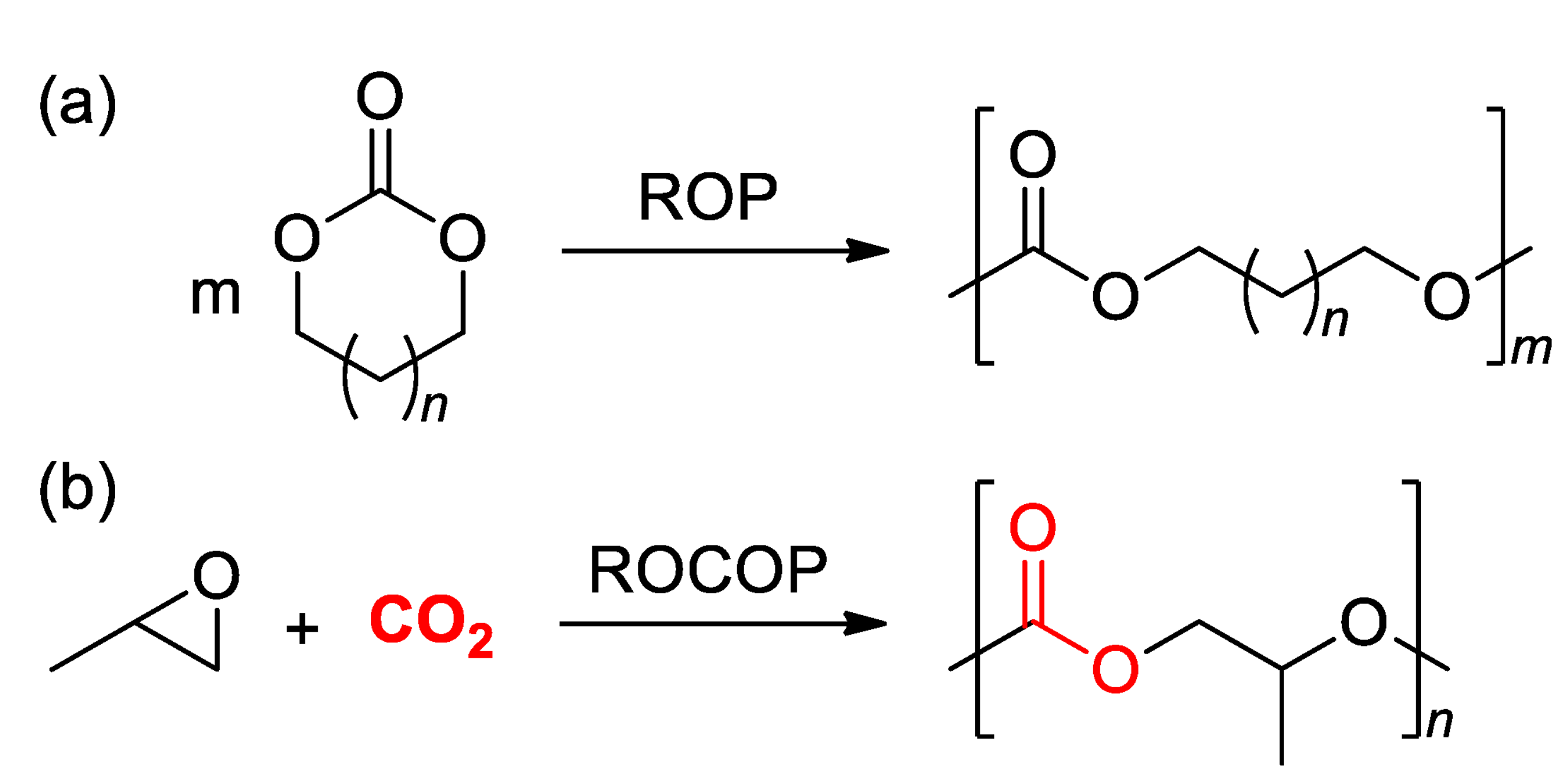
3.1. ROP of Cyclic Carbonates
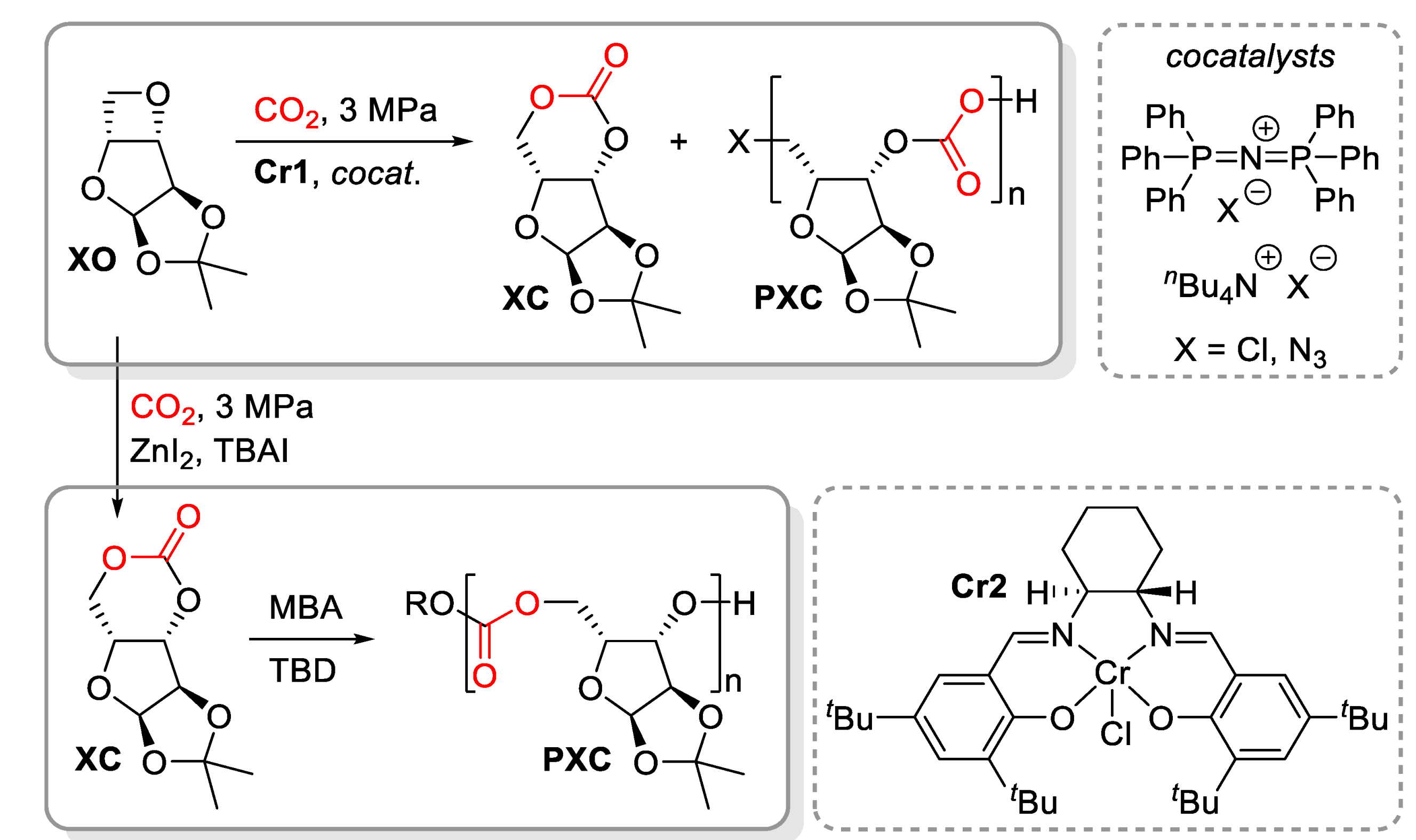
3.2. ROCOP of Carbon Dioxide and Oxiranes
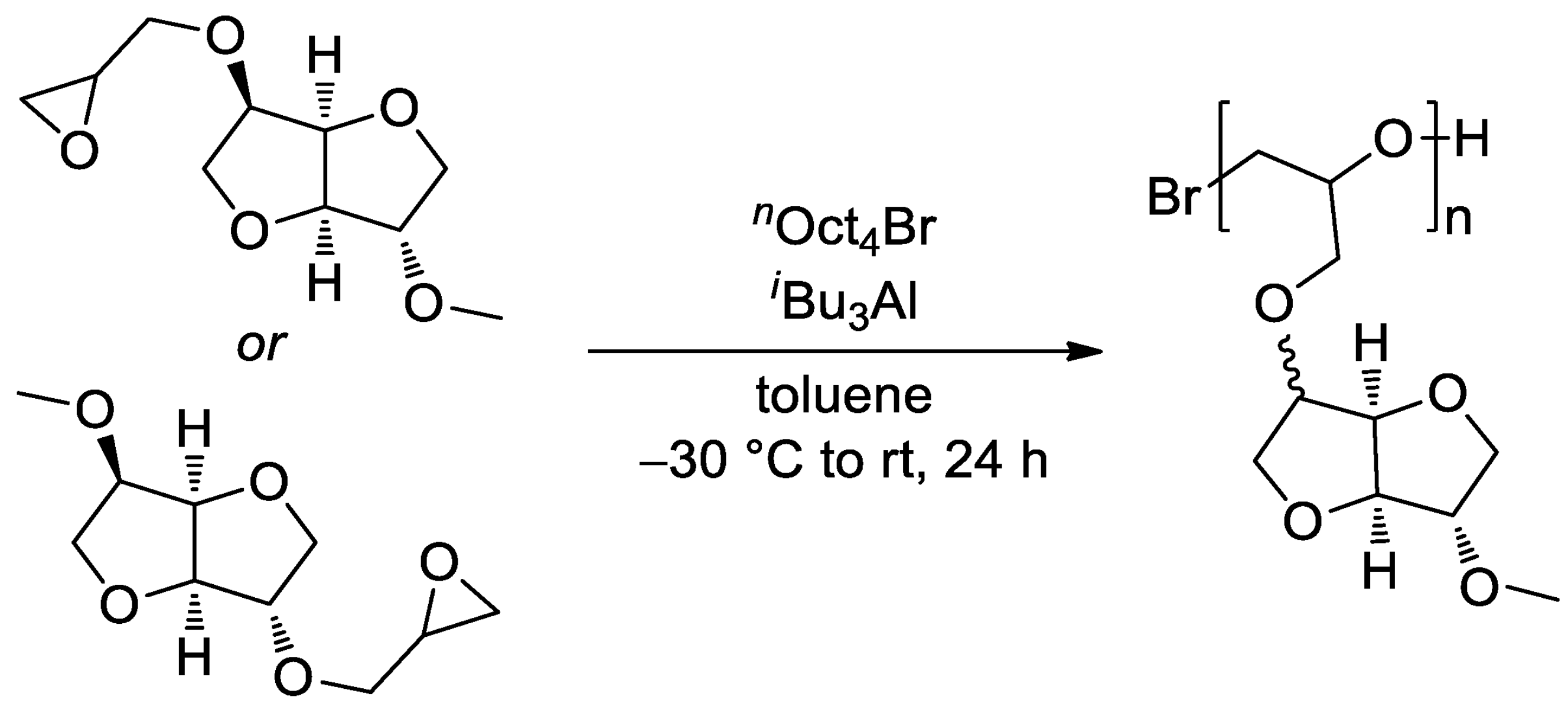
4. Polyethers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buchanan, R.A. History of Technology. Available online: https://www.britannica.com/technology/history-of-technology (accessed on 31 March 2022).
- Zalasiewicz, J.; Waters, C.N.; Ivar do Sul, J.A.; Corcoran, P.L.; Barnosky, A.D.; Cearreta, A.; Edgeworth, M.; Gałuszka, A.; Jeandel, C.; Leinfelder, R.; et al. The geological cycle of plastics and their use as a stratigraphic indicator of the Anthropocene. Anthropocene 2016, 13, 4–17. [Google Scholar] [CrossRef] [Green Version]
- Riechers, M.; Fanini, L.; Apicella, A.; Galván, B.C.; Blondel, E.; Espiña, B.; Kefer, S.; Keroullé, T.; Klun, K.; Pereira, T.R.; et al. Plastics in our ocean as transdisciplinary challenge. Mar. Pollut. Bull. 2021, 164, 112051. [Google Scholar] [CrossRef] [PubMed]
- Jehanno, C.; Alty, J.W.; Roosen, M.; De Meester, S.; Dove, A.P.; Chen, E.Y.-X.; Leibfarth, F.A.; Sardon, H. Critical advances and future opportunities in upcycling commodity polymers. Nature 2022, 603, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Della Monica, F.; Kleij, A.W. From terpenes to sustainable and functional polymers. Polym. Chem. 2020, 11, 5109–5127. [Google Scholar] [CrossRef]
- Galbis, J.A.; de García-Martín, M.G.; de Paz, M.V.; Galbis, E. Synthetic Polymers from Sugar-Based Monomers. Chem. Rev. 2016, 116, 1600–1636. [Google Scholar] [CrossRef]
- de Espinosa, M.L.; Meier, M.A.R. Plant oils: The perfect renewable resource for polymer science?! Eur. Polym. J. 2011, 47, 837–852. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Fridrich, B.; de Santi, A.; Elangovan, S.; Barta, K. Bright Side of Lignin Depolymerisation: Toward New Platform Chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef] [Green Version]
- Isikgora, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef] [Green Version]
- Jiang, T.; Duan, Q.; Zhu, J.; Liu, H.; Yu, L. Starch-based biodegradable materials: Challenges and opportunities. Adv. Ind. Eng. Polym. Res. 2020, 3, 8–18. [Google Scholar] [CrossRef]
- Winnacker, M. Polyhydroxyalkanoates: Recent Advances in Their Synthesis and Applications. Eur. J. Lipid Sci. Technol. 2019, 121, 1900101. [Google Scholar] [CrossRef] [Green Version]
- Wahlen, C.; Frey, H. Anionic Polymerisation of Terpene Monomers: New Options for Bio- Based Thermoplastic Elastomers. Macromolecules 2021, 54, 7323–7336. [Google Scholar] [CrossRef]
- Lamparelli, D.H.; Winnacker, M.; Capacchione, C. Stereoregular Polymerisation of Acyclic Terpenes. ChemPlusChem 2022, 87, e202100366. [Google Scholar] [CrossRef] [PubMed]
- Veith, C.; Diot-Néant, F.; Miller, S.A. Synthesis and polymerisation of bio-based acrylates: A review. Polym. Chem. 2020, 11, 7452–7470. [Google Scholar] [CrossRef]
- Gomez-Lopez, A.; Panchireddy, S.; Grignard, B.; Calvo, I.; Jerome, C.; Detrembleur, C.; Sardon, H. Poly(hydroxyurethane) Adhesives and Coatings: State-of-the-Art and Future Directions. ACS Sustain. Chem. Eng. 2021, 9, 9541–9562. [Google Scholar] [CrossRef]
- Coates, G.W.; Moore, D.R. Discrete metal-based catalysts for the copolymerisation of CO2 and epoxides: Discovery, reactivity, optimization, and mechanism. Angew. Chem. Int. Ed. 2004, 43, 6618–6639. [Google Scholar] [CrossRef] [PubMed]
- Klaus, S.; Lehenmeier, M.W.; Anderson, C.E.; Rieger, B. Recent advances in CO2/epoxide copolymerisation—New strategies and cooperative mechanisms. Coord. Chem. Rev. 2011, 255, 1460–1479. [Google Scholar] [CrossRef]
- Lu, X.-B.; Darensbourg, D.J. Cobalt catalysts for the coupling of CO2 and epoxides to provide polycarbonates and cyclic carbonates. Chem. Soc. Rev. 2012, 41, 1462–1484. [Google Scholar] [CrossRef]
- Paul, S.; Zhu, Y.; Romain, C.; Brooks, R.; Saini, P.K.; Williams, C.K. Ring-opening copolymerisation (ROCOP): Synthesis and properties of polyesters and polycarbonates. Chem. Commun. 2015, 51, 6459–6479. [Google Scholar] [CrossRef] [Green Version]
- Kozak, C.M.; Ambrose, K.; Anderson, T.S. Copolymerisation of carbon dioxide and epoxides by metal coordination complexes. Coord. Chem. Rev. 2018, 376, 565–587. [Google Scholar] [CrossRef]
- Xiao, L.; Zhao, Y.; Qiao, S.; Sun, Z.; Santoro, O.; Redshaw, C. Synthesis and structures of mono- and di-nuclear aluminium and zinc complexes bearing α-diimine and related ligands, and their use in the ring opening polymerization of cyclic ester. Dalton Trans. 2020, 49, 1456–1472. [Google Scholar] [CrossRef] [PubMed]
- Dubois, P.; Coulembier, O.; Raquez, J.-M. Handbook of Ring-Opening Polymerisation; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2009. [Google Scholar]
- Santoro, O.; Redshaw, C. Use of titanium complexes bearing diphenolate or calix[n]arene ligands in α-olefin polymerisation and the ROP of cyclic esters. Catalysts 2020, 10, 210. [Google Scholar] [CrossRef] [Green Version]
- Vert, M.; Li, S.M.; Spenlehauer, G.; Guerin, P. Bioresorbability and biocompatibility of aliphatic polyesters. J. Mater. Sci. Mater. Med. 1992, 3, 432–446. [Google Scholar] [CrossRef]
- Gross, R.A.; Kalra, B. Biodegradable Polymers for the Environment. Science 2002, 297, 803–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buntara, T.; Noel, S.; Phua, P.H.; Melián-Cabrera, I.; de Vries, J.G.; Heeres, H.J. Although ε-CL is currently obtained mainly from petroleum, promising sustainable processes have been developed. Caprolactam from Renewable Resources: Catalytic Conversion of 5-Hydroxymethylfurfural into Caprolactone. Angew. Chem. Int. Ed. 2011, 50, 7083–7087. [Google Scholar] [CrossRef]
- Hillmyer, M.A.; Tolman, W.B. Aliphatic Polyester Block Polymers: Renewable, Degradable, and Sustainable. Acc. Chem. Res. 2014, 47, 2390–2396. [Google Scholar] [CrossRef]
- Duparc, H.V.; Shakaroun, R.M.; Slawinski, M.; Carpentier, J.F.; Guillaume, S.M. Ring-opening (co)polymerisation of six-membered substituted δ-valerolactones with alkali metal alkoxides. Eur. Polym. J. 2020, 134, 10985–109864. [Google Scholar] [CrossRef]
- Shakaroun, R.M.; Li, H.; Jéhan, P.; Blot, M.; Alaaeddine, A.; Carpentier, J.-F.; Guillaume, S.M. Stereoselective ring-opening polymerisation of functional β-lactones: Influence of the exocyclic side-group. Polym. Chem. 2021, 12, 4022–4034. [Google Scholar] [CrossRef]
- Albertson, A.C.; Varma, I.K. Degradable Polymer Microspheres for Controlled Drug Delivery. Adv. Polym. Sci. 2002, 157, 67–112. [Google Scholar]
- Bogaert, J.-C.; Coszach, P. Poly(lactic acids): A potential solution to plastic waste dilemma. Macromol. Symp. 2000, 153, 287–303. [Google Scholar] [CrossRef]
- Masutani, K.; Kimura, Y. Chapter 1: PLA Synthesis. From the Monomer to the Polymer. In Poly(Lactic Acid) Science and Technology: Processing, Properties, Additives and Applications; The Royal Society of Chemistry: London, UK, 2015; pp. 1–36. [Google Scholar] [CrossRef]
- Fuoco, T.; Pappalardo, D. Aluminum Alkyl Complexes Bearing Salicylaldiminato Ligands: Versatile Initiators in the Ring-Opening Polymerisation of Cyclic Esters. Catalysts 2017, 7, 64. [Google Scholar] [CrossRef]
- Ksiazkiewicz, A.N.; Pich, A.; Kögerler, P.; Monakhov, K.Y.; Herres-Pawlis, S. Mononuclear zinc(II) Schiff base complexes as catalysts for the ring-opening polymerisation of lactide. Eur. Polym. J. 2020, 122, 109302–109308. [Google Scholar] [CrossRef]
- Santoro, O.; Zhang, X.; Redshaw, C. Synthesis of Biodegradable Polymers: A Review on the Use of Schiff-Base Metal Complexes as Catalysts for the Ring Opening Polymerisation (ROP) of Cyclic Esters. Catalysts 2020, 10, 800. [Google Scholar] [CrossRef]
- O’Keefe, B.J.; Monnier, S.M.; Hillmyer, M.A.; Tolman, W.B. Rapid and Controlled Polymerisation of Lactide by Structurally Characterized Ferric Alkoxides. J. Am. Chem. Soc. 2001, 123, 339–340. [Google Scholar] [CrossRef]
- O’Keefe, B.J.; Breyfogle, L.E.; Hillmyer, M.A.; Tolman, W.B. Mechanistic Comparison of Cyclic Ester Polymerisations by Novel Iron(III)−Alkoxide Complexes: Single vs Multiple Site Catalysis. J. Am. Chem. Soc. 2002, 124, 4384–4393. [Google Scholar] [CrossRef]
- Gibson, V.C.; Marshall, E.L.; Navarro-Llobet, D.; White, A.J.P.; Williams, D.J. A well-defined iron(ii) alkoxide initiator for the controlled polymerisation of lactide. J. Chem. Soc. Dalton Trans. 2002, 4321–4322. [Google Scholar] [CrossRef]
- McGuinness, D.S.; Marshall, E.L.; Gibson, V.C.; Steed, J.W. Anionic iron(II) alkoxides as initiators for the controlled ring-opening polymerisation of lactide. J. Polym. Sci. Part A 2003, 41, 3798–3803. [Google Scholar] [CrossRef]
- Biernesser, A.B.; Li, B.; Byers, J.A. Redox-Controlled Polymerisation of Lactide Catalyzed by Bis(imino)pyridine Iron Bis(alkoxide) Complexes. J. Am. Chem. Soc. 2013, 135, 16553–16560. [Google Scholar] [CrossRef]
- Manna, C.M.; Kaplan, H.Z.; Li, B.; Byer, J.A. High molecular weight poly(lactic acid) produced by an efficient iron catalyst bearing a bis(amidinato)-N-heterocyclic carbene ligand. Polyhedron 2014, 84, 160–167. [Google Scholar] [CrossRef]
- Herber, U.; Hegner, K.; Wolters, D.; Siris, R.; Wrobel, K.; Hoffmann, A.; Lochenie, C.; Weber, B.; Kuckling, D.; Herres-Pawlis, S. Iron(II) and Zinc(II) Complexes with Tetradentate Bis(pyrazolyl)methane Ligands as Catalysts for the Ring-Opening Polymerisation of rac-Lactide. Eur. J. Inorg. Chem. 2017, 2017, 1341–1354. [Google Scholar] [CrossRef]
- Rittinghaus, R.D.; Schfer, P.M.; Albrecht, P.; Conrads, C.; Hoffmann, A.; Ksiazkiewicz, A.N.; Bienemann, O.; Pich, A.; Herres-Pawlis, S. New Kids in Lactide Polymerisation: Highly Active and Robust Iron Guanidine Complexes as Superior Catalysts. ChemSusChem 2019, 12, 2161–2165. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.A.; Wekesa, F.S.; Unruh, D.K.; Findlater, M.; Long, B.K. BIAN-Fe(η6-C6H6): Synthesis, Characterization, and L-Lactide Polymerisation. J. Polym. Sci. Part A 2017, 55, 2824–2830. [Google Scholar] [CrossRef]
- Liang, Y.; Duan, R.-L.; Hu, C.-Y.; Lia, L.-L.; Pang, X.; Zhang, W.-X.; Chen, X.S. Salen-iron Complexes: Synthesis, Characterization and Their Reactivity with Lactide. Chin. J. Polym. Sci. 2018, 36, 185–189. [Google Scholar] [CrossRef]
- Duan, R.; Hu, C.; Li, X.; Pang, X.; Sun, Z.; Chen, X.; Wang, X. Air-Stable Salen−Iron Complexes: Stereoselective Catalysts for Lactide and ε-Caprolactone Polymerisation through in Situ Initiation. Macromolecules 2017, 50, 9188–9195. [Google Scholar] [CrossRef]
- Semlyen, J.A. Cyclic Polymers, 2nd ed.; Kluwer: Dordrecht, The Netherlands, 2000. [Google Scholar]
- Chang, Y.A.; Waymouth, R.M. Recent progress on the synthesis of cyclic polymers via ring-expansion strategies. J. Polym. Sci. Part A 2017, 55, 2892–2902. [Google Scholar] [CrossRef]
- Weil, J.; Mathers, R.T.; Getzler, Y.D.Y.L. Lactide Cyclopolymerisation by an Alumatrane-Inspired Catalyst. Macromolecules 2012, 45, 1118–1121. [Google Scholar] [CrossRef]
- Castro-Osma, J.A.; Alonso-Moreno, C.; García-Martinez, J.C.; Fernández-Baeza, J.; Sánchez-Barba, L.F.; Lara-Sánchez, A.; Otero, A. Ring-Opening (ROP) versus Ring-Expansion (REP) Polymerisation of ε-Caprolactone to Give Linear or Cyclic Polycaprolactones. Macromolecules 2013, 46, 6388–6394. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Weidner, S.M.; Meyer, A. High Tm poly(Llactide)s via REP or ROPPOC of L-lactide. Polym. Chem. 2020, 11, 2182–2193. [Google Scholar] [CrossRef] [Green Version]
- Kricheldorf, H.R.; Weidner, S.M. High molar mass cyclic poly(L-lactide) via ring-expansion polymerisation with cyclic dibutyltin bisphenoxides. Eur. Polym. J. 2018, 105, 158–166. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Weidner, S.M. High molar mass cyclic poly(L-lactide) obtained by means of neat tin(II) 2-ethylhexanoate. Polym. Chem. 2020, 11, 5249–5260. [Google Scholar] [CrossRef]
- Impemba, S.; Della Monica, F.; Grassi, A.; Capacchione, C.; Milione, S. Cyclic Polyester Formation with an [OSSO]-Type Iron(III) Catalyst. ChemSusChem 2020, 13, 141–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Della Monica, F.; Maity, B.; Pehl, T.; Buonerba, A.; De Nisi, A.; Monari, M.; Grassi, A.; Rieger, B.; Cavallo, L.; Capacchione, C. [OSSO]-Type Iron(III) Complexes for the Low-Pressure Reaction of Carbon Dioxide with Epoxides: Catalytic Activity, Reaction Kinetics, and Computational Study. ACS Catal. 2018, 8, 6882–6893. [Google Scholar] [CrossRef]
- Della Monica, F.; Ricciardi, M.; Proto, A.; Cucciniello, R.; Capacchione, C. Regioselective Ring-Opening of Glycidol to Monoalkyl Glyceryl Ethers Promoted by an [OSSO]-FeIII Triflate Complex. ChemSusChem 2019, 12, 3448–3452. [Google Scholar] [CrossRef] [PubMed]
- Kerr, R.W.F.; Ewing, P.M.D.A.; Raman, S.K.; Smith, A.D.; Williams, C.K.; Arnold, P.L. Ultrarapid Cerium(III)–NHC Catalysts for High Molar Mass Cyclic Polylactide. ACS Catal. 2021, 11, 1563–1569. [Google Scholar] [CrossRef]
- Santoro, O.; Elsegood, M.R.J.; Teat, S.J.; Yamato, T.; Redshaw, C. Lithium calix[4]arenes: Structural studies and use in the ring opening polymerisation of cyclic esters. RSC Adv. 2021, 11, 11304–11317. [Google Scholar] [CrossRef]
- Santoro, O.; Elsegood, M.R.J.; Bedwell, E.V.; Pryce, J.A.; Redshaw, C. INSIGHTS into the structures adopted by titanocalix[6 and 8]arenes and their use in the ring opening polymerisation of cyclic esters. Dalton Trans. 2020, 49, 11978–11996. [Google Scholar] [CrossRef]
- Gruszka, W.; Garden, J.A. Advances in heterometallic ring-opening (co)polymerisation catalysis. Nat. Commun. 2021, 12, 3252. [Google Scholar] [CrossRef]
- Focarete, M.L.; Scandola, M.; Kumar, A.; Gross, R.A. Flat polymer ribbons and other shapes by electrospinning. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 1721–1729. [Google Scholar] [CrossRef]
- Huang, H.-C.; Wang, B.; Chen, X.-L.; Pan, L.; Li, Y.-S. Ring-opening polymerisation of (Macro)lactones by highly active mononuclear salen–aluminum complexes bearing cyclic β-ketoiminato ligand. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 973–981. [Google Scholar] [CrossRef]
- Naddeo, M.; D’Auria, I.; Viscusi, G.; Gorrasi, G.; Pellecchia, C.; Pappalardo, D. Tuning the thermal properties of poly(ethylene)-like poly(esters) by copolymerisation of ε-caprolactone with macrolactones, in the presence of a pyridylamidozinc(II) complex. J. Polym. Sci. 2020, 58, 528–539. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Shlyakhtin, A.V.; Tavtorkin, A.N.; Ivchenko, P.V.; Borisov, R.S.; Churakov, A.V. Monomeric and dimeric magnesium mono-BHT complexes as effective ROP catalysts. Catal. Commun. 2016, 87, 106–111. [Google Scholar] [CrossRef]
- Wilson, J.A.; Hopkins, S.A.; Wright, P.M.; Dove, A.P. Synthesis of ω-Pentadecalactone Copolymers with Independently Tunable Thermal and Degradation Behavior. Macromolecules 2015, 48, 950–958. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Zhen, N.; Gu, J.; Zhang, H.; Dong, B.; Wang, F.; Liu, H. Sodium complexes bearing cavity-like conformations: A highly active and well-controlled catalytic system for macrolactone homo- and copolymerisation. Polym. Chem. 2021, 12, 1957. [Google Scholar] [CrossRef]
- Zhao, N.; Ren, C.; Shen, Y.; Liu, S.; Li, Z. Facile Synthesis of Aliphatic ω-Pentadecalactone Containing Diblock Copolyesters via Sequential ROP with l-Lactide, ε-Caprolactone, and δ-Valerolactone Catalyzed by Cyclic Trimeric Phosphazene Base with Inherent Tribasic Characteristics. Macromolecules 2019, 52, 1083–1091. [Google Scholar] [CrossRef]
- Ladelta, V.; Bilalis, P.; Gnanou, Y.; Hadjichristidis, N. Ring-opening polymerisation of ω-pentadecalactone catalyzed by phosphazene superbases. Polym. Chem. 2017, 8, 511–515. [Google Scholar] [CrossRef]
- Naumann, S.; Scholten, P.B.V.; Wilson, J.A.; Dove, A.P. Dual Catalysis for Selective Ring-Opening Polymerisation of Lactones: Evolution toward Simplicity. J. Am. Chem. Soc. 2015, 137, 14439–14445. [Google Scholar] [CrossRef]
- Longo, J.M.; Sanford, M.J.; Coates, G.W. Ring-Opening Copolymerisation of Epoxides and Cyclic Anhydrides with Discrete Metal Complexes: Structure−Property Relationships. Chem. Rev. 2016, 116, 15167–15197. [Google Scholar] [CrossRef]
- Robert, C.; de Montigny, F.; Thomas, C.M. Tandem synthesis of alternating polyesters from renewable resources. Nat. Commun. 2011, 2, 586. [Google Scholar] [CrossRef]
- Fournier, L.; Robert, C.; Pourchet, S.; Gonzalez, A.; Williams, L.; Prunet, J.; Thomas, C.M. Facile and efficient chemical functionalization of aliphatic polyesters by cross metathesis. Polym. Chem. 2016, 7, 3700–3704. [Google Scholar] [CrossRef] [Green Version]
- Van Zee, N.J.; Coates, G.W. Alternating Copolymerisation of Propylene Oxide with Biorenewable Terpene-Based Cyclic Anhydrides: A Sustainable Route to Aliphatic Polyesters with High Glass Transition Temperatures. Angew. Chem. Int. Ed. 2015, 54, 2665–2668. [Google Scholar] [CrossRef]
- Sanford, M.J.; Peña Carrodeguas, L.; Van Zee, N.J.; Kleij, A.W.; Coates, G.W. Alternating Copolymerisation of Propylene Oxide and Cyclohexene Oxide with Tricyclic Anhydrides: Access to Partially Renewable Aliphatic Polyesters with High Glass Transition Temperatures. Macromolecules 2016, 49, 6394–6400. [Google Scholar] [CrossRef]
- Nejad, E.H.; Paoniasari, A.; van Melis, C.G.W.; Koning, C.E.; Duchateau, R. Catalytic Ring-Opening Copolymerisation of Limonene Oxide and Phthalic Anhydride: Toward Partially Renewable Polyesters. Macromolecules 2013, 46, 631–637. [Google Scholar] [CrossRef]
- Carrodeguas, P.L.; Martín, C.; Kleij, A.W. Semiaromatic Polyesters Derived from Renewable Terpene Oxides with High Glass Transitions. Macromolecules 2017, 50, 5337–5345. [Google Scholar] [CrossRef]
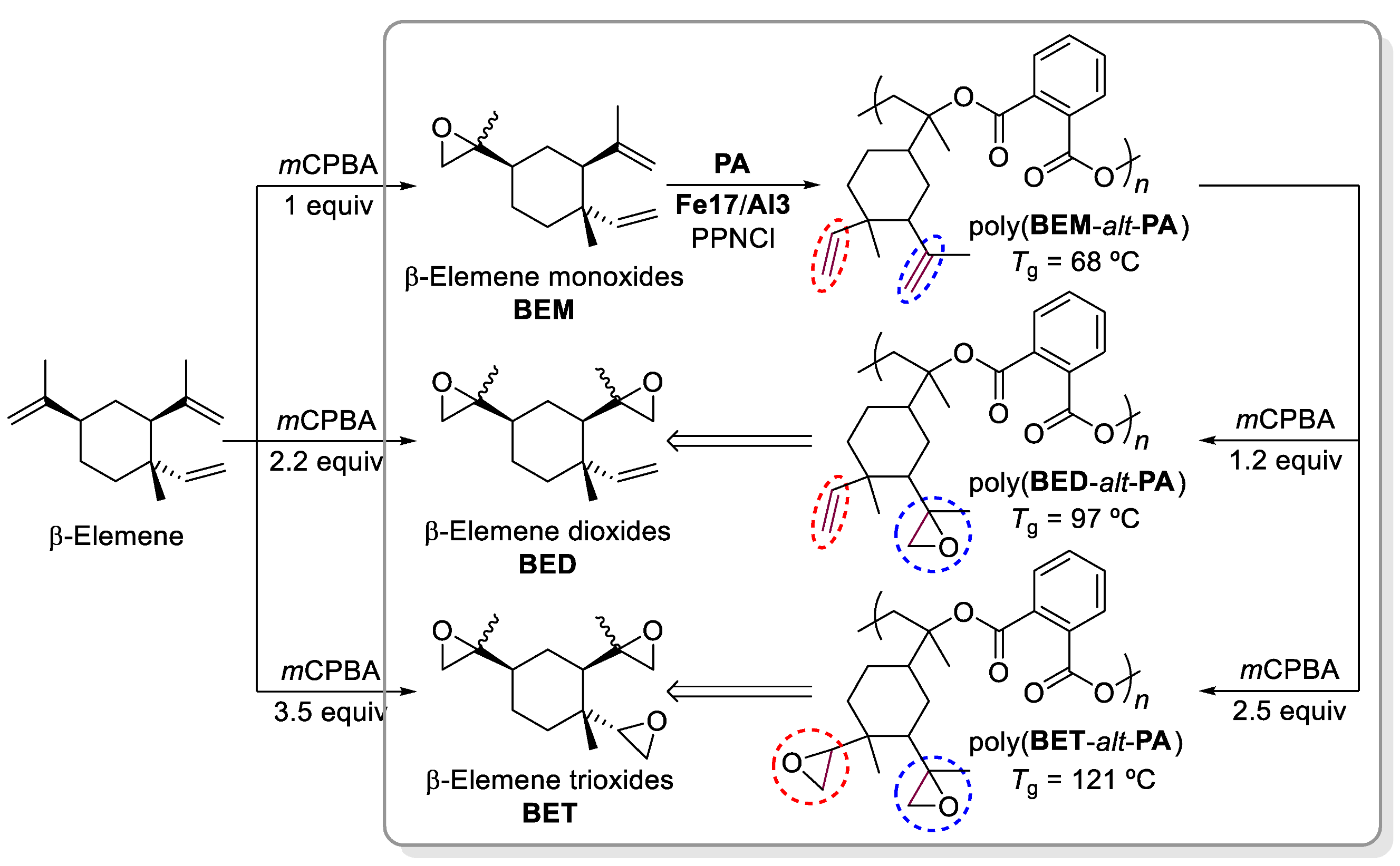
- Della Monica, F.; Kleij, A.W. Synthesis and Characterization of Bio-based Polyesters with Tunable Tg by ROCOP of Beta-Elemene Oxides and Phthalic Anhydride. ACS Sustain. Chem. Eng. 2021, 9, 2619–2625. [Google Scholar] [CrossRef]
- Adio, A.M. (−)-trans-β-Elemene and related compounds: Occurrence, synthesis, and anticancer activity. Tetrahedron 2009, 65, 5145–5159. [Google Scholar] [CrossRef]
- Biermann, U.; Bornscheuer, U.T.; Feussner, I.; Meier, M.A.R.; Metzger, J.O. Fatty Acids and their Derivatives as Renewable Platform Molecules for the Chemical Industry. Angew. Chem. Int. Ed. 2021, 60, 20144–20165. [Google Scholar] [CrossRef]
- Nomura, K.; Awang, B.N.W. Synthesis of Bio-Based Aliphatic Polyesters from Plant Oils by Efficient Molecular Catalysis: A Selected Survey from Recent Reports. ACS Sustain. Chem. Eng. 2021, 9, 5486–5505. [Google Scholar] [CrossRef]
- Yadav, N.; Seidi, F.; Crespy, D.; D’Elia, V. Polymers Based on Cyclic Carbonates as Trait d’Union Between Polymer Chemistry and Sustainable CO2 Utilization. ChemSusChem 2019, 12, 724–754. [Google Scholar] [CrossRef] [Green Version]
- Vatcharaporn, A.; Cristòfol, À.; Della Monica, F.; Limburg, B.; D’Elia, V.; Kleij, A.W. Recent progress in the catalytic transformation of carbon dioxide into biosourced organic carbonates. Green Chem. 2021, 23, 1077–1113. [Google Scholar]
- Hosney, H.; Nadiem, B.; Ashour, I.; Mustafa, I.; El-Shibiny, A. Epoxidized vegetable oil and bio-based materials as PVC plasticizer. J. Appl. Polym. Sci. 2018, 135, 46270. [Google Scholar] [CrossRef] [Green Version]
- Clark, A.J.; Hoong, S.S. Copolymers of tetrahydrofuran and epoxidized vegetable oils: Application to elastomeric polyurethanes. Polym. Chem. 2014, 5, 3238–3244. [Google Scholar] [CrossRef]
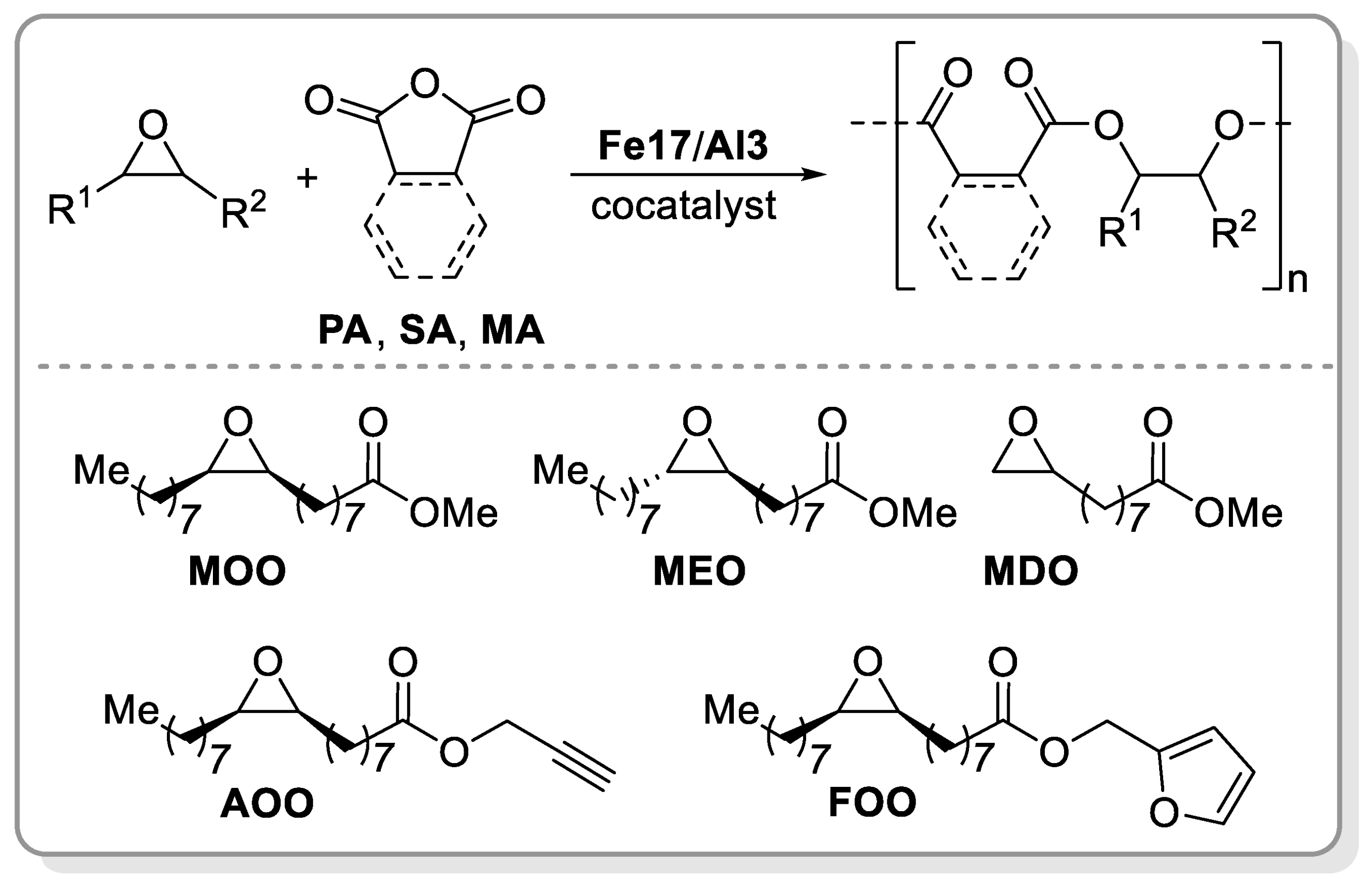
- Brandolese, A.; Della Monica, F.; Pericàs, M.À; Kleij, A.W. Catalytic Ring-Opening Copolymerisation of Fatty Acid Epoxides: Access to Functional Biopolyesters. Macromolecules 2022, 55, 2566–2573. [Google Scholar] [CrossRef] [PubMed]
- Morin, N.; Arp, H.P.H.; Hale, S.E. Bisphenol A in Solid Waste Materials, Leachate Water, and Air Particles from Norwegian Waste-Handling Facilities: Presence and Partitioning Behavior. Environ. Sci. Technol. 2015, 49, 7675–7683. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Feng, E.; Song, J. Renaissance of aliphatic polycarbonates: New techniques and biomedical applications. J. Appl. Polym. Sci. 2014, 131, 39822. [Google Scholar] [CrossRef] [Green Version]
- Artham, T.; Doble, M. Biodegradation of aliphatic and aromatic polycarbonates. Macromol. Biosci. 2008, 8, 14–24. [Google Scholar] [CrossRef]
- Luinstra, G.A.; Borchardt, E. Biodegradation of aliphatic and aromatic polycarbonates. Adv. Polym. Sci. 2012, 245, 29–48. [Google Scholar]
- Dove, P. Organic Catalysis for Ring-Opening Polymerisation Andrew. ACS Macro Lett. 2012, 12, 1409–1412. [Google Scholar] [CrossRef]
- Fukushima, K.; Nozaki, K. Organocatalysis: A Paradigm Shift in the Synthesis of Aliphatic Polyesters and Polycarbonates. Macromolecules 2020, 13, 5018–5022. [Google Scholar] [CrossRef]
- Tezuka, K.; Komatsu, K.; Haba, O. The anionic ring-opening polymerisation of five-membered cyclic carbonates fused to the cyclohexane ring. Polym. J. 2013, 45, 1183–1187. [Google Scholar] [CrossRef] [Green Version]
- Guerin, W.; Diallo, A.K.; Kirilov, E.; Helou, M.; Slawinski, M.; Brusson, J.-M.; Carpentier, J.-F.; Guillaume, S.M. Enantiopure Isotactic PCHC Synthesized by Ring-Opening Polymerisation of Cyclohexene Carbonate. Macromolecules 2014, 47, 4230–4235. [Google Scholar] [CrossRef]
- Della Monica, F.; Kleij, A.W. Mechanistic guidelines in nonreductive conversion of CO2: The case of cyclic carbonates. Catal. Sci. Technol. 2020, 10, 3483–3501. [Google Scholar] [CrossRef]
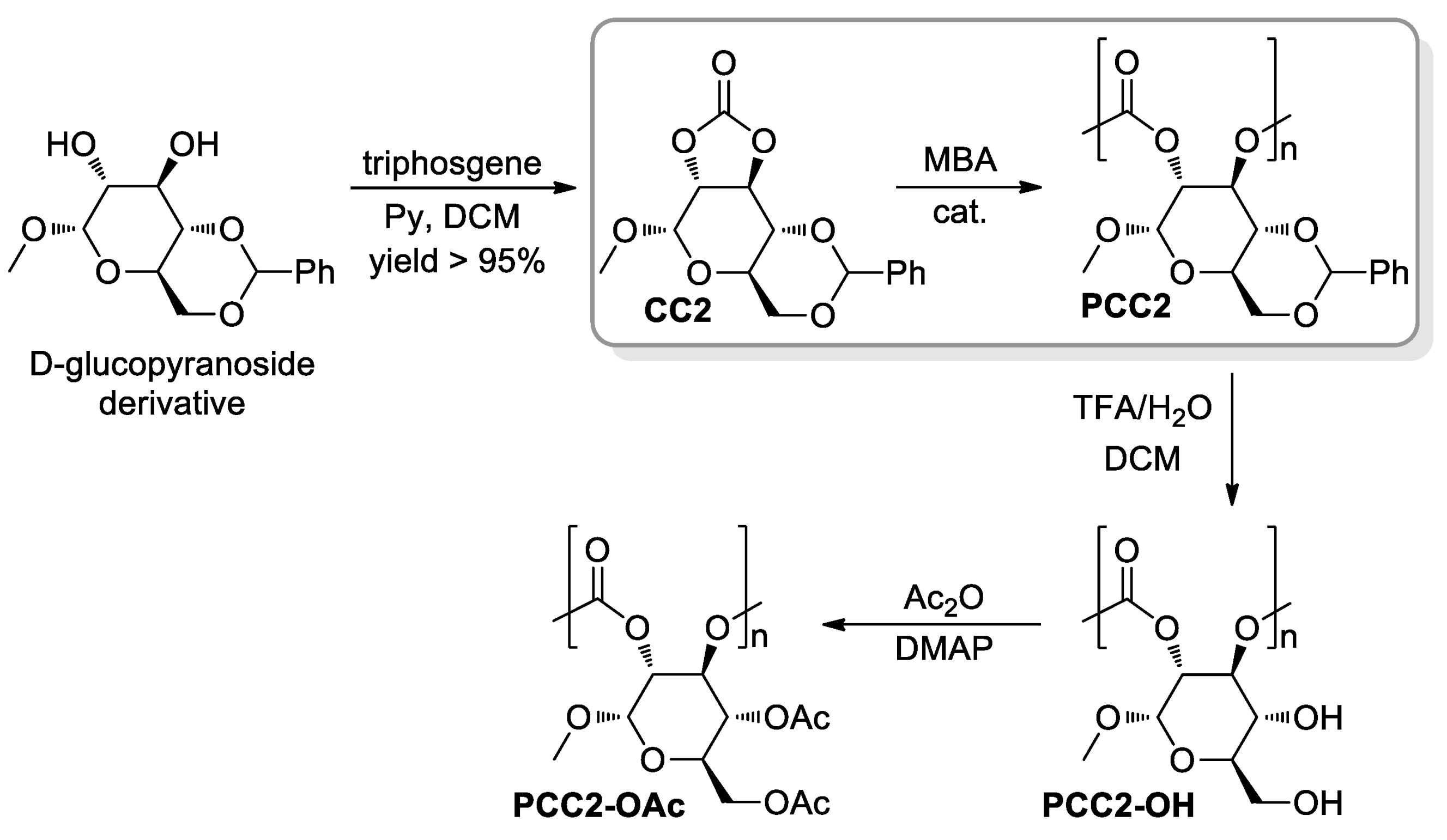
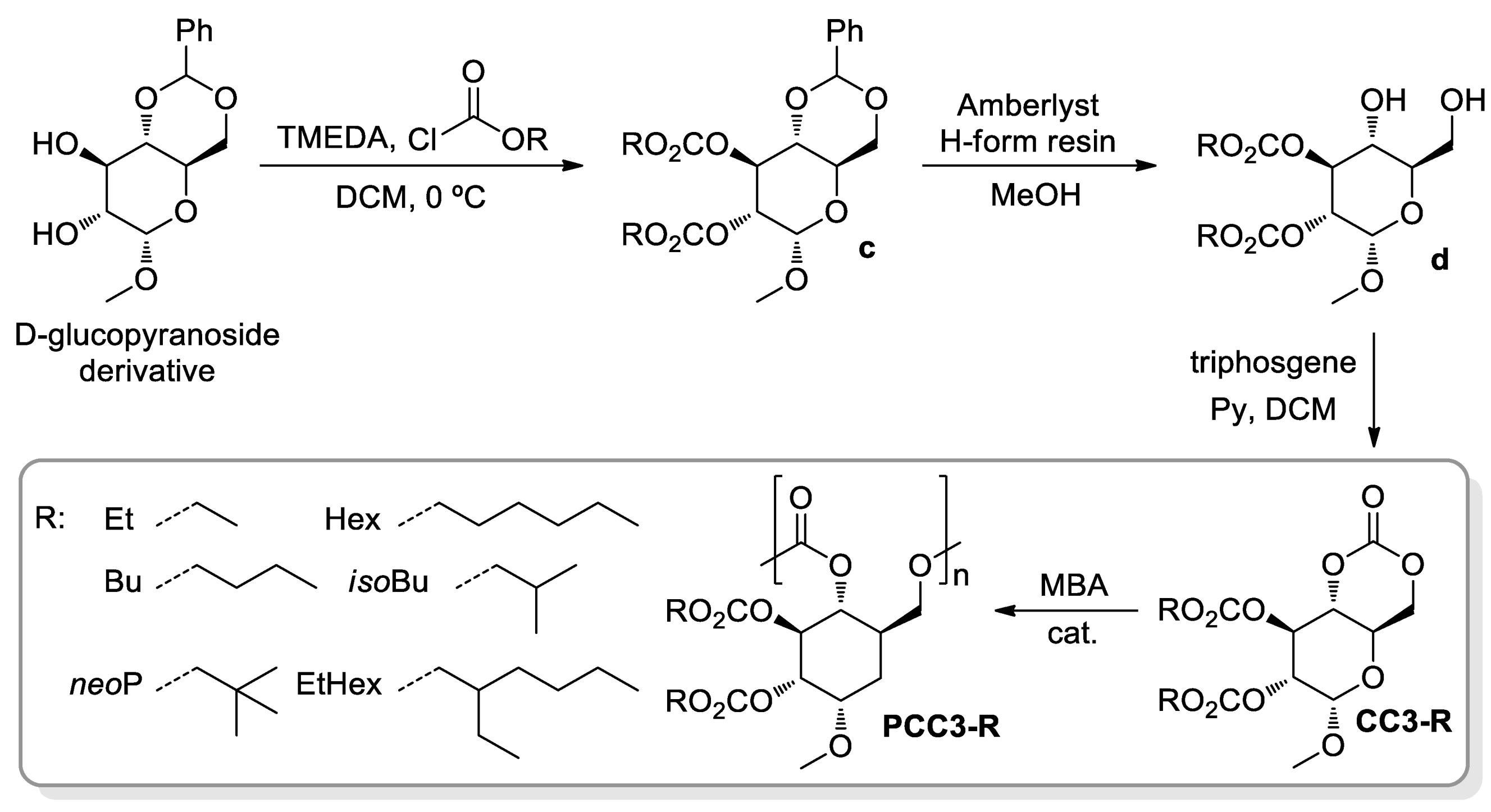
- Gregory, G.L.; López-Vidal, E.M.; Buchard, A. Polymers from sugars: Cyclic monomer synthesis, ring-opening polymerisation, material properties and applications. Chem. Commun. 2017, 53, 2198–2217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
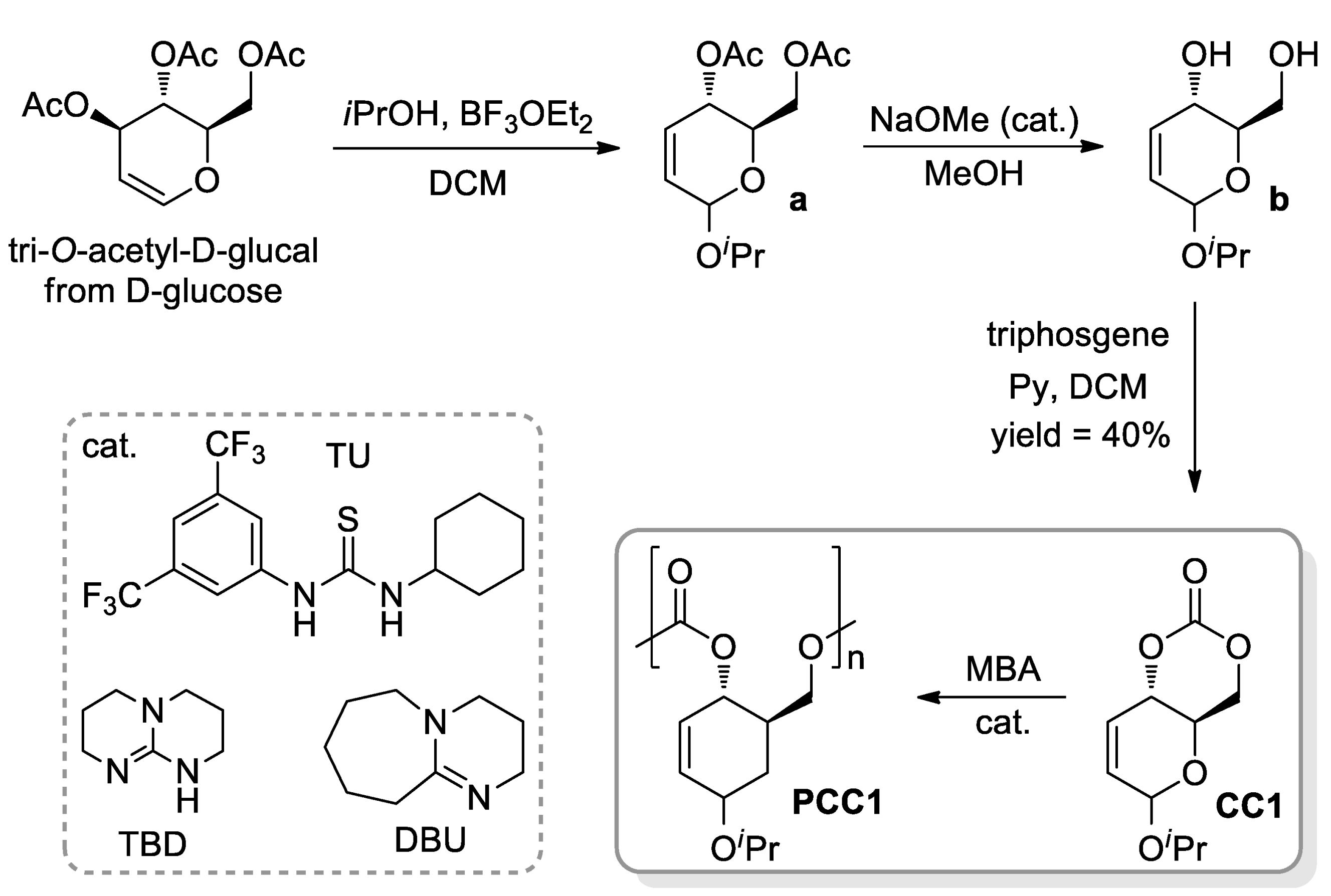
- Lonnecker, A.T.; Lim, Y.H.; Wooley, K.L. Functional Polycarbonate of a D-Glucal-Derived Bicyclic Carbonate via Organocatalytic Ring-Opening Polymerisation. ACS Macro Lett. 2017, 6, 748–753. [Google Scholar] [CrossRef] [Green Version]
- Felder, S.E.; Redding, M.J.; Noel, A.; Grayson, S.M.; Wooley, K.L. Organocatalyzed ROP of a Glucopyranoside Derived Five-Membered Cyclic Carbonate. Macromolecules 2018, 51, 1787–1797. [Google Scholar] [CrossRef]
- Song, Y.; Ji, X.; Dong, M.; Li, R.; Lin, Y.-N.; Wang, H.; Wooley, K.L. Advancing the Development of Highly-Functionalizable Glucose-Based Polycarbonates by Tuning of the Glass Transition Temperature. J. Am. Chem. Soc. 2018, 140, 16053–16057. [Google Scholar] [CrossRef] [PubMed]
- Fox, T.G., Jr.; Flory, P.J. Second-order transition temperatures and related properties of polystyrene. I. Influence of molecular weight. J. Appl. Phys. 1950, 21, 581–591. [Google Scholar] [CrossRef]
- Song, Y.; Yang, X.; Shen, Y.; Dong, M.; Lin, Y.-N.; Hall, M.B.; Wooley, K.L. Invoking Side-Chain Functionality for the Mediation of Regioselectivity during Ring-Opening Polymerisation of Glucose Carbonates. J. Am. Chem. Soc. 2020, 142, 16974–16981. [Google Scholar] [CrossRef]
- Gregory, G.L.; Ulmann, M.; Buchard, A. Synthesis of 6-membered cyclic carbonates from 1,3-diols and low CO2 pressure: A novel mild strategy to replace phosgene reagents. RSC Adv. 2015, 5, 39404–39408. [Google Scholar] [CrossRef] [Green Version]
- McGuire, T.M.; López-Vidal, E.M.; Gregory, G.L.; Buchard, A. Synthesis of 5- to 8-membered cyclic carbonates from diols and CO2: A one-step, atmospheric pressure and ambient temperature procedure. J. CO2 Util. 2018, 27, 283–288. [Google Scholar] [CrossRef]
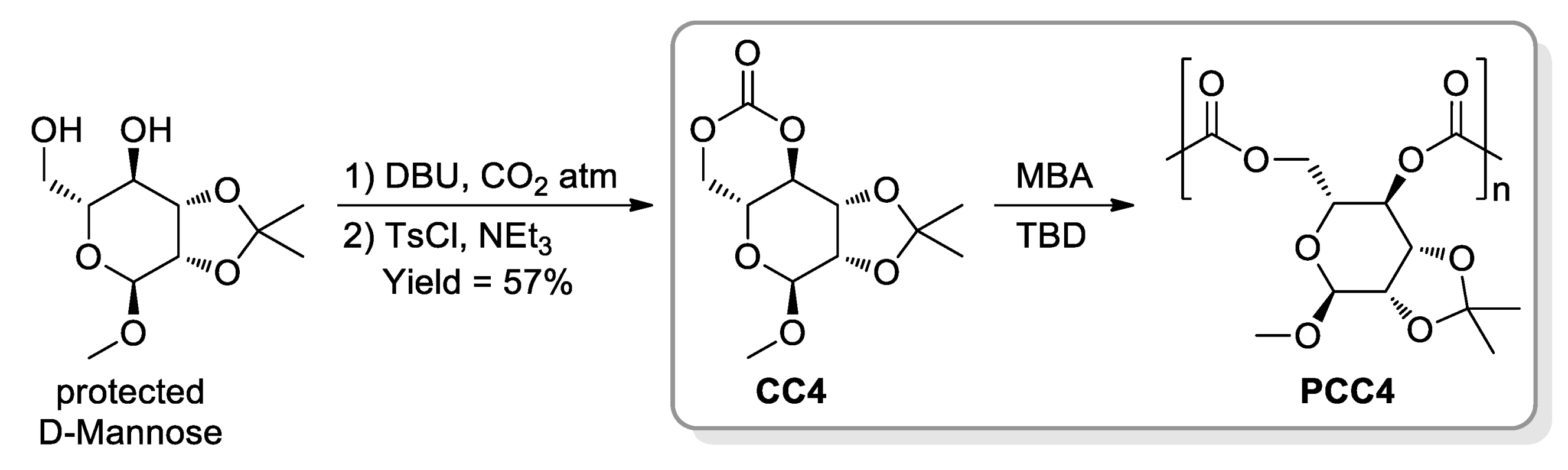
- Gregory, G.L.; Jenisch, L.M.; Charles, B.; Kociok-Köhn, G.; Buchard, A. Polymers from Sugars and CO2: Synthesis and Polymerisation of a D-Mannose-Based Cyclic Carbonate. Macromolecules 2016, 49, 7165–7169. [Google Scholar] [CrossRef] [Green Version]
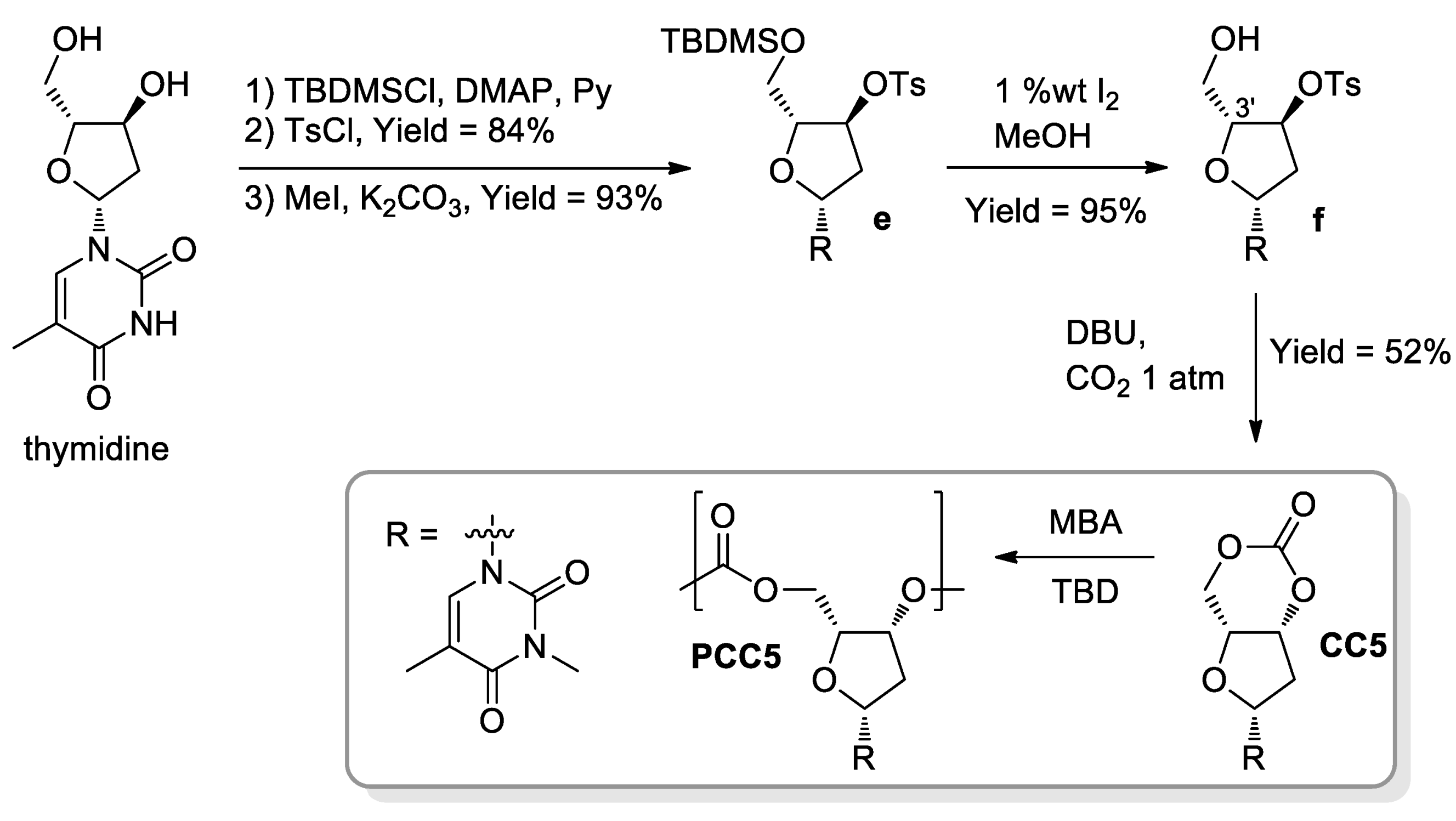
- Gregory, G.L.; Hierons, E.M.; Kociok-Köhn, G.; Sharma, R.I.; Buchard, A. CO2-Driven stereochemical inversion of sugars to create thymidine-based polycarbonates by ring-opening polymerisation. Polym. Chem. 2017, 8, 1714–1721. [Google Scholar] [CrossRef] [Green Version]
- Gregory, G.L.; Kociok-Köhnc, G.; Buchard, A. Polymers from sugars and CO2: Ring-opening polymerisation and copolymerisation of cyclic carbonates derived from 2-deoxy-D-ribose. Polym. Chem. 2017, 8, 2093–2104. [Google Scholar] [CrossRef] [Green Version]
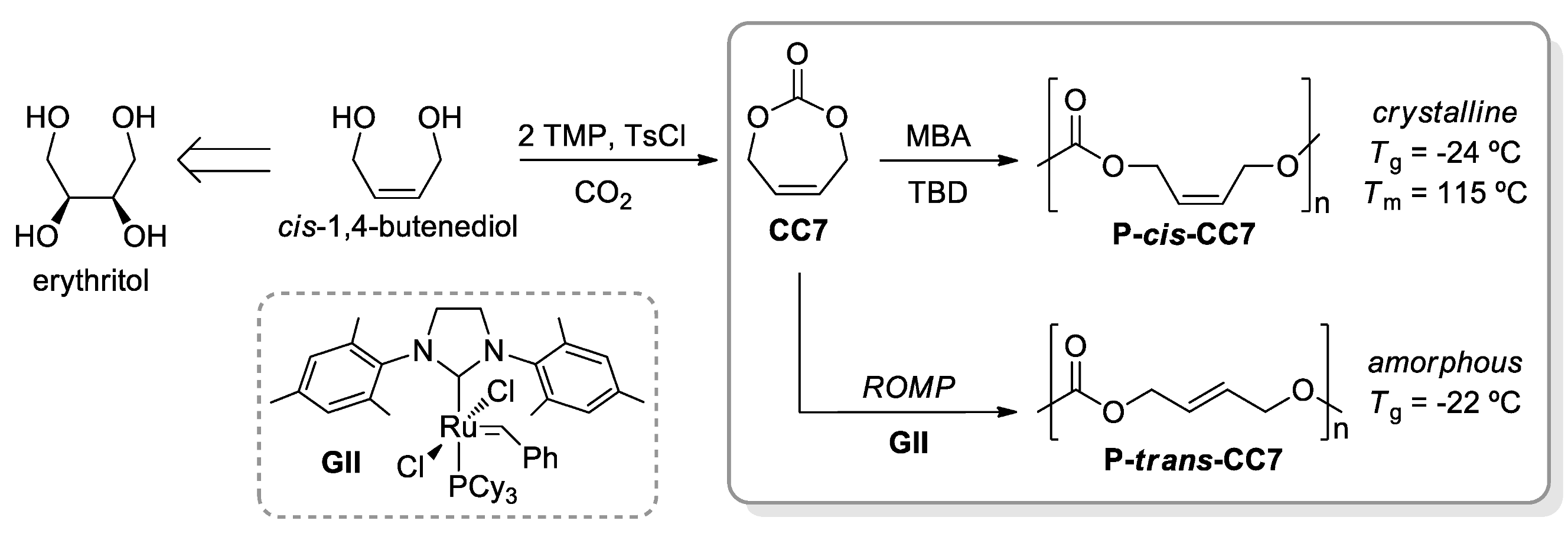
- McGuire, T.M.; Pérale, C.; Castaing, R.; Kociok-Köhn, G.; Buchard, A. Divergent Catalytic Strategies for the Cis/Trans Stereoselective Ring-Opening Polymerisation of a Dual Cyclic Carbonate/Olefin Monomer. J. Am. Chem. Soc. 2019, 141, 13301–13305. [Google Scholar] [CrossRef] [Green Version]
- Burgard, A.; Burk, M.J.; Osterhout, R.; Van Dien, S.; Yim, H. Development of a Commercial Scale Process for Production of 1,4- Butanediol from Sugar. Curr. Opin. Biotechnol. 2016, 42, 118–125. [Google Scholar] [CrossRef] [PubMed]
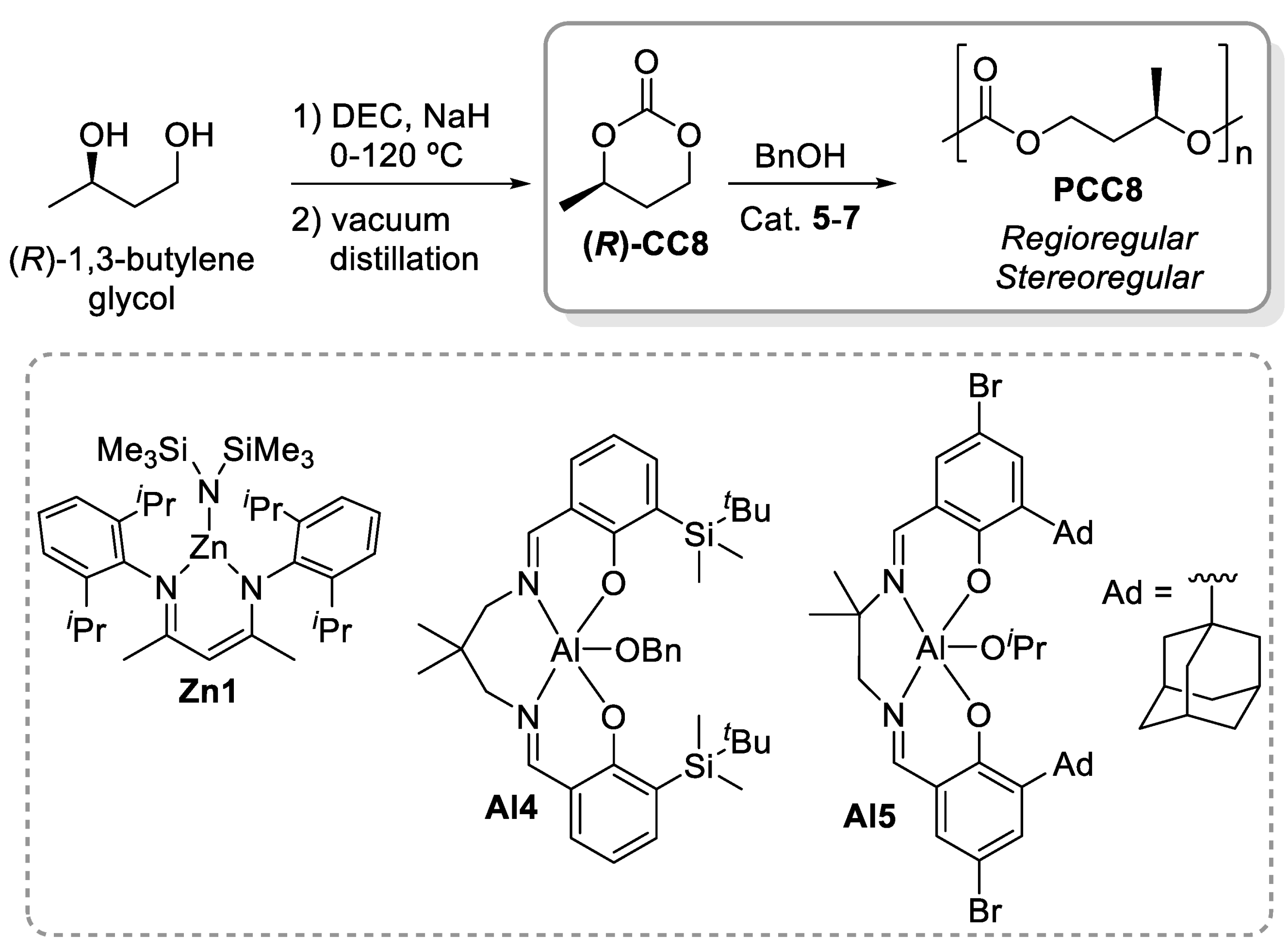
- DeRosa, C.A.; Luke, A.M.; Anderson, K.; Reineke, T.M.; Tolman, W.B.; Bates, F.S.; Hillmyer, M.A. Regioregular Polymers from Bio-based (R)-1,3-Butylene Carbonate. Macromolecules 2021, 54, 5974–5984. [Google Scholar] [CrossRef]
- Yasuda, H.; Aludin, M.-S.; Kitamura, N.; Tanabe, M.; Sirahama, H. Syntheses and Physical Properties of Novel Optically Active Poly(Ester-Carbonate)s by Copolymerisation of Substituted Trimethylene Carbonate with ε-Caprolactone and Their Biodegradation Behavior. Macromolecules 1999, 32, 6047–6057. [Google Scholar] [CrossRef]
- Tsutsumi, C.; Yasuda, H. Biodegradation of Copolymers Composed of Optically Active L-Lactide and (R)- or (S)-1-Methyltrimethylene Carbonate. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 3916–3927. [Google Scholar] [CrossRef]
- Brignou, P.; Carpentier, J.-F.; Guillaume, S.M. Metal—And Organo-Catalyzed Ring-Opening Polymerisation of α-Methyl-Trimethylene Carbonate: Insights into the Microstructure of the Polycarbonate. Macromolecules 2011, 44, 5127–5135. [Google Scholar] [CrossRef]
- Hua, G.; Franzén, J.; Odelius, K. Phosphazene-Catalyzed Regioselective Ring-Opening Polymerisation of Rac-1-Methyl Trimethylene Carbonate: Colder and Less Is Better. Macromolecules 2019, 52, 2681–2690. [Google Scholar] [CrossRef]
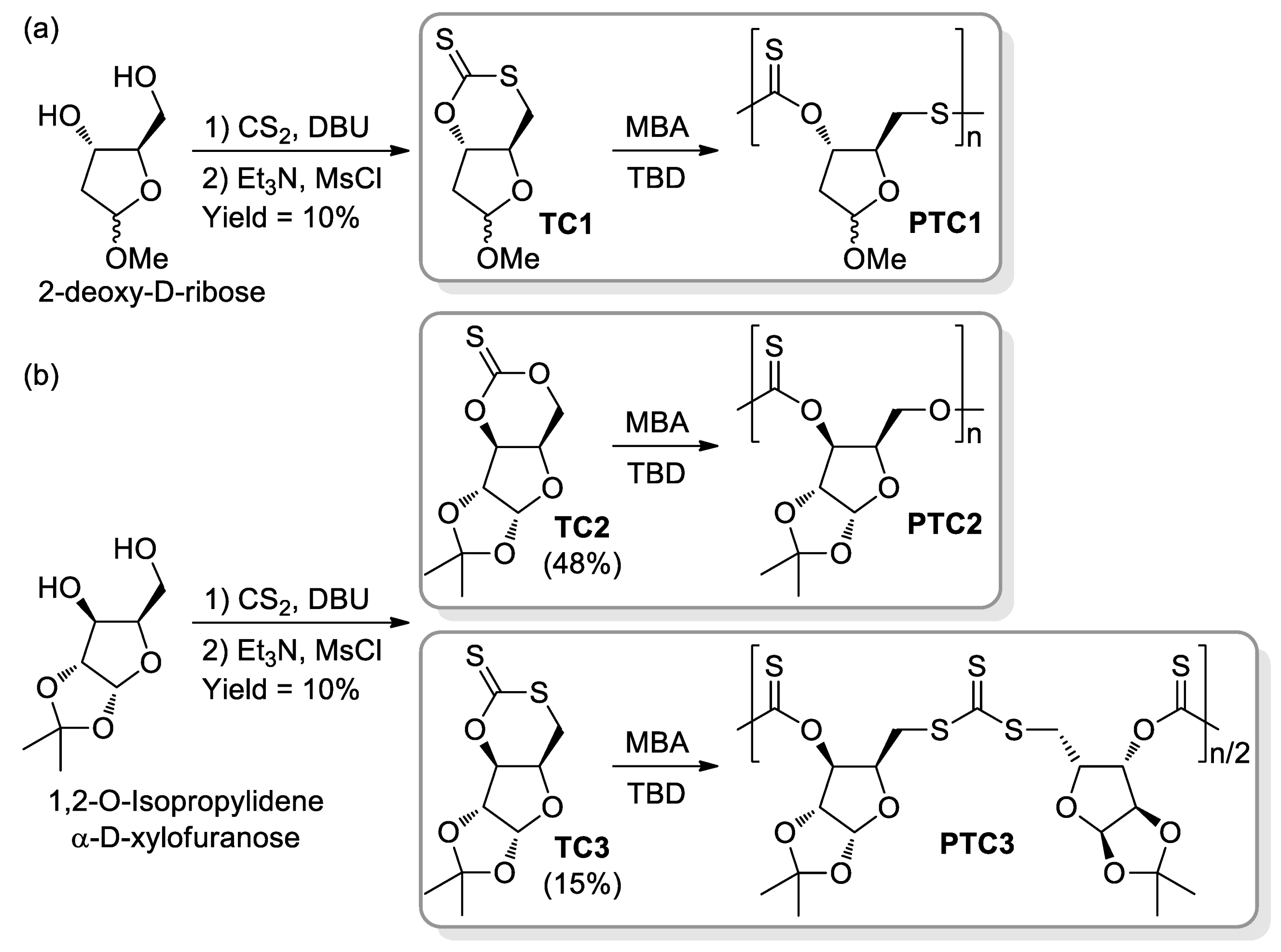
- López-Vidal, E.M.; Gregory, G.L.; Kociok-Köhnc, G.; Buchard, A. Polymers from sugars and CS2: Synthesis and ring-opening polymerisation of sulfur-containing monomers derived from 2-deoxy-D-ribose and D-xylose. Polym. Chem. 2018, 9, 1577–1582. [Google Scholar] [CrossRef] [Green Version]
- Overberger, C.G.; Weise, J.K.J. Anionic ring-opening polymerization of thiolactones. Am. Chem. Soc. 1968, 90, 3533–3537. [Google Scholar] [CrossRef]
- Wu, H.-L.; Yang, J.-L.; Luo, M.; Wang, R.-Y.; Xu, J.-T.; Du, B.-Y.; Zhang, X.-H.; Darensbourg, D.J. Poly(trimethylene monothiocarbonate) from the Alternating Copolymerization of COS and Oxetane: A Semicrystalline Copolymer. Macromolecules 2016, 49, 8863–8868. [Google Scholar] [CrossRef]
- Luo, M.; Zhang, X.-H.; Darensbourg, D.J. Poly (monothiocarbonate)s from the alternating and regioselective copolymerization of carbonyl sulfide with epoxides. Acc. Chem. Res. 2016, 49, 2209–2219. [Google Scholar] [CrossRef] [PubMed]
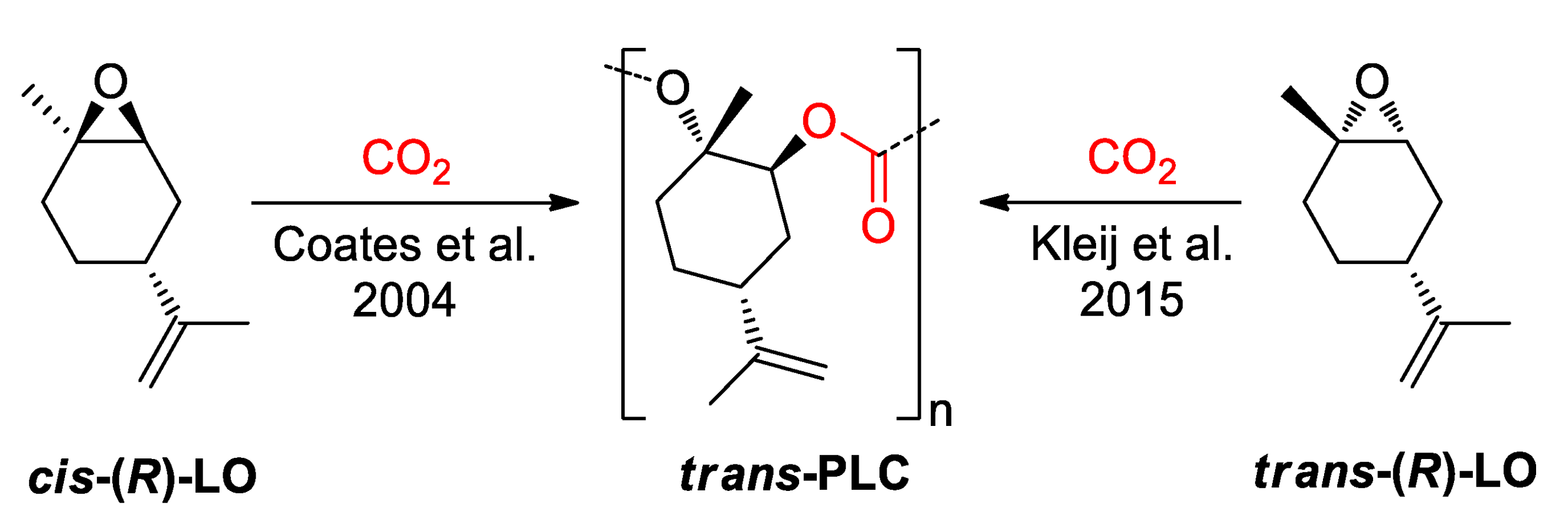
- Byrne, C.M.; Allen, S.D.; Lobkovsky, E.B.; Coates, G.W.J. Alternating Copolymerization of Limonene Oxide and Carbon Dioxide. Am. Chem. Soc. 2004, 126, 11404–11405. [Google Scholar] [CrossRef] [Green Version]
- Carrodeguas, P.L.; González-Fabra, J.; Castro-Gómez, F.; Bo, C.; Kleij, A.W. Al(III) -catalysed formation of poly(limonene)carbonate: DFT analysis of the origin of stereoregularity. Chem. Eur. J. 2015, 21, 6115–6122. [Google Scholar] [CrossRef]
- Wambach, A.; Agarwal, S.; Greiner, A. Synthesis of Bio-based Polycarbonate by Copolymerisation of Menth-2-ene Oxide and CO2 with Exceptional Thermal Stability. ACS Sustain. Chem. Eng. 2020, 8, 14690–14693. [Google Scholar] [CrossRef]
- Tran, D.K.; Rashad, A.Z.; Darensbourg, D.J.; Wooley, K.L. Sustainable synthesis of CO2-derived polycarbonates from D-xylose. Polym. Chem. 2021, 12, 5271–5278. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Moncada, A.I. Tuning the Selectivity of the Oxetane and CO2 Coupling Process Catalyzed by (Salen)CrCl/n-Bu4NX: Cyclic Carbonate Formation vs Aliphatic Polycarbonate Production. Macromolecules 2010, 43, 5996–6003. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Moncada, A.I.; Wei, S.-H. Aliphatic Polycarbonates Produced from the Coupling of Carbon Dioxide and Oxetanes and Their Depolymerisation via Cyclic Carbonate Formation. Macromolecules 2011, 44, 2568–2576. [Google Scholar] [CrossRef]
- Park, H.J.; Ryu, C.Y.; Crivello, J.V. Photoinitiated cationic polymerisation of limonene 1,2-oxide and a-pinene oxide J. Polym. Sci. Part A Polym. Chem. 2013, 51, 109–117. [Google Scholar] [CrossRef]
- Sessini, V.; Palenzuela, M.; Damian, J.; Mosquera, M.E.G. Bio-based polyether from limonene oxide catalytic ROP as green polymeric plasticizer for PLA. Polymer 2020, 210, 123003. [Google Scholar] [CrossRef]
- Saxon, D.J.; Nasiri, M.; Mandal, M.; Maduskar, S.; Dauenhauer, P.J.; Cramer, C.J.; LaPointe, A.M.; Reineke, T.M. Architectural Control of Isosorbide-Based Polyethers via Ring-Opening Polymerisation. J. Am. Chem. Soc. 2019, 141, 5107–5111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matt, L.; Liblikas, I.; Bonjour, O.; Jannasch, P.; Vares, L. Synthesis and anionic polymerisation of isosorbide mono-epoxides for linear bio-based polyethers. Polym. Chem. 2021, 12, 5937–5941. [Google Scholar] [CrossRef]
- Gandini, A.; Lacerda, T.M. From monomers to polymers from renewable resources: Recent advances. Prog. Polym. Sci. 2015, 48, 1–39. [Google Scholar] [CrossRef]
- Harvey, B.J.; Meylemans, H.A. The role of butanol in the development of sustainable fuel technologies. J. Chem. Technol. Biotechnol. 2011, 86, 2–9. [Google Scholar] [CrossRef]
- Herzberger, J.; Niederer, K.; Pohlit, H.; Seiwert, J.; Worm, M.; Wurm, F.R.; Frey, H. Polymerisation of Ethylene Oxide, Propylene Oxide, and Other Alkylene Oxides: Synthesis, Novel Polymer Architectures, and Bioconjugation. Chem. Rev. 2016, 116, 2170–2243. [Google Scholar] [CrossRef] [Green Version]
- PolyTHF® - Polytetrahydrofuran. Available online: https://chemicals.basf.com/global/en/Intermediates/Product_groups/PolyTHF.html (accessed on 31 March 2022).
- Sardon, H.; Mecerreyes, D.; Basterretxea, A.; Avérous, L.; Jehanno, C. From Lab to Market: Current Strategies for the Production of Bio-based Polyols. ACS Sustain. Chem. Eng. 2021, 9, 10664–10677. [Google Scholar] [CrossRef]
- Jacobsen, S.; Degee, P.H.; Fritz, H.G.; Jerome, D.R. Polylactide (PLA)-A New Way of Production. Polym. Eng. Sci. 1999, 39, 1311–1319. [Google Scholar] [CrossRef]
- Pradhan, R.; Reddy, M.; Diebel, W.; Erickson, L.; Misra, M.; Mohanty, A. Comparative compostability and biodegradation studies of various components of green composites and their blends in simulated aerobic composting bioreactor. Int. J. Plast. Technol. 2010, 14, 45–50. [Google Scholar] [CrossRef]


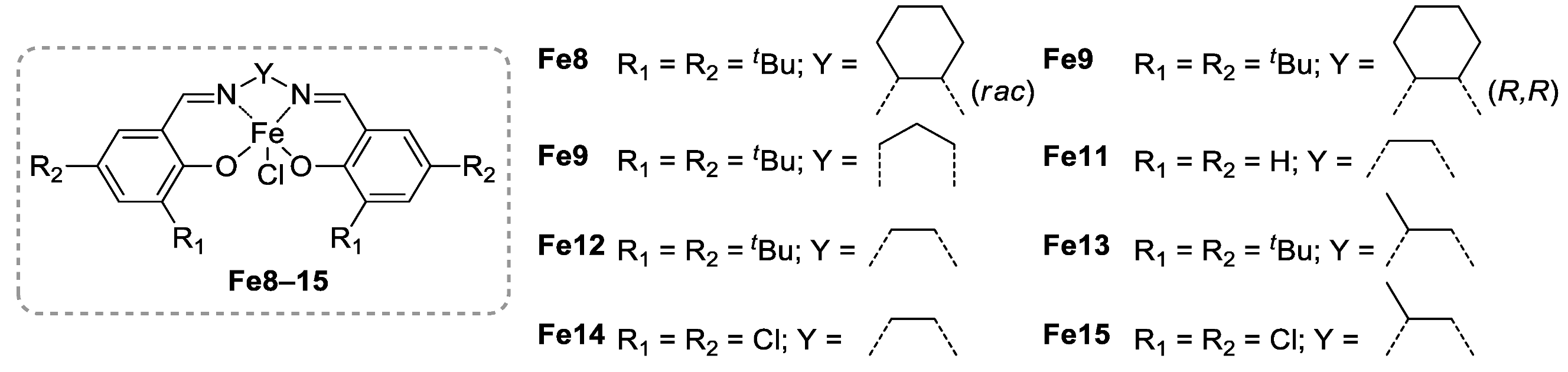









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santoro, O.; Izzo, L.; Della Monica, F. Recent Advances in RO(CO)P of Bio-Based Monomers. Sustain. Chem. 2022, 3, 259-285. https://doi.org/10.3390/suschem3020017
Santoro O, Izzo L, Della Monica F. Recent Advances in RO(CO)P of Bio-Based Monomers. Sustainable Chemistry. 2022; 3(2):259-285. https://doi.org/10.3390/suschem3020017
Chicago/Turabian StyleSantoro, Orlando, Lorella Izzo, and Francesco Della Monica. 2022. "Recent Advances in RO(CO)P of Bio-Based Monomers" Sustainable Chemistry 3, no. 2: 259-285. https://doi.org/10.3390/suschem3020017
APA StyleSantoro, O., Izzo, L., & Della Monica, F. (2022). Recent Advances in RO(CO)P of Bio-Based Monomers. Sustainable Chemistry, 3(2), 259-285. https://doi.org/10.3390/suschem3020017






