Correlation Between Conductivity and Oxygen Evolution Reaction Activity in Perovskite Oxides CaMnO3-δ, Ca0.5Sr0.5MnO3-δ and SrMnO3-δ
Abstract
:1. Introduction
- M + OH− → M–OH + e−
- M–OH + OH− → M–O + H2O + e−
- M–O + OH− → M–OOH + e−
- M–OOH + OH− → M + H2O + O2 + e−
2. Experimental Method
3. Result and Discussion
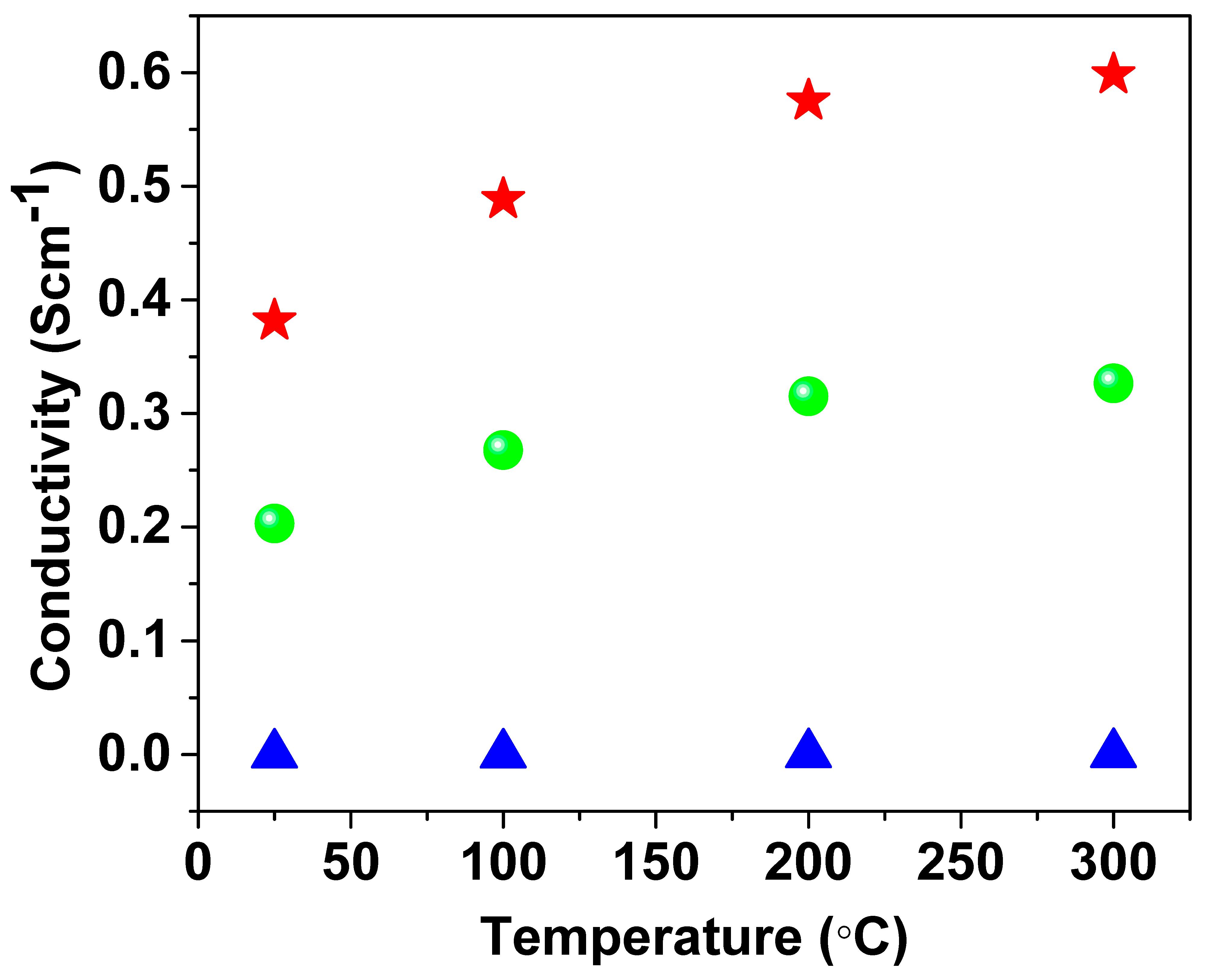
3.1. Electrical Conductivity
3.2. Correlation Between OER and Conductivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, L.; Li, J.; Le, Z.; Nie, P.; Guo, Y.; Wang, H.; Xu, T.; Xue, X. Perovskite-type CaMnO3 anode material for highly efficient and stable lithium ion storage. J. Colloid Interface Sci. 2021, 584, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Hona, R.K.; Ramezanipour, F. Structure-dependence of electrical conductivity and electrocatalytic properties of Sr2Mn2O6 and CaSrMn2O6. J. Chem. Sci. 2019, 131, 109. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, W.; Sunarso, J.; Zhong, Y.; Shao, Z. Phosphorus-Doped Perovskite Oxide as Highly Efficient Water Oxidation Electrocatalyst in Alkaline Solution. Adv. Funct. Mater. 2016, 26, 5862–5872. [Google Scholar] [CrossRef]
- Rong, X.; Parolin, J.; Kolpak, A.M. A Fundamental Relationship between Reaction Mechanism and Stability in Metal Oxide Catalysts for Oxygen Evolution. ACS Catal. 2016, 6, 1153–1158. [Google Scholar] [CrossRef]
- Larson, A.C.; Von Dreele, A.C. General Structure Analysis System (GSAS); Los Alamos National Laboratory Report LAUR; Los Alamos National Laboratory: Los Alamos, NM, USA, 1994; pp. 86–748.
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Hona, R.K.; Ramezanipour, F. Disparity in electrical and magnetic properties of isostructural oxygen-deficient perovskites BaSrCo2O6−δ and BaSrCoFeO6−δ. J. Mater. Sci. Mater. Electron. 2018, 29, 13464–13473. [Google Scholar] [CrossRef]
- Galinsky, N.; Sendi, M.; Bowers, L.; Li, F. CaMn1−xBxO3-δ (B=Al, V, Fe, Co, and Ni) perovskite based oxygen carriers for chemical looping with oxygen uncoupling (CLOU). Appl. Energy 2016, 174, 80–87. [Google Scholar] [CrossRef]
- Sato, T.; Takagi, S.; Deledda, S.; Hauback, B.C.; Orimo, S.-I. Extending the applicability of the Goldschmidt tolerance factor to arbitrary ionic compounds. Sci. Rep. 2016, 6, 23592. [Google Scholar] [CrossRef]
- Roth, R.S. Classification of perovskite and other ABO3-type compounds. J. Res. Natl. Bur. Stand. 1957, 58, 75. [Google Scholar] [CrossRef]
- Taguchi, H.; Nagao, M.; Sato, T.; Shimada, M. High-temperature phase transition of CaMnO3-δ. J. Solid State Chem. 1989, 78, 312–315. [Google Scholar] [CrossRef]
- Aschauer, U.; Pfenninger, R.; Selbach, S.M.; Grande, T.; Spaldin, N.A. Strain-controlled oxygen vacancy formation and ordering in CaMnO3. Phys. Rev. B 2013, 88, 054111. [Google Scholar] [CrossRef]
- Tilley, R.J.D. Defects in Solids; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Asenath-Smith, E.; Misture, S.T.; Edwards, D.D. Structural behavior and thermoelectric properties of the brownmillerite system Ca2(ZnxFe2−x)O5. J. Solid State Chem. 2011, 184, 2167–2177. [Google Scholar] [CrossRef]
- Töpfer, J.; Pippardt, U.; Voigt, I.; Kriegel, R. Structure, nonstoichiometry and magnetic properties of the perovskites Sr1−xCaxMnO3-δ. Solid State Sci. 2004, 6, 647–654. [Google Scholar] [CrossRef]
- Belik, A.A.; Matsushita, Y.; Katsuya, Y.; Tanaka, M.; Kolodiazhnyi, T.; Isobe, M.; Takayama-Muromachi, E. Crystal structure and magnetic properties of 6H-SrMnO3. Phys. Rev. B 2011, 84, 094438. [Google Scholar] [CrossRef]
- Søndenå, R.; Ravindran, P.; Stølen, S.; Grande, T.; Hanfland, M. Electronic structure and magnetic properties of cubic and hexagonal SrMnO3. Phy. Rev. B 2006, 74, 144102. [Google Scholar] [CrossRef]
- Asenath-Smith, E.; Lokuhewa, I.N.; Misture, S.T.; Edwards, D.D. p-Type thermoelectric properties of the oxygen-deficient perovskite Ca2Fe2O5 in the brownmillerite structure. J. Solid State Chem. 2010, 183, 1670–1677. [Google Scholar] [CrossRef]
- Durán, A.; Verdin, E.; Escamilla, R.; Morales, F.; Escudero, R. Mechanism of small-polaron formation in the biferroic YCrO3 doped with calcium. Mater. Chem. Phys. 2012, 133, 1011–1017. [Google Scholar] [CrossRef]
- Du, J.; Zhang, T.; Cheng, F.; Chu, W.; Wu, Z.; Chen, J. Nonstoichiometric perovskite CaMnO3-δ for oxygen electrocatalysis with high activity. Inorg. Chem. 2014, 53, 9106–9114. [Google Scholar] [CrossRef]
- Singh, Y. Electrical Resistivity Measurements: A Review. Int. J. Modern Phys. Conf. Ser. 2013, 22, 745–756. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, Z.; Wang, L.; Gao, S.; Yuan, S. Structural and electromagnetic properties driven by oxygen vacancy in Sr2FeMoO6−δ double perovskite. J. Alloys Compd. 2015, 649, 1151–1155. [Google Scholar] [CrossRef]
- Kozhevnikov, V.L.; Leonidov, I.A.; Mitberg, E.B.; Patrakeev, M.V.; Petrov, A.N.; Poeppelmeier, K.R. Conductivity and carrier traps in La1−xSrxCo1−zMnzO3-δ (x = 0.3; z = 0 and 0.25). J. Solid State Chem. 2003, 172, 296–304. [Google Scholar] [CrossRef]
- Hona, R.K.; Huq, A.; Ramezanipour, F. Magnetic structure of CaSrFeCoO6–δ: Correlations with structural order. Mater. Res. Bull. 2018, 106, 131–136. [Google Scholar] [CrossRef]
- Kao, K.C. Dielectric Phenomena in Solids; Academic Press: Cambridge, MA, USA, 2004; p. 402. ISBN 0-12-396561-6. [Google Scholar]
- Cheng, X.; Fabbri, E.; Nachtegaal, M.; Castelli, I.E.; El Kazzi, M.; Haumont, R.; Marzari, N.; Schmidt, T.J. Oxygen evolution reaction on La1–xSrxCoO3 perovskites: A combined experimental and theoretical study of their structural, electronic, and electrochemical properties. Chem. Mater. 2015, 27, 7662–7672. [Google Scholar] [CrossRef]
- Hona, R.K.; Huq, A.; Ramezanipour, F. Unraveling the Role of Structural Order in the Transformation of Electrical Conductivity in Ca2FeCoO6−δ, CaSrFeCoO6−δ, and Sr2FeCoO6−δ. Inorg. Chem. 2017, 56, 14494–14505. [Google Scholar] [CrossRef]
- Hona, R.K.; Huq, A.; Mulmi, S.; Ramezanipour, F. Transformation of Structure, Electrical Conductivity, and Magnetism in AA′Fe2O6−δ, A = Sr, Ca and A′ = Sr. Inorg. Chem. 2017, 56, 9716–9724. [Google Scholar] [CrossRef]
- Yáng, Z.; Harvey, A.S.; Infortuna, A.; Schoonman, J.; Gauckler, L.J. Electrical conductivity and defect chemistry of BaxSr1−xCoyFe1−yO3-δ perovskites. J. Solid State Electrochem. 2011, 15, 277–284. [Google Scholar] [CrossRef]
- Patrakeev, M.V.; Leonidov, I.A.; Kozhevnikov, V.L.; Poeppelmeier, K.R. p-Type electron transport in La1−xSrxFeO3-δ at high temperatures. J. Solid State Chem. 2005, 178, 921–927. [Google Scholar] [CrossRef]
- Lee, J.G.; Hwang, J.; Hwang, H.J.; Jeon, O.S.; Jang, J.; Kwon, O.; Lee, Y.; Han, B.; Shul, Y.G. A new family of perovskite catalysts for oxygen-evolution reaction in alkaline media: BaNiO3 and BaNi0.83O2.5. J. Am. Chem. Soc. 2016, 138, 3541–3547. [Google Scholar] [CrossRef]
- Kim, J.; Yin, X.; Tsao, K.; Fang, S.; Yang, H. Ca2Mn2O5 as oxygen-deficient perovskite electrocatalyst for oxygen evolution reaction. J. Am. Chem. Soc. 2014, 136, 14646–14649. [Google Scholar] [CrossRef]
- Jin, C.; Cao, X.; Zhang, L.; Zhang, C.; Yang, R. Preparation and electrochemical properties of urchin-like La0.8Sr0.2MnO3 perovskite oxide as a bifunctional catalyst for oxygen reduction and oxygen evolution reaction. J. Power Sources 2013, 241, 225–230. [Google Scholar] [CrossRef]
- May, K.J.; Carlton, C.E.; Stoerzinger, K.A.; Risch, M.; Suntivich, J.; Lee, Y.-L.; Grimaud, A.; Shao-Horn, Y. Influence of oxygen evolution during water oxidation on the surface of perovskite oxide catalysts. J. Phys. Chem. Lett. 2012, 3, 3264–3270. [Google Scholar] [CrossRef]
- Malkhandi, S.; Trinh, P.; Manohar, A.K.; Jayachandrababu, K.C.; Kindler, A.; Prakash, G.S.; Narayanan, S.R. Electrocatalytic activity of transition metal oxide-carbon composites for oxygen reduction in alkaline batteries and fuel cells. J. Electrochem. Soc. 2013, 160, 943–952. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Wang, H.; Zhou, J.; Wang, J.; Regier, T.; Dai, H. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011, 10, 780–786. [Google Scholar] [CrossRef]
- Mohamed, R.; Cheng, X.; Fabbri, E.; Levecque, P.; Kötz, R.; Conrad, O.; Schmidt, T.J. Electrocatalysis of perovskites: The influence of carbon on the oxygen evolution activity. J. Electrochem. Soc. 2015, 162, 579–586. [Google Scholar] [CrossRef]
- Fabbri, E.; Nachtegaal, M.; Cheng, X.; Schmidt, T.J. Superior bifunctional electrocatalytic activity of Ba0.5Sr0.5Co0.8Fe0.2O3-δ/carbon composite electrodes: Insight into the local electronic structure. Adv. Energy Mater. 2015, 5, 1402033. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, Y.; Li, X.; Liu, Y.; Liu, C. Nanostructured nickel sulfides: Phase evolution, characterization and electrocatalytic properties for the hydrogen evolution reaction. RSC Adv. 2015, 5, 104740–104749. [Google Scholar] [CrossRef]
- Oh, S.; Kim, H.; Kwon, Y.; Kim, M.; Cho, E.; Kwon, H. Porous Co–P foam as an efficient bifunctional electrocatalyst for hydrogen and oxygen evolution reactions. J. Mater. Chem. A 2016, 4, 18272–18277. [Google Scholar] [CrossRef]
- Song, F.; Hu, X. Ultrathin cobalt–manganese layered double hydroxide Is an efficient oxygen evolution catalyst. J. Am. Chem. Soc. 2014, 136, 16481–16484. [Google Scholar] [CrossRef]
- Moir, J.; Soheilnia, N.; O’brien, P.; Ali, F.M.; Grozea, C.M.; Faulkner, D.; Helander, M.G.; Ozin, G.A. Enhanced hematite water electrolysis using a 3D antimony-doped tin oxide electrode. ACS Nano 2013, 7, 4261–4274. [Google Scholar] [CrossRef]
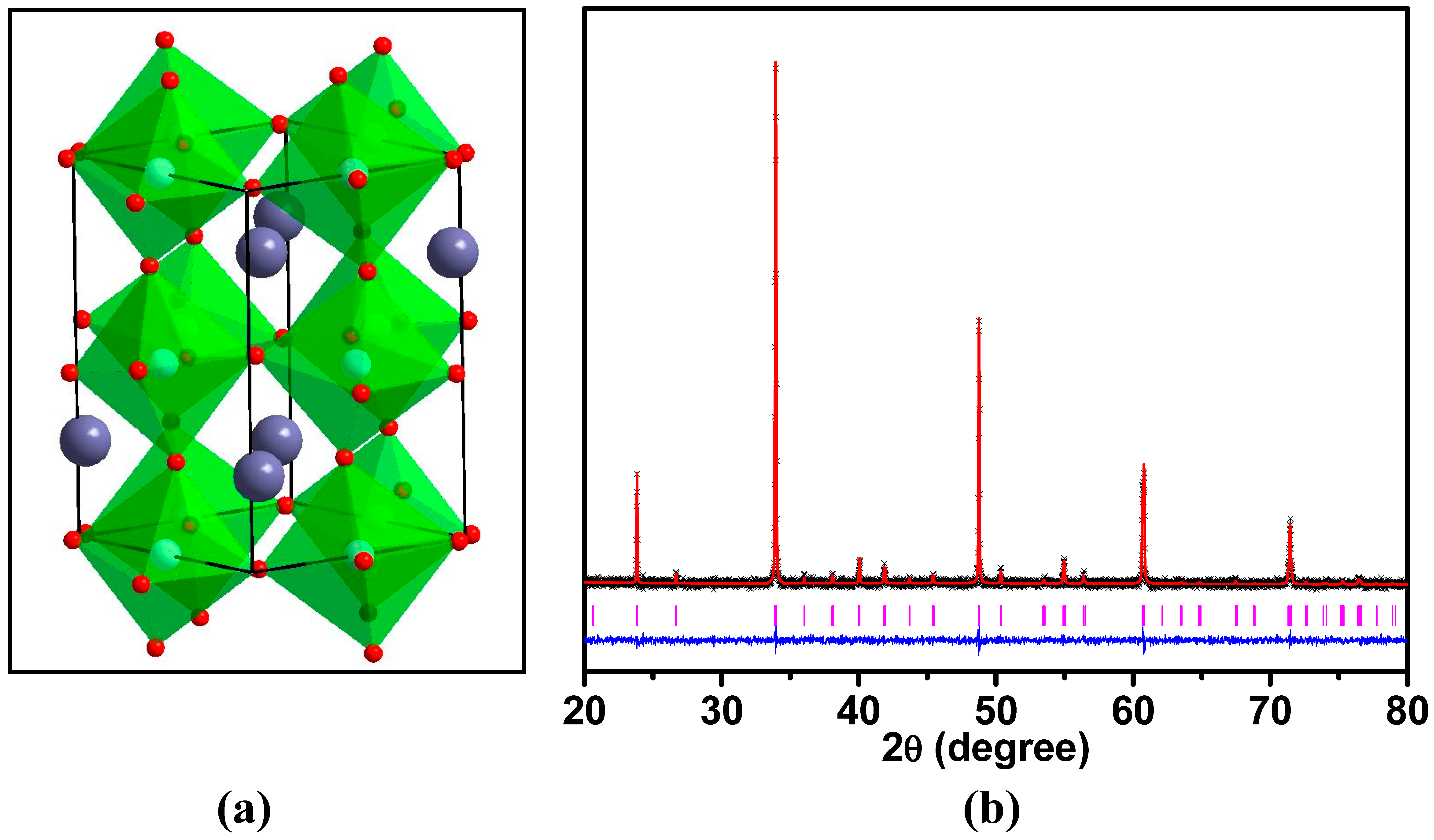

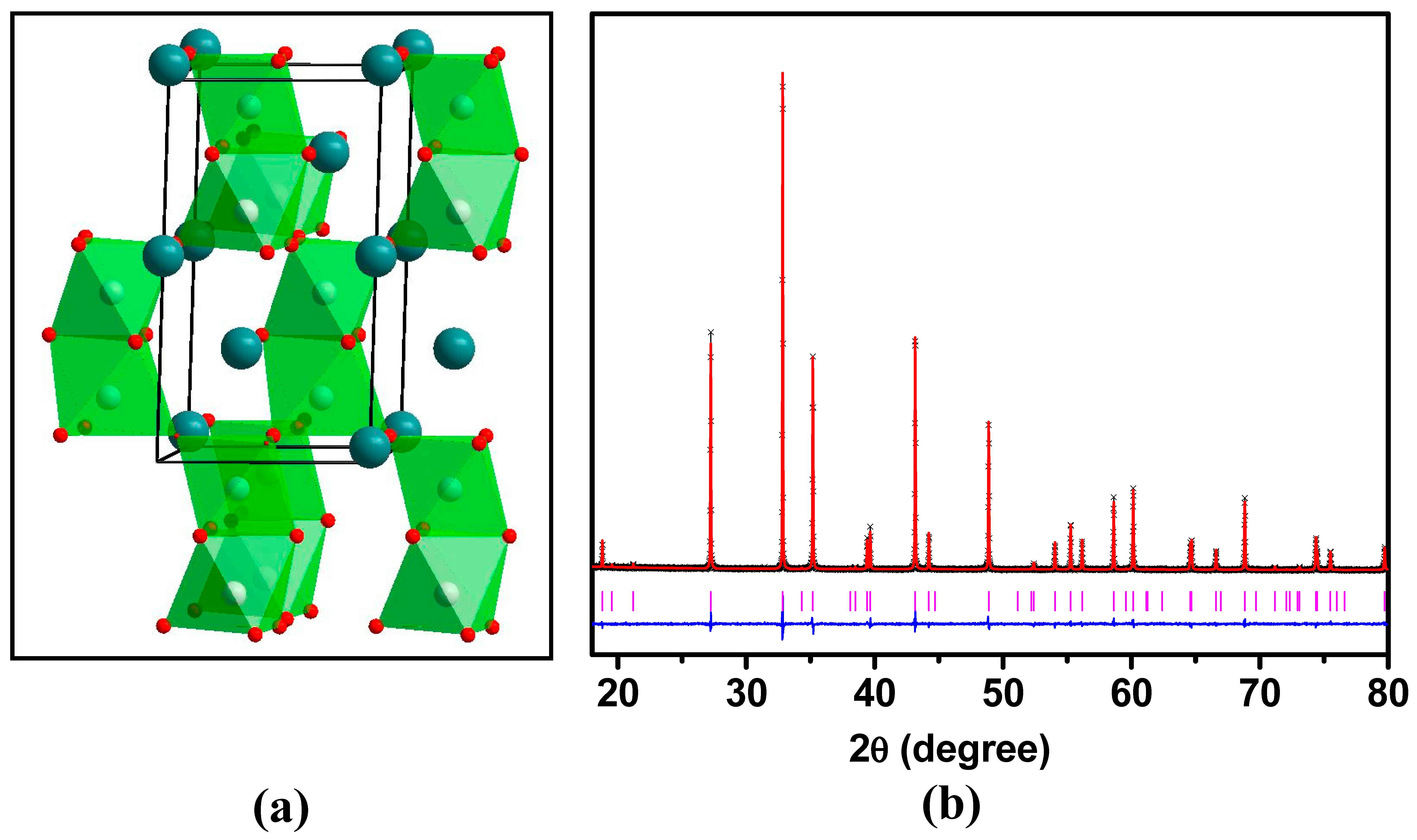


| Element | x | y | z | Uiso | Occupancy | Multiplicity |
|---|---|---|---|---|---|---|
| O1 | 0.4969(31) | 0.25 | 0.071(4) | 0.012(7) | 1 | 4 |
| O2 | 0.294(2) | 0.030(3) | 0.718(3) | 0.005(4) | 1 | 8 |
| Mn1 | 0.0 | 0.0 | 0.5 | 0.002(1) | 1 | 4 |
| Ca1 | 0.033(1) | 0.25 | −0.006(3) | 0.007(2) | 1 | 4 |
| Element | x | y | z | Uiso | Occupancy | Multiplicity |
|---|---|---|---|---|---|---|
| Ca1 | 0.5 | 0.5 | 0.5 | 0.025(2) | 0.5 | 1 |
| Sr1 | 0.5 | 0.5 | 0.5 | 0.025(2) | 0.5 | 1 |
| Mn1 | 0.0 | 0.0 | 0.0 | 0.036(3) | 1 | 1 |
| O1 | 0.5 | 0.5 | 0.0 | 0.068(3) | 0.9867 | 3 |
| Element | x | y | z | Uiso | Occupancy | Multiplicity |
|---|---|---|---|---|---|---|
| Sr1 | 0.0 | 0.0 | −0.197(7) | 0.020(6) | 0.5 | 4 |
| Sr2 | 0.333(3) | 0.666(7) | 0.250 | 0.020(6) | 1 | 2 |
| Mn1 | 0.333(3) | 0.666(7) | 0.572(7) | 0.031(4) | 1 | 4 |
| O1 | 0.5 | 0.0 | 0.0 | 0.020(6) | 0.9000 | 6 |
| O2 | −0.898055 | 0.898055 | 0.750000 | 0.020(6) | 0.9000 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinson, A.; Guinn, M.; Mortensen, P.; Hona, R.K. Correlation Between Conductivity and Oxygen Evolution Reaction Activity in Perovskite Oxides CaMnO3-δ, Ca0.5Sr0.5MnO3-δ and SrMnO3-δ. Sustain. Chem. 2025, 6, 3. https://doi.org/10.3390/suschem6010003
Martinson A, Guinn M, Mortensen P, Hona RK. Correlation Between Conductivity and Oxygen Evolution Reaction Activity in Perovskite Oxides CaMnO3-δ, Ca0.5Sr0.5MnO3-δ and SrMnO3-δ. Sustainable Chemistry. 2025; 6(1):3. https://doi.org/10.3390/suschem6010003
Chicago/Turabian StyleMartinson, Amara, Mandy Guinn, Peter Mortensen, and Ram Krishna Hona. 2025. "Correlation Between Conductivity and Oxygen Evolution Reaction Activity in Perovskite Oxides CaMnO3-δ, Ca0.5Sr0.5MnO3-δ and SrMnO3-δ" Sustainable Chemistry 6, no. 1: 3. https://doi.org/10.3390/suschem6010003
APA StyleMartinson, A., Guinn, M., Mortensen, P., & Hona, R. K. (2025). Correlation Between Conductivity and Oxygen Evolution Reaction Activity in Perovskite Oxides CaMnO3-δ, Ca0.5Sr0.5MnO3-δ and SrMnO3-δ. Sustainable Chemistry, 6(1), 3. https://doi.org/10.3390/suschem6010003







