Blood-Spinal Cord Barrier: Its Role in Spinal Disorders and Emerging Therapeutic Strategies
Abstract
1. Introduction
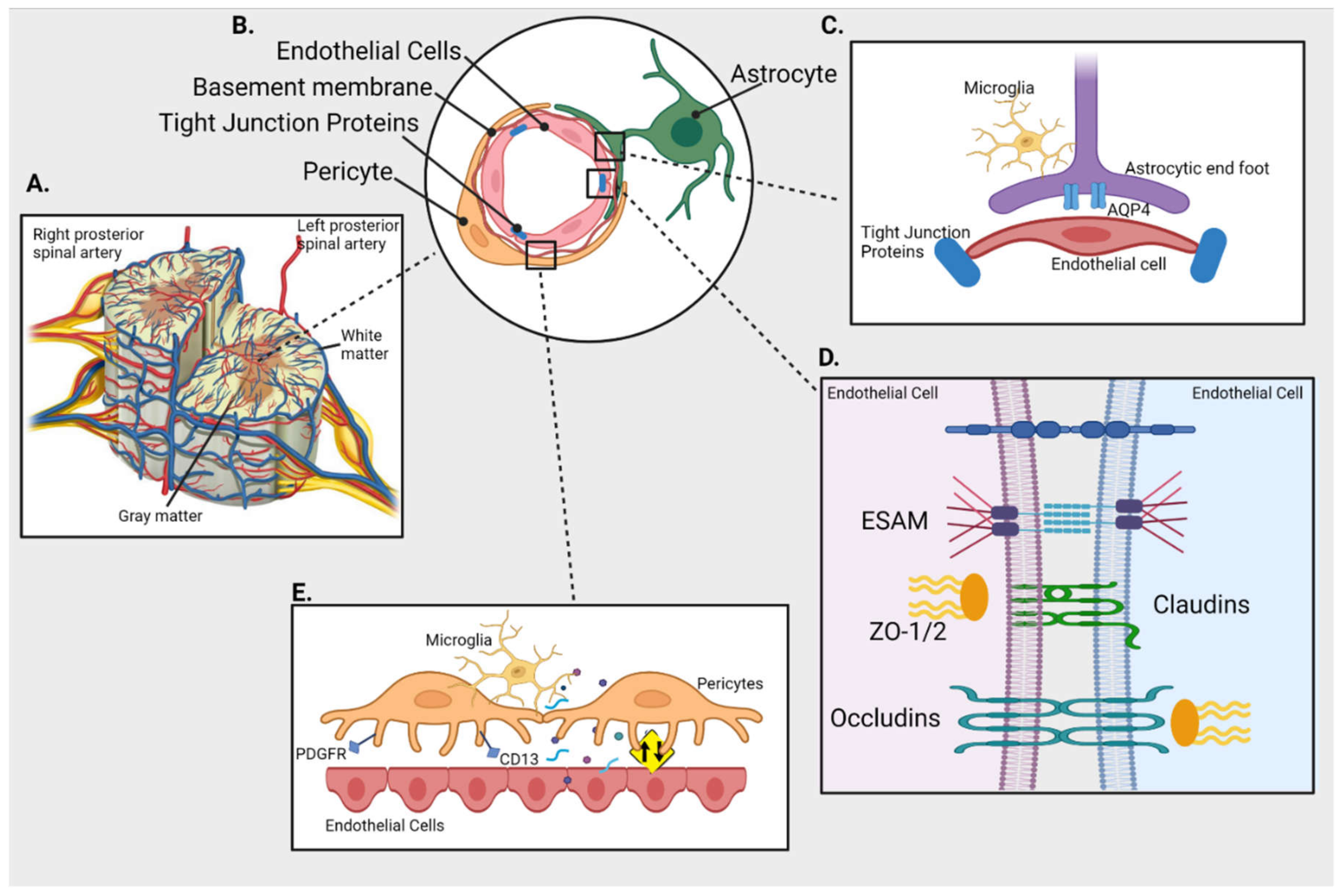
2. Anatomy of BSCB
2.1. Spinal Cord Microvascular Endothelial Cells (SCMECs)
2.2. Basal Membrane
2.3. Pericytes
2.4. Astrocytic Feet
2.5. TJ Proteins
2.6. Transporters
3. Methods to Assess BSCB Impairment
3.1. Dye Extravasation
3.2. Contrast Magnetic Resonance imaging (MRI)
3.3. Immunohistochemistry (IHC)
3.4. Electron Microscopy
4. Spinal Cord Disorders
4.1. SCI
4.2. ALS
4.3. PNI
4.4. IRI
4.5. MS
4.6. SCM
4.7. DCM
4.8. Cancers
5. Risk Factors
5.1. Aging
5.2. Obesity and Metabolic Syndrome
5.3. Lifestyle
5.4. Infection and Auto-Immunity
5.5. Environment
6. Conclusions
7. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Daneman, R.; Zhou, L.; Kebede, A.A.; Barres, B.A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 2010, 468, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Verheggen, I.C.M.; de Jong, J.J.A.; van Boxtel, M.P.J.; Gronenschild, E.; Palm, W.M.; Postma, A.A.; Jansen, J.F.A.; Verhey, F.R.J.; Backes, W.H. Increase in blood-brain barrier leakage in healthy, older adults. Geroscience 2020, 42, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, M. Natalizumab: A new treatment for relapsing remitting multiple sclerosis. Ther. Clin. Risk Manag. 2007, 3, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.J.; Atwal, J.K.; Zhang, Y.; Tong, R.K.; Wildsmith, K.R.; Tan, C.; Bien-Ly, N.; Hersom, M.; Maloney, J.A.; Meilandt, W.J.; et al. Therapeutic bispecific antibodies cross the blood-brain barrier in nonhuman primates. Sci. Transl. Med. 2014, 6, 261ra154. [Google Scholar] [CrossRef]
- Carpentier, A.; Canney, M.; Vignot, A.; Reina, V.; Beccaria, K.; Horodyckid, C.; Karachi, C.; Leclercq, D.; Lafon, C.; Chapelon, J.Y.; et al. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci. Transl. Med. 2016, 8, 343re342. [Google Scholar] [CrossRef]
- Nzou, G.; Wicks, R.T.; VanOstrand, N.R.; Mekky, G.A.; Seale, S.A.; El-Taibany, A.; Wicks, E.E.; Nechtman, C.M.; Marrotte, E.J.; Makani, V.S.; et al. Multicellular 3D Neurovascular Unit Model for Assessing Hypoxia and Neuroinflammation Induced Blood-Brain Barrier Dysfunction. Sci. Rep. 2020, 10, 9766. [Google Scholar] [CrossRef]
- Bartanusz, V.; Jezova, D.; Alajajian, B.; Digicaylioglu, M. The blood-spinal cord barrier: Morphology and clinical implications. Ann. Neurol. 2011, 70, 194–206. [Google Scholar] [CrossRef]
- Sauer, R.S.; Kirchner, J.; Yang, S.; Hu, L.; Leinders, M.; Sommer, C.; Brack, A.; Rittner, H.L. Blood-spinal cord barrier breakdown and pericyte deficiency in peripheral neuropathy. Ann. N. Y. Acad. Sci. 2017, 1405, 71–88. [Google Scholar] [CrossRef]
- Garbuzova-Davis, S.; Saporta, S.; Haller, E.; Kolomey, I.; Bennett, S.P.; Potter, H.; Sanberg, P.R. Evidence of compromised blood-spinal cord barrier in early and late symptomatic SOD1 mice modeling ALS. PLoS ONE 2007, 2, e1205. [Google Scholar] [CrossRef]
- Armulik, A.; Genove, G.; Mae, M.; Nisancioglu, M.H.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K.; et al. Pericytes regulate the blood-brain barrier. Nature 2010, 468, 557–561. [Google Scholar] [CrossRef]
- Bell, R.D.; Winkler, E.A.; Sagare, A.P.; Singh, I.; LaRue, B.; Deane, R.; Zlokovic, B.V. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 2010, 68, 409–427. [Google Scholar] [CrossRef]
- Whetstone, W.D.; Hsu, J.Y.; Eisenberg, M.; Werb, Z.; Noble-Haeusslein, L.J. Blood-spinal cord barrier after spinal cord injury: Relation to revascularization and wound healing. J. Neurosci. Res. 2003, 74, 227–239. [Google Scholar] [CrossRef]
- Bauer, H.C.; Krizbai, I.A.; Bauer, H.; Traweger, A. “You Shall Not Pass”-tight junctions of the blood brain barrier. Front. Neurosci. 2014, 8, 392. [Google Scholar] [CrossRef]
- Katsuno, T.; Umeda, K.; Matsui, T.; Hata, M.; Tamura, A.; Itoh, M.; Takeuchi, K.; Fujimori, T.; Nabeshima, Y.; Noda, T.; et al. Deficiency of zonula occludens-1 causes embryonic lethal phenotype associated with defected yolk sac angiogenesis and apoptosis of embryonic cells. Mol. Biol. Cell. 2008, 19, 2465–2475. [Google Scholar] [CrossRef]
- Koehn, L.M.; Noor, N.M.; Dong, Q.; Er, S.Y.; Rash, L.D.; King, G.F.; Dziegielewska, K.M.; Saunders, N.R.; Habgood, M.D. Selective inhibition of ASIC1a confers functional and morphological neuroprotection following traumatic spinal cord injury. F1000Res. 2016, 5, 1822. [Google Scholar] [CrossRef]
- Nishimura, M.; Naito, S. Tissue-specific mRNA expression profiles of human ATP-binding cassette and solute carrier transporter superfamilies. Drug Metab. Pharmacokinet. 2005, 20, 452–477. [Google Scholar] [CrossRef]
- Uchida, Y.; Yagi, Y.; Takao, M.; Tano, M.; Umetsu, M.; Hirano, S.; Usui, T.; Tachikawa, M.; Terasaki, T. Comparison of Absolute Protein Abundances of Transporters and Receptors among Blood-Brain Barriers at Different Cerebral Regions and the Blood-Spinal Cord Barrier in Humans and Rats. Mol. Pharm. 2020, 17, 2006–2020. [Google Scholar] [CrossRef]
- Halsey, A.M.; Conner, A.C.; Bill, R.M.; Logan, A.; Ahmed, Z. Aquaporins and Their Regulation after Spinal Cord Injury. Cells 2018, 7, 174. [Google Scholar] [CrossRef]
- Prockop, L.D.; Naidu, K.A.; Binard, J.E.; Ransohoff, J. Selective permeability of [3H]-D-mannitol and [14C]-carboxyl-inulin across the blood-brain barrier and blood-spinal cord barrier in the rabbit. J. Spinal Cord Med. 1995, 18, 221–226. [Google Scholar] [CrossRef]
- Ge, S.; Pachter, J.S. Isolation and culture of microvascular endothelial cells from murine spinal cord. J. Neuroimmunol. 2006, 177, 209–214. [Google Scholar] [CrossRef]
- Winkler, E.A.; Sengillo, J.D.; Bell, R.D.; Wang, J.; Zlokovic, B.V. Blood-spinal cord barrier pericyte reductions contribute to increased capillary permeability. J. Cereb. Blood Flow Metab. 2012, 32, 1841–1852. [Google Scholar] [CrossRef]
- Wilhelm, I.; Nyul-Toth, A.; Suciu, M.; Hermenean, A.; Krizbai, I.A. Heterogeneity of the blood-brain barrier. Tissue Barriers 2016, 4, e1143544. [Google Scholar] [CrossRef]
- Pan, W.; Banks, W.A.; Kastin, A.J. Permeability of the blood-brain and blood-spinal cord barriers to interferons. J. Neuroimmunol. 1997, 76, 105–111. [Google Scholar] [CrossRef]
- Patterson, C.E.; Rhoades, R.A.; Garcia, J.G. Evans blue dye as a marker of albumin clearance in cultured endothelial monolayer and isolated lung. J. Appl. Physiol. 1992, 72, 865–873. [Google Scholar] [CrossRef]
- Yue, Y.; Zhao, J.; Li, X.; Zhang, L.; Su, Y.; Fan, H. Involvement of Shh/Gli1 signaling in the permeability of blood-spinal cord barrier and locomotion recovery after spinal cord contusion. Neurosci. Lett. 2020, 728, 134947. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, X.; Gong, L.; Cui, L.; Zhang, H.; Zhao, W.; Jiang, P.; Hou, G.; Hou, Y. Tetramethylpyrazine Protects Blood-Spinal Cord Barrier Integrity by Modulating Microglia Polarization Through Activation of STAT3/SOCS3 and Inhibition of NF-small ka, CyrillicB Signaling Pathways in Experimental Autoimmune Encephalomyelitis Mice. Cell. Mol. Neurobiol. 2021, 41, 717–731. [Google Scholar] [CrossRef]
- Saunders, N.R.; Dziegielewska, K.M.; Mollgard, K.; Habgood, M.D. Markers for blood-brain barrier integrity: How appropriate is Evans blue in the twenty-first century and what are the alternatives? Front. Neurosci. 2015, 9, 385. [Google Scholar] [CrossRef]
- Kumar, V.; Lee, J.D.; Coulson, E.J.; Woodruff, T.M. A validated quantitative method for the assessment of neuroprotective barrier impairment in neurodegenerative disease models. J. Neurochem. 2021, 158, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Blume, C.; Geiger, M.F.; Brandenburg, L.O.; Muller, M.; Mainz, V.; Kalder, J.; Albanna, W.; Clusmann, H.; Mueller, C.A. Patients with degenerative cervical myelopathy have signs of blood spinal cord barrier disruption, and its magnitude correlates with myelopathy severity: A prospective comparative cohort study. Eur. Spine J. 2020, 29, 986–993. [Google Scholar] [CrossRef]
- Winkler, E.A.; Sengillo, J.D.; Sullivan, J.S.; Henkel, J.S.; Appel, S.H.; Zlokovic, B.V. Blood-spinal cord barrier breakdown and pericyte reductions in amyotrophic lateral sclerosis. Acta Neuropathol. 2013, 125, 111–120. [Google Scholar] [CrossRef]
- Bilgen, M.; Dogan, B.; Narayana, P.A. In vivo assessment of blood-spinal cord barrier permeability: Serial dynamic contrast enhanced MRI of spinal cord injury. Magn. Reson. Imaging 2002, 20, 337–341. [Google Scholar] [CrossRef]
- Bakhsheshian, J.; Strickland, B.A.; Mack, W.J.; Zlokovic, B.V. Investigating the blood-spinal cord barrier in preclinical models: A systematic review of in vivo imaging techniques. Spinal Cord 2021, 59, 596–612. [Google Scholar] [CrossRef] [PubMed]
- Cahill, L.S.; Laliberte, C.L.; Liu, X.J.; Bishop, J.; Nieman, B.J.; Mogil, J.S.; Sorge, R.E.; Jones, C.D.; Salter, M.W.; Henkelman, R.M. Quantifying blood-spinal cord barrier permeability after peripheral nerve injury in the living mouse. Mol. Pain 2014, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Hellenbrand, D.J.; Reichl, K.A.; Travis, B.J.; Filipp, M.E.; Khalil, A.S.; Pulito, D.J.; Gavigan, A.V.; Maginot, E.R.; Arnold, M.T.; Adler, A.G.; et al. Sustained interleukin-10 delivery reduces inflammation and improves motor function after spinal cord injury. J. Neuroinflamm. 2019, 16, 93. [Google Scholar] [CrossRef]
- Yamadera, M.; Fujimura, H.; Inoue, K.; Toyooka, K.; Mori, C.; Hirano, H.; Sakoda, S. Microvascular disturbance with decreased pericyte coverage is prominent in the ventral horn of patients with amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Frontotemporal Degener. 2015, 16, 393–401. [Google Scholar] [CrossRef]
- Smith, J.R.; Lee, J.; Winkelstein, B.A. Nerve Root Compression Increases Spinal Astrocytic Vimentin in Parallel With Sustained Pain and Endothelial Vimentin in Association With Spinal Vascular Reestablishment. Spine 2017, 42, 1434–1439. [Google Scholar] [CrossRef]
- Allnoch, L.; Baumgartner, W.; Hansmann, F. Impact of Astrocyte Depletion upon Inflammation and Demyelination in a Murine Animal Model of Multiple Sclerosis. Int. J. Mol. Sci. 2019, 20, 3922. [Google Scholar] [CrossRef]
- Shrestha, B.; Ge, S.; Pachter, J.S. Resolution of central nervous system astrocytic and endothelial sources of CCL2 gene expression during evolving neuroinflammation. Fluids Barriers CNS 2014, 11, 6. [Google Scholar] [CrossRef]
- Sasaki, S. Alterations of the blood-spinal cord barrier in sporadic amyotrophic lateral sclerosis. Neuropathology 2015, 35, 518–528. [Google Scholar] [CrossRef]
- Ying, X.; Xie, Q.; Yu, X.; Li, S.; Wu, Q.; Chen, X.; Yue, J.; Zhou, K.; Tu, W.; Jiang, S. Water treadmill training protects the integrity of the blood-spinal cord barrier following SCI via the BDNF/TrkB-CREB signalling pathway. Neurochem. Int. 2021, 143, 104945. [Google Scholar] [CrossRef]
- Cawston, T.E.; Young, D.A. Proteinases involved in matrix turnover during cartilage and bone breakdown. Cell Tissue Res. 2010, 339, 221–235. [Google Scholar] [CrossRef]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a005058. [Google Scholar] [CrossRef]
- Lee, J.Y.; Choi, H.Y.; Ahn, H.J.; Ju, B.G.; Yune, T.Y. Matrix metalloproteinase-3 promotes early blood-spinal cord barrier disruption and hemorrhage and impairs long-term neurological recovery after spinal cord injury. Am. J. Pathol. 2014, 184, 2985–3000. [Google Scholar] [CrossRef]
- Lee, J.Y.; Na, W.H.; Choi, H.Y.; Lee, K.H.; Ju, B.G.; Yune, T.Y. Jmjd3 mediates blood-spinal cord barrier disruption after spinal cord injury by regulating MMP-3 and MMP-9 expressions. Neurobiol. Dis. 2016, 95, 66–81. [Google Scholar] [CrossRef]
- Kumar, H.; Jo, M.J.; Choi, H.; Muttigi, M.S.; Shon, S.; Kim, B.J.; Lee, S.H.; Han, I.B. Matrix Metalloproteinase-8 Inhibition Prevents Disruption of Blood-Spinal Cord Barrier and Attenuates Inflammation in Rat Model of Spinal Cord Injury. Mol. Neurobiol. 2018, 55, 2577–2590. [Google Scholar] [CrossRef]
- Wells, J.E.; Rice, T.K.; Nuttall, R.K.; Edwards, D.R.; Zekki, H.; Rivest, S.; Yong, V.W. An adverse role for matrix metalloproteinase 12 after spinal cord injury in mice. J. Neurosci. 2003, 23, 10107–10115. [Google Scholar] [CrossRef]
- Kumar, H.; Lim, C.S.; Choi, H.; Joshi, H.P.; Kim, K.T.; Kim, Y.H.; Park, C.K.; Kim, H.M.; Han, I.B. Elevated TRPV4 Levels Contribute to Endothelial Damage and Scarring in Experimental Spinal Cord Injury. J. Neurosci. 2020, 40, 1943–1955. [Google Scholar] [CrossRef]
- Jiang, X.; Yu, M.; Ou, Y.; Cao, Y.; Yao, Y.; Cai, P.; Zhang, F. Downregulation of USP4 Promotes Activation of Microglia and Subsequent Neuronal Inflammation in Rat Spinal Cord After Injury. Neurochem. Res. 2017, 42, 3245–3253. [Google Scholar] [CrossRef]
- Zhang, Q.; Bian, G.; Chen, P.; Liu, L.; Yu, C.; Liu, F.; Xue, Q.; Chung, S.K.; Song, B.; Ju, G.; et al. Aldose Reductase Regulates Microglia/Macrophages Polarization Through the cAMP Response Element-Binding Protein After Spinal Cord Injury in Mice. Mol. Neurobiol. 2016, 53, 662–676. [Google Scholar] [CrossRef]
- Pan, Y.L.; Guo, Y.; Ma, Y.; Wang, L.; Zheng, S.Y.; Liu, M.M.; Huang, G.C. Aquaporin-4 expression dynamically varies after acute spinal cord injury-induced disruption of blood spinal cord barrier in rats. Neuropathology 2019, 39, 181–186. [Google Scholar] [CrossRef]
- Yoshizaki, S.; Tamaru, T.; Hara, M.; Kijima, K.; Tanaka, M.; Konno, D.J.; Matsumoto, Y.; Nakashima, Y.; Okada, S. Microglial inflammation after chronic spinal cord injury is enhanced by reactive astrocytes via the fibronectin/beta1 integrin pathway. J. Neuroinflamm. 2021, 18, 12. [Google Scholar] [CrossRef]
- Kitchen, P.; Salman, M.M.; Halsey, A.M.; Clarke-Bland, C.; MacDonald, J.A.; Ishida, H.; Vogel, H.J.; Almutiri, S.; Logan, A.; Kreida, S.; et al. Targeting Aquaporin-4 Subcellular Localization to Treat Central Nervous System Edema. Cell 2020, 181, 784–799 e719. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, H.; Xia, H.; Wang, B.; Zhang, R.; Zeng, Q.; Guo, L.; Shen, K.; Wang, B.; Zhong, Y.; et al. CD8 T cell-derived perforin aggravates secondary spinal cord injury through destroying the blood-spinal cord barrier. Biochem. Biophys. Res. Commun. 2019, 512, 367–372. [Google Scholar] [CrossRef]
- Ouali Alami, N.; Tang, L.; Wiesner, D.; Commisso, B.; Bayer, D.; Weishaupt, J.; Dupuis, L.; Wong, P.; Baumann, B.; Wirth, T.; et al. Multiplexed chemogenetics in astrocytes and motoneurons restore blood-spinal cord barrier in ALS. Life Sci. Alliance 2020, 3, e201900571. [Google Scholar] [CrossRef]
- Garbuzova-Davis, S.; Haller, E.; Saporta, S.; Kolomey, I.; Nicosia, S.V.; Sanberg, P.R. Ultrastructure of blood-brain barrier and blood-spinal cord barrier in SOD1 mice modeling ALS. Brain Res. 2007, 1157, 126–137. [Google Scholar] [CrossRef]
- Mohamed, L.A.; Markandaiah, S.S.; Bonanno, S.; Pasinelli, P.; Trotti, D. Excess glutamate secreted from astrocytes drives upregulation of P-glycoprotein in endothelial cells in amyotrophic lateral sclerosis. Exp. Neurol. 2019, 316, 27–38. [Google Scholar] [CrossRef]
- Chan, G.N.; Evans, R.A.; Banks, D.B.; Mesev, E.V.; Miller, D.S.; Cannon, R.E. Selective induction of P-glycoprotein at the CNS barriers during symptomatic stage of an ALS animal model. Neurosci. Lett. 2017, 639, 103–113. [Google Scholar] [CrossRef]
- Garbuzova-Davis, S.; Woods, R.L., 3rd; Louis, M.K.; Zesiewicz, T.A.; Kuzmin-Nichols, N.; Sullivan, K.L.; Miller, A.M.; Hernandez-Ontiveros, D.G.; Sanberg, P.R. Reduction of circulating endothelial cells in peripheral blood of ALS patients. PLoS ONE 2010, 5, e10614. [Google Scholar] [CrossRef]
- Jablonski, M.R.; Jacob, D.A.; Campos, C.; Miller, D.S.; Maragakis, N.J.; Pasinelli, P.; Trotti, D. Selective increase of two ABC drug efflux transporters at the blood-spinal cord barrier suggests induced pharmacoresistance in ALS. Neurobiol. Dis. 2012, 47, 194–200. [Google Scholar] [CrossRef]
- Nicaise, C.; Mitrecic, D.; Demetter, P.; De Decker, R.; Authelet, M.; Boom, A.; Pochet, R. Impaired blood-brain and blood-spinal cord barriers in mutant SOD1-linked ALS rat. Brain Res. 2009, 1301, 152–162. [Google Scholar] [CrossRef]
- Sasaki, S.; Iguchi, Y.; Katsuno, M.; Sobue, G. Alterations in the blood-spinal cord barrier in TDP-43 conditional knockout mice. Neurosci. Lett. 2015, 598, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Kang, Y.; Zhou, Y.; Li, X.; Lan, J.; Wu, L.; Feng, X.; Peng, Y. ALS-causing SOD1 mutants regulate occludin phosphorylation/ubiquitination and endocytic trafficking via the ITCH/Eps15/Rab5 axis. Neurobiol. Dis. 2021, 153, 105315. [Google Scholar] [CrossRef]
- Zhong, Z.; Deane, R.; Ali, Z.; Parisi, M.; Shapovalov, Y.; O’Banion, M.K.; Stojanovic, K.; Sagare, A.; Boillee, S.; Cleveland, D.W.; et al. ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nat. Neurosci. 2008, 11, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Echeverry, S.; Shi, X.Q.; Rivest, S.; Zhang, J. Peripheral nerve injury alters blood-spinal cord barrier functional and molecular integrity through a selective inflammatory pathway. J. Neurosci. 2011, 31, 10819–10828. [Google Scholar] [CrossRef]
- Zhang, J.; De Koninck, Y. Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. J. Neurochem. 2006, 97, 772–783. [Google Scholar] [CrossRef]
- Oklinski, M.K.; Choi, H.J.; Kwon, T.H. Peripheral nerve injury induces aquaporin-4 expression and astrocytic enlargement in spinal cord. Neuroscience 2015, 311, 138–152. [Google Scholar] [CrossRef]
- Tsymbalyuk, O.; Gerzanich, V.; Mumtaz, A.; Andhavarapu, S.; Ivanova, S.; Makar, T.K.; Sansur, C.A.; Keller, A.; Nakamura, Y.; Bryan, J.; et al. SUR1, newly expressed in astrocytes, mediates neuropathic pain in a mouse model of peripheral nerve injury. Mol. Pain 2021, 17, 17448069211006603. [Google Scholar] [CrossRef]
- Blume, C.; Geiger, M.F.; Muller, M.; Clusmann, H.; Mainz, V.; Kalder, J.; Brandenburg, L.O.; Mueller, C.A. Decreased angiogenesis as a possible pathomechanism in cervical degenerative myelopathy. Sci. Rep. 2021, 11, 2497. [Google Scholar] [CrossRef]
- Vidal, P.M.; Ulndreaj, A.; Tetreault, L.; Hong, J.; Fehlings, M.G. The changes in systemic monocytes in humans undergoing surgical decompression for degenerative cervical myelopathy may influence clinical neurological recovery. J. Neuroimmunol. 2019, 336, 577024. [Google Scholar] [CrossRef]
- Uchida, Y.; Sumiya, T.; Tachikawa, M.; Yamakawa, T.; Murata, S.; Yagi, Y.; Sato, K.; Stephan, A.; Ito, K.; Ohtsuki, S.; et al. Involvement of Claudin-11 in Disruption of Blood-Brain, -Spinal Cord, and -Arachnoid Barriers in Multiple Sclerosis. Mol. Neurobiol. 2019, 56, 2039–2056. [Google Scholar] [CrossRef]
- Aube, B.; Levesque, S.A.; Pare, A.; Chamma, E.; Kebir, H.; Gorina, R.; Lecuyer, M.A.; Alvarez, J.I.; De Koninck, Y.; Engelhardt, B.; et al. Neutrophils mediate blood-spinal cord barrier disruption in demyelinating neuroinflammatory diseases. J. Immunol. 2014, 193, 2438–2454. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Q.; Lv, H.W.; Tan, W.F.; Fang, B.; Wang, H.; Ma, H. Role of the TLR4 pathway in blood-spinal cord barrier dysfunction during the bimodal stage after ischemia/reperfusion injury in rats. J. Neuroinflamm. 2014, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Q.; Chen, F.S.; Tan, W.F.; Fang, B.; Zhang, Z.L.; Ma, H. Elevated microRNA-129-5p level ameliorates neuroinflammation and blood-spinal cord barrier damage after ischemia-reperfusion by inhibiting HMGB1 and the TLR3-cytokine pathway. J. Neuroinflamm. 2017, 14, 205. [Google Scholar] [CrossRef]
- Chen, F.; Li, X.; Li, Z.; Zhou, Y.; Qiang, Z.; Ma, H. The roles of chemokine (C-X-C motif) ligand 13 in spinal cord ischemia-reperfusion injury in rats. Brain Res. 2020, 1727, 146489. [Google Scholar] [CrossRef]
- Jia, H.; Ma, H.; Li, Z.; Chen, F.; Fang, B.; Cao, X.; Chang, Y.; Qiang, Z. Downregulation of LncRNA TUG1 Inhibited TLR4 Signaling Pathway-Mediated Inflammatory Damage After Spinal Cord Ischemia Reperfusion in Rats via Suppressing TRIL Expression. J. Neuropathol. Exp. Neurol. 2019, 78, 268–282. [Google Scholar] [CrossRef]
- Wang, X.; Campos, C.R.; Peart, J.C.; Smith, L.K.; Boni, J.L.; Cannon, R.E.; Miller, D.S. Nrf2 upregulates ATP binding cassette transporter expression and activity at the blood-brain and blood-spinal cord barriers. J. Neurosci. 2014, 34, 8585–8593. [Google Scholar] [CrossRef]
- Wang, C.; Xu, K.; Wang, Y.; Mao, Y.; Huang, Y.; Liang, Y.; Liu, Y.; Hao, J.; Gu, X.; Ma, Z.; et al. Spinal cannabinoid receptor 2 activation reduces hypersensitivity associated with bone cancer pain and improves the integrity of the blood-spinal cord barrier. Reg. Anesth. Pain Med. 2020, 45, 783–791. [Google Scholar] [CrossRef]
- Sharma, H.S. Neuroprotective effects of neurotrophins and melanocortins in spinal cord injury: An experimental study in the rat using pharmacological and morphological approaches. Ann. N. Y. Acad. Sci. 2005, 1053, 407–421. [Google Scholar] [CrossRef]
- Sharma, H.S.; Sjoquist, P.O.; Mohanty, S.; Wiklund, L. Post-injury treatment with a new antioxidant compound H-290/51 attenuates spinal cord trauma-induced c-fos expression, motor dysfunction, edema formation, and cell injury in the rat. Acta Neurochir. Suppl. 2006, 96, 322–328. [Google Scholar] [CrossRef]
- Sharma, H.S. A bradykinin BK2 receptor antagonist HOE-140 attenuates blood-spinal cord barrier permeability following a focal trauma to the rat spinal cord. An experimental study using Evans blue, [131]I-sodium and lanthanum tracers. Acta Neurochir. Suppl. 2000, 76, 159–163. [Google Scholar] [CrossRef]
- Lee, J.Y.; Choi, H.Y.; Na, W.H.; Ju, B.G.; Yune, T.Y. Ghrelin inhibits BSCB disruption/hemorrhage by attenuating MMP-9 and SUR1/TrpM4 expression and activation after spinal cord injury. Biochim. Biophys. Acta 2014, 1842, 2403–2412. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Choi, H.Y.; Na, W.H.; Ju, B.G.; Yune, T.Y. 17beta-estradiol inhibits MMP-9 and SUR1/TrpM4 expression and activation and thereby attenuates BSCB disruption/hemorrhage after spinal cord injury in male rats. Endocrinology 2015, 156, 1838–1850. [Google Scholar] [CrossRef] [PubMed]
- Park, C.S.; Lee, J.Y.; Choi, H.Y.; Ju, B.G.; Youn, I.; Yune, T.Y. Protocatechuic acid improves functional recovery after spinal cord injury by attenuating blood-spinal cord barrier disruption and hemorrhage in rats. Neurochem. Int. 2019, 124, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Xu, J.; Yu, T.; Chen, Z.; Xiao, Z.; Wang, J.; Hu, Y.; Wu, Y.; Zhu, D. Flufenamic acid inhibits secondary hemorrhage and BSCB disruption after spinal cord injury. Theranostics 2018, 8, 4181–4198. [Google Scholar] [CrossRef]
- Shechter, R.; Miller, O.; Yovel, G.; Rosenzweig, N.; London, A.; Ruckh, J.; Kim, K.W.; Klein, E.; Kalchenko, V.; Bendel, P.; et al. Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity 2013, 38, 555–569. [Google Scholar] [CrossRef]
- Kunis, G.; Baruch, K.; Rosenzweig, N.; Kertser, A.; Miller, O.; Berkutzki, T.; Schwartz, M. IFN-gamma-dependent activation of the brain’s choroid plexus for CNS immune surveillance and repair. Brain 2013, 136, 3427–3440. [Google Scholar] [CrossRef]
- Chen, S.; Ye, J.; Chen, X.; Shi, J.; Wu, W.; Lin, W.; Lin, W.; Li, Y.; Fu, H.; Li, S. Valproic acid attenuates traumatic spinal cord injury-induced inflammation via STAT1 and NF-kappaB pathway dependent of HDAC3. J. Neuroinflamm. 2018, 15, 150. [Google Scholar] [CrossRef]
- Wang, J.L.; Ren, C.H.; Feng, J.; Ou, C.H.; Liu, L. Oleanolic acid inhibits mouse spinal cord injury through suppressing inflammation and apoptosis via the blockage of p38 and JNK MAPKs. Biomed. Pharmacother. 2020, 123, 109752. [Google Scholar] [CrossRef]
- Yu, D.S.; Wang, Y.S.; Bi, Y.L.; Guo, Z.P.; Yuan, Y.J.; Tong, S.M.; Su, R.C.; Ge, L.H.; Wang, J.; Pan, Y.L.; et al. Salvianolic acid A ameliorates the integrity of blood-spinal cord barrier via miR-101/Cul3/Nrf2/HO-1 signaling pathway. Brain Res. 2017, 1657, 279–287. [Google Scholar] [CrossRef]
- Sahib, S.; Niu, F.; Sharma, A.; Feng, L.; Tian, Z.R.; Muresanu, D.F.; Nozari, A.; Sharma, H.S. Potentiation of spinal cord conduction and neuroprotection following nanodelivery of DL-3-n-butylphthalide in titanium implanted nanomaterial in a focal spinal cord injury induced functional outcome, blood-spinal cord barrier breakdown and edema formation. Int. Rev. Neurobiol. 2019, 146, 153–188. [Google Scholar] [CrossRef]
- Sharma, H.S.; Feng, L.; Muresanu, D.F.; Castellani, R.J.; Sharma, A. Neuroprotective effects of a potent bradykinin B2 receptor antagonist HOE-140 on microvascular permeability, blood flow disturbances, edema formation, cell injury and nitric oxide synthase upregulation following trauma to the spinal cord. Int. Rev. Neurobiol. 2019, 146, 103–152. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, Y.; Wu, L.; Wang, Y.; Du, C.; Li, C.; Wang, Z.; Wang, Y. Brilliant Blue G Inhibits Inflammasome Activation and Reduces Disruption of Blood-Spinal Cord Barrier Induced by Spinal Cord Injury in Rats. Med. Sci. Monit. 2019, 25, 6359–6366. [Google Scholar] [CrossRef]
- Chio, J.C.T.; Wang, J.; Badner, A.; Hong, J.; Surendran, V.; Fehlings, M.G. The effects of human immunoglobulin G on enhancing tissue protection and neurobehavioral recovery after traumatic cervical spinal cord injury are mediated through the neurovascular unit. J. Neuroinflamm. 2019, 16, 141. [Google Scholar] [CrossRef]
- Chio, J.C.T.; Wang, J.; Surendran, V.; Li, L.; Zavvarian, M.M.; Pieczonka, K.; Fehlings, M.G. Delayed administration of high dose human immunoglobulin G enhances recovery after traumatic cervical spinal cord injury by modulation of neuroinflammation and protection of the blood spinal cord barrier. Neurobiol. Dis. 2021, 148, 105187. [Google Scholar] [CrossRef]
- Lin, Y.; Vreman, H.J.; Wong, R.J.; Tjoa, T.; Yamauchi, T.; Noble-Haeusslein, L.J. Heme oxygenase-1 stabilizes the blood-spinal cord barrier and limits oxidative stress and white matter damage in the acutely injured murine spinal cord. J. Cereb. Blood Flow Metab. 2007, 27, 1010–1021. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Han, W.; Li, J.; Xu, K.; Li, Z.; Wang, Q.; Xu, K.; Liu, Y.; Xie, L.; et al. Hydrogen Sulfide Ameliorates Blood-Spinal Cord Barrier Disruption and Improves Functional Recovery by Inhibiting Endoplasmic Reticulum Stress-Dependent Autophagy. Front. Pharmacol. 2018, 9, 858. [Google Scholar] [CrossRef]
- Park, C.S.; Lee, J.Y.; Choi, H.Y.; Lee, K.; Heo, Y.; Ju, B.G.; Choo, H.P.; Yune, T.Y. Gallic acid attenuates blood-spinal cord barrier disruption by inhibiting Jmjd3 expression and activation after spinal cord injury. Neurobiol. Dis. 2020, 145, 105077. [Google Scholar] [CrossRef]
- Zheng, B.; Zhou, Y.; Zhang, H.; Yang, G.; Hong, Z.; Han, D.; Wang, Q.; He, Z.; Liu, Y.; Wu, F.; et al. Dl-3-n-butylphthalide prevents the disruption of blood-spinal cord barrier via inhibiting endoplasmic reticulum stress following spinal cord injury. Int. J. Biol. Sci. 2017, 13, 1520–1531. [Google Scholar] [CrossRef]
- He, Z.; Zhou, Y.; Wang, Q.; Li, J.; Zheng, Z.; Chen, J.; Zhang, H.; Wang, Z.; Xu, H.; Xiao, J. Inhibiting endoplasmic reticulum stress by lithium chloride contributes to the integrity of blood-spinal cord barrier and functional recovery after spinal cord injury. Am. J. Transl. Res. 2017, 9, 1012–1024. [Google Scholar]
- Tong, M.; He, Z.; Lin, X.; Zhou, Y.; Wang, Q.; Zheng, Z.; Chen, J.; Xu, H.; Tian, N. Lithium chloride contributes to blood-spinal cord barrier integrity and functional recovery from spinal cord injury by stimulating autophagic flux. Biochem. Biophys. Res. Commun. 2018, 495, 2525–2531. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.; Gu, Z.; Zhang, Q.; Zheng, H. Effect of lycopene on the blood-spinal cord barrier after spinal cord injury in mice. Biosci. Trends 2016, 10, 288–293. [Google Scholar] [CrossRef][Green Version]
- He, Z.; Zou, S.; Yin, J.; Gao, Z.; Liu, Y.; Wu, Y.; He, H.; Zhou, Y.; Wang, Q.; Li, J.; et al. Inhibition of Endoplasmic Reticulum Stress Preserves the Integrity of Blood-Spinal Cord Barrier in Diabetic Rats Subjected to Spinal Cord Injury. Sci. Rep. 2017, 7, 7661. [Google Scholar] [CrossRef]
- Zhou, Y.; Ye, L.; Zheng, B.; Zhu, S.; Shi, H.; Zhang, H.; Wang, Z.; Wei, X.; Chen, D.; Li, X.; et al. Phenylbutyrate prevents disruption of blood-spinal cord barrier by inhibiting endoplasmic reticulum stress after spinal cord injury. Am. J. Transl. Res. 2016, 8, 1864–1875. [Google Scholar]
- Sanchez-Ventura, J.; Amo-Aparicio, J.; Navarro, X.; Penas, C. BET protein inhibition regulates cytokine production and promotes neuroprotection after spinal cord injury. J. Neuroinflamm. 2019, 16, 124. [Google Scholar] [CrossRef]
- Miranpuri, G.S.; Nguyen, J.; Moreno, N.; Yutuc, N.A.; Kim, J.; Buttar, S.; Brown, G.R.; Sauer, S.E.; Singh, C.K.; Kumar, S.; et al. Folic Acid Modulates Matrix Metalloproteinase-9 Expression Following Spinal Cord Injury. Ann. Neurosci. 2019, 26, 60–65. [Google Scholar] [CrossRef]
- Fan, Z.K.; Lv, G.; Wang, Y.F.; Li, G.; Yu, D.S.; Wang, Y.S.; Zhang, Y.Q.; Mei, X.F.; Cao, Y. The protective effect of salvianolic acid B on blood-spinal cord barrier after compression spinal cord injury in rats. J. Mol. Neurosci. 2013, 51, 986–993. [Google Scholar] [CrossRef]
- Lee, J.Y.; Choi, H.Y.; Yune, T.Y. Fluoxetine and vitamin C synergistically inhibits blood-spinal cord barrier disruption and improves functional recovery after spinal cord injury. Neuropharmacology 2016, 109, 78–87. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, H.S.; Choi, H.Y.; Oh, T.H.; Yune, T.Y. Fluoxetine inhibits matrix metalloprotease activation and prevents disruption of blood-spinal cord barrier after spinal cord injury. Brain 2012, 135, 2375–2389. [Google Scholar] [CrossRef]
- Gao, K.; Shen, Z.; Yuan, Y.; Han, D.; Song, C.; Guo, Y.; Mei, X. Simvastatin inhibits neural cell apoptosis and promotes locomotor recovery via activation of Wnt/beta-catenin signaling pathway after spinal cord injury. J. Neurochem. 2016, 138, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.W.; Liang, C.L.; Yeh, L.R.; Liu, K.Y.; Chen, C.C.; Chen, J.S.; Chen, H.J.; Wang, H.K. Simvastatin-Ezetimibe enhances growth factor expression and attenuates neuron loss in the hippocampus in a model of intracerebral hemorrhage. Fundam. Clin. Pharmacol. 2020, 35, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, H.; Zheng, B.; Ye, L.; Zhu, S.; Johnson, N.R.; Wang, Z.; Wei, X.; Chen, D.; Cao, G.; et al. Retinoic Acid Induced-Autophagic Flux Inhibits ER-Stress Dependent Apoptosis and Prevents Disruption of Blood-Spinal Cord Barrier after Spinal Cord Injury. Int. J. Biol. Sci. 2016, 12, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, A.M.; Sozbilen, M.C.; Sevgili, E.; Dagci, T.; Ozyalcin, H.; Armagan, G. Epidermal growth factor regulates apoptosis and oxidative stress in a rat model of spinal cord injury. Injury 2018, 49, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Q.; Cai, H.; He, Z.; Wang, H.; Chen, J.; Zheng, Z.; Yin, J.; Liao, Z.; Xu, H.; et al. FGF1 improves functional recovery through inducing PRDX1 to regulate autophagy and anti-ROS after spinal cord injury. J. Cell Mol. Med. 2018, 22, 2727–2738. [Google Scholar] [CrossRef] [PubMed]
- Pena, L.A.; Fuks, Z.; Kolesnick, R.N. Radiation-induced apoptosis of endothelial cells in the murine central nervous system: Protection by fibroblast growth factor and sphingomyelinase deficiency. Cancer Res. 2000, 60, 321–327. [Google Scholar] [PubMed]
- Cabrera-Aldana, E.E.; Ruelas, F.; Aranda, C.; Rincon-Heredia, R.; Martinez-Cruz, A.; Reyes-Sanchez, A.; Guizar-Sahagun, G.; Tovar, Y.R.L.B. Methylprednisolone Administration Following Spinal Cord Injury Reduces Aquaporin 4 Expression and Exacerbates Edema. Mediators Inflamm. 2017, 2017, 4792932. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.K.; Wang, Y.F.; Cao, Y.; Zhang, M.C.; Zhang, Z.; Lv, G.; Lu, W.; Zhang, Y.Q. The effect of aminoguanidine on compression spinal cord injury in rats. Brain Res. 2010, 1342, 1–10. [Google Scholar] [CrossRef]
- Wang, C.; Wang, P.; Zeng, W.; Li, W. Tetramethylpyrazine improves the recovery of spinal cord injury via Akt/Nrf2/HO-1 pathway. Bioorg. Med. Chem. Lett. 2016, 26, 1287–1291. [Google Scholar] [CrossRef]
- Yu, D.S.; Cao, Y.; Mei, X.F.; Wang, Y.F.; Fan, Z.K.; Wang, Y.S.; Lv, G. Curcumin improves the integrity of blood-spinal cord barrier after compressive spinal cord injury in rats. J. Neurol. Sci. 2014, 346, 51–59. [Google Scholar] [CrossRef]
- Ma, L.; Mu, Y.; Zhang, Z.; Sun, Q. Eugenol promotes functional recovery and alleviates inflammation, oxidative stress, and neural apoptosis in a rat model of spinal cord injury. Restor. Neurol. Neurosci. 2018, 36, 659–668. [Google Scholar] [CrossRef]
- Lee, J.Y.; Choi, H.Y.; Park, C.S.; Ju, B.G.; Yune, T.Y. Mithramycin A Improves Functional Recovery by Inhibiting BSCB Disruption and Hemorrhage after Spinal Cord Injury. J. Neurotrauma 2018, 35, 508–520. [Google Scholar] [CrossRef]
- Joshi, H.P.; Kumar, H.; Choi, U.Y.; Lim, Y.C.; Choi, H.; Kim, J.; Kyung, J.W.; Sohn, S.; Kim, K.T.; Kim, J.K.; et al. CORM-2-Solid Lipid Nanoparticles Maintain Integrity of Blood-Spinal Cord Barrier After Spinal Cord Injury in Rats. Mol. Neurobiol. 2020, 57, 2671–2689. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Zheng, F.; Luo, Z.; Ma, H.; Zheng, D.; Xiang, G.; Xu, C.; Zhou, Y.; Wu, Y.; Tian, N.; et al. CO-Releasing Molecule (CORM)-3 Ameliorates Spinal Cord-Blood Barrier Disruption Following Injury to the Spinal Cord. Front. Pharmacol. 2020, 11, 761. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhou, Y.; Zhang, R.; Wen, L.; Wu, K.; Li, Y.; Yao, Y.; Duan, R.; Jia, Y. Bone Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote Recovery Following Spinal Cord Injury via Improvement of the Integrity of the Blood-Spinal Cord Barrier. Front. Neurosci. 2019, 13, 209. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.J.; Liu, Y.; Wang, H.J.; Ban, D.X.; Cheng, S.Z.; Ning, G.Z.; Wang, L.L.; Chang, J.; Feng, S.Q. New approach to treating spinal cord injury using PEG-TAT-modified, cyclosporine-A-loaded PLGA/polymeric liposomes. J. Drug Target. 2017, 25, 75–82. [Google Scholar] [CrossRef]
- Gao, Y.; Vijayaraghavalu, S.; Stees, M.; Kwon, B.K.; Labhasetwar, V. Evaluating accessibility of intravenously administered nanoparticles at the lesion site in rat and pig contusion models of spinal cord injury. J. Control. Release 2019, 302, 160–168. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, C.Y.; Kong, X.H.; Wang, H.J.; Chang, J.; Zhang, D.P.; Ban, D.X.; Feng, S.Q. Novel multifunctional polyethylene glycol-transactivating-transduction protein-modified liposomes cross the blood-spinal cord barrier after spinal cord injury. J. Drug Target. 2010, 18, 420–429. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Q.; Wang, S.; Huang, Y.; Tian, N.; Wu, Y.; Wu, Y.; Zhou, Y.; Xu, H.; Zhang, X. Astragoloside IV Loaded Polycaprolactone Membrane Repairs Blood Spinal Cord Barrier and Recovers Spinal Cord Function in Traumatic Spinal Cord Injury. J. Biomed. Nanotechnol. 2019, 15, 799–812. [Google Scholar] [CrossRef]
- Matsushita, T.; Lankford, K.L.; Arroyo, E.J.; Sasaki, M.; Neyazi, M.; Radtke, C.; Kocsis, J.D. Diffuse and persistent blood-spinal cord barrier disruption after contusive spinal cord injury rapidly recovers following intravenous infusion of bone marrow mesenchymal stem cells. Exp. Neurol. 2015, 267, 152–164. [Google Scholar] [CrossRef]
- Kang, C.E.; Baumann, M.D.; Tator, C.H.; Shoichet, M.S. Localized and sustained delivery of fibroblast growth factor-2 from a nanoparticle-hydrogel composite for treatment of spinal cord injury. Cells Tissues Organs 2013, 197, 55–63. [Google Scholar] [CrossRef]
- Wang, J.; Nie, Z.; Zhao, H.; Gao, K.; Cao, Y. MiRNA-125a-5p attenuates blood-spinal cord barrier permeability under hypoxia in vitro. Biotechnol. Lett. 2020, 42, 25–34. [Google Scholar] [CrossRef]
- Sun, R.; Ge, L.; Cao, Y.; Wu, W.; Wu, Y.; Zhu, H.; Li, J.; Yu, D. MiR-429 regulates blood-spinal cord barrier permeability by targeting Kruppel-like factor 6. Biochem. Biophys. Res. Commun. 2020, 525, 740–746. [Google Scholar] [CrossRef]
- Sun, R.; Yu, D. Inhibitory effect of miR-429 on expressions of ZO-1, Occludin, and Claudin-5 proteins to improve the permeability of blood spinal cord barrier in vitro. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2020, 34, 1163–1169. [Google Scholar] [CrossRef]
- Chang, S.; Bi, Y.; Meng, X.; Qu, L.; Cao, Y. Adenovirus-delivered GFP-HO-1C[INCREMENT]23 attenuates blood-spinal cord barrier permeability after rat spinal cord contusion. Neuroreport 2018, 29, 402–407. [Google Scholar] [CrossRef]
- Winkler, E.A.; Sengillo, J.D.; Sagare, A.P.; Zhao, Z.; Ma, Q.; Zuniga, E.; Wang, Y.; Zhong, Z.; Sullivan, J.S.; Griffin, J.H.; et al. Blood-spinal cord barrier disruption contributes to early motor-neuron degeneration in ALS-model mice. Proc. Natl. Acad. Sci. USA 2014, 111, E1035–E1042. [Google Scholar] [CrossRef]
- Zhong, Z.; Ilieva, H.; Hallagan, L.; Bell, R.; Singh, I.; Paquette, N.; Thiyagarajan, M.; Deane, R.; Fernandez, J.A.; Lane, S.; et al. Activated protein C therapy slows ALS-like disease in mice by transcriptionally inhibiting SOD1 in motor neurons and microglia cells. J. Clin. Investig. 2009, 119, 3437–3449. [Google Scholar] [CrossRef]
- Rabinovich-Nikitin, I.; Ezra, A.; Barbiro, B.; Rabinovich-Toidman, P.; Solomon, B. Chronic administration of AMD3100 increases survival and alleviates pathology in SOD1(G93A) mice model of ALS. J. Neuroinflamm. 2016, 13, 123. [Google Scholar] [CrossRef]
- Eve, D.J.; Steiner, G.; Mahendrasah, A.; Sanberg, P.R.; Kurien, C.; Thomson, A.; Borlongan, C.V.; Garbuzova-Davis, S. Reduction of microhemorrhages in the spinal cord of symptomatic ALS mice after intravenous human bone marrow stem cell transplantation accompanies repair of the blood-spinal cord barrier. Oncotarget 2018, 9, 10621–10634. [Google Scholar] [CrossRef][Green Version]
- Garbuzova-Davis, S.; Haller, E.; Navarro, S.; Besong, T.E.; Boccio, K.J.; Hailu, S.; Khatib, M.; Sanberg, P.R.; Appel, S.H.; Borlongan, C.V. Transplantation of human bone marrow stem cells into symptomatic ALS mice enhances structural and functional blood-spinal cord barrier repair. Exp. Neurol. 2018, 310, 33–47. [Google Scholar] [CrossRef]
- Garbuzova-Davis, S.; Kurien, C.; Thomson, A.; Falco, D.; Ahmad, S.; Staffetti, J.; Steiner, G.; Abraham, S.; James, G.; Mahendrasah, A.; et al. Endothelial and Astrocytic Support by Human Bone Marrow Stem Cell Grafts into Symptomatic ALS Mice towards Blood-Spinal Cord Barrier Repair. Sci. Rep. 2017, 7, 884. [Google Scholar] [CrossRef]
- Garbuzova-Davis, S.; Kurien, C.; Haller, E.; Eve, D.J.; Navarro, S.; Steiner, G.; Mahendrasah, A.; Hailu, S.; Khatib, M.; Boccio, K.J.; et al. Human Bone Marrow Endothelial Progenitor Cell Transplantation into Symptomatic ALS Mice Delays Disease Progression and Increases Motor Neuron Survival by Repairing Blood-Spinal Cord Barrier. Sci. Rep. 2019, 9, 5280. [Google Scholar] [CrossRef]
- Magota, H.; Sasaki, M.; Kataoka-Sasaki, Y.; Oka, S.; Ukai, R.; Kiyose, R.; Onodera, R.; Kocsis, J.D.; Honmou, O. Intravenous infusion of mesenchymal stem cells delays disease progression in the SOD1G93A transgenic amyotrophic lateral sclerosis rat model. Brain Res. 2021, 1757, 147296. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.J.; Huang, J.X.; Yang, J.; Yuan, F.; Zhang, S.S.; Yu, Q.J.; Hu, J. Propofol protects against blood-spinal cord barrier disruption induced by ischemia/reperfusion injury. Neural Regen. Res. 2017, 12, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Li, X.Q.; Bi, B.; Tan, W.F.; Liu, G.; Zhang, Y.; Ma, H. Dexmedetomidine attenuates blood-spinal cord barrier disruption induced by spinal cord ischemia reperfusion injury in rats. Cell Physiol. Biochem. 2015, 36, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, S.; Fan, X.; Yuan, F.; Dai, J.; Hu, J. Dexmedetomidine Preconditioning Ameliorates Inflammation and Blood-Spinal Cord Barrier Damage After Spinal Cord Ischemia-Reperfusion Injury by Down-Regulation High Mobility Group Box 1-Toll-Like Receptor 4-Nuclear Factor kappaB Signaling Pathway. Spine 2019, 44, E74–E81. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Li, X.M.; Sun, X.J.; Bao, N.R.; Ren, X.Y.; Lv, H.W.; Ma, H. Ischemic preconditioning protects against spinal cord ischemia-reperfusion injury in rabbits by attenuating blood spinal cord barrier disruption. Int. J. Mol. Sci. 2013, 14, 10343–10354. [Google Scholar] [CrossRef]
- Jing, N.; Fang, B.; Wang, Z.L.; Ma, H. Remote Ischemia Preconditioning Attenuates Blood-Spinal Cord Barrier Breakdown in Rats Undergoing Spinal Cord Ischemia Reperfusion Injury: Associated with Activation and Upregulation of CB1 and CB2 Receptors. Cell Physiol. Biochem. 2017, 43, 2516–2524. [Google Scholar] [CrossRef]
- Li, X.Q.; Cao, X.Z.; Wang, J.; Fang, B.; Tan, W.F.; Ma, H. Sevoflurane preconditioning ameliorates neuronal deficits by inhibiting microglial MMP-9 expression after spinal cord ischemia/reperfusion in rats. Mol. Brain 2014, 7, 69. [Google Scholar] [CrossRef]
- Fang, B.; Wang, H.; Sun, X.J.; Li, X.Q.; Ai, C.Y.; Tan, W.F.; White, P.F.; Ma, H. Intrathecal transplantation of bone marrow stromal cells attenuates blood-spinal cord barrier disruption induced by spinal cord ischemia-reperfusion injury in rabbits. J. Vasc. Surg. 2013, 58, 1043–1052. [Google Scholar] [CrossRef]
- Yasuda, N.; Sasaki, M.; Kataoka-Sasaki, Y.; Nagahama, H.; Kocsis, J.D.; Kawaharada, N.; Honmou, O. Intravenous delivery of mesenchymal stem cells protects both white and gray matter in spinal cord ischemia. Brain Res. 2020, 1747, 147040. [Google Scholar] [CrossRef]
- Wang, D.; Chen, F.; Fang, B.; Zhang, Z.; Dong, Y.; Tong, X.; Ma, H. MiR-128-3p Alleviates Spinal Cord Ischemia/Reperfusion Injury Associated Neuroinflammation and Cellular Apoptosis via SP1 Suppression in Rat. Front. Neurosci. 2020, 14, 609613. [Google Scholar] [CrossRef]
- Li, X.Q.; Fang, B.; Tan, W.F.; Wang, Z.L.; Sun, X.J.; Zhang, Z.L.; Ma, H. miR-320a affects spinal cord edema through negatively regulating aquaporin-1 of blood-spinal cord barrier during bimodal stage after ischemia reperfusion injury in rats. BMC Neurosci. 2016, 17, 10. [Google Scholar] [CrossRef]
- Li, X.Q.; Lv, H.W.; Wang, Z.L.; Tan, W.F.; Fang, B.; Ma, H. MiR-27a ameliorates inflammatory damage to the blood-spinal cord barrier after spinal cord ischemia: Reperfusion injury in rats by downregulating TICAM-2 of the TLR4 signaling pathway. J. Neuroinflamm. 2015, 12, 25. [Google Scholar] [CrossRef]
- De Oliveira, L.R.C.; Mimura, L.A.N.; Fraga-Silva, T.F.C.; Ishikawa, L.L.W.; Fernandes, A.A.H.; Zorzella-Pezavento, S.F.G.; Sartori, A. Calcitriol Prevents Neuroinflammation and Reduces Blood-Brain Barrier Disruption and Local Macrophage/Microglia Activation. Front. Pharmacol. 2020, 11, 161. [Google Scholar] [CrossRef]
- Lu, K.; Liu, L.; Xu, X.; Zhao, F.; Deng, J.; Tang, X.; Wang, X.; Zhao, B.Q.; Zhang, X.; Zhao, Y. ADAMTS13 ameliorates inflammatory responses in experimental autoimmune encephalomyelitis. J. Neuroinflamm. 2020, 17, 67. [Google Scholar] [CrossRef]
- Mondal, S.; Dasarathi, S.; Pahan, K. Glyceryl Tribenzoate: A Flavoring Ingredient, Inhibits the Adoptive Transfer of Experimental Allergic Encephalomyelitis via TGF-beta: Implications for Multiple Sclerosis Therapy. J. Clin. Cell Immunol. 2017, 8, 488. [Google Scholar] [CrossRef]
- Mondal, S.; Pahan, K. Cinnamon ameliorates experimental allergic encephalomyelitis in mice via regulatory T cells: Implications for multiple sclerosis therapy. PLoS ONE 2015, 10, e0116566. [Google Scholar] [CrossRef]
- Hou, Y.; Heon Ryu, C.; Jun, J.A.; Kim, S.M.; Jeong, C.H.; Jeun, S.S. Interferon beta-secreting mesenchymal stem cells combined with minocycline attenuate experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2014, 274, 20–27. [Google Scholar] [CrossRef]
- Shao, Y.; Sang, J.; Fu, J. On human pluripotent stem cell control: The rise of 3D bioengineering and mechanobiology. Biomaterials 2015, 52, 26–43. [Google Scholar] [CrossRef]
- Fanciullino, R.; Ciccolini, J.; Milano, G. Challenges, expectations and limits for nanoparticles-based therapeutics in cancer: A focus on nano-albumin-bound drugs. Crit. Rev. Oncol. Hemato.l 2013, 88, 504–513. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, R.I. Blood-Brain Delivery Methods Using Nanotechnology. Pharmaceutics 2018, 10, 269. [Google Scholar] [CrossRef]
- Zottel, A.; Videtic Paska, A.; Jovcevska, I. Nanotechnology Meets Oncology: Nanomaterials in Brain Cancer Research, Diagnosis and Therapy. Materials 2019, 12, 1588. [Google Scholar] [CrossRef]
- Guo, S.; Perets, N.; Betzer, O.; Ben-Shaul, S.; Sheinin, A.; Michaelevski, I.; Popovtzer, R.; Offen, D.; Levenberg, S. Intranasal Delivery of Mesenchymal Stem Cell Derived Exosomes Loaded with Phosphatase and Tensin Homolog siRNA Repairs Complete Spinal Cord Injury. ACS Nano 2019, 13, 10015–10028. [Google Scholar] [CrossRef]
- Yuan, X.; Wu, Q.; Wang, P.; Jing, Y.; Yao, H.; Tang, Y.; Li, Z.; Zhang, H.; Xiu, R. Exosomes Derived From Pericytes Improve Microcirculation and Protect Blood-Spinal Cord Barrier After Spinal Cord Injury in Mice. Front. Neurosci. 2019, 13, 319. [Google Scholar] [CrossRef]
- Zuidema, J.M.; Gilbert, R.J.; Osterhout, D.J. Nanoparticle Technologies in the Spinal Cord. Cells Tissues Organs 2016, 202, 102–115. [Google Scholar] [CrossRef]
- Kim, Y.T.; Caldwell, J.M.; Bellamkonda, R.V. Nanoparticle-mediated local delivery of Methylprednisolone after spinal cord injury. Biomaterials 2009, 30, 2582–2590. [Google Scholar] [CrossRef]
- Ren, H.; Han, M.; Zhou, J.; Zheng, Z.F.; Lu, P.; Wang, J.J.; Wang, J.Q.; Mao, Q.J.; Gao, J.Q.; Ouyang, H.W. Repair of spinal cord injury by inhibition of astrocyte growth and inflammatory factor synthesis through local delivery of flavopiridol in PLGA nanoparticles. Biomaterials 2014, 35, 6585–6594. [Google Scholar] [CrossRef]
- Baumann, M.D.; Kang, C.E.; Tator, C.H.; Shoichet, M.S. Intrathecal delivery of a polymeric nanocomposite hydrogel after spinal cord injury. Biomaterials 2010, 31, 7631–7639. [Google Scholar] [CrossRef]
- Figley, S.A.; Khosravi, R.; Legasto, J.M.; Tseng, Y.F.; Fehlings, M.G. Characterization of vascular disruption and blood-spinal cord barrier permeability following traumatic spinal cord injury. J. Neurotrauma 2014, 31, 541–552. [Google Scholar] [CrossRef]
- Cohen, D.M.; Patel, C.B.; Ahobila-Vajjula, P.; Sundberg, L.M.; Chacko, T.; Liu, S.J.; Narayana, P.A. Blood-spinal cord barrier permeability in experimental spinal cord injury: Dynamic contrast-enhanced MRI. NMR Biomed. 2009, 22, 332–341. [Google Scholar] [CrossRef]
- Hardiman, O.; Al-Chalabi, A.; Chio, A.; Corr, E.M.; Logroscino, G.; Robberecht, W.; Shaw, P.J.; Simmons, Z.; van den Berg, L.H. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers 2017, 3, 17085. [Google Scholar] [CrossRef]
- Rosen, D.R.; Siddique, T.; Patterson, D.; Figlewicz, D.A.; Sapp, P.; Hentati, A.; Donaldson, D.; Goto, J.; O’Regan, J.P.; Deng, H.X.; et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 1993, 362, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Rowland, L.P.; Shneider, N.A. Amyotrophic lateral sclerosis. N. Engl. J. Med. 2001, 344, 1688–1700. [Google Scholar] [CrossRef] [PubMed]
- Li, H.F.; Wu, Z.Y. Genotype-phenotype correlations of amyotrophic lateral sclerosis. Transl. Neurodegener. 2016, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Guiloff, R.J.; McGregor, B.; Thompson, E.; Blackwood, W.; Paul, E. Motor neurone disease with elevated cerebrospinal fluid protein. J. Neurol. Neurosurg. Psychiatry 1980, 43, 390–396. [Google Scholar] [CrossRef]
- Leonardi, A.; Abbruzzese, G.; Arata, L.; Cocito, L.; Vische, M. Cerebrospinal fluid (CSF) findings in amyotrophic lateral sclerosis. J. Neurol. 1984, 231, 75–78. [Google Scholar] [CrossRef]
- Meucci, G.; Rossi, G.; Bettini, R.; Montanaro, D.; Gironelli, L.; Voci, L.; Bianchi, F. Laser nephelometric evaluation of albumin, IgG and alpha 2-macroglobulin: Applications to the study of alterations of the blood-brain barrier. J. Neurol. Sci. 1993, 118, 73–78. [Google Scholar] [CrossRef]
- Pirttila, T.; Vanhatalo, S.; Turpeinen, U.; Riikonen, R. Cerebrospinal fluid insulin-like growth factor-1, insulin growth factor binding protein-2 or nitric oxide are not increased in MS or ALS. Acta Neurol. Scand. 2004, 109, 337–341. [Google Scholar] [CrossRef]
- Annunziata, P.; Volpi, N. High levels of C3c in the cerebrospinal fluid from amyotrophic lateral sclerosis patients. Acta Neurol. Scand. 1985, 72, 61–64. [Google Scholar] [CrossRef]
- Apostolski, S.; Nikolic, J.; Bugarski-Prokopljevic, C.; Miletic, V.; Pavlovic, S.; Filipovic, S. Serum and CSF immunological findings in ALS. Acta Neurol. Scand. 1991, 83, 96–98. [Google Scholar] [CrossRef]
- Brettschneider, J.; Petzold, A.; Sussmuth, S.D.; Ludolph, A.C.; Tumani, H. Axonal damage markers in cerebrospinal fluid are increased in ALS. Neurology 2006, 66, 852–856. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.H.; Wu, J.J.; Ren, H.M.; Wang, J.; Ding, Z.T.; Jiang, Y.P. Proteomic analysis of cerebrospinal fluid in amyotrophic lateral sclerosis. Exp. Ther. Med. 2016, 11, 2095–2106. [Google Scholar] [CrossRef]
- Li, W.; Maeda, Y.; Yuan, R.R.; Elkabes, S.; Cook, S.; Dowling, P. Beneficial effect of erythropoietin on experimental allergic encephalomyelitis. Ann. Neurol. 2004, 56, 767–777. [Google Scholar] [CrossRef]
- Kim, Y.J.; Park, H.J.; Lee, G.; Bang, O.Y.; Ahn, Y.H.; Joe, E.; Kim, H.O.; Lee, P.H. Neuroprotective effects of human mesenchymal stem cells on dopaminergic neurons through anti-inflammatory action. Glia 2009, 57, 13–23. [Google Scholar] [CrossRef]
- Palmer, A.M. Multiple sclerosis and the blood-central nervous system barrier. Cardiovasc. Psychiatry Neurol. 2013, 2013, 530356. [Google Scholar] [CrossRef]
- Schellenberg, A.E.; Buist, R.; Yong, V.W.; Del Bigio, M.R.; Peeling, J. Magnetic resonance imaging of blood-spinal cord barrier disruption in mice with experimental autoimmune encephalomyelitis. Magn. Reson. Med. 2007, 58, 298–305. [Google Scholar] [CrossRef]
- Wu, F.; Cao, W.; Yang, Y.; Liu, A. Extensive infiltration of neutrophils in the acute phase of experimental autoimmune encephalomyelitis in C57BL/6 mice. Histochem. Cell Biol. 2010, 133, 313–322. [Google Scholar] [CrossRef]
- Liu, X.; Yao, D.L.; Webster, H. Insulin-like growth factor I treatment reduces clinical deficits and lesion severity in acute demyelinating experimental autoimmune encephalomyelitis. Mult. Scler. 1995, 1, 2–9. [Google Scholar] [CrossRef]
- Manouchehri, N.; Stuve, O. Choroid plexus volumetrics and brain inflammation in multiple sclerosis. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef]
- Canavero, S.; Pagni, C.A.; Duca, S.; Bradac, G.B. Spinal intramedullary cavernous angiomas: A literature meta-analysis. Surg. Neurol. 1994, 41, 381–388. [Google Scholar] [CrossRef]
- El-Koussy, M.; Stepper, F.; Spreng, A.; Lukes, A.; Gralla, J.; Brekenfeld, C.; Sturzenegger, M.; Schroth, G. Incidence, clinical presentation and imaging findings of cavernous malformations of the CNS. A twenty-year experience. Swiss Med. Wkly. 2011, 141, w13172. [Google Scholar] [CrossRef]
- Gross, B.A.; Du, R.; Popp, A.J.; Day, A.L. Intramedullary spinal cord cavernous malformations. Neurosurg. Focus 2010, 29, E14. [Google Scholar] [CrossRef]
- McCormick, P.C.; Michelsen, W.J.; Post, K.D.; Carmel, P.W.; Stein, B.M. Cavernous malformations of the spinal cord. Neurosurgery 1988, 23, 459–463. [Google Scholar] [CrossRef]
- Mitha, A.P.; Turner, J.D.; Abla, A.A.; Vishteh, A.G.; Spetzler, R.F. Outcomes following resection of intramedullary spinal cord cavernous malformations: A 25-year experience. J. Neurosurg. Spine 2011, 14, 605–611. [Google Scholar] [CrossRef]
- Reitz, M.; Burkhardt, T.; Vettorazzi, E.; Raimund, F.; Fritzsche, E.; Schmidt, N.O.; Regelsberger, J.; Westphal, M.; Eicker, S.O. Intramedullary spinal cavernoma: Clinical presentation, microsurgical approach, and long-term outcome in a cohort of 48 patients. Neurosurg. Focus 2015, 39, E19. [Google Scholar] [CrossRef] [PubMed]
- Anson, J.A.; Spetzler, R.F. Surgical resection of intramedullary spinal cord cavernous malformations. J. Neurosurg. 1993, 78, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Khalatbari, M.R.; Hamidi, M.; Moharamzad, Y. Pediatric intramedullary cavernous malformation of the conus medullaris: Case report and review of the literature. Child’s Nerv Syst. 2011, 27, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Liu, A.; Zhang, J.T.; Sun, B.; Zhao, Y.L. Surgical management of brain-stem cavernous malformations: Report of 137 cases. Surg. Neurol. 2003, 59, 444–454, discussion 454. [Google Scholar] [CrossRef]
- Clatterbuck, R.E.; Eberhart, C.G.; Crain, B.J.; Rigamonti, D. Ultrastructural and immunocytochemical evidence that an incompetent blood-brain barrier is related to the pathophysiology of cavernous malformations. J. Neurol. Neurosurg. Psychiatry 2001, 71, 188–192. [Google Scholar] [CrossRef]
- Choi, J.P.; Wang, R.; Yang, X.; Wang, X.; Wang, L.; Ting, K.K.; Foley, M.; Cogger, V.; Yang, Z.; Liu, F.; et al. Ponatinib (AP24534) inhibits MEKK3-KLF signaling and prevents formation and progression of cerebral cavernous malformations. Sci. Adv. 2018, 4, eaau0731. [Google Scholar] [CrossRef]
- Davies, B.M.; Mowforth, O.D.; Smith, E.K.; Kotter, M.R. Degenerative cervical myelopathy. BMJ 2018, 360, k186. [Google Scholar] [CrossRef]
- Martin-Vaquero, P.; da Costa, R.C.; Allen, M.J.; Moore, S.A.; Keirsey, J.K.; Green, K.B. Proteomic analysis of cerebrospinal fluid in canine cervical spondylomyelopathy. Spine 2015, 40, 601–612. [Google Scholar] [CrossRef]
- Bartlett, R.D.; Eleftheriadou, D.; Evans, R.; Choi, D.; Phillips, J.B. Mechanical properties of the spinal cord and brain: Comparison with clinical-grade biomaterials for tissue engineering and regenerative medicine. Biomaterials 2020, 258, 120303. [Google Scholar] [CrossRef]
- Li, Y.Q.; Chen, P.; Haimovitz-Friedman, A.; Reilly, R.M.; Wong, C.S. Endothelial apoptosis initiates acute blood-brain barrier disruption after ionizing radiation. Cancer Res. 2003, 63, 5950–5956. [Google Scholar]
- Ljubimova, N.V.; Levitman, M.K.; Plotnikova, E.D.; Eidus, L. Endothelial cell population dynamics in rat brain after local irradiation. Br. J. Radiol. 1991, 64, 934–940. [Google Scholar] [CrossRef]
- Lossinsky, A.S.; Mossakowski, M.J.; Pluta, R.; Wisniewski, H.M. Intercellular adhesion molecule-1 (ICAM-1) upregulation in human brain tumors as an expression of increased blood-brain barrier permeability. Brain Pathol. 1995, 5, 339–344. [Google Scholar] [CrossRef]
- Carlos, T.M.; Clark, R.S.; Franicola-Higgins, D.; Schiding, J.K.; Kochanek, P.M. Expression of endothelial adhesion molecules and recruitment of neutrophils after traumatic brain injury in rats. J. Leukoc. Biol. 1997, 61, 279–285. [Google Scholar] [CrossRef]
- Clark, W.M.; Lauten, J.D.; Lessov, N.; Woodward, W.; Coull, B.M. Time course of ICAM-1 expression and leukocyte subset infiltration in rat forebrain ischemia. Mol. Chem. Neuropathol. 1995, 26, 213–230. [Google Scholar] [CrossRef]
- Nordal, R.A.; Wong, C.S. Intercellular adhesion molecule-1 and blood-spinal cord barrier disruption in central nervous system radiation injury. J. Neuropathol. Exp. Neurol. 2004, 63, 474–483. [Google Scholar] [CrossRef]
- Li, Y.Q.; Ballinger, J.R.; Nordal, R.A.; Su, Z.F.; Wong, C.S. Hypoxia in radiation-induced blood-spinal cord barrier breakdown. Cancer Res. 2001, 61, 3348–3354. [Google Scholar]
- Tsao, M.N.; Li, Y.Q.; Lu, G.; Xu, Y.; Wong, C.S. Upregulation of vascular endothelial growth factor is associated with radiation-induced blood-spinal cord barrier breakdown. J. Neuropathol. Exp. Neurol. 1999, 58, 1051–1060. [Google Scholar] [CrossRef]
- Piekarz, K.M.; Bhaskaran, S.; Sataranatarajan, K.; Street, K.; Premkumar, P.; Saunders, D.; Zalles, M.; Gulej, R.; Khademi, S.; Laurin, J.; et al. Molecular changes associated with spinal cord aging. Geroscience 2020, 42, 765–784. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A. The Blood-Brain Barrier Interface in Diabetes Mellitus: Dysfunctions, Mechanisms and Approaches to Treatment. Curr. Pharm. Des. 2020, 26, 1438–1447. [Google Scholar] [CrossRef]
- Cragg, J.J.; Noonan, V.K.; Dvorak, M.; Krassioukov, A.; Mancini, G.B.; Borisoff, J.F. Spinal cord injury and type 2 diabetes: Results from a population health survey. Neurology 2013, 81, 1864–1868. [Google Scholar] [CrossRef]
- Carrino, D.; Branca, J.J.V.; Becatti, M.; Paternostro, F.; Morucci, G.; Gulisano, M.; Di Cesare Mannelli, L.; Pacini, A. Alcohol-Induced Blood-Brain Barrier Impairment: An In Vitro Study. Int. J. Environ. Res. Public Health 2021, 18, 2683. [Google Scholar] [CrossRef]
- Wei, J.; Dai, Y.; Wen, W.; Li, J.; Ye, L.L.; Xu, S.; Duan, D.D. Blood-brain barrier integrity is the primary target of alcohol abuse. Chem. Biol. Interact. 2021, 337, 109400. [Google Scholar] [CrossRef]
- Valkov, T.; Hristova, J.; Tcherveniakova, T.; Svinarov, D. Blood-Brain Barrier and Intrathecal Immune Response in patients with neuroinfections. Infez. Med. 2017, 25, 320–325. [Google Scholar]
- Wang, Y.; Liu, X.; Liu, Q. NOD2 Expression in Streptococcus pneumoniae Meningitis and Its Influence on the Blood-Brain Barrier. Can. J. Infect. Dis. Med. Microbiol. 2018, 2018, 7292084. [Google Scholar] [CrossRef]
- Thompson, D.; Sorenson, J.; Greenmyer, J.; Brissette, C.A.; Watt, J.A. The Lyme disease bacterium, Borrelia burgdorferi, stimulates an inflammatory response in human choroid plexus epithelial cells. PLoS ONE 2020, 15, e0234993. [Google Scholar] [CrossRef]
- Carloni, S.; Bertocchi, A.; Mancinelli, S.; Bellini, M.; Erreni, M.; Borreca, A.; Braga, D.; Giugliano, S.; Mozzarelli, A.M.; Manganaro, D.; et al. Identification of a choroid plexus vascular barrier closing during intestinal inflammation. Science 2021, 374, 439–448. [Google Scholar] [CrossRef]
- Marques, F.; Sousa, J.C. The choroid plexus is modulated by various peripheral stimuli: Implications to diseases of the central nervous system. Front. Cell Neurosci. 2015, 9, 136. [Google Scholar] [CrossRef][Green Version]
- Bertrand, L.; Cho, H.J.; Toborek, M. Blood-brain barrier pericytes as a target for HIV-1 infection. Brain 2019, 142, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Rhea, E.M.; Logsdon, A.F.; Hansen, K.M.; Williams, L.M.; Reed, M.J.; Baumann, K.K.; Holden, S.J.; Raber, J.; Banks, W.A.; Erickson, M.A. The S1 protein of SARS-CoV-2 crosses the blood-brain barrier in mice. Nat. Neurosci. 2021, 24, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, L.; Albecka, A.; Mallery, D.L.; Kellner, M.J.; Paul, D.; Carter, A.P.; James, L.C.; Lancaster, M.A. SARS-CoV-2 Infects the Brain Choroid Plexus and Disrupts the Blood-CSF Barrier in Human Brain Organoids. Cell Stem Cell 2020, 27, 951–961 e955. [Google Scholar] [CrossRef] [PubMed]
- Christie, M.J.; Irving, A.T.; Forster, S.C.; Marsland, B.J.; Hansbro, P.M.; Hertzog, P.J.; Nold-Petry, C.A.; Nold, M.F. Of bats and men: Immunomodulatory treatment options for COVID-19 guided by the immunopathology of SARS-CoV-2 infection. Sci. Immunol. 2021, 6, eabd0205. [Google Scholar] [CrossRef]
- Buzhdygan, T.P.; DeOre, B.J.; Baldwin-Leclair, A.; Bullock, T.A.; McGary, H.M.; Khan, J.A.; Razmpour, R.; Hale, J.F.; Galie, P.A.; Potula, R.; et al. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood-brain barrier. Neurobiol. Dis. 2020, 146, 105131. [Google Scholar] [CrossRef]
- Leda, A.R.; Bertrand, L.; Andras, I.E.; El-Hage, N.; Nair, M.; Toborek, M. Selective Disruption of the Blood-Brain Barrier by Zika Virus. Front. Microbiol. 2019, 10, 2158. [Google Scholar] [CrossRef]
- Shao, Q.; Herrlinger, S.; Yang, S.L.; Lai, F.; Moore, J.M.; Brindley, M.A.; Chen, J.F. Zika virus infection disrupts neurovascular development and results in postnatal microcephaly with brain damage. Development 2016, 143, 4127–4136. [Google Scholar] [CrossRef]
- Suwannasual, U.; Lucero, J.; Davis, G.; McDonald, J.D.; Lund, A.K. Mixed Vehicle Emissions Induces Angiotensin II and Cerebral Microvascular Angiotensin Receptor Expression in C57Bl/6 Mice and Promotes Alterations in Integrity in a Blood-Brain Barrier Coculture Model. Toxicol. Sci. 2019, 170, 525–535. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, C.; Zhang, X.; Zhu, J.; Wang, L.; Ji, M.; Zhang, Z.; Ji, X.M.; Wang, S.L. Perfluorooctane sulfonate disrupts the blood brain barrier through the crosstalk between endothelial cells and astrocytes in mice. Environ. Pollut. 2020, 256, 113429. [Google Scholar] [CrossRef]
- Garate-Velez, L.; Escudero-Lourdes, C.; Salado-Leza, D.; Gonzalez-Sanchez, A.; Alvarado-Morales, I.; Bahena, D.; Labrada-Delgado, G.J.; Rodriguez-Lopez, J.L. Anthropogenic Iron Oxide Nanoparticles Induce Damage to Brain Microvascular Endothelial Cells Forming the Blood-Brain Barrier. J. Alzheimers Dis. 2020, 76, 1527–1539. [Google Scholar] [CrossRef]
- Miyazaki, W.; Fujiwara, Y.; Katoh, T. The effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on the development and function of the blood-brain barrier. Neurotoxicology 2016, 52, 64–71. [Google Scholar] [CrossRef]
- Calderon-Garciduenas, L.; Vojdani, A.; Blaurock-Busch, E.; Busch, Y.; Friedle, A.; Franco-Lira, M.; Sarathi-Mukherjee, P.; Martinez-Aguirre, X.; Park, S.B.; Torres-Jardon, R.; et al. Air pollution and children: Neural and tight junction antibodies and combustion metals, the role of barrier breakdown and brain immunity in neurodegeneration. J. Alzheimers Dis. 2015, 43, 1039–1058. [Google Scholar] [CrossRef]
- Rosell, A.; Cuadrado, E.; Ortega-Aznar, A.; Hernandez-Guillamon, M.; Lo, E.H.; Montaner, J. MMP-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke 2008, 39, 1121–1126. [Google Scholar] [CrossRef]
- Ries, C. Cytokine functions of TIMP-1. Cell. Mol. Life Sci. 2014, 71, 659–672. [Google Scholar] [CrossRef]
- Li, D.D.; Song, J.N.; Huang, H.; Guo, X.Y.; An, J.Y.; Zhang, M.; Li, Y.; Sun, P.; Pang, H.G.; Zhao, Y.L.; et al. The roles of MMP-9/TIMP-1 in cerebral edema following experimental acute cerebral infarction in rats. Neurosci. Lett. 2013, 550, 168–172. [Google Scholar] [CrossRef]
- Tang, J.; Kang, Y.; Huang, L.; Wu, L.; Peng, Y. TIMP1 preserves the blood-brain barrier through interacting with CD63/integrin beta 1 complex and regulating downstream FAK/RhoA signaling. Acta Pharm. Sin. B. 2020, 10, 987–1003. [Google Scholar] [CrossRef]
- Souza, P.S.; Goncalves, E.D.; Pedroso, G.S.; Farias, H.R.; Junqueira, S.C.; Marcon, R.; Tuon, T.; Cola, M.; Silveira, P.C.L.; Santos, A.R.; et al. Physical Exercise Attenuates Experimental Autoimmune Encephalomyelitis by Inhibiting Peripheral Immune Response and Blood-Brain Barrier Disruption. Mol. Neurobiol. 2017, 54, 4723–4737. [Google Scholar] [CrossRef]
- Mokhtarzade, M.; Motl, R.; Negaresh, R.; Zimmer, P.; Khodadoost, M.; Baker, J.S.; Patel, D.; Majdinasab, N.; Ranjbar, R. Exercise-induced changes in neurotrophic factors and markers of blood-brain barrier permeability are moderated by weight status in multiple sclerosis. Neuropeptides 2018, 70, 93–100. [Google Scholar] [CrossRef]
- Kumarasamy, M.; Sosnik, A. Heterocellular spheroids of the neurovascular blood-brain barrier as a platform for personalized nanoneuromedicine. iScience 2021, 24, 102183. [Google Scholar] [CrossRef]
- Zhuang, X.; Xiang, X.; Grizzle, W.; Sun, D.; Zhang, S.; Axtell, R.C.; Ju, S.; Mu, J.; Zhang, L.; Steinman, L.; et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol. Ther. 2011, 19, 1769–1779. [Google Scholar] [CrossRef]
| Feature | Blood-Brain Barrier (BBB) | Blood-Spinal Cord Barrier (BSCB) | References |
|---|---|---|---|
| Permeability | Low | High: 3H-mannitol and 14C-inulin | [19] |
| Tight Junction proteins | High | Low: ZO-1, occludin, β-catenin, VE-cadherin | [20] |
| Number of pericytes | High | Low | [21] |
| Glycogen Deposits | Low | High | [7] |
| Cells | Cause/Effects of BSCB Impairment | Refs. |
|---|---|---|
| Spinal Cord Injury (SCI) | ||
| Microglia | Jmjd3 ↑ → NF-κB → MMP3 ↑ and MMP9 ↑ | [44] |
| TRPV4 ↑ → spinal cord scarring, endothelial damage, BSCB damage | [47] | |
| MMP-8 ↑ → occludin ↓ and ZO-1 ↓ | [45] | |
| MMP-12 → functional recovery↓, BSCB permeability ↓ | [46] | |
| USP4 ↓; NF-κB → TRFAF6 → Neuronal inflammation | [48] | |
| AR deficiency → M2 response → locomotion recovery AR inhibition → HNE accumulation → phosphorylation of CREB → Arg1 ↑ | [49] | |
| AQP4 ↑ → BSCB permeability ↑ | [50] | |
| Reactive astrocytes (RAs) | Shh/Gli ↑ → BSCB permeability ↑, locomotor recovery ↓ | [25] |
| Ras → fibronectin/β1 integrin pathway → microglial inflammation | [51] | |
| Calmodulin → AQP4 ↑ | [52] | |
| Macrophages | Perforin ↑ → BSCB permeability ↑ → cytokine and inflammatory cell infiltration | [49,51,53] |
| Neutrophils | MMP-3 ↑ → NF-κB → occludin ↓ and ZO-1 ↓ | [43] |
| Amyotrophic Lateral Sclerosis (ALS) | ||
| Astrocytes | Wnt7a ↓, Wnt5a ↓ Gi signalling in astrocytes restores BSCB integrity | [54] |
| Swollen astrocyte foot processes Degenerating astrocytes | [55] | |
| Glutamate ↑ → EC P-gp ↑, NMDA ↑ | [56] | |
| Neurons | Motor neuron loss | [9,30,35,54,55,56,57,58,59,60,61,62,63] |
| PDGFC ↑ → BSCB dysfunction | [57] | |
| Immune cells | Erythrocyte extravasation | [30] |
| Pericyte | Reduction in pericytes | [30,35] |
| Endothelial cells | Glut-1 ↓, CD146 ↓ | [9] |
| Claudin 5 ↓, occludin ↓, ZO-1↓ | [60,62,63] | |
| Cytoplasmic vacuoles | [61] | |
| Mitochondrial degeneration | [55] | |
| P-gp ↑, BCRP ↑, MRP2 ↑ | [56,57,59] | |
| ROS ↑ | [62] | |
| Circulating ECs ↓ | [58] | |
| ECM | Agrin ↓ | [60] |
| Peripheral Nerve Injury (PNI) | ||
| Microglia | MCP-1 ↑; EB extravasation ↑; IL-1β ↑; TGF-β1 ↑; ZO-1, occludin ↓ | [64] |
| MCP-1 → microglial activation → neuropathic pain → delayed astrocyte activation | [65] | |
| Astrocytes | AQP4 ↑ → length and volume of astrocytic processes ↑ | [66] |
| SUR1-TRPM4 ↑ → dorsal horn astrocytes | [67] | |
| Degenerative Cervical Myelopathy (DCM) | ||
| Immune cells | Angiopoietin 2 ↓, VEGF C ↓ | [68] |
| Peripheral monocytes ↑ | [69] | |
| IgGA ↑, IgGQ ↑, BSCB permeability ↑ | [29] | |
| Multiple Sclerosis | ||
| Endothelial cells | Claudin-11 ↓; BSCB permeability ↑ | [70] |
| Microglia | TMP → STAT3/SOC3 → NF-κB → M1 to M2 polarization TNF-α ↑, IL-1β ↑, IL-4 ↓, IL-10 ↓ | [26] |
| Neutrophils | IL-R1 → adhesion of neutrophils to inflamed SC | [71] |
| Spinal Cord Ischemia | ||
| Microglia | TLR4/MyD88/TRIF ↓ Inflammation ↓ | [72] |
| HMGB1 ↑ | [73] | |
| CXCL13/CXCR5 ↑ → ERK | [74] | |
| TUG ↓ → TRIL ↓ → NF-κB/IL-1β ↓ | [75] | |
| Nrf2 ↑ → p53/p38/MAPK/NF-κB → ABC transporters | [76] | |
| Cancer | ||
| Microglia | ZO-1 ↓, claudin-5 ↓ | [77] |
| Intervention | Mechanisms | Refs. | |
|---|---|---|---|
| DRUGS | Spinal Cord Injury (SCI) | ||
| Valproic acid | M2 polarization HDAC3, STAT1 ↓ TNF-α, IL-1β, IL-6, IFN-γ ↓ | [87] | |
| DL-3-n-butylphthalide (DL-NBP) | Motor function ↑, oedema ↓ | [90] | |
| Bradykinin B2 receptor antagonist- HOE-140 | SC blood flow ↑; nNOS ↑ BSCB disruption ↓, oedema formation ↓ | [91] | |
| Protocatechuic acid (PCA) | Apoptotic cell death of neurons and oligodendrocytes ↓ Infiltration of neutrophils and macrophages ↓ MMP-9 ↓ TNFα, IL-1β, cyclooxygenase-2, inducible nitric oxide synthase and Chemokines ↓ | [83] | |
| Brilliant blue G (BBG) | P2X7, NLRP3, ASC, cleaved XIAP, caspase-1, caspase-11, IL-1ß, IL-18 ↑ | [92] | |
| Human immunoglobulin G (hIgG) | Antagonize neutrophil infiltration Neutrophil chemo-attractants ↑ | [93,94] | |
| Haem oxygenase (HO)-1 | 4-Hydroxynonenal (4-HNE), malondialdehyde (MDA) ↑ | [95] | |
| NaHS (H2S donor) | TJ proteins ↑ BSCB permeability ↓ | [96] | |
| Gallic acid (GA) | Jmjd3 ↓, MMP9 ↓ Neutrophil and macrophage infiltration ↓ | [97] | |
| Dl-3-n-butylphthalide (NBP) | ER stress ↓ Occludin, p120-Catenin, β-Catenin, claudin-5 ↑ | [98] | |
| Lithium chloride (LiCl) | Occludin, claudin-5 ↑ ER stress ↓ LC3-II, ATG-5 ↑ p62 ↓ | [99,100] | |
| Lycopene | Water content ↓ TNF-α and NF-kB ↓ ZO-1, claudin-5 ↑ | [101] | |
| Phenylbutyrate (PBA) | p120, β-catenin, occludin, claudin5 ↑ ER stress ↓ BSCB permeability ↓ | [102,103] | |
| Bromodomain and extra-terminal domain (BET) proteins | Pro-inflammatory mediators ↓ Anti-inflammatory cytokines ↑ Reactivity of microglia/macrophages ↓ Neuroprotection and functional recovery ↑ | [104] | |
| Folic acid (FA) | MMP2 ↓ | [105] | |
| Flufenamic acid | TrpM4 ↓ MMP2 ↓, MMP9 ↓ | [84] | |
| Valproic acid | Microglia polarization; ↓TNF-α, IL-1β, IL-6, INF-γ | [87] | |
| Oleanolic acid (OA) | Caspase-3 ↓ Pro-inflammatory response ↓ MAPKs, NF-κB ↓ | [88] | |
| Salvianolic acid (A and B) | ZO-1, occludin ↑ TNF-α and NF-κB ↓ miR-101/Cul3/Nrf2/HO-1 ↑ | [89,106] | |
| Fluoxetine | MMP2 ↓, MMP9 ↓ ZO-1, occludin ↑ Groα, MIP1α and 1β ↓ Infiltration of neutrophils and macrophage ↓ | [107,108] | |
| Simvastatin-ezetimibe | ICAM-1 ↓ Endothelial inflammatory response ↓ Wnt/β-catenin ↑ | [109,110] | |
| Retinoic acid (RA) | P120, β-catenin, occludin and claudin5 ↑ CHOP ↓, caspase12 ↓ | [111] | |
| Epidermal growth factor (EGF) | Bax ↓, Bcl-2 ↑ Superoxide dismutase (SOD) ↑ glutathione peroxidase (GPx) ↑ | [112] | |
| Basic fibroblast growth factor (bFGF) | MMP9 ↓ Caveolin-1, TJs, including occludin, claudin-5, p120-catenin and β-catenin ↑ PRDX1 ↑→ autophagy Neuroprotection ↑, axonal regeneration | [113,114] | |
| Methylprednisolone (MP) and aminoguanidine (AG) | AQP4 ↓ iNOS ↓ | [115,116] | |
| Tetramethylpyrazine (TMP) | BSCB permeability ↓ IL-1β, TNFα, IL-18, TUNEL-positive cells, caspase 3/9 ↓ | [117] | |
| Curcumin | TNF-α and NF-κB ↓ ZO-1, occludin ↑ | [118] | |
| Eugenol | NF-κB, p38 MAPK ↓ Inflammation, oxidative stress ↓ | [119] | |
| Mithramycin A (MA) Ghrelin 17β-estradiol (E2) | MMP9 ↓ SUR1/TRPM4 ↓ TJs ↑ | [81,120] | |
| NANOPARTICLES | Carbon monoxide-releasing molecule-2 (CORM-2) | ZO-1, ZO-2, occludin and claudin-1 ↑ BSCB permeability ↓ | [121] |
| CORM-3 | TJs ↑, MMP9 ↓ | [122] | |
| Bone mesenchymal stem cell-derived extracellular vesicles (BMSC-EV) | Brain cell death ↓ Neuronal survival ↑, motor function ↑ Pericyte migration ↓ | [123] | |
| Poly (d,l-lactide co-glycolide, PLGA)-based NPs | Localization at lesion site | [124,125,126] | |
| BIOMATERIALS | Astragoloside IV Loaded Polycaprolactone Membrane | Caspase3 ↓, Bax/Bcl-2 ↓ Occludin, claudin5, ZO-1 ↑ MMP9 ↓, neutrophil infiltration ↓ BSCB permeability ↓ | [127] |
| MSCs | BSCB leakage ↓ von Willebrand factor (vWF) ↑ Locomotor function ↑ | [128] | |
| HAMC/PLGA/FGF2 | FGF2 ↑ | [129] | |
| miRNAs | miRNA-125a-5p | ZO-1, occludin, VE-cadherin ↑ BSCB permeability ↓ | [130] |
| miR-429 | ZO-1, occludin and claudin-5 ↑ Krüppel-like factor 6 (KLF6) ↓ | [131,132] | |
| Ad-GFP-HO-1C[INCREMENT]23 | Hindlimb function ↑ TJs ↑ | [133] | |
| Amyotrophic Lateral Sclerosis (ALS) | |||
| DRUGS | APC (Activated protein C) | IgG and iron deposition ↓ ZO-1, occludin ↑ | [134,135] |
| AMD3100 | CXCR4/CXCL12 ↓ Microglial pathology ↑ Proinflammatory cytokines ↓ | [136] | |
| BIOMATERIALS | Unmodified human bone marrow CD34+ (hBM34+) stem cells | EB extravasation ↓ Restored capillary ultrastructure Engrafted widely into capillaries of the gray/white matter SC and brain Motor cortex/brainstem structural and functional repair of BSCB impairment | [137,138,139] |
| Human bone marrow-derived endothelial progenitor cells (hBMEPCs) | VEGF-A and angiogenin-1 ↑ EC phenotype ↑ ZO-1, occludin ↑ | [140] | |
| Mesenchymal stem cells (MSCs) | Motor neuron loss ↓ Locomotor activity ↑ Neurturin ↑ | [141] | |
| Spinal Cord Ischemia | |||
| DRUGS | Propofol | BSCB permeability ↓, MMP9 ↓, NF-kB ↓ Occludin ↑, claudin-5 ↑ | [142] |
| Dexmedetomidine (Dex) | HMGB1-TLR4-NF-κB signalling pathway ↓ MMP9 ↓, angiopoietin-1 (Ang1) and Tie2 ↑ | [143,144] | |
| Remote ischemic preconditioning (RIPC) | Cannabinoid-1,2 receptors ↑ BSCB integrity ↑ ZO-1 ↑ MMP9 ↓, TNF-α ↓ | [145,146] | |
| Sevoflurane | MMP9 ↓ CXCL10, CCL2 ↓ IL-1β ↓ | [147] | |
| BIOMATERIALS | BM-MSCs | EB extravasation ↓ MMP9 ↓, TNF-α ↓ | [148,149] |
| miRNA | miR-128-3p | Specificity protein 1 (SP1) ↓ Neuroinflammation ↓ | [150] |
| miR-320a | AQP1 ↓ | [151] | |
| miR-27a | TICAM-2 ↓→NF-κB ↓ | [152] | |
| Multiple Sclerosis | |||
| DRUGS | Tetramethylpyrazine (TMP) | TNF-α, IL-1β ↓ IL-4, IL-10 ↑; TJs ↑ STAT3/SOCS3 ↑→NF-кB ↓→M2 polarization | [117] |
| Calcitriol (vitamin D analog) | NLRP3, caspase-1, (IL)-1β, CX3CR1, CCL17, RORc, Tbx21 ↓ MHCII ↓ ZO-1 ↑ | [153] | |
| ADAMTS13 | VWF ↓ Demyelination ↓ T lymphocyte, neutrophil and monocyte infiltration ↓ | [154] | |
| Glyceryl tribenzoate (GTB) and Cinnamon | Perivascular cuffing ↓ Inflammation ↓ TGF-β, regulatory T cells (Tregs) in splenocytes ↑ | [155,156] | |
| BIOMATERIALS | MSCs-IFN-β+minocycline | IFN-γ, TNF-α ↓ IL-4, IL-10 ↑ MMP9 ↓ | [157] |
| Peripheral Nerve Injury (PNI) | |||
| Salmon thrombin | TNF-α-induced endothelial permeability ↓ BSCB breakdown ↓ | [158] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chopra, N.; Menounos, S.; Choi, J.P.; Hansbro, P.M.; Diwan, A.D.; Das, A. Blood-Spinal Cord Barrier: Its Role in Spinal Disorders and Emerging Therapeutic Strategies. NeuroSci 2022, 3, 1-27. https://doi.org/10.3390/neurosci3010001
Chopra N, Menounos S, Choi JP, Hansbro PM, Diwan AD, Das A. Blood-Spinal Cord Barrier: Its Role in Spinal Disorders and Emerging Therapeutic Strategies. NeuroSci. 2022; 3(1):1-27. https://doi.org/10.3390/neurosci3010001
Chicago/Turabian StyleChopra, Neha, Spiro Menounos, Jaesung P. Choi, Philip M. Hansbro, Ashish D. Diwan, and Abhirup Das. 2022. "Blood-Spinal Cord Barrier: Its Role in Spinal Disorders and Emerging Therapeutic Strategies" NeuroSci 3, no. 1: 1-27. https://doi.org/10.3390/neurosci3010001
APA StyleChopra, N., Menounos, S., Choi, J. P., Hansbro, P. M., Diwan, A. D., & Das, A. (2022). Blood-Spinal Cord Barrier: Its Role in Spinal Disorders and Emerging Therapeutic Strategies. NeuroSci, 3(1), 1-27. https://doi.org/10.3390/neurosci3010001







