Fast and Efficient Mechanosynthesis of Aldonamides by Aminolysis of Unprotected Sugar Lactones
Abstract
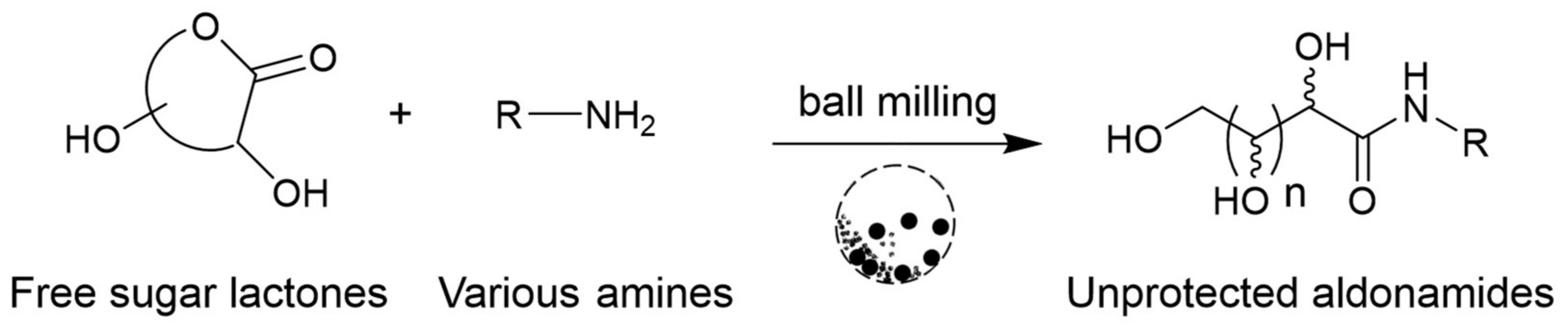
:1. Introduction
2. Materials and Methods
2.1. General
2.2. General Optimized Procedure
2.3. Crudes Characterization Data
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gallezot, P. Conversion of biomass to selected chemical products. Chem. Soc. Rev. 2012, 41, 1538. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, C.C. Sugar-Based Surfactants: Fundamentals and Applications; CRC Press: Boca Raton, FL, USA, 2008; pp. 245–306. [Google Scholar]
- Kahn, G.F. Hair Compositions, Comprising a Glucose-Based Hair Conditioning Agent and an Unsaturated Cationic Surfactant. HENKEL AG & CO. Patent KGAA—DE102020206655, 2 December 2021. [Google Scholar]
- Zielińska, K.; Wilk, K.A.; Jezierski, A.; Jesionowski, T. Microstructure and structural transition in microemulsions stabilized by aldonamide-type surfactants. J. Colloid Interface Sci. 2008, 321, 408. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, K.; Pietkiewicz, J.; Saczko, J.; Wilk, K.A. Microemulsion stabilized by gemini, dicephalic and single-head single tail sugar surfactants as biologically important systems: Hemolytic activity and cytotoxic studies. Prog. Colloid Polym. Sci. 2011, 138, 193. [Google Scholar]
- Ohsedo, Y.; Oono, M.; Saruhashi, K.; Watanabe, H. Onset of mixing-induced thixotropy in hydrogels by mixing two homologues of low-molecular-weight hydrogelators. RSC Adv. 2014, 4, 43560. [Google Scholar] [CrossRef]
- Pilakowska-Pietras, D.; Lunkenheimer, K.; Piasecki, A. Investigations on foamability of surface-chemically pure aqueous solutions of functionalized alkylaldonamides. J. Colloid Interface Sci. 2006, 294, 423. [Google Scholar] [CrossRef] [PubMed]
- Piasecki, A.; Pilakowska-Pietras, D. Synthesis and properties of functionalized alkylaldonamides. J. Surfactants Deterg. 2007, 10, 125. [Google Scholar] [CrossRef]
- Capicciotti, C.J.; Leclère, M.; Perras, F.A.; Bryce, D.L.; Paulin, H.; Harden, J.; Liu, Y.; Ben, R.N. Potent inhibition of ice recrystallization by low molecular weight carbohydrate-based surfactants and hydrogelators. Chem. Sci. 2012, 3, 1408. [Google Scholar] [CrossRef]
- Briard, J.G.; Jahan, S.; Chandran, P.; Allan, D.; Pineault, N.; Ben, R.N. Small-molecule ice recrystallization inhibitors improve the post-thaw function of hematopoietic stem and progenitor cells. ACS Omega 2016, 1, 1010. [Google Scholar] [CrossRef] [Green Version]
- Wilk, K.A.; Syper, L.; Domagalska, B.W.; Komorek, U.; Maliszewska, I.; Gancarz, R. Aldonamide-type gemini surfactants: Synthesis, structural analysis, and biological properties. J. Surfactants Deterg. 2002, 5, 235. [Google Scholar] [CrossRef]
- Reis, R.C.N.; Oda, S.C.; De Almeida, M.V.; Lourenço, M.C.S.; Vicente, F.R.C.; Barbosa, N.R.; Trevizani, R.; Santos, P.L.C.; Le Hyaric, M. Synthesis and antimicrobial activity of amphiphilic carbohydrate derivatives. J. Braz. Chem. Soc. 2008, 19, 1065. [Google Scholar] [CrossRef] [Green Version]
- Acharya, G.; Park, K.; Thompson, D.H. Synthesis and evaluation of α-cyclodextrin-aldonamide conjugates for D-glucose recognition. J. Drug Deliv. Sci. Technol. 2006, 16, 45. [Google Scholar] [CrossRef]
- Adokoh, C.K.; Quan, S.; Hitt, M.; Darkwa, J.; Kumar, P.; Narain, R. Synthesis and evaluation of glycopolymeric decorated gold nanoparticles functionalized with gold-triphenyl phosphine as anti-cancer agents. Biomacromolecules 2014, 15, 3802. [Google Scholar] [CrossRef]
- Aoyama, Y. Macrocyclic glycoclusters: From amphiphiles through nanoparticles to glycoviruses. Chem. Eur. J. 2004, 10, 588. [Google Scholar] [CrossRef]
- Abe, H.; Kenmoku, A.; Yamaguch, N.; Hattori, K. Structural effects of oligosaccharide-branched cyclodextrins on the dual recognition toward lectin and drug. J. Incl. Phenom. Macrocycl. Chem. 2002, 44, 39. [Google Scholar] [CrossRef]
- Lönngren, J.; Goldstein, I.I.; Niederhuber, J.E. Aldonate coupling, a simple procedure for the preparation of carbohydrate-protein conjugates for studies of carbohydrate-binding proteins. Arch. Biochem. Biophys. 1976, 175, 661. [Google Scholar] [CrossRef] [Green Version]
- Lu, B.; Vayssade, M.; Miao, Y.; Chagnault, V.; Grand, E.; Wadouachi, A.; Postel, D.; Drelich, A.; Egles, C.; Pezron, I. Physico-chemical properties and cytotoxic effects of sugar-based surfactants: Impact of structural variations. Colloids Surf. B 2016, 145, 79. [Google Scholar] [CrossRef]
- Bois, R.; Abdellahi, B.; Mika, B.; Golonu, S.; Vigneron, P.; Chagnault, V.; Drelich, A.; Pourceau, G.; Wadouachi, A.; Vayssade, M.; et al. Physicochemical, foaming and biological properties of lowly irritant anionic sugar-based surfactants. Colloid Surf. A 2020, 607, 125525. [Google Scholar] [CrossRef]
- Abdellahi, B.; Bois, R.; Golonu, S.; Pourceau, G.; Lesur, D.; Chagnault, V.; Drelich, A.; Pezron, I.; Nesterenko, A.; Wadouachi, A. Synthesis and interfacial properties of new 6-sulfate sugar-based anionic surfactants. Tetrahedron Lett. 2021, 74, 153113. [Google Scholar] [CrossRef]
- Colombeau, L.; Traoré, T.; Compain, P.; Martin, O.R. Metal-free one-pot oxidative amidation of aldoses with functionalized amines. J. Org. Chem. 2008, 73, 8647. [Google Scholar] [CrossRef]
- Fusaro, M.; Chagnault, V.; Postel, D. Synthesis of glycosylamines and glyconamides using molecular iodine. Tetrahedron 2013, 69, 542. [Google Scholar] [CrossRef]
- Cho, C.C.; Liu, J.N.; Chien, C.H.; Shie, J.J.; Chen, Y.C.; Fang, J.M. Direct amidation of aldoses and decarboxylative amidation of α-keto acids: An efficient conjugation method for unprotected carbohydrate molecules. J. Org. Chem. 2009, 74, 1549. [Google Scholar] [CrossRef] [PubMed]
- Reis, M.I.P.; Gonçalves, A.D.; da Silva, F.C.; Jordão, A.K.; Alves, R.J.; de Andrade, S.F.; Resende, J.A.L.C.; Rocha, A.A.; Ferreira, V.F. Synthesis and evaluation of D-gluconamides as green mineral scales. Carbohydr. Res. 2012, 353, 6. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, J.; Yang, S.; Zhang, W.; Niu, X.; Chen, Y.; Ran, F. Hydrophilicity and anti-fouling performance of polyethersulfone membrane modified by grafting block glycosyl copolymers via surface initiated electrochemically mediated atom transfer radical polymerization. New J. Chem. 2018, 42, 2692. [Google Scholar] [CrossRef]
- Cerrada, M.L.; Sánchez-Chaves, M.; Ruiz, C.; Fernández-García, M. Recognition abilities and development of heat-induced entangled networks in lactone-derived glycopolymers obtained from ethylene-vinyl alcohol copolymers. Biomacromolecules 2009, 10, 1828. [Google Scholar] [CrossRef]
- Wang, G.-W. Mechanochemical organic synthesis. Chem. Soc. Rev. 2013, 42, 7668. [Google Scholar] [CrossRef]
- Rightmire, N.R.; Hanusa, T.P. Advances in organometallic synthesis with mechanochemical methods. Dalton Trans. 2016, 45, 2352. [Google Scholar] [CrossRef]
- Howard, J.L.; Cao, Q.; Browne, D.L. Mechanochemistry as an emerging tool for molecular synthesis: What can it offer? Chem. Sci. 2018, 9, 3080. [Google Scholar] [CrossRef] [Green Version]
- Tan, D.; Friščić, T. Mechanochemistry for Organic Chemists: An Update. Eur. J. Org. Chem. 2018, 2018, 18–33. [Google Scholar] [CrossRef]
- Avila-Ortiz, C.G.; Juaristi Egorov, E. Novel Methodologies for Chemical Activation in Organic Synthesis under Solvent-Free Reaction Conditions. Molecules 2020, 25, 3579. [Google Scholar] [CrossRef]
- Egorov, I.N.; Santra, S.; Kopchuk, D.S.; Kovalev, I.S.; Zyryanov, G.V.; Majee, A.; Ranu, B.C.; Rusinov, V.L.; Chupakhin, O.N. Ball milling: An efficient and green approach for asymmetric organic syntheses. Green Chem. 2020, 22, 302. [Google Scholar] [CrossRef]
- Ardila-Fierro, K.J.; Hernández, J.G. Sustainability Assessment of Mechanochemistry by Using the Twelve Principles of Green Chemistry. ChemSusChem 2021, 14, 2145. [Google Scholar] [CrossRef]
- Yang, X.; Wu, C.; Su, W.; Yu, J. Mechanochemical C−X/C−H Functionalization: An Alternative Strategic Access to Pharmaceuticals. Eur. J. Org. Chem. 2022, 2022, e202101440. [Google Scholar] [CrossRef]
- Bento, O.; Luttringer, F.; Mohy El Dine, T.; Pétry, N.; Bantreil, X.; Lamaty, F. Sustainable Mechanosynthesis of Biologically Active Molecules. Eur. J. Org. Chem. 2022, 2022, e202101516. [Google Scholar] [CrossRef]
- Lingome, C.E.; Pourceau, G.; Gobert-Deveaux, V.; Wadouachi, A. Efficient synthesis of glycosylamines in solventless conditions promoted by mechanical milling. RSC Adv. 2014, 4, 36350. [Google Scholar] [CrossRef]
- Epoune Lingome, C.; Wadouachi, A.; Pourceau, G.; Beury, A.; Gobert-Deveaux, V. Novel Method for Synthesising n-alkyl-glycosyl(di)amine Derivatives and Uses of Same against Phytopathogens. PCT International Application WO 2014195828 A1, 11 December 2014. [Google Scholar]
- Nicholson, W.I.; Barreteau, F.; Leitch, J.A.; Payne, R.; Priestley, I.; Godineau, E.; Battilocchio, C.; Browne, D.L. Direct amidation of esters in ball milling. Angew. Chem. Int. Ed. 2021, 60, 21868. [Google Scholar] [CrossRef]
- Frankel, D.A.; O’Brien, D.F. Supramolecular Assemblies of Diacetylenic Aldonamides. J. Am. Chem. Soc. 1994, 116, 10057. [Google Scholar]
- Falentin-Daudre, C.; Beaupère, D.; Stasik-Boutbaiba, I. Synthesis of new N-substituted 3,4,5-trihydroxypiperidin-2-ones from d-ribono-1,4-lactone. Carbohydr. Res. 2010, 345, 1983–1987. [Google Scholar] [CrossRef]
- Zhi, L.; Li, J.; Li, X.; Chen, Y.; Song, Y.; Yu, J.; Zhang, Q. Enhancing water solubility of N-dodecyl-D-gluconamide surfactant using borax. Chem. Phys. Lett. 2019, 725, 87. [Google Scholar] [CrossRef]
- Ryu, E.-H.; Zhao, Y. Efficient synthesis of water-soluble calixarenes using click chemistry. Org. Lett. 2005, 7, 1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adokoh, C.K.; Obuah, C.; Kinfe, H.H.; Zinyemba, O.; Darkwa, J. Novel bio-friendly and non-toxic thiocarbohydrate stabilizers of gold nanoparticles. New J. Chem. 2015, 39, 5249. [Google Scholar] [CrossRef]
- Friščić, T.; Childs, S.L.; Rizvi, S.A.; Jones, W. The role of solvent in mechanochemical and sonochemical cocrystal formation: A solubility-based approach for predicting cocrystallisation outcome. CrystEngComm 2009, 11, 418. [Google Scholar] [CrossRef]
- Ying, P.; Yu, J.; Su, W. Liquid-Assisted Grinding Mechanochemistry in the Synthesis of Pharmaceuticals. Adv. Synth. Catal. 2021, 363, 1246. [Google Scholar] [CrossRef]
- Pratt, M.R.; Bertozzi, C.R. Synthetic glycopeptides and glycoproteins as tools for biology. Chem. Soc. Rev. 2005, 34, 58. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.G. Synthesis of glycoproteins. Chem. Rev. 2002, 102, 579. [Google Scholar] [CrossRef]
- Grogan, M.J.; Pratt, M.R.; Marcaurelle, L.A.; Bertozzi, C.R. Homogeneous Glycopeptides and Glycoproteins for Biological Investigation. Annu. Rev. Biochem. 2002, 71, 593. [Google Scholar] [CrossRef]



| Entry | Conditions | LAG b | t (min) | Isolated Yield (%) |
|---|---|---|---|---|
| 1 | MeOH a | - | 30 | 62 |
| 2 | SPEX ball mill | - | 30 | 69 |
| 3 | - | 10 | 34 | |
| 4 | H2O | 10 | 86 | |
| 5 | H2O | 5 | 84 | |
| 6 | H2O | 15 | 83 | |
| 7 | Mortar | H2O | 10 | 83 |
| Entry | LAG | V (mL) | Yield (%) |
|---|---|---|---|
| 1 | EtOAc | 0.25 | 50 |
| 2 | H2O | 82 | |
| 3 | EtOH | 86 | |
| 4 | EtOH | 0.50 | 83 |
| 5 | 0.75 | 84 |
| Entry | Sugar Lactone | Product | Isolated Yield (%) |
|---|---|---|---|
| 1 |  |  | 90 |
| 2 |  |  | 91 |
| 3 |  |  | 96 |
| Entry | Amines | Product | Conv. (%) |
|---|---|---|---|
| 1 |  |  | Quant. |
| 2 |  |  | 84 |
| 3 |  |  | 24 |
| 4 |  |  | 89 |
| 5 |  |  | Quant. |
| 6 |  |  | - |
| 7 |  |  | 89 |
| 8 |  | Mixture of   | Quant. b Isolated yield of 4i′: 39 |
| 9 |  |  | 80 |
| 10 |  |   | Quant. 4k/4k′: ~80/20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bil, A.; Abdellahi, B.; Pourceau, G.; Wadouachi, A. Fast and Efficient Mechanosynthesis of Aldonamides by Aminolysis of Unprotected Sugar Lactones. Sustain. Chem. 2022, 3, 300-311. https://doi.org/10.3390/suschem3030019
Bil A, Abdellahi B, Pourceau G, Wadouachi A. Fast and Efficient Mechanosynthesis of Aldonamides by Aminolysis of Unprotected Sugar Lactones. Sustainable Chemistry. 2022; 3(3):300-311. https://doi.org/10.3390/suschem3030019
Chicago/Turabian StyleBil, Abed, Bemba Abdellahi, Gwladys Pourceau, and Anne Wadouachi. 2022. "Fast and Efficient Mechanosynthesis of Aldonamides by Aminolysis of Unprotected Sugar Lactones" Sustainable Chemistry 3, no. 3: 300-311. https://doi.org/10.3390/suschem3030019
APA StyleBil, A., Abdellahi, B., Pourceau, G., & Wadouachi, A. (2022). Fast and Efficient Mechanosynthesis of Aldonamides by Aminolysis of Unprotected Sugar Lactones. Sustainable Chemistry, 3(3), 300-311. https://doi.org/10.3390/suschem3030019






