Saccharide-Derived Zinc Oxide Nanoparticles with High Photocatalytic Activity for Water Decontamination and Sanitation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemical–Physical Characterization of ZnO NPs
2.3. Synthesis of ZnO NPs
2.4. Methyl Orange (MO) Photodegradation Reaction Procedure
2.5. Fluorescence Test for the Hydroxyl Radicals Detection
3. Results and Discussion
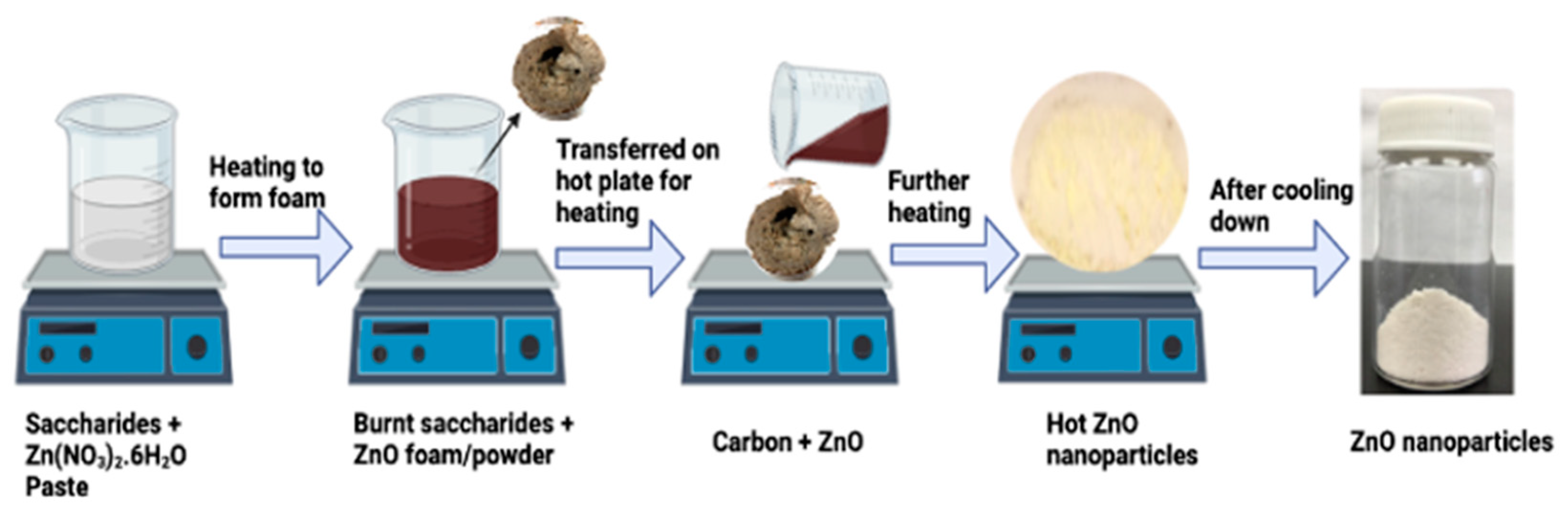
3.1. Synthesis of the ZnO NPs
3.2. ZnO NPs Characterization
3.2.1. TEM Analysis of the ZnO NPs
3.2.2. SEM Image and EDX Spectrum of the ZnO NPs
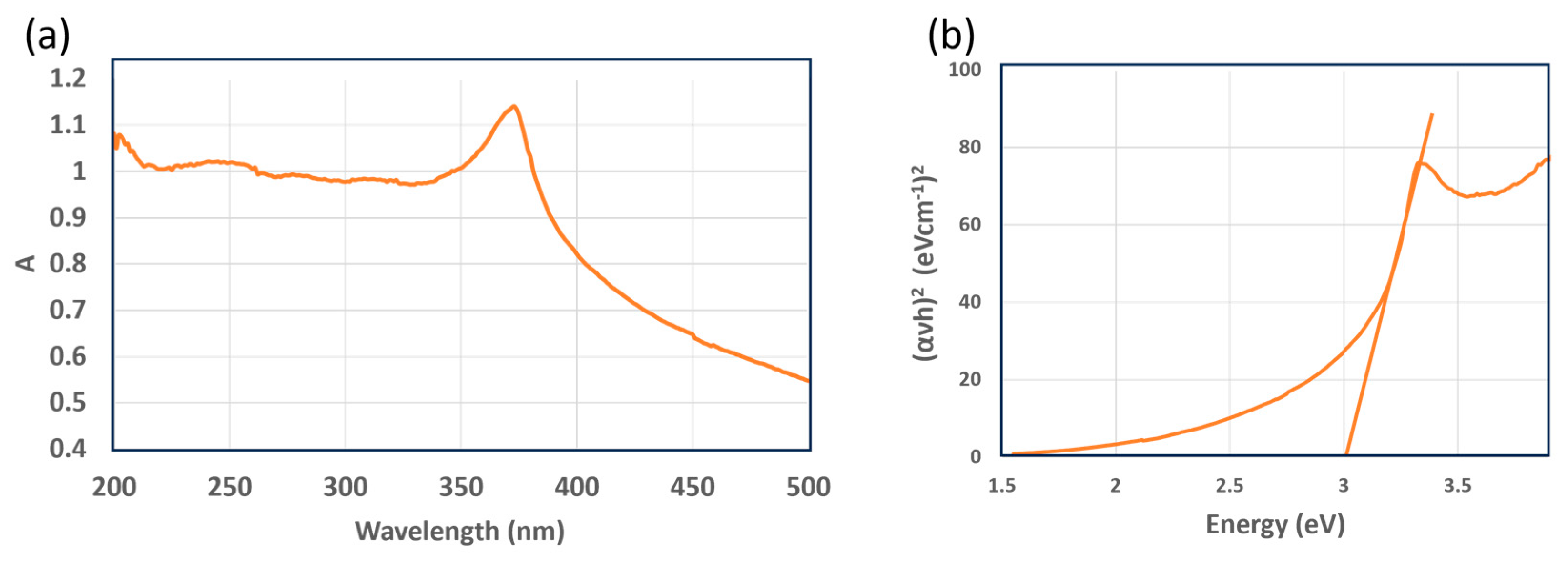
3.2.3. UV–Visible Spectrum of the ZnO NPs
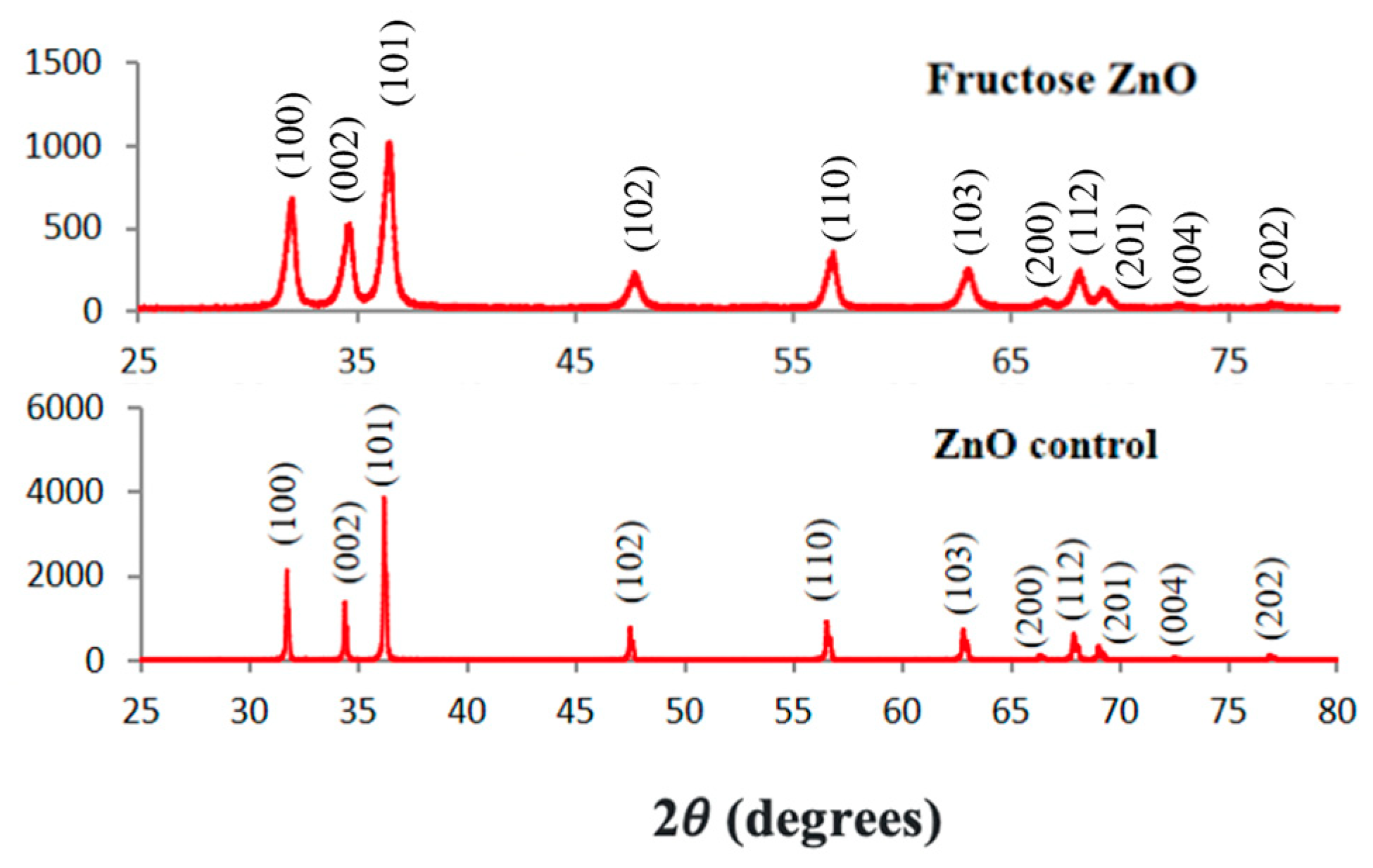
3.2.4. XRPD Analysis of the ZnO NPs
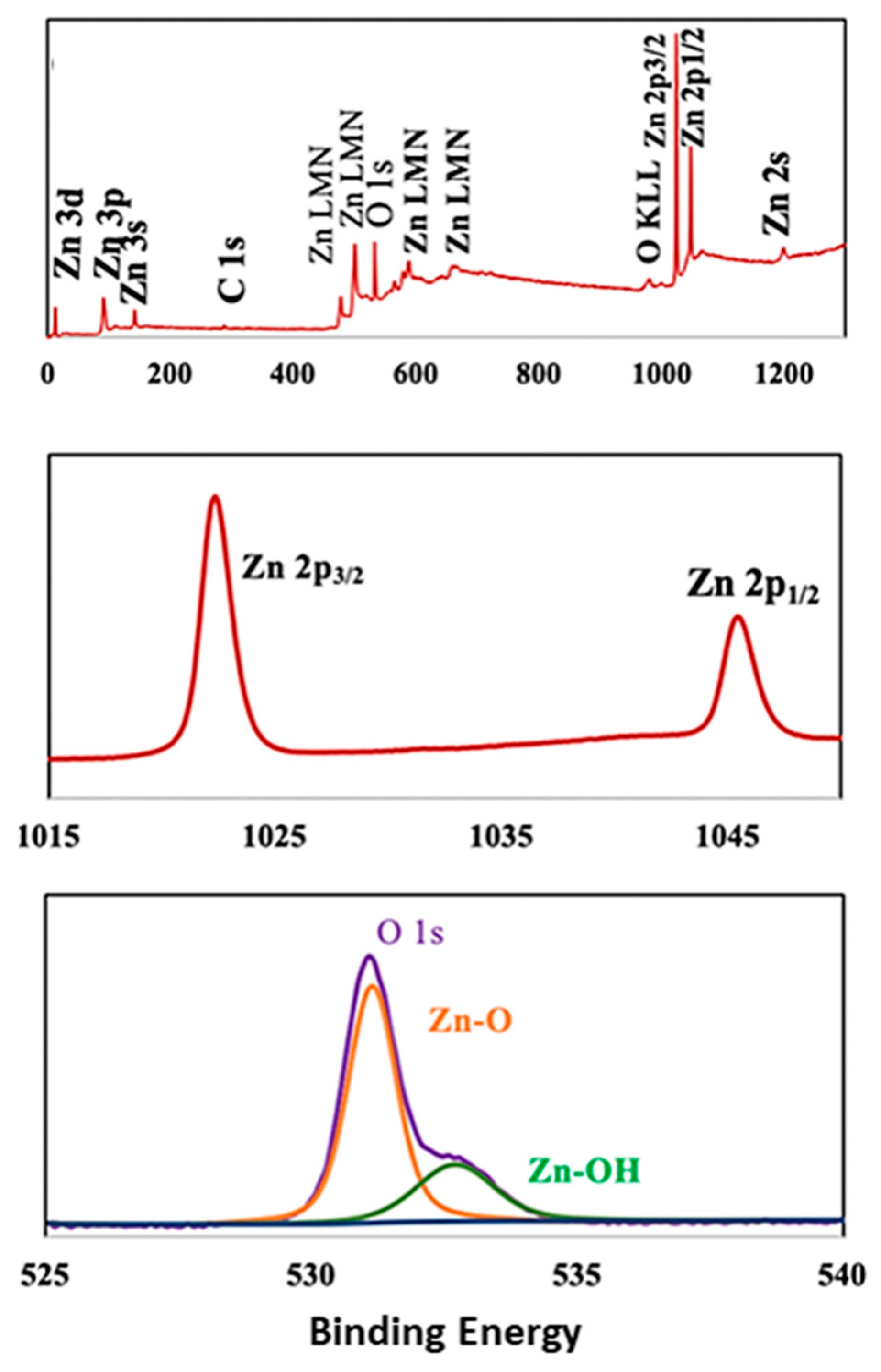
3.2.5. XPS Analysis of the ZnO NPs
3.2.6. Photocatalytic Degradation of Methyl Orange (MO)
3.2.7. Determination of ROS Generation by the ZnO NPs
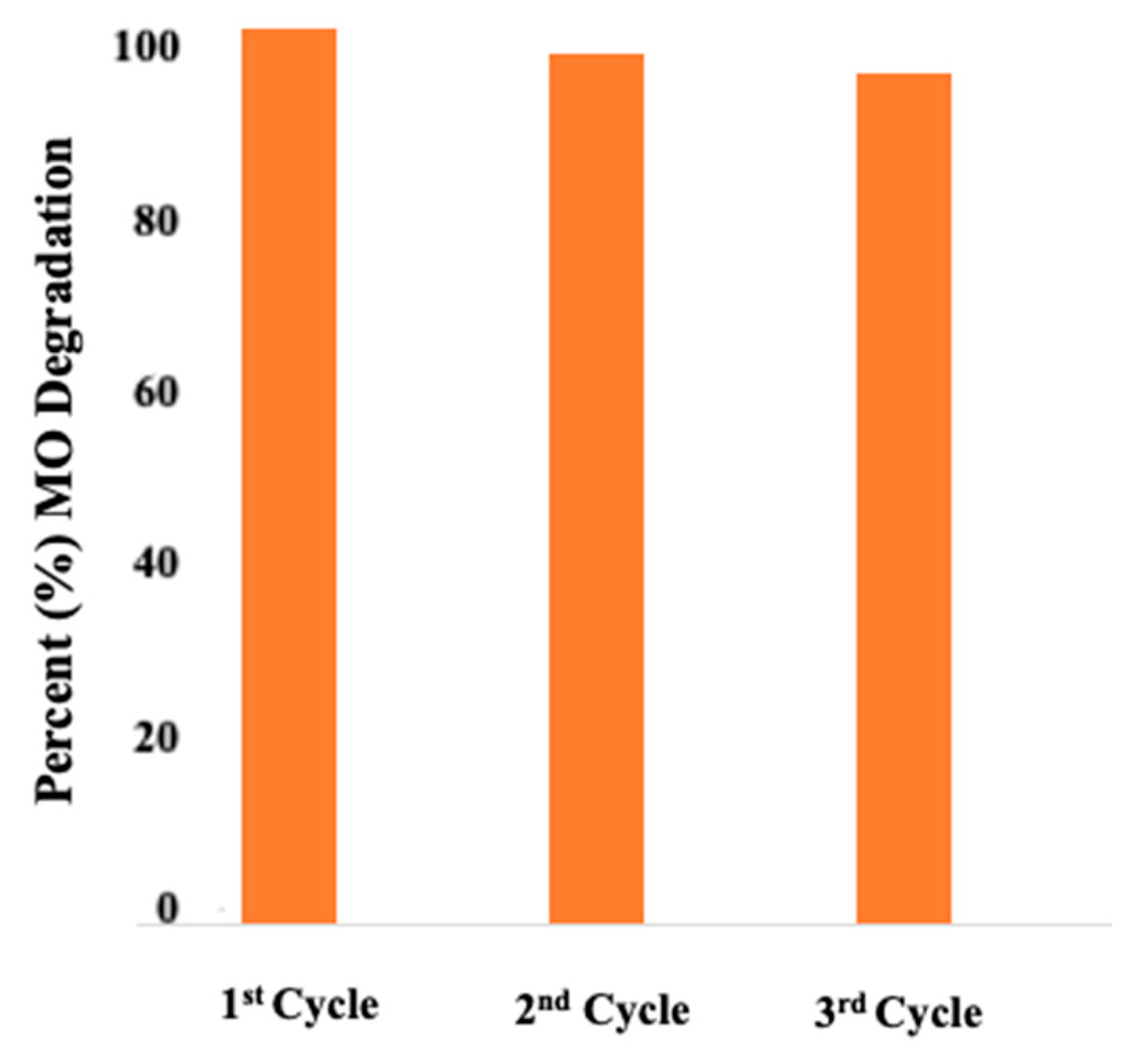
3.2.8. Cyclic Stability of the ZnO NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Fan, Z.; Zhang, Z.; Niu, W.; Li, C.; Yang, N.; Zhang, H. Two-dimensional metal nanomaterials: Synthesis, properties, and applications. Chem. Rev. 2018, 118, 6409–6455. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Peng, L.; Fang, Z.; Yan, C.; Zhang, X.; Yu, G. Structural engineering of 2D nanomaterials for energy storage and catalysis. Adv. Mater. 2018, 30, 1706347. [Google Scholar] [CrossRef] [PubMed]
- Giljohann, D.A.; Seferos, D.S.; Daniel, W.L.; Massich, M.D.; Patel, P.C.; Mirkin, C.A. Gold nanoparticles for biology and medicine. Angew. Chem. Int. Ed. 2010, 49, 3280–3294. [Google Scholar] [CrossRef] [PubMed]
- Campelo, J.M.; Luna, D.; Luque, R.; Marinas, J.M.; Romero, A.A. Sustainable preparation of supported metal nanoparticles and their applications in catalysis. ChemSusChem 2009, 2, 18–45. [Google Scholar] [CrossRef] [PubMed]
- Sathyavathi, R.; Krishna, M.B.; Rao, S.V.; Saritha, R.; Rao, D.N. Biosynthesis of silver nanoparticles using Coriandrum sativum leaf extract and their application in nonlinear optics. Adv. Sci. Lett. 2010, 3, 138–143. [Google Scholar] [CrossRef]
- Kim, T.Y.; Song, D.; Barea, E.M.; Lee, J.H.; Kim, Y.R.; Cho, W.; Kang, Y.S. Origin of the high open-circuit voltage in solid-state dye-sensitized solar cells employing polymer electrolyte. Nano Energy 2016, 28, 455–461. [Google Scholar] [CrossRef]
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive contributions to electrochemical energy storage in TiO2 (anatase) nanoparticles. J. Phys. Chem. C 2007, 111, 14925–14931. [Google Scholar] [CrossRef]
- Luo, X.; Morrin, A.; Killard, A.J.; Smyth, M.R. Application of nanoparticles in electrochemical sensors and biosensors. Electroanalysis 2006, 18, 319–326. [Google Scholar] [CrossRef]
- He, Z.; Zhang, Z.; Bi, S. Nanoparticles for organic electronics applications. Mater. Res. Express 2020, 7, 012004. [Google Scholar] [CrossRef]
- Huang, X.; Neretina, S.; El-Sayed, M.A. Gold nanorods: From synthesis and properties to biological and biomedical applications. Adv. Mater. 2009, 21, 4880–4910. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A.; Ghanem, A.F.; Yassin, M.A.; Youssef, A.M.; Rehim, M.H.A. Utilization and characterization of cellulose nanocrystals decorated with silver and zinc oxide nanoparticles for removal of lead ion from wastewater. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100501. [Google Scholar] [CrossRef]
- Das, T.K.; Poater, A. Review on the use of heavy metal deposits from water treatment waste towards catalytic chemical syntheses. Int. J. Mol. Sci. 2021, 22, 13383. [Google Scholar] [CrossRef] [PubMed]
- Somu, P.; Paul, S. Casein-based biogenic-synthesized zinc oxide nanoparticles simultaneously decontaminate heavy metals, dyes, and pathogenic microbes: A rational strategy for wastewater treatment. J. Chem. Technol. Biotechnol. 2018, 93, 2962–2976. [Google Scholar] [CrossRef]
- Hariharan, C. Photocatalytic degradation of organic contaminants in water by ZnO nanoparticles: Revisited. Appl. Catal. A Gen. 2006, 304, 55–61. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, Y.; Sun, Z.; Wei, J.; Zheng, G.; Asiri, A.M.; Zhao, D. Hierarchical Cu2S micro sponges constructed from nanosheets for efficient photocatalysis. Small 2013, 9, 2702–2708. [Google Scholar] [CrossRef]
- Kumar, S.G.; Rao, K.K. Zinc oxide based photocatalysis: Tailoring surface-bulk structure and related interfacial charge carrier dynamics for better environmental applications. Rsc Adv. 2015, 5, 3306–3351. [Google Scholar] [CrossRef]
- Hossain, M.A.; Elias, M.; Sarker, D.R.; Diba, Z.R.; Mithun, J.M.; Azad, M.A.K.; Uddin, M.N. Synthesis of Fe-or Ag-doped TiO2–MWCNT nanocomposite thin films and their visible-light-induced catalysis of dye degradation and antibacterial activity. Res. Chem. Intermed. 2018, 44, 2667–2683. [Google Scholar] [CrossRef]
- Wahid, K.A.; Rahim, I.A.; Safri, S.N.; Ariffin, A.H. Synthesis of ZnO nanorods at very low temperatures using ultrasonically pre-treated growth solution. Processes 2023, 11, 708. [Google Scholar] [CrossRef]
- Lv, M.S.; Li, C.; Li, Y.N.; Zhang, X.F.; Deng, Z.P.; Cheng, X.L.; Gao, S. Facilely controlled synthesis of porous ZnO nanotubes with rich oxygen vacancies for highly sensitive and selective detection of NO2 at low temperatures. Sens. Actuators B Chem. 2023, 375, 132865. [Google Scholar] [CrossRef]
- Wang, X.; Song, J.; Wang, Z.L. Nanowire and nanobelt arrays of zinc oxide from synthesis to properties and to novel devices. J. Mater. Chem. 2007, 17, 711–720. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, G.; Umar, A. ZnO nano-mushrooms for photocatalytic degradation of methyl orange. Mater. Lett. 2013, 97, 100–103. [Google Scholar] [CrossRef]
- Sharma, H.; Kaur, N.; Singh, N.; Jang, D.O. Synergetic catalytic effect of ionic liquids and ZnO nanoparticles on the selective synthesis of 1, 2-disubstituted benzimidazoles using a ball-milling technique. Green Chem. 2015, 17, 4263–4270. [Google Scholar] [CrossRef]
- Krobthong, S.; Rungsawang, T.; Wongrerkdee, S. Comparison of ZnO Nanoparticles Prepared by Precipitation and Combustion for UV and Sunlight-Driven Photocatalytic Degradation of Methylene Blue. Toxics 2023, 11, 266. [Google Scholar] [CrossRef]
- Jalali, T.; Ghanavati, F.; Osfouri, S. Green synthesis of ZnO nanoparticles using marine brown algae (Cystoseira) extract comprising sol–gel, and combustion techniques based on dye-sensitized solar cells application. Int. J. Mod. Phys. B 2023, 245017. [Google Scholar] [CrossRef]
- Alberti, S.; Basciu, I.; Vocciante, M.; Ferretti, M. Experimental and physicochemical comparison of ZnO nanoparticles’ activity for photocatalytic applications in wastewater treatment. Catalysts 2021, 11, 678. [Google Scholar] [CrossRef]
- Yu, J.; Liu, X.; Wang, G.; Zhi, H.; Dong, J.; Hao, J.; Zhang, X.; Liu, B. Synthesis of the porous ZnO nanosheets and tio2/zno/fto composite films by a low-temperature hydrothermal method and their applications in photocatalysis and electrochromism. Coatings 2022, 12, 695. [Google Scholar]
- Sansenya, T.; Masri, N.; Chankhanittha, T.; Senasu, T.; Piriyanon, J.; Mukdasai, S.; Nanan, S. Hydrothermal synthesis of ZnO photocatalyst for detoxification of anionic azo dyes and antibiotics. J. Phys. Chem. Solids 2022, 160, 110353. [Google Scholar] [CrossRef]
- Ding, J.; Chen, S.; Han, N.; Shi, Y.; Hu, P.; Li, H.; Wang, J. Aerosol-assisted chemical vapor deposition of nanostructured ZnO thin films for NO2 and ethanol monitoring. Ceram. Int. 2020, 46, 15152–15158. [Google Scholar] [CrossRef]
- Ponja, S.D.; Sathasivam, S.; Parkin, I.P.; Carmalt, C.J. Highly conductive and transparent gallium-doped zinc oxide thin films via chemical vapor deposition. Sci. Rep. 2020, 10, 638. [Google Scholar] [CrossRef]
- Shkir, M.; Palanivel, B.; Khan, A.; Kumar, M.; Chang, J.H.; Mani, A.; AlFaify, S. Enhanced photocatalytic activities of facile auto-combustion synthesized ZnO nanoparticles for wastewater treatment: An impact of Ni doping. Chemosphere 2022, 291, 132687. [Google Scholar] [CrossRef]
- Sultana, K.A.; Islam, M.T.; Silva, J.A.; Turley, R.S.; Hernandez-Viezcas, J.A.; Gardea-Torresdey, J.L.; Noveron, J.C. Sustainable synthesis of zinc oxide nanoparticles for photocatalytic degradation of organic pollutant and generation of hydroxyl radical. J. Mol. Liq. 2020, 307, 112931. [Google Scholar] [CrossRef]
- Naik, M.A.; Mishra, B.G.; Dubey, A. Combustion synthesized WO3–ZrO2 nanocomposites as catalysts for the solvent-free synthesis of coumarins. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 234–238. [Google Scholar] [CrossRef]
- Ngulube, T.; Gumbo, J.R.; Masindi, V.; Maity, A. An update on synthetic dye adsorption onto clay-based minerals: A state-of-art review. J. Environ. Manag. 2017, 191, 35–57. [Google Scholar] [CrossRef]
- Morcillo-Martín, R.; Espinosa, E.; Rabasco-Vílchez, L.; Sanchez, L.M.; de Haro, J.; Rodríguez, A. Cellulose nanofiber-based aerogels from wheat straw: Influence of surface load and lignin content on their properties and dye removal capacity. Biomolecules 2022, 12, 232. [Google Scholar] [CrossRef] [PubMed]
- Maleki, A.; Moradi, F.; Shahmoradi, B.; Rezaee, R.; Lee, S.M. The photocatalytic removal of diazinon from aqueous solutions using tungsten oxide doped zinc oxide nanoparticles immobilized on a glass substrate. J. Mol. Liq. 2020, 297, 111918. [Google Scholar] [CrossRef]
- Szostak, K.; Banach, M. Sorption and photocatalytic degradation of methylene blue on bentonite-ZnO-CuO nanocomposite. J. Mol. Liq. 2019, 286, 110859. [Google Scholar] [CrossRef]
- Kausar, A.; Iqbal, M.; Javed, A.; Aftab, K.; Bhatti, H.N.; Nouren, S. Dyes adsorption using clay and modified clay: A review. J. Mol. Liq. 2018, 256, 395–407. [Google Scholar] [CrossRef]
- Chen, Y.; He, F.; Ren, Y.; Peng, H.; Huang, K. Fabrication of chitosan/PAA multilayer onto magnetic microspheres by LbL method for removal of dyes. J. Chem. Eng. 2014, 249, 79–92. [Google Scholar] [CrossRef]
- Adeel, M.; Saeed, M.; Khan, I.; Muneer, M.; Akram, N. Synthesis and characterization of Co–ZnO, and evaluation of its photocatalytic activity for photodegradation of methyl orange. ACS Omega 2021, 6, 1426–1435. [Google Scholar] [CrossRef]
- Aljuaid, A.; Almehmadi, M.; Alsaiari, A.A.; Allahyani, M.; Abdulaziz, O.; Alsharif, A.; Alsaiari, J.A.; Saih, M.; Alotaibi, R.T.; Khan, I. g-C3N4 Based Photocatalyst for the Efficient Photodegradation of Toxic Methyl Orange Dye: Recent Modifications and Future Perspectives. Molecules 2023, 28, 3199. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, A.; Kadam, J.; Gade, T.; Sonawane, K.; Garadkar, K. Efficient photodegradation of methyl orange and bactericidal activity of Ag-doped ZnO nanoparticles. J. Indian Chem. Soc. 2023, 100, 100920. [Google Scholar] [CrossRef]
- Khan, S.; Naushad, M.; Govarthanan, M.; Iqbal, J.; Alfadul, S.M. Emerging contaminants of high concern for the environment: Current trends and future research. Environ. Res. 2022, 207, 112609. [Google Scholar] [CrossRef]
- Hasnat, M.A.; Safwan, J.A.; Islam, M.S.; Rahman, Z.; Karim, M.R.; Pirzada, T.J.; Rahman, M.M. Electrochemical decolorization of Methylene blue at Pt electrode in KCl solution for environmental remediation. J. Ind. Eng. Chem. 2015, 21, 787–791. [Google Scholar] [CrossRef]
- Ljubas, D.; Smoljanić, G.; Juretić, H. Degradation of Methyl Orange and Congo Red dyes using TiO2 nanoparticles activated by the solar and the solar-like radiation. J. Environ. Manag. 2015, 161, 83–91. [Google Scholar] [CrossRef]
- Piaskowski, K.; Świderska-Dąbrowska, R.; Zarzycki, P.K. Dye removal from water and wastewater using various physical, chemical, and biological processes. J. AOAC Int. 2018, 101, 1371–1384. [Google Scholar] [CrossRef]
- Islam, M.T.; Jing, H.; Yang, T.; Zubia, E.; Goos, A.G.; Bernal, R.A.; Noveron, J.C. Fullerene stabilized gold nanoparticles supported on titanium dioxide for enhanced photocatalytic degradation of methyl orange and catalytic reduction of 4-nitrophenol. J. Environ. Chem. Eng. 2018, 6, 3827–3836. [Google Scholar] [CrossRef]
- Islam, M.T.; Dominguez, A.; Turley, R.S.; Kim, H.; Sultana, K.A.; Shuvo, M.A.I.; Noveron, J.C. Development of photocatalytic paint based on TiO2 and photopolymer resin for the degradation of organic pollutants in water. Sci. Total Environ. 2020, 704, 135406. [Google Scholar] [CrossRef]
- Islam, M.T.; Dominguez, A.; Alvarado-Tenorio, B.; Bernal, R.A.; Montes, M.O.; Noveron, J.C. Sucrose-mediated fast synthesis of zinc oxide nanoparticles for the photocatalytic degradation of organic pollutants in water. ACS Omega 2019, 4, 6560–6572. [Google Scholar] [CrossRef]
- Meena, P.L.; Poswal, K.; Surela, A.K. Facile synthesis of ZnO nanoparticles for the effective photodegradation of malachite green dye in aqueous solution. Water Environ. J. 2022, 36, 513–524. [Google Scholar] [CrossRef]
- Becheri, A.; Dürr, M.; Lo Nostro, P.; Baglioni, P. Synthesis and characterization of zinc oxide nanoparticles: Application to textiles as UV-absorbers. J. Nanopart. Res. 2008, 10, 679–689. [Google Scholar] [CrossRef]
- Tripathy, N.; Ahmad, R.; Song, J.E.; Ko, H.A.; Hahn, Y.B.; Khang, G. Photocatalytic degradation of methyl orange dye by ZnO nanoneedle under UV irradiation. Mater. Lett. 2014, 136, 171–174. [Google Scholar] [CrossRef]
- Salem, M.; Salem, J.; Ghannam, H.; Massoudi, I.; Bourguiba, F.; Gaidi, M. Optical and passivation properties of ZnO: Fe on silicon substrates. J. Mater. Sci. Mater. Electron. 2023, 34, 332. [Google Scholar] [CrossRef]
- Raoufi, D. Synthesis, and microstructural properties of ZnO nanoparticles prepared by precipitation method. Renew. Energy 2013, 50, 932–937. [Google Scholar] [CrossRef]
- Bindu, P.; Thomas, S. Estimation of lattice strain in ZnO nanoparticles: X-ray peak profile analysis. J. Theor. Appl. Phys. 2014, 8, 123–134. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Davar, F.; Khansari, A. Nanosphericals and nano bundles of ZnO: Synthesis and characterization. J. Alloys Compd. 2011, 509, 61–65. [Google Scholar] [CrossRef]
- Claros, M.; Setka, M.; Jimenez, Y.P.; Vallejos, S. AACVD synthesis and characterization of iron and copper oxides modified ZnO structured films. Nanomaterials 2020, 10, 471. [Google Scholar] [CrossRef]
- Lupan, O.; Emelchenko, G.A.; Ursaki, V.V.; Chai, G.; Redkin, A.N.; Gruzintsev, A.N.; Yakimov, E.E. Synthesis, and characterization of ZnO nanowires for nanosensor applications. Mater. Res. Bull. 2010, 45, 1026–1032. [Google Scholar] [CrossRef]
- Molla, M.A.I.; Furukawa, M.; Tateishi, I.; Katsumata, H.; Kaneko, S. Studies of effects of calcination temperature on the crystallinity and optical properties of Ag-doped ZnO nanocomposites. J. Compos. Sci. 2019, 3, 18. [Google Scholar] [CrossRef]
- Kaur, J.; Bansal, S.; Singhal, S. Photocatalytic degradation of methyl orange using ZnO nanopowders synthesized via thermal decomposition of oxalate precursor method. Phys. B Condens. Matter 2013, 416, 33–38. [Google Scholar] [CrossRef]
- Balcha, A.; Yadav, O.P.; Dey, T. Photocatalytic degradation of methylene blue dye by zinc oxide nanoparticles obtained from precipitation and sol-gel methods. Environ. Sci. Pollut. Res. 2016, 23, 25485–25493. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran, E.K.; Pillay, K. One-step synthesis of AgClNPs/PANI/D-dextrose nanocomposite by interfacial polymerization method and its catalytic and photocatalytic applications. J. Mol. Liq. 2019, 283, 6–29. [Google Scholar] [CrossRef]
- He, J.; Zhang, Y.; Guo, Y.; Rhodes, G.; Yeom, J.; Li, H.; Zhang, W. Photocatalytic degradation of cephalexin by ZnO nanowires under simulated sunlight: Kinetics, influencing factors, and mechanisms. Environ. Int. 2019, 132, 105105. [Google Scholar] [CrossRef] [PubMed]
- Neelgund, G.M.; Oki, A. ZnO conjugated graphene: An efficient sunlight driven photocatalyst for degradation of organic dyes. Mater. Res. Bull. 2020, 129, 110911. [Google Scholar] [CrossRef]
- Shen, J.H.; Chiang, T.H.; Tsai, C.K.; Jiang, Z.W.; Horng, J.J. Mechanistic insights into hydroxyl radical formation of Cu-doped ZnO/g-C3N4 composite photocatalysis for enhanced degradation of ciprofloxacin under visible light: Efficiency, kinetics, products identification and toxicity evaluation. J. Environ. Chem. Eng. 2022, 10, 107352. [Google Scholar] [CrossRef]



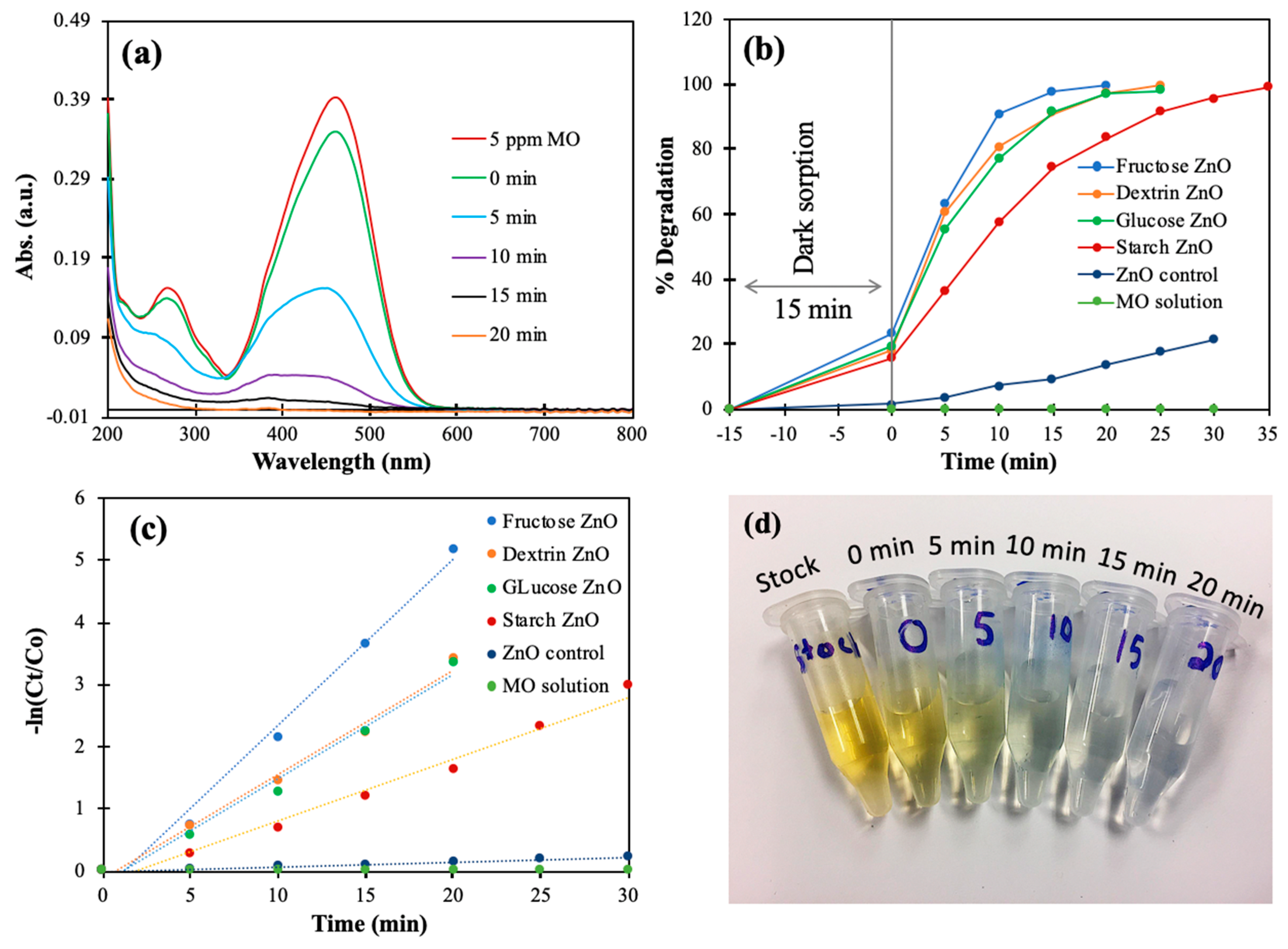
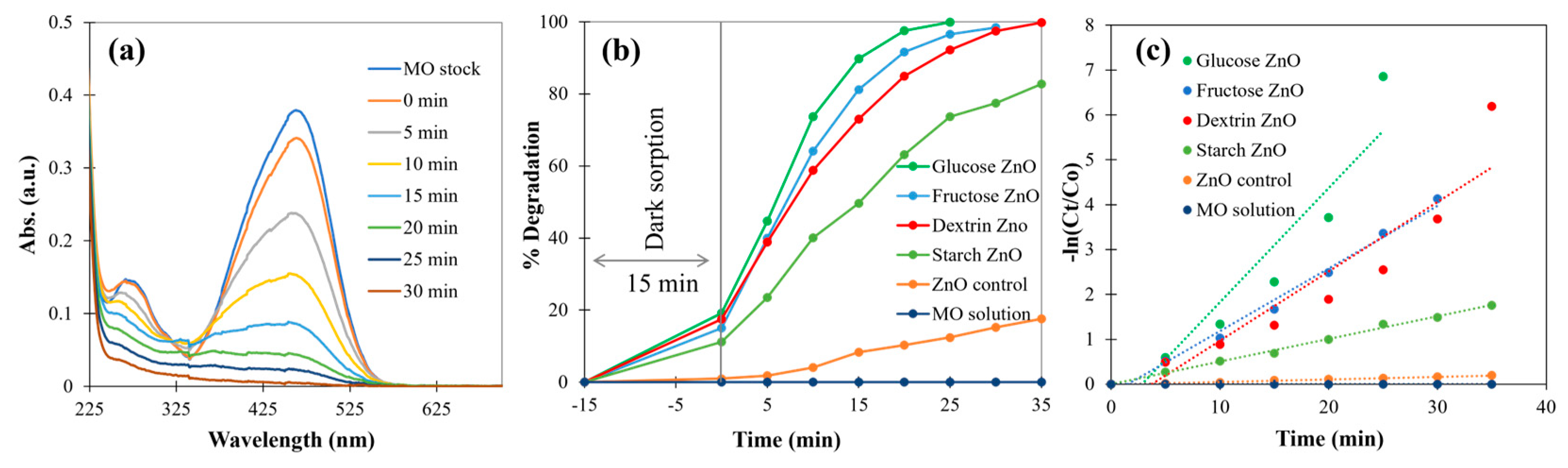

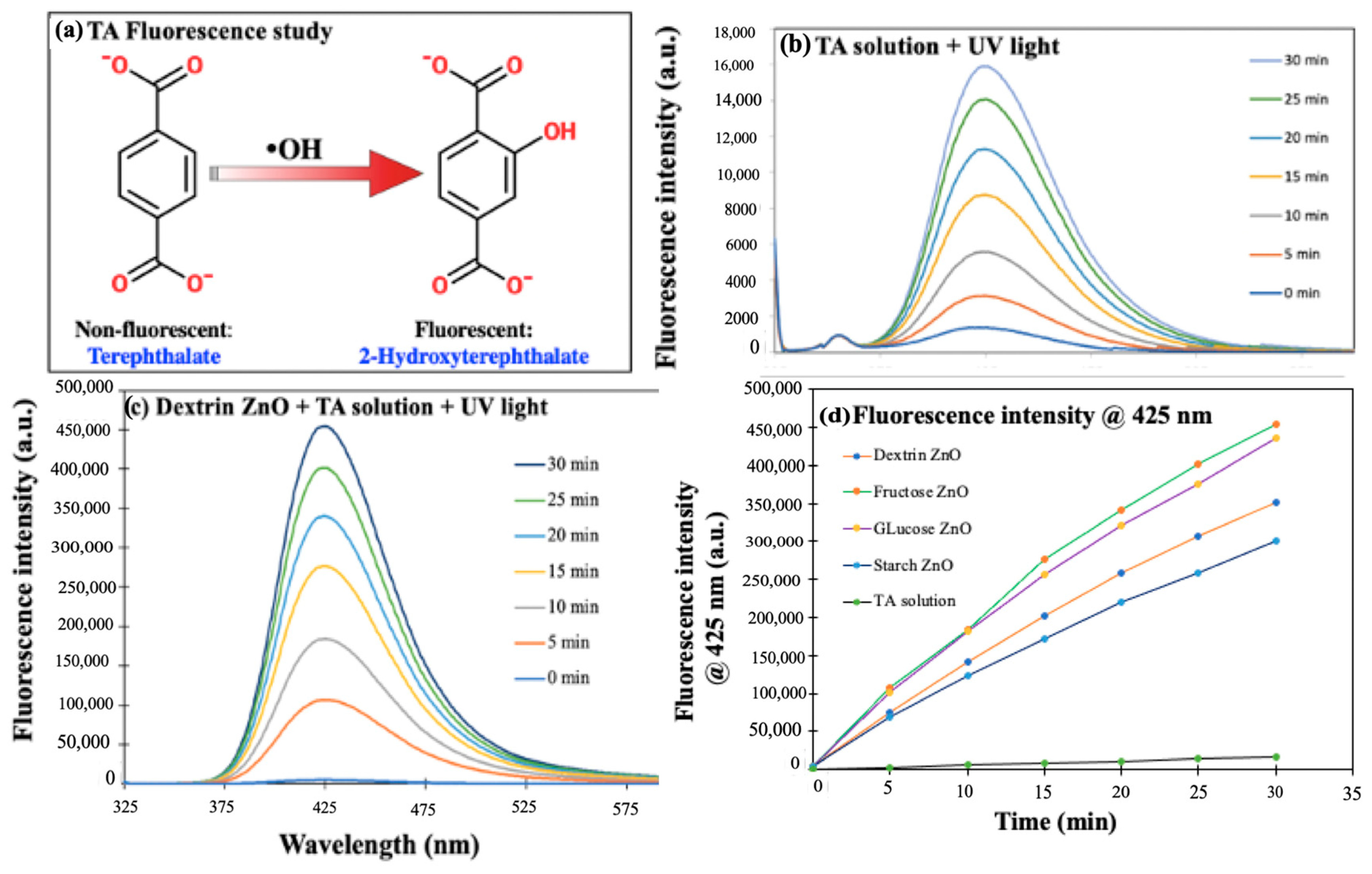
| ZnO Sample | MO Conc. | Water Matrix | Light Source | Rate Constant (min−1) | R-squared Value |
|---|---|---|---|---|---|
| Glucose-ZnO | 5 ppm | DIW | UV | 1.62 × 10−1 | 0.98 |
| Fructose-ZnO | 5 ppm | DIW | UV | 2.65 × 10−1 | 0.98 |
| Dextrin-ZnO | 5 ppm | DIW | UV | 2.00 × 10−1 | 0.99 |
| Starch-ZnO | 5 ppm | DIW | UV | 1.19 × 10−1 | 0.98 |
| ZnO-Control | 5 ppm | DIW | UV | 7.62 × 10−3 | 0.98 |
| Glucose-ZnO | 5 ppm | FDW | UV | 2.54 × 10−1 | 0.89 |
| Fructose-ZnO | 5 ppm | FDW | UV | 1.39 × 10−1 | 0.98 |
| Dextrin-ZnO | 5 ppm | FDW | UV | 1.54 × 10−1 | 0.87 |
| Starch-ZnO | 5 ppm | FDW | UV | 5.03 × 10−2 | 0.99 |
| ZnO-Control | 5 ppm | FDW | UV | 5.57 × 10−3 | 0.99 |
| Glucose-ZnO | 5 ppm | DIW | Sunlight | 1.28 × 10−1 | 0.95 |
| Fructose-ZnO | 5 ppm | DIW | Sunlight | 2.82 × 10−1 | 0.99 |
| Dextrin-ZnO | 5 ppm | DIW | Sunlight | 2.29 × 10−1 | 0.99 |
| Starch-ZnO | 5 ppm | DIW | Sunlight | 1.32 × 10−1 | 0.99 |
| ZnO-Control | 5 ppm | DIW | Sunlight | 3.50 × 10−3 | 0.95 |
| MO Solution | 5 ppm | DIW and FDW | UV | 0.0 | NA |
| MO Solution | 5 ppm | DIW | Sunlight | 0.0 | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sultana, K.A.; Hernandez Ortega, J.; Islam, M.T.; Dorado, Z.N.; Alvarado-Tenorio, B.; Galindo-Esquivel, I.R.; Noveron, J.C. Saccharide-Derived Zinc Oxide Nanoparticles with High Photocatalytic Activity for Water Decontamination and Sanitation. Sustain. Chem. 2023, 4, 321-338. https://doi.org/10.3390/suschem4040023
Sultana KA, Hernandez Ortega J, Islam MT, Dorado ZN, Alvarado-Tenorio B, Galindo-Esquivel IR, Noveron JC. Saccharide-Derived Zinc Oxide Nanoparticles with High Photocatalytic Activity for Water Decontamination and Sanitation. Sustainable Chemistry. 2023; 4(4):321-338. https://doi.org/10.3390/suschem4040023
Chicago/Turabian StyleSultana, Kazi Afroza, Javier Hernandez Ortega, Md Tariqul Islam, Zayra N. Dorado, Bonifacio Alvarado-Tenorio, Ignacio Rene Galindo-Esquivel, and Juan C. Noveron. 2023. "Saccharide-Derived Zinc Oxide Nanoparticles with High Photocatalytic Activity for Water Decontamination and Sanitation" Sustainable Chemistry 4, no. 4: 321-338. https://doi.org/10.3390/suschem4040023
APA StyleSultana, K. A., Hernandez Ortega, J., Islam, M. T., Dorado, Z. N., Alvarado-Tenorio, B., Galindo-Esquivel, I. R., & Noveron, J. C. (2023). Saccharide-Derived Zinc Oxide Nanoparticles with High Photocatalytic Activity for Water Decontamination and Sanitation. Sustainable Chemistry, 4(4), 321-338. https://doi.org/10.3390/suschem4040023








