P2Y1 Receptor as a Catalyst of Brain Neurodegeneration
Abstract
:1. Introduction
2. The Multimodal P2Y1 Receptor
3. P2Y1 Receptor in Neurodegenerative Disorders
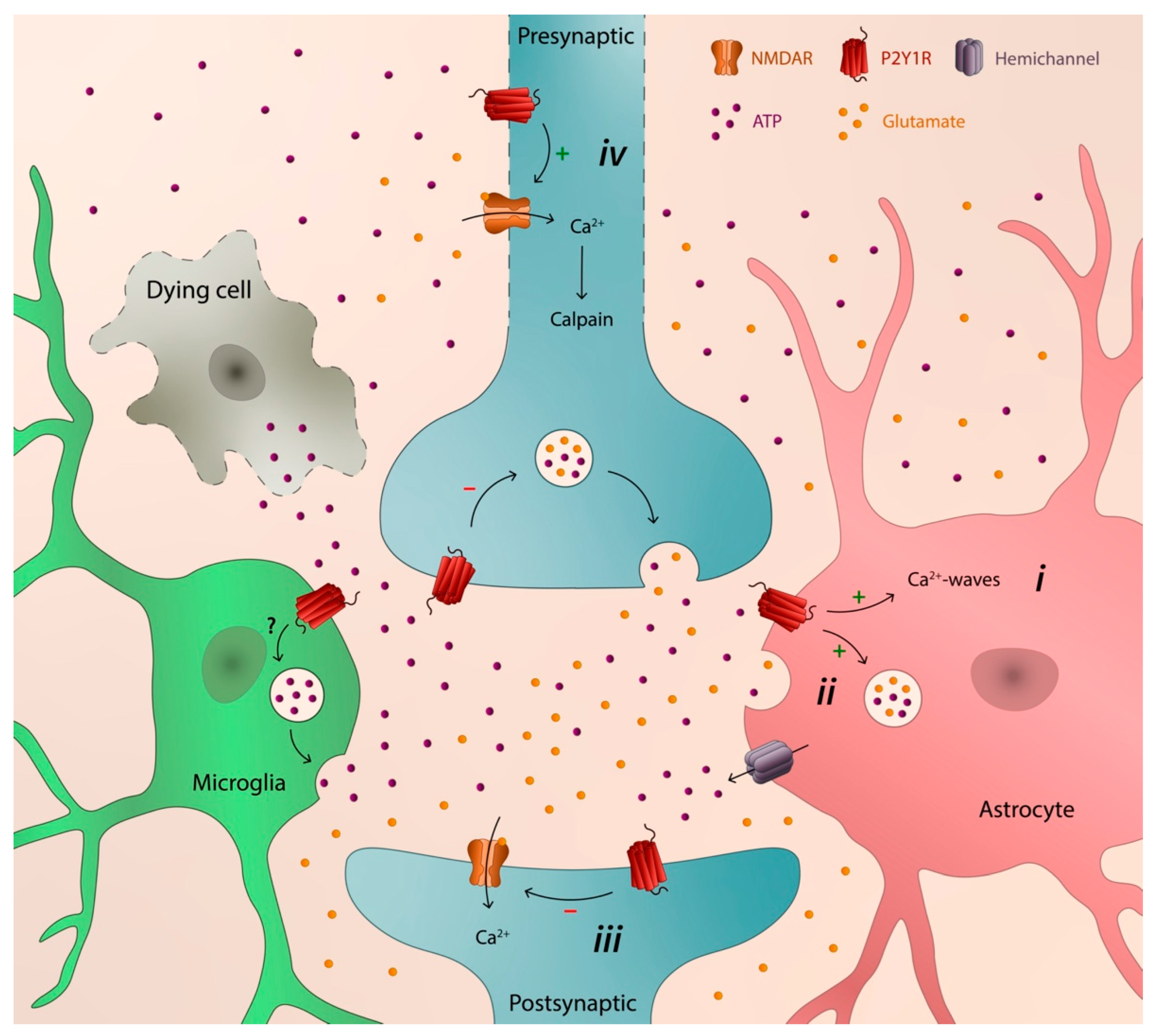
4. P2Y1 Receptor as a Catalyst of Neurodegeneration
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Olney, J.W. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science 1969, 164, 719–721. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.W. Glutamate neurotoxicity in cortical cell culture is calcium dependent. Neurosci. Lett. 1985, 58, 293–297. [Google Scholar] [CrossRef]
- Lipton, S.A.; Rosenberg, P.A. Excitatory amino acids as a final common pathway for neurologic disorders. N. Engl. J. Med. 1994, 330, 613–622. [Google Scholar] [PubMed]
- Ikonomidou, C.; Turski, L. Excitotoxicity and neurodegenerative diseases. Curr. Opin. Neurol. 1995, 8, 487–497. [Google Scholar] [CrossRef]
- Dong, X.X.; Wang, Y.; Qin, Z.H. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol. Sin. 2009, 30, 379–387. [Google Scholar] [CrossRef] [Green Version]
- Lewerenz, J.; Maher, P. Chronic Glutamate Toxicity in Neurodegenerative Diseases-What is the Evidence? Front. Neurosci. 2015, 9, 469. [Google Scholar] [CrossRef] [Green Version]
- Choi, D.W. Calcium and excitotoxic neuronal injury. Ann. N. Y. Acad. Sci. 1994, 747, 162–171. [Google Scholar] [CrossRef]
- Rothman, S.M.; Olney, J.W. Excitotoxicity and the NMDA receptor–still lethal after eight years. Trends Neurosci. 1995, 18, 57–58. [Google Scholar]
- Vanderklish, P.W.; Bahr, B.A. The pathogenic activation of calpain: A marker and mediator of cellular toxicity and disease states. Int. J. Exp. Pathol. 2000, 81, 323–339. [Google Scholar] [CrossRef]
- Dawson, V.L.; Dawson, T.M. Deadly conversations: Nuclear-mitochondrial cross-talk. J. Bioenerg. Biomembr. 2004, 36, 287–294. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Sheng, M. NMDA receptors in nervous system diseases. Neuropharmacology 2013, 74, 69–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yildiz-Unal, A.; Korulu, S.; Karabay, A. Neuroprotective strategies against calpain-mediated neurodegeneration. Neuropsychiatr. Dis. Treat. 2015, 11, 297–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davalos, D.; Grutzendler, J.; Yang, G.; Kim, J.V.; Zuo, Y.; Jung, S.; Littman, D.R.; Dustin, M.L.; Gan, W.B. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 2005, 8, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Choo, A.M.; Miller, W.J.; Chen, Y.C.; Nibley, P.; Patel, T.P.; Goletiani, C.; Morrison, B., 3rd; Kutzing, M.K.; Firestein, B.L.; Sul, J.Y.; et al. Antagonism of purinergic signalling improves recovery from traumatic brain injury. Brain 2013, 136, 65–80. [Google Scholar] [CrossRef] [Green Version]
- Faroqi, A.H.; Lim, M.J.; Kee, E.C.; Lee, J.H.; Burgess, J.D.; Chen, R.; Di Virgilio, F.; Delenclos, M.; McLean, P.J. In vivo detection of extracellular adenosine triphosphate in a mouse model of traumatic brain injury. J. Neurotrauma 2021, 38, 655–664. [Google Scholar] [CrossRef]
- Moro, N.; Ghavim, S.S.; Sutton, R.L. Massive efflux of adenosine triphosphate into the extracellular space immediately after experimental traumatic brain injury. Exp. Ther. Med. 2021, 21, 575. [Google Scholar] [CrossRef]
- Koizumi, S.; Fujishita, K.; Tsuda, M.; Shigemoto-Mogami, Y.; Inoue, K. Dynamic inhibition of excitatory synaptic transmission by astrocyte-derived ATP in hippocampal cultures. Proc. Natl. Acad. Sci. USA 2003, 100, 11023–11028. [Google Scholar] [CrossRef] [Green Version]
- White, T.D. Direct detection of depolarisation-induced release of AYP from a synaptosomal preparation. Nature 1977, 267, 67–68. [Google Scholar] [CrossRef]
- Pankratov, Y.; Lalo, U.; Verkhratsky, A.; North, R.A. Vesicular release of aTP at central synapses. Pflugers Arch. 2006, 452, 589–597. [Google Scholar] [CrossRef]
- Wieraszko, A.; Goldsmith, G.; Seyfried, T.N. Stimulation-dependent release of adenosine triphosphate from hippocampal slices. Brain Res. 1989, 485, 244–250. [Google Scholar] [CrossRef]
- Cunha, R.A.; Correia-de-Sá, P.; Sebastião, A.M.; Ribeiro, J.A. Preferential activation of excitatory adenosine receptors at rat hippocampal and neuromuscular synapses by adenosine formed from released adenine nucleotides. Br. J. Pharmacol. 1996, 119, 253–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebola, N.; Lujan, R.; Cunha, R.A.; Mulle, C. Adenosine A2A receptors are essential for long-term potentiation of NMDA-EPSCs at hippocampal mossy fiber synapses. Neuron 2008, 57, 121–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wieraszko, A.; Seyfried, T.N. ATP-induced synaptic potentiation in hippocampal slices. Brain Res. 1989, 491, 356–359. [Google Scholar] [CrossRef]
- Dale, N.; Frenguelli, B.G. Release of adenosine and ATP during ischemia and epilepsy. Curr. Neuropharmacol. 2009, 7, 160–179. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Beleza, R.O.; Gonçalves, F.Q.; Valbuena, S.; Alçada-Morais, S.; Gonçalves, N.; Magalhães, J.; Rocha, J.M.M.; Ferreira, S.; Figueira, A.S.G.; et al. Adenosine A2A receptors control synaptic remodeling in the adult brain. Sci. Rep. 2022, 12, 14690. [Google Scholar] [CrossRef]
- Lutz, P.L.; Kabler, S. Release of adenosine and ATP in the brain of the freshwater turtle (Trachemys Scripta) during long-term anoxia. Brain Res. 1997, 769, 281–286. [Google Scholar] [CrossRef]
- Juranyi, Z.; Sperlagh, B.; Vizi, E.S. Involvement of P2 purinoceptors and the nitric oxide pathway in [3H] purine outflow evoked by short-term hypoxia and hypoglycemia in rat hippocampal slices. Brain Res. 1999, 823, 183–190. [Google Scholar] [CrossRef]
- Melani, A.; Turchi, D.; Vannucchi, M.G.; Cipriani, S.; Gianfriddo, M.; Pedata, F. ATP extracellular concentrations are increased in the rat striatum during in vivo ischemia. Neurochem. Int. 2005, 47, 442–448. [Google Scholar] [CrossRef]
- Frenguelli, B.G.; Wigmore, G.; Llaudet, E.; Dale, N. Temporal and mechanistic dissociation of ATP and adenosine release during ischaemia in the mammalian hippocampus. J. Neurochem. 2007, 101, 1400–1413. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, F.Q.; Lopes, J.P.; Silva, H.B.; Lemos, C.; Silva, A.C.; Gonçalves, N.; Tomé, A.R.; Ferreira, S.G.; Canas, P.M.; Rial, D.; et al. Synaptic and memory dysfunction in a β-amyloid model of early Alzheimer’s disease depends on increased formation of ATP-derived extracellular adenosine. Neurobiol. Dis. 2019, 132, 104570. [Google Scholar] [CrossRef] [PubMed]
- Coco, S.; Calegari, F.; Pravettoni, E.; Pozzi, D.; Taverna, E.; Rosa, P.; Matteoli, M.; Verderio, C. Storage and release of ATP from astrocytes in culture. J. Biol. Chem. 2003, 278, 1354–1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montana, V.; Malarkey, E.B.; Verderio, C.; Matteoli, M.; Parpura, V. Vesicular transmitter release from astrocytes. Glia 2006, 54, 700–715. [Google Scholar] [CrossRef] [PubMed]
- Bowser, D.N.; Khakh, B.S. Vesicular ATP is the predominant cause of intercellular calcium waves in astrocytes. J. Gen. Physiol. 2007, 129, 485–491. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Chen, G.; Zhou, W.; Song, A.; Xu, T.; Luo, Q.; Wang, W.; Gu, X.S.; Duan, S. Regulated ATP release from astrocytes through lysosome exocytosis. Nat. Cell Biol. 2007, 9, 945–953. [Google Scholar] [CrossRef]
- Iwabuchi, S.; Kawahara, K. Functional significance of the negative-feedback regulation of ATP release via pannexin-1 hemichannels under ischemic stress in astrocytes. Neurochem. Int. 2011, 58, 376–384. [Google Scholar] [CrossRef] [Green Version]
- Bao, L.; Sachs, F.; Dahl, G. Connexins are mechanosensitive. Am. J. Physiol. Cell Physiol. 2004, 287, C1389–C1395. [Google Scholar] [CrossRef] [Green Version]
- Reigada, D.; Lu, W.; Zhang, M.; Mitchell, C.H. Elevated pressure triggers a physiological release of ATP from the retina: Possible role for pannexin hemichannels. Neuroscience 2008, 157, 396–404. [Google Scholar] [CrossRef] [Green Version]
- Cotrina, M.L.; Lin, J.H.; López-García, J.C.; Naus, C.C.; Nedergaard, M. ATP-mediated glia signaling. J. Neurosci. 2000, 20, 2835–2844. [Google Scholar] [CrossRef] [Green Version]
- Stout, C.E.; Constantin, J.L.; Naus, C.C.G.; Charles, A.C. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J. Biol. Chem. 2002, 277, 10482–10488. [Google Scholar] [CrossRef] [Green Version]
- Orellana, J.A.; Shoji, K.F.; Abudara, V.; Ezan, P.; Amigou, E.; Saez, P.J.; Jiang, J.X.; Naus, C.C.; Saez, J.C.; Giaume, C. Amyloid β-induced death in neurons involves glial and neuronal hemichannels. J. Neurosci. 2011, 31, 4962–4977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, E.S.; An, K.; Hong, S.H.; Kim, J.-H.; Mook-Jung, I. Astrocyte-originated ATP protects Aβ1-42-induced impairment of synaptic plasticity. J. Neurosci. 2012, 32, 3081–3087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madeira, D.; Dias, L.; Santos, P.; Cunha, R.A.; Canas, P.M.; Agostinho, P. Association between adenosine A2A receptors and connexin 43 regulates hemichannels activity and ATP release in astrocytes exposed to amyloid-β peptides. Mol. Neurobiol. 2021, 58, 6232–6248. [Google Scholar] [CrossRef] [PubMed]
- Delekate, A.; Fuchtemeier, M.; Schumacher, T.; Ulbrich, C.; Foddis, M.; Petzold, G.C. Metabotropic P2Y1 receptor signalling mediates astrocytic hyperactivity in vivo in an alzheimer’s disease mouse model. Nat. Commun. 2014, 5, 5422. [Google Scholar] [CrossRef] [Green Version]
- Vessey, D.A.; Li, L.; Kelley, M. P2X7 receptor agonists pre- and postcondition the heart against ischemia-reperfusion injury by opening pannexin-1/P2X7 channels. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H881–H887. [Google Scholar] [CrossRef]
- Shestopalov, V.I.; Slepak, V.Z. Molecular pathways of pannexin1-mediated neurotoxicity. Front. Physiol. 2014, 5, 23. [Google Scholar] [CrossRef] [Green Version]
- Dossi, E.; Blauwblomme, T.; Moulard, J.; Chever, O.; Vasile, F.; Guinard, E.; Le Bert, M.; Couillin, I.; Pallud, J.; Capelle, L.; et al. Pannexin-1 channels contribute to seizure generation in human epileptic brain tissue and in a mouse model of epilepsy. Sci. Transl. Med. 2018, 10, eaar3796. [Google Scholar] [CrossRef] [Green Version]
- Wellmann, M.; Álvarez-Ferradas, C.; Maturana, C.J.; Sáez, J.C.; Bonansco, C. Astroglial Ca2+-dependent hyperexcitability requires P2Y1 purinergic receptors and pannexin-1 channel activation in a chronic model of epilepsy. Front. Cell Neurosci. 2018, 12, 446. [Google Scholar] [CrossRef] [Green Version]
- Thompson, R.J.; Zhou, N.; MacVicar, B.A. Ischemia opens neuronal gap junction hemichannels. Science 2006, 312, 924–927. [Google Scholar] [CrossRef]
- Liu, H.T.; Sabirov, R.Z.; Okada, Y. Oxygen-glucose deprivation induces ATP release via maxi-anion channels in astrocytes. Purinergic Signal. 2008, 4, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Duan, S.; Neary, J.T. P2X7 receptors: Properties and relevance to CNS function. Glia 2006, 54, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Cisneros-Mejorado, A.; Pérez-Samartín, A.; Gottlieb, M.; Matute, C. ATP signaling in brain: Release, excitotoxicity and potential therapeutic targets. Cell. Mol. Neurobiol. 2015, 35, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Locovei, S.; Scemes, E.; Qiu, F.; Spray, D.C.; Dahl, G. Pannexin1 is part of the pore forming unit of the P2X(7) receptor death complex. FEBS Lett. 2007, 581, 483–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iglesias, R.; Locovei, S.; Roque, A.; Alberto, A.P.; Dahl, G.; Spray, D.C.; Scemes, E. P2X7 receptor-pannexin1 complex: Pharmacology and signaling. Am. J. Physiol. Cell Physiol. 2008, 295, C752–C760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imura, Y.; Morizawa, Y.; Komatsu, R.; Shibata, K.; Shinozaki, Y.; Kasai, H.; Moriishi, K.; Moriyama, Y.; Koizumi, S. Microglia release ATP by exocytosis. Glia 2013, 61, 1320–1330. [Google Scholar] [CrossRef]
- Kim, S.Y.; Moon, J.H.; Lee, H.G.; Kim, S.U.; Lee, Y.B. ATP released from beta-amyloid-stimulated microglia induces reactive oxygen species production in an autocrine fashion. Exp. Mol. Med. 2007, 39, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.M.; Chiozzi, P.; Ferrari, D.; Colaianna, M.; Idzko, M.; Falzoni, S.; Fellin, R.; Trabace, L.; Di Virgilio, F. Activation of microglia by amyloid {beta} requires P2X7 receptor expression. J. Immunol. 2009, 182, 4378–4385. [Google Scholar] [CrossRef] [Green Version]
- Di Virgilio, F. Dr. Jekyll/Mr. Hyde: The dual role of extracellular ATP. J. Auton. Nerv. Syst. 2000, 81, 59–63. [Google Scholar] [CrossRef]
- Rodrigues, R.J.; Tomé, A.R.; Cunha, R.A. ATP as a multi-target danger signal in the brain. Front. Neurosci. 2015, 9, 148. [Google Scholar] [CrossRef] [Green Version]
- Burnstock, G.; Krügel, U.; Abbracchio, M.P.; Illes, P. Purinergic signalling: From normal behaviour to pathological brain function. Prog. Neurobiol. 2011, 95, 229–274. [Google Scholar] [CrossRef]
- Weisman, G.A.; Ajit, D.; Garrad, R.; Peterson, T.S.; Woods, L.T.; Thebeau, C.; Camden, J.M.; Erb, L. Neuroprotective roles of the P2Y(2) receptor. Purinergic Signal. 2012, 8, 559–578. [Google Scholar] [CrossRef] [PubMed]
- Illes, P. P2X7 receptors amplify CNS damage in neurodegenerative diseases. Int. J. Mol. Sci. 2020, 21, 5996. [Google Scholar] [CrossRef]
- Forster, D.; Reiser, J. Supportive or detrimental roles of P2Y receptors in brain pathology?—The two faces of P2Y receptors in stroke and neurodegeneration detected in neural cell and in animal model studies. Purinergic Signal. 2015, 11, 441–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunha, R.A. How does adenosine control neuronal dysfunction and neurodegeneration? J. Neurochem. 2016, 139, 1019–1055. [Google Scholar] [CrossRef]
- Miras-Portugal, M.T.; Queipo, M.J.; Gil-Redondo, J.C.; Ortega, F.; Gómez-Villafuertes, R.; Gualix, J.; Delicado, E.G.; Pérez-Sen, R. P2 receptor interaction and signalling cascades in neuroprotection. Brain Res. Bull. 2019, 151, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Engel, T.; Smith, J.; Alves, M. Targeting neuroinflammation via purinergic P2 receptors for disease modification in drug-refractory epilepsy. J. Inflamm. Res. 2021, 14, 3367–3392. [Google Scholar] [CrossRef]
- Rodrigues, R.J.; Almeida, T.; Richardson, P.J.; Oliveira, C.R.; Cunha, R.A. Dual presynaptic control by ATP of glutamate release via facilitatory P2X1, P2X2/3, and P2X3 and inhibitory P2Y1, P2Y2, and/or P2Y4 receptors in the rat hippocampus. J. Neurosci. 2005, 25, 6286–6295. [Google Scholar] [CrossRef] [Green Version]
- Simões, A.P.; Silva, C.G.; Marques, J.M.; Pochmann, D.; Porciúncula, L.O.; Ferreira, S.; Oses, J.P.; Beleza, R.O.; Real, J.I.; Köfalvi, A.; et al. Glutamate-induced and NMDA receptor-mediated neurodegeneration entails P2Y1 receptor activation. Cell Death Dis. 2018, 9, 297. [Google Scholar] [CrossRef]
- Mendoza-Fernandez, V.; Andrew, R.D.; Barajas-López, C. ATP inhibits glutamate synaptic release by acting at P2Y receptors in pyramidal neurons of hippocampal slices. J. Pharmacol. Exp. Ther. 2000, 293, 172–179. [Google Scholar]
- Donato, R.; Rodrigues, R.J.; Takahashi, M.; Tsai, M.C.; Soto, D.; Miyagi, K.; Villafuertes, R.G.; Cunha, R.A.; Edwards, F.A. GABA release by basket cells onto Purkinje cells, in rat cerebellar slices, is directly controlled by presynaptic purinergic receptors, modulating Ca2+ influx. Cell Calcium 2008, 44, 521–532. [Google Scholar] [CrossRef] [Green Version]
- Filippov, A.K.; Brown, D.A.; Barnard, E.A. The P2Y1 receptor closes the N-type Ca2+ channel in neurons, with both adenosine triphosphates and diphosphates as potent agonists. Br. J. Pharmacol. 2000, 129, 1063–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerevich, Z.; Borvendeg, S.J.; Schroder, W.; Franke, H.; Wirkner, K.; Norenberg, W.; Furst, S.; Gillen, C.; Illes, P. Inhibition of N-type voltage-activated calcium channels in rat dorsal root ganglion neurons by P2Y receptors is a possible mechanism of ADP-induced analgesia. J. Neurosci. 2004, 24, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Luthardt, J.; Borvendeg, S.J.; Sperlagh, B.; Poelchen, W.; Wirkner, K.; Illes, P. P2Y1 receptor activation inhibits NMDA receptor-channels in layer V pyramidal neurons of the rat prefrontal and parietal cortex. Neurochem. Int. 2003, 42, 161–172. [Google Scholar] [CrossRef]
- Guzman, S.J.; Gerevich, Z.; Hengstler, J.G.; Illes, P.; Kleemann, W. P2Y1 receptors inhibit both strength and plasticity of glutamatergic synaptic neurotransmission in the rat prefrontal cortex. Synapse 2005, 57, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Guzman, S.J.; Schmidt, H.; Franke, H.; Krugel, U.; Eilers, J.; Illes, P.; Gerevich, Z. P2y1 Receptors Inhibit Long-Term Depression in the Prefrontal Cortex. Neuropharmacology 2010, 59, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Saitow, F.; Murakoshi, T.; Suzuki, H.; Konishi, S. Metabotropic P2Y purinoceptor-mediated presynaptic and postsynaptic enhancement of cerebellar GABAergic transmission. J. Neurosci. 2005, 25, 2108–2116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowser, D.N.; Khakh, B.S. ATP excites interneurons and astrocytes to increase synaptic inhibition in neuronal networks. J. Neurosci. 2004, 24, 8606–8620. [Google Scholar] [CrossRef] [Green Version]
- Kawamura, M.; Gachet, C.; Inoue, K.; Kato, F. Direct excitation of inhibitory interneurons by extracellular ATP mediated by P2Y1 receptors in the hippocampal slice. J. Neurosci. 2004, 24, 10835–41085. [Google Scholar] [CrossRef] [Green Version]
- Coppi, E.; Pedata, F.; Gibb, A.J. P2Y1 receptor modulation of Ca2+-activated K+ currents in medium-sized neurons from neonatal rat striatal slices. J. Neurophysiol. 2012, 107, 1009–1021. [Google Scholar] [CrossRef] [Green Version]
- Tan, Z.; Liu, Y.; Xi, W.; Lou, H.F.; Zhu, L.; Guo, Z.; Mei, L.; Duan, S. Glia-derived ATP inversely regulates excitability of pyramidal and CCK-positive neurons. Nat. Commun. 2017, 8, 13772. [Google Scholar] [CrossRef] [Green Version]
- Filippov, A.K.; Choi, R.C.Y.; Simon, J.; Barnard, E.A.; Brown, D.A. Activation of P2Y1 nucleotide receptors induces inhibition of the M-type K+ current in rat hippocampal pyramidal neurons. J. Neurosci. 2006, 26, 9340–9348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franke, H.; Krugel, U.; Illes, P. P2 receptor-mediated proliferative effects on astrocytes in vivo. Glia, 1999; 28, 190–200. [Google Scholar]
- Jourdain, P.; Bergersen, L.H.; Bhaukaurally, K.; Bezzi, P.; Santello, M.; Domercq, M.; Matute, C.; Tonello, F.; Gundersen, V.; Volterra, A. Glutamate exocytosis from astrocytes controls synaptic strength. Nat. Neurosci. 2007, 10, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Kuboyama, K.; Harada, H.; Tozaki-Saitoh, H.; Tsuda, M.; Ushijima, K.; Inoue, K. Astrocytic P2Y(1) receptor is involved in the regulation of cytokine/chemokine transcription and cerebral damage in a rat model of cerebral ischemia. J. Cereb. Blood Flow Metab. 2011, 31, 1930–1941. [Google Scholar] [CrossRef] [Green Version]
- Pascual, O.; Achour, S.B.; Rostaing, P.; Triller, A.; Bessis, A. Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proc. Natl. Acad. Sci. USA 2012, 109, E197–E205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fam, S.R.; Gallagher, C.J.; Kalia, L.V.; Salter, M.W. Differential frequency dependence of P2Y1- and P2Y2-mediated Ca2+ signaling in astrocytes. J. Neurosci. 2003, 23, 4437–4444. [Google Scholar] [CrossRef] [Green Version]
- Neary, J.T.; Kang, Y.; Willoughby, K.A.; Ellis, E.F. Activation of extracellular signal-regulated kinase by stretch-induced injury in astrocytes involves extracellular ATP and P2 purinergic receptors. J. Neurosci. 2003, 23, 2348–2356. [Google Scholar] [CrossRef] [Green Version]
- Domercq, M.; Brambilla, L.; Pilati, E.; Marchaland, J.; Volterra, A.; Bezzi, P. P2Y1 receptor-evoked glutamate exocytosis from astrocytes: Control by tumor necrosis factor-alpha and prostaglandins. J. Biol. Chem. 2006, 281, 30684–93066. [Google Scholar] [CrossRef] [Green Version]
- Santello, M.; Bezzi, P.; Volterra, A. TNFα controls glutamatergic gliotransmission in the hippocampal dentate gyrus. Neuron 2011, 69, 988–1001. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.; Nikolic, L.; Meunier, C.; Pfrieger, F.; Audinat, E. An autocrine purinergic signaling controls astrocyte-induced neuronal excitation. Sci. Rep. 2017, 7, 11280. [Google Scholar] [CrossRef]
- Locovei, S.; Wang, J.; Dahl, G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006, 580, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.M.; Wang, H.K.; Ye, C.Q.; Ge, W.; Chen, Y.; Jiang, Z.L.; Wu, C.P.; Poo, M.M.; Duan, S. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron 2003, 40, 971–982. [Google Scholar] [CrossRef] [Green Version]
- Jacob, P.F.; Vaz, S.H.; Ribeiro, J.A.; Sebastiao, A.M. P2Y1 receptor inhibits GABA transport through a calcium signalling-dependent mechanism in rat cortical astrocytes. Glia 2014, 62, 1211–1226. [Google Scholar] [CrossRef] [PubMed]
- Boucsein, C.; Zacharias, R.; Farber, K.; Pavlovic, S.; Hanisch, U.K.; Kettenmann, H. Purinergic receptors on microglial cells: Functional expression in acute brain slices and modulation of microglial activation in vitro. Eur. J. Neurosci. 2003, 17, 2267–2276. [Google Scholar] [CrossRef] [PubMed]
- Bianco, F.; Fumagalli, M.; Pravettoni, E.; D’Ambrosi, N.; Volonte, C.; Matteoli, M.; Abbracchio, M.P.; Verderio, C. Pathophysiological roles of extracellular nucleotides in glial cells: Differential expression of purinergic receptors in resting and activated microglia. Brain Res. Rev. 2005, 48, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Ballerini, P.; Di Iorio, P.; Ciccarelli, R.; Caciagli, F.; Poli, A.; Beraudi, A.; Buccella, S.; D’Alimonte, I.; D’Auro, M.; Nargi, E.; et al. P2Y1 and cysteinyl leukotriene receptors mediate purine and cysteinyl leukotriene co-release in primary cultures of rat microglia. Int. J. Immunopathol. Pharmacol. 2005, 18, 255–268. [Google Scholar] [CrossRef]
- Koizumi, S.; Ohsawa, K.; Inoue, K.; Kohsaka, S. Purinergic receptors in microglia: Functional modal shifts of microglia mediated by P2 and P1 receptors. Glia 2013, 61, 47–54. [Google Scholar] [CrossRef]
- Eyo, U.B.; Peng, J.; Swiatkowski, P.; Mukherjee, A.; Bispo, A.; Wu, L.J. Neuronal hyperactivity recruits microglial processes via neuronal NMDA receptors and microglial P2Y12 receptors after status epilepticus. J. Neurosci. 2014, 34, 10528–10540. [Google Scholar] [CrossRef] [Green Version]
- Milior, G.; Morin-Brureau, M.; Chali, F.; Le Duigou, C.; Savary, E.; Huberfeld, G.; Rouach, N.; Pallud, J.; Capelle, L.; Navarro, V.; et al. Distinct P2Y receptors mediate extension and retraction of microglial processes in epileptic and peritumoral human tissue. J. Neurosci. 2020, 40, 1373–1388. [Google Scholar] [CrossRef]
- Padrão, R.A.; Ariza, C.B.; Canzian, M.; Porcionatto, M.; Araffljo, M.G.L.; Cavalheiro, E.A. The P2 purinergic receptors are increased in the hippocampus of patients with temporal lobe epilepsy: What is the relevance to the epileptogenesis? Purinergic Signal. 2011, 7, 127. [Google Scholar]
- Alves, M.; Gomez-Villafuertes, R.; Delanty, N.; Farrell, M.A.; O’Brien, D.F.; Miras-Portugal, M.T.; Hernandez, M.D.; Henshall, D.C.; Engel, T. Expression and function of the metabotropic purinergic P2Y receptor family in experimental seizure models and patients with drug-refractory epilepsy. Epilepsia 2017, 58, 1603–1614. [Google Scholar] [CrossRef] [Green Version]
- Alves, M.; Smith, J.; Engel, T. Differential expression of the metabotropic P2Y receptor family in the cortex following status epilepticus and neuroprotection via P2Y1 antagonism in mice. Front. Pharmacol. 2020, 10, 1558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franke, H.; Krugel, U.; Grosche, J.; Heine, C.; Hartig, W.; Allgaier, C.; Illes, P. P2Y receptor expression on astrocytes in the nucleus accumbens of rats. Neuroscience 2004, 127, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.; Iritani, S.; Chambers, J.; Emson, P. Immunohistochemical localization of the P2Y1 purinergic receptor in Alzheimer’s disease. Neuroreport 2000, 11, 3799–3803. [Google Scholar] [CrossRef] [PubMed]
- Traini, C.; Pedata, F.; Cipriani, S.; Mello, T.; Galli, A.; Giovannini, M.G.; Cerbai, F.; Volpini, R.; Cristalli, G.; Pugliese, A.M. P2 receptor antagonists prevent synaptic failure and extracellular signal-regulated kinase 1/2 activation induced by oxygen and glucose deprivation in rat CA1 hippocampus in vitro. Eur. J. Neurosci. 2011, 33, 2203–2215. [Google Scholar] [CrossRef]
- Maraula, G.; Lana, D.; Coppi, E.; Gentile, F.; Mello, T.; Melani, A.; Galli, A.; Giovannini, M.G.; Pedata, F.; Pugliese, A.M. The selective antagonism of P2X7 and P2Y1 receptors prevents synaptic failure and affects cell proliferation induced by oxygen and glucose deprivation in rat dentate gyrus. PLoS ONE 2014, 9, e115273. [Google Scholar] [CrossRef]
- Chin, Y.; Kishi, M.; Sekino, M.; Nakajo, F.; Abe, Y.; Terazono, Y.; Hiroyuki, O.; Kato, F.; Koizumi, S.; Gachet, C.; et al. Involvement of glial P2Y1 receptors in cognitive deficit after focal cerebral stroke in a rodent model. J. Neuroinflamm. 2013, 10, 95. [Google Scholar] [CrossRef]
- Sun, J.J.; Liu, Y.; Ye, Z.R. Effects of P2Y1 receptor on glial fibrillary acidic protein and glial cell line-derived neurotrophic factor production of astrocytes under ischemic condition and the related signaling pathways. Neurosci. Bull. 2008, 24, 231–243. [Google Scholar] [CrossRef] [Green Version]
- Carmo, M.R.; Simões, A.P.; Fonteles, A.A.; Souza, C.M.; Cunha, R.A.; Andrade, G.M. ATP P2Y1 receptors control cognitive deficits and neurotoxicity but not glial modifications induced by brain ischemia in mice. Eur. J. Neurosci. 2014, 39, 614–622. [Google Scholar] [CrossRef]
- Fukumoto, Y.; Tanaka, K.F.; Parajuli, B.; Shibata, K.; Yoshioka, H.; Kanemaru, K.; Gachet, C.; Ikenaka, K.; Koizumi, S.; Kinouchi, H. Neuroprotective effects of microglial P2Y1 receptors against ischemic neuronal injury. J. Cereb. Blood Flow Metab. 2019, 39, 2144–2156. [Google Scholar] [CrossRef]
- Zheng, W.; Watts, L.T.; Holstein, D.M.; Wewer, J.; Lechleiter, J.D. P2Y1R-initiated, IP3R-dependent stimulation of astrocyte mitochondrial metabolism reduces and partially reverses ischemic neuronal damage in mouse. J. Cereb. Blood Flow Metab. 2013, 33, 600–611. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.; Watts, L.T.; Holstein, D.M.; Prajapati, S.I.; Keller, C.; Grass, E.H.; Walter, C.A.; Lechleiter, J.D. Purinergic receptor stimulation reduces cytotoxic edema and brain infarcts in mouse induced by photothrombosis by energizing glial mitochondria. PLoS ONE 2010, 5, e14401. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Tozaki-Saitoh, H.; Inoue, K. P2Y1 receptor signaling enhances neuroprotection by astrocytes against oxidative stress via IL-6 release in hippocampal cultures. Glia 2009, 57, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Maiolino, M.; O’Neill, N.; Lariccia, V.; Amoroso, S.; Sylantyev, S.; Angelova, P.R.; Abramov, A.Y. Inorganic polyphosphate regulates AMPA and NMDA receptors and protects against glutamate excitotoxicity via activation of P2Y receptors. J. Neurosci. 2018, 39, 6038–6048. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.; De Diego Garcia, L.; Conte, G.; Jimenez-Mateos, E.M.; D’Orsi, B.; Sanz-Rodriguez, A.; Prehn, J.H.M.; Henshall, D.C.; Engel, T. Context-specific switch from anti- to pro-epileptogenic function of the P2Y1 receptor in experimental epilepsy. J. Neurosci. 2019, 39, 5377–5392. [Google Scholar] [CrossRef] [Green Version]
- Nikolic, L.; Shen, W.; Nobili, P.; Virenque, A.; Ulmann, L.; Audinat, E. Blocking TNFα-driven astrocyte purinergic signaling restores normal synaptic activity during epileptogenesis. Glia 2018, 66, 2673–2683. [Google Scholar] [CrossRef]
- Nobili, P.; Shen, W.; Milicevic, K.; Bogdanovic Pristov, J.; Audinat, E.; Nikolic, L. Therapeutic potential of astrocyte purinergic signalling in epilepsy and multiple sclerosis. Front. Pharmacol. 2022, 13, 900337. [Google Scholar]
- Su, L.; Bai, X.; Niu, T.; Zhuang, X.; Dong, B.; Wang, G.; Yu, Y. P2Y1 purinergic receptor inhibition attenuated remifentanil-induced postoperative hyperalgesia via decreasing NMDA receptor phosphorylation in dorsal root ganglion. Brain Res. Bull. 2021, 177, 352–362. [Google Scholar] [CrossRef]
- Nedergaard, M.; Dirnagl, U. Role of glial cells in cerebral ischemia. Glia 2005, 50, 281–286. [Google Scholar] [CrossRef]
- Shinozaki, Y.; Shibata, K.; Yoshida, K.; Shigetomi, E.; Gachet, C.; Ikenaka, K.; Tanaka, K.F.; Koizumi, S. Transformation of astrocytes to a neuroprotective phenotype by microglia via P2Y1 receptor downregulation. Cell Rep. 2017, 19, 1151–1164. [Google Scholar] [CrossRef] [Green Version]
- Martorell, A.; Wellmann, M.; Guiffa, F.; Fuenzalida, M.; Bonansco, C. P2Y1 receptor inhibition rescues impaired synaptic plasticity and astroglial Ca2+-dependent activity in the epileptic hippocampus. Neurobiol. Dis. 2020, 146, 105132. [Google Scholar] [CrossRef]
- Kumagawa, T.; Moro, N.; Maeda, T.; Kobayashi, M.; Furukawa, Y.; Shijo, K.; Yoshino, A. Anti-inflammatory effect of P2Y1 receptor blocker MRS2179 in a rat model of traumatic brain injury. Brain Res. Bull. 2022, 181, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Kuchibhotla, K.V.; Lattarulo, C.R.; Hyman, B.T.; Bacskai, B.J. Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science 2009, 323, 1211–1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reichenbach, N.; Delekate, A.; Breithausen, B.; Keppler, K.; Poll, S.; Schulte, T.; Peter, J.; Plescher, M.; Hansen, J.N.; Blank, N.; et al. P2Y1 receptor blockade normalizes network dysfunction and cognition in an Alzheimer’s disease model. J. Exp. Med. 2018, 215, 1649–1663. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.; Bespalov, A.; Drescher, K.; Franke, H.; Krügel, U. Impaired cognition after stimulation of P2Y1 receptors in the rat medial prefrontal cortex. Neuropsychopharmacology 2015, 40, 305–314. [Google Scholar] [CrossRef]
- Shi, A.; Petrache, A.L.; Shi, J.; Ali, A.B. Preserved calretinin interneurons in an APP model of Alzheimer’s disease disrupt hippocampal inhibition via upregulated P2Y1 purinoreceptors. Cereb. Cortex 2020, 30, 1272–1290. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, Y.; Koizumi, S.; Ishida, S.; Sawada, J.; Ohno, Y.; Inoue, K. Cytoprotection against oxidative stress-induced damage of astrocytes by extracellular ATP via P2Y1 receptors. Glia 2005, 49, 288–300. [Google Scholar] [CrossRef]
- Shinozaki, Y.; Koizumi, S.; Ohno, Y.; Nagao, T.; Inoue, K. Extracellular ATP counteracts the ERK1/2-mediated death-promoting signaling cascades in astrocytes. Glia 2006, 54, 606–618. [Google Scholar] [CrossRef]
- Guo, H.; Liu, Z.Q.; Zhou, H.; Wang, Z.L.; Tao, Y.H.; Tong, Y. P2Y1 receptor antagonists mitigate oxygen and glucose deprivation-induced astrocyte injury. Mol. Med. Rep. 2018, 17, 1819–1824. [Google Scholar] [CrossRef] [Green Version]
- Miller, W.J.; Leventhal, I.; Scarsella, D.; Haydon, P.G.; Janmey, P.; Meaney, D.F. Mechanically induced reactive gliosis causes ATP-mediated alterations in astrocyte stiffness. J. Neurotrauma 2009, 26, 789–797. [Google Scholar] [CrossRef] [Green Version]
- Guzman, S.J.; Gerevich, Z. P2Y receptors in synaptic transmission and plasticity: Therapeutic potential in cognitive dysfunction. Neural Plast. 2016, 2016, 1207393. [Google Scholar] [CrossRef] [Green Version]
- Woods, L.T.; Ajit, D.; Camden, J.M.; Erb, L.; Weisman, G.A. Purinergic receptors as potential therapeutic targets in Alzheimer’s disease. Neuropharmacology 2016, 104, 169–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quintas, C.; Vale, N.; Gonçalves, J.; Queiroz, G. Microglia P2Y13 receptors prevent astrocyte proliferation mediated by P2Y1 receptors. Front. Pharmacol. 2018, 9, 418. [Google Scholar] [CrossRef] [PubMed]
- Dias, L.; Madeira, D.; Dias, R.; Tomé, Â.R.; Cunha, R.A.; Agostinho, P. Aβ1-42 peptides blunt the adenosine A2A receptor-mediated control of the interplay between P2X7 and P2Y1 receptors mediated calcium responses in astrocytes. Cell. Mol. Life Sci. 2022, 79, 457. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, R.J.; Figueira, A.S.; Marques, J.M. P2Y1 Receptor as a Catalyst of Brain Neurodegeneration. NeuroSci 2022, 3, 604-615. https://doi.org/10.3390/neurosci3040043
Rodrigues RJ, Figueira AS, Marques JM. P2Y1 Receptor as a Catalyst of Brain Neurodegeneration. NeuroSci. 2022; 3(4):604-615. https://doi.org/10.3390/neurosci3040043
Chicago/Turabian StyleRodrigues, Ricardo J., Ana S. Figueira, and Joana M. Marques. 2022. "P2Y1 Receptor as a Catalyst of Brain Neurodegeneration" NeuroSci 3, no. 4: 604-615. https://doi.org/10.3390/neurosci3040043
APA StyleRodrigues, R. J., Figueira, A. S., & Marques, J. M. (2022). P2Y1 Receptor as a Catalyst of Brain Neurodegeneration. NeuroSci, 3(4), 604-615. https://doi.org/10.3390/neurosci3040043






