Stress-Induced Hyperglycemia Predicts Poor Outcomes in Primary Intracerebral Hemorrhage Patients
Abstract
:1. Introduction
2. Materials and Methods
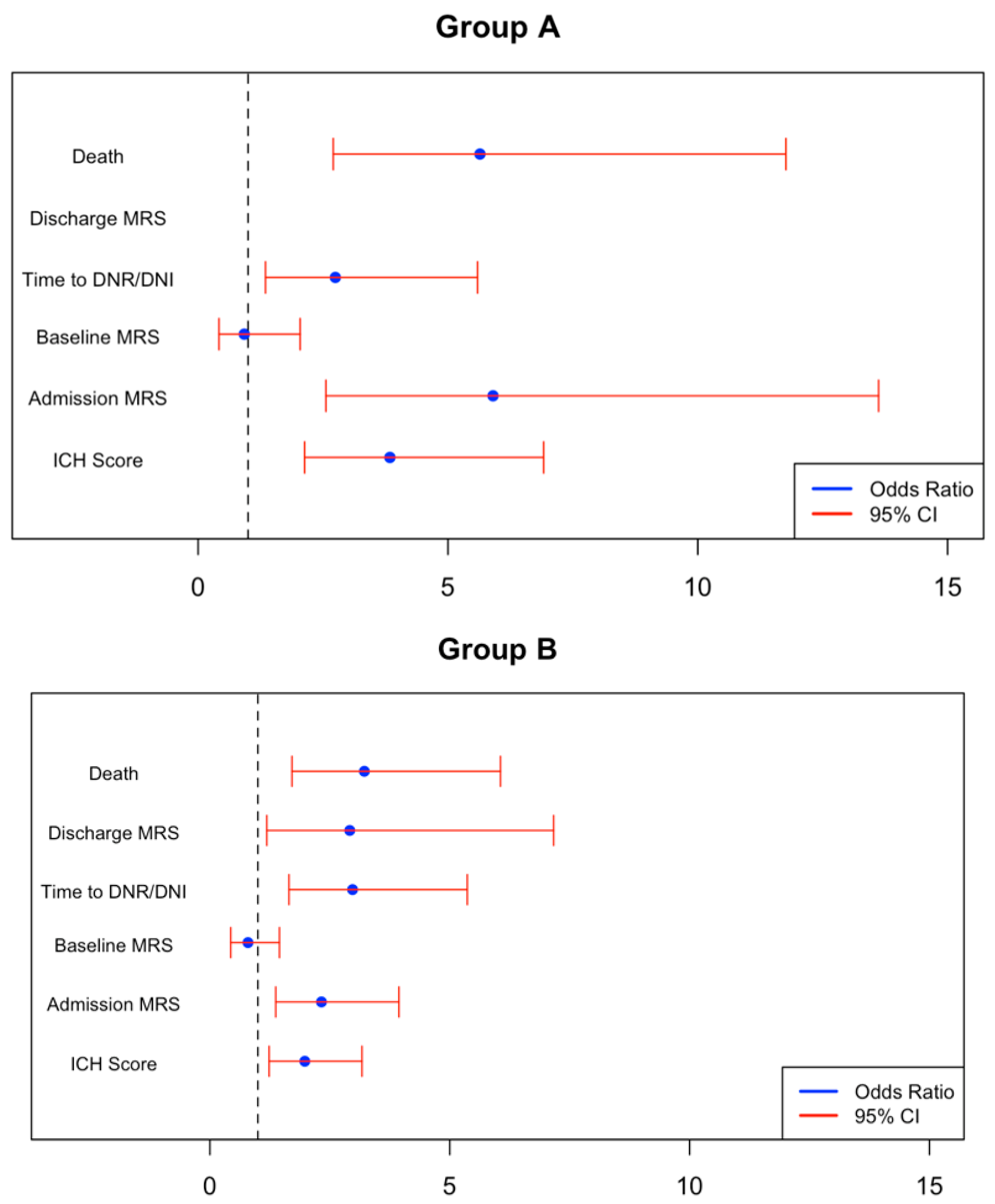
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Van Asch, C.J.; Luitse, M.J.; Rinkel, G.J.; Van Der Tweel, I.; Algra, A.; Klijn, C.J. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: A systematic review and meta-analysis. Lancet Neurol. 2010, 9, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.; Misselwitz, B.; Hamann, G.F.; Scharbrodt, W.; Schummer, D.I.; Oertel, M.F. Intracerebral Hemorrhage in the Very Old: Future Demographic Trends of an Aging Population. Stroke 2012, 43, 1126–1128. [Google Scholar] [CrossRef] [PubMed]
- Sacco, S.; Marini, C.; Toni, D.; Olivieri, L.; Carolei, A. Incidence and 10-Year Survival of Intracerebral Hemorrhage in a Population-Based Registry. Stroke 2009, 40, 394–399. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Smith, C.; Beaton, A.; Boehme, A.; Buxton, A.; et al. Heart Disease and Stroke Statistics—2023 Update: A Report From the American Heart Association. Circulation 2023, 147, e93–e621. Available online: https://www.ahajournals.org/doi/10.1161/CIR.0000000000001123 (accessed on 1 February 2024). [CrossRef]
- McCowen, K.C.; Malhotra, A.; Bistrian, B.R. Stress-Induced Hyperglycemia. Crit. Care Clin. 2001, 17, 107–124. [Google Scholar] [CrossRef]
- Dungan, K.M.; Braithwaite, S.S.; Preiser, J.C. Stress hyperglycaemia. Lancet 2009, 373, 1798–1807. [Google Scholar] [CrossRef] [PubMed]
- Vedantam, D.; Poman, D.S.; Motwani, L.; Asif, N.; Patel, A.; Anne, K.K. Stress-Induced Hyperglycemia: Consequences and Management. Cureus 2022, 14, e26714. Available online: https://www.cureus.com/articles/100945-stress-induced-hyperglycemia-consequences-and-management (accessed on 19 April 2024). [CrossRef] [PubMed]
- Kimura, K.; Iguchi, Y.; Inoue, T.; Shibazaki, K.; Matsumoto, N.; Kobayashi, K.; Yamashita, S. Hyperglycemia independently increases the risk of early death in acute spontaneous intracerebral hemorrhage. J. Neurol. Sci. 2007, 255, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Stöllberger, C.; Exner, I.; Finsterer, J.; Slany, J.; Steger, C. Stroke in diabetic and non--diabetic patients: Course and prognostic value of admission serum glucose. Ann. Med. 2005, 37, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Fogelholm, R. Admission blood glucose and short term survival in primary intracerebral haemorrhage: A population based study. J. Neurol. Neurosurg. Psychiatry 2005, 76, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Wang, Y.; Chen, D.; Teng, Y.; Xu, F.; Yang, P. Predictive value of hyperglycemia on prognosis in spontaneous intracerebral hemorrhage patients. Heliyon 2023, 9, e14290. [Google Scholar] [CrossRef]
- Chu, H.; Huang, C.; Tang, Y.; Dong, Q.; Guo, Q. The stress hyperglycemia ratio predicts early hematoma expansion and poor outcomes in patients with spontaneous intracerebral hemorrhage. Ther. Adv. Neurol. Disord. 2022, 15, 175628642110706. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Wang, W.; Zhang, Q.; Wang, A.; Zhao, X. Stress hyperglycemia is predictive of clinical outcomes in patients with spontaneous intracerebral hemorrhage. BMC Neurol. 2022, 22, 236. [Google Scholar] [CrossRef]
- Chen, S.; Wan, Y.; Guo, H.; Shen, J.; Li, M.; Xia, Y.; Zhang, L.; Sun, Z.; Chen, X.; Li, G.; et al. Diabetic and stress-induced hyperglycemia in spontaneous intracerebral hemorrhage: A multicenter prospective cohort (CHEERY) study. CNS Neurosci. Ther. 2023, 29, 979–987. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, Y.; Luo, Z.; Yu, M.; Zhan, C.; Xu, H.; Lin, R.; Bian, S.; Yang, Y.; Jiang, Z.; Tao, X.; et al. Acute Phase Blood Glucose Levels and Functional Outcomes in Patients with Spontaneous Intracerebral Hemorrhage. Neuropsychiatr. Dis. Treat. 2023, 19, 2697–2707. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, G.; Wang, S.; Xiong, Y.; Gu, H.; Jiang, Y.; Yang, X.; Wang, C.; Wang, C.; Li, Z.; Zhao, X. Higher fasting blood glucose was associated with worse in-hospital clinical outcomes in patients with primary intracerebral hemorrhage: From a large-scale nationwide longitudinal registry. CNS Neurosci. Ther. 2022, 28, 2260–2267. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saxena, A.; Anderson, C.S.; Wang, X.; Sato, S.; Arima, H.; Chan, E.; Muñoz-Venturelli, P.; Delcourt, C.; Robinson, T.; Stapf, C.; et al. INTERACT2 Investigators. Prognostic Significance of Hyperglycemia in Acute Intracerebral Hemorrhage: The INTERACT2 Study. Stroke 2016, 47, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Yu, Z.; Ma, L.; Guo, R.; Lin, S.; You, C.; Li, H. Association Between Blood Glucose and Functional Outcome in Intracerebral Hemorrhage: A Systematic Review and Meta-Analysis. World Neurosurg. 2018, 114, e756–e765. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, H.; Zhang, Z.; Li, S.; Zhang, L.; Zhang, J.; Han, G. Hyperglycemia and Mortality Risk in Patients with Primary Intracerebral Hemorrhage: A Meta-Analysis. Mol. Neurobiol. 2016, 53, 2269–2275. [Google Scholar] [CrossRef]
- Sun, S.; Pan, Y.; Zhao, X.; Liu, L.; Li, H.; He, Y.; Wang, Y.; Wang, Y.; Guo, L. Prognostic Value of Admission Blood Glucose in Diabetic and Non-diabetic Patients with Intracerebral Hemorrhage. Sci. Rep. 2016, 6, 32342. [Google Scholar] [CrossRef]
- Capes, S.E.; Hunt, D.; Malmberg, K.; Pathak, P.; Gerstein, H.C. Stress Hyperglycemia and Prognosis of Stroke in Nondiabetic and Diabetic Patients: A Systematic Overview. Stroke 2001, 32, 2426–2432. [Google Scholar] [CrossRef]
- Stead, L.G.; Jain, A.; Bellolio, M.F.; Odufuye, A.; Gilmore, R.M.; Rabinstein, A.; Chandra, R.; Dhillon, R.; Manivannan, V.; Serrano, L.A.; et al. Emergency Department hyperglycemia as a predictor of early mortality and worse functional outcome after intracerebral hemorrhage. Neurocrit Care 2010, 13, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Godoy, D.A.; Piñero, G.R.; Svampa, S.; Papa, F.; Di Napoli, M. Hyperglycemia and Short-term Outcome in Patients with Spontaneous Intracerebral Hemorrhage. Neurocrit Care 2008, 9, 217–229. [Google Scholar] [CrossRef]
- Wu, T.Y.; Putaala, J.; Sharma, G.; Strbian, D.; Tatlisumak, T.; Davis, S.M.; Meretoja, A. Persistent Hyperglycemia Is Associated With Increased Mortality After Intracerebral Hemorrhage. J. Am. Heart Assoc. 2017, 6, e005760. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, J.; Zhao, H.; Ding, Y.; Zhou, J.; Zhang, Y. The association between hyperglycemia and the prognosis of acute spontaneous intracerebral hemorrhage. Neurol. Res. 2017, 39, 152–157. [Google Scholar] [CrossRef]
- Kang, K.; Lu, J.; Ju, Y.; Wang, W.; Shen, Y.; Wang, A.; Cao, Z.; Zhao, X. Association of pre- and post-stroke glycemic status with clinical outcome in spontaneous intracerebral hemorrhage. Sci. Rep. 2019, 9, 19054. [Google Scholar] [CrossRef]
- Fang, M.; Wang, D.; Coresh, J.; Selvin, E. Undiagnosed diabetes in U.S. Adults: Prevalence and trends. Diabetes Care 2022, 45, 1994–2002. [Google Scholar] [CrossRef]
- Saver, J.L.; Chaisinanunkul, N.; Campbell, B.C.V.; Grotta, J.C.; Hill, M.D.; Khatri, P.; Landen, J.; Lansberg, M.G.; Venkatasubramanian, C.; Albers, G.W. XIth Stroke Treatment Academic Industry Roundtable. Standardized Nomenclature for Modified Rankin Scale Global Disability Outcomes: Consensus Recommendations from Stroke Therapy Academic Industry Roundtable XI. Stroke 2021, 52, 3054–3062. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, J.C.; Bonovich, D.C.; Besmertis, L.; Manley, G.T.; Johnston, S.C. The ICH Score: A Simple, Reliable Grading Scale for Intracerebral Hemorrhage. Stroke 2001, 32, 891–897. [Google Scholar] [CrossRef]
- Qureshi, A.I.; Huang, W.; Lobanova, I.; Chandrasekaran, P.N.; Hanley, D.F.; Hsu, C.Y.; Martin, R.; Steiner, T.; Suarez, J.; Yamamoto, H.; et al. Effect of Moderate and Severe Persistent Hyperglycemia on Outcomes in Patients with Intracerebral Hemorrhage. Stroke 2022, 53, 1226–1234. [Google Scholar] [CrossRef]
- Yee, T.W. The VGAM package for negative binomial regression. Aust. N. Z. J. Stat. 2020, 62, 116–131. [Google Scholar] [CrossRef]
- Yee, T.W.; Stoklosa, J.; Huggins, R.M. The VGAM Package for Capture-Recapture Data Using the Conditional Likelihood. J. Stat. Softw. 2015, 65, 1–33. Available online: http://www.jstatsoft.org/v65/i05/ (accessed on 19 April 2024). [CrossRef]
- Zhang, M.Y.; Mlynash, M.; Sainani, K.L.; Albers, G.W.; Lansberg, M.G. Ordinal Prediction Model of 90-Day Modified Rankin Scale in Ischemic Stroke. Front. Neurol. 2021, 12, 727171. [Google Scholar] [CrossRef]
- Chiu, C.D.; Chen, C.C.V.; Shen, C.C.; Chin, L.T.; Ma, H.I.; Chuang, H.Y.; Cho, D.Y.; Chu, C.H.; Chang, C. Hyperglycemia Exacerbates Intracerebral Hemorrhage via the Downregulation of Aquaporin-4: Temporal Assessment with Magnetic Resonance Imaging. Stroke 2013, 44, 1682–1689. [Google Scholar] [CrossRef]
- Liu, J.; Gao, B.B.; Clermont, A.C.; Blair, P.; Chilcote, T.J.; Sinha, S.; Flaumenhaft, R.; Feener, E. Hyperglycemia-induced cerebral hematoma expansion is mediated by plasma kallikrein. Nat. Med. 2011, 17, 206–210. [Google Scholar] [CrossRef]
- Fuentes, B.; Ntaios, G.; Putaala, J.; Thomas, B.; Turc, G.; Díez-Tejedor, E. European Stroke Organisation (ESO) guidelines on glycaemia management in acute stroke. Eur. Stroke, J. 2018, 3, 5–21. [Google Scholar] [CrossRef]
- Bilotta, F.; Spinelli, A.; Giovannini, F.; Doronzio, A.; Delfini, R.; Rosa, G. The Effect of Intensive Insulin Therapy on Infection Rate, Vasospasm, Neurologic Outcome, and Mortality in Neurointensive Care Unit After Intracranial Aneurysm Clipping in Patients with Acute Subarachnoid Hemorrhage: A Randomized Prospective Pilot Trial. J. Neurosurg. Anesthesiol. 2007, 19, 156–160. [Google Scholar] [CrossRef]
- Kramer, A.H.; Roberts, D.J.; Zygun, D.A. Optimal glycemic control in neurocritical care patients: A systematic review and meta-analysis. Crit. Care 2012, 16, R203. [Google Scholar] [CrossRef]
- Song, E.C.; Chu, K.; Jeong, S.W.; Jung, K.H.; Kim, S.H.; Kim, M.; Yoon, B. Hyperglycemia Exacerbates Brain Edema and Perihematomal Cell Death After Intracerebral Hemorrhage. Stroke 2003, 34, 2215–2220. [Google Scholar] [CrossRef]
- Broderick, J.P.; Diringer, M.N.; Hill, M.D.; Brun, N.C.; Mayer, S.A.; Steiner, T.; Skolnick, B.; Davis, S. Determinants of Intracerebral Hemorrhage Growth: An Exploratory Analysis. Stroke 2007, 38, 1072–1075. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Liu, S.; Gao, M.; Wang, W.; Chen, K.; Huang, L.; Liu, Y. Diabetic vascular diseases: Molecular mechanisms and therapeutic strategies. Sig. Transduct. Target. Ther. 2023, 8, 152. [Google Scholar] [CrossRef] [PubMed]
- Saliba, W.; Barnett-Griness, O.; Gronich, N.; Molad, J.; Naftali, J.; Rennert, G.; Auriel, E. Association of Diabetes and Glycated Hemoglobin with the Risk of Intracerebral Hemorrhage: A Population-Based Cohort Study. Diabetes Care 2019, 42, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, A.; Luengo-Fernandez, R.; Wharton, R.M.; Rothwell, P.M. Ordinal vs dichotomous analyses of modified Rankin Scale, 5-year outcome, and cost of stroke. Neurology 2018, 201, 91. Available online: https://www.neurology.org/doi/10.1212/WNL.0000000000006554 (accessed on 27 May 2024). [CrossRef] [PubMed]


| Demographic Information (N = 636) | Group A—Hyperglycemia Without DM (SIH) (N = 42) | Group B—Hyperglycemia with DM (N = 70) | Group C—Normoglycemia with DM (N = 101) | Group D—Normoglycemia Without DM (N = 423) |
|---|---|---|---|---|
| Median age | 74 | 71 | 75 | 74 |
| Sex | ||||
| Female | 17 (40.5%) | 40 (57.1%) | 59 (58.4%) | 214 (50.6%) |
| Male | 25 (59.5%) | 30 (42.9%) | 42 (41.6%) | 209 (49.4%) |
| Race/Ethnicity | ||||
| African American | 0 (0%) | 8 (11.4%) | 10 (9.9%) | 33 (7.8%) |
| Asian | 0 (0%) | 1 (1.4%) | 7 (6.9%) | 6 (1.4%) |
| Hispanic | 0 (0%) | 3 (4.3%) | 3 (3.0%) | 7 (1.7%) |
| White | 42 (100%) | 58 (82.9%) | 81 (80.2%) | 377 (89.1%) |
| Clinical Outcomes (N = 636) | ||||
| Inpatient mortality | 19 (45.2%) | 22 (31.4%) | 14 (13.9%) | 51 (12.1%) |
| mRS discharge 0–2 | 0 (0.0%) | 7 (10.0%) | 20 (19.8%) | 98 (23.2%) |
| mRS discharge | 42 (100%) | 63 (90.0%) | 81 (80.2%) | 323 (76.4%) |
| Outcome | Group A—Hyperglycemia Without DM (SIH) (95% CI) | p-Value | Group B—Hyperglycemia with DM (95% CI) | p-Value | Group C—Normoglycemia with DM (95% CI) | p-Value |
|---|---|---|---|---|---|---|
| Inpatient mortality | 1.730 (0.995, 2.465) | <0.001 | 1.169 (0.537, 1.801) | <0.001 | −0.110 (−0.801, 0.580) | 0.754 |
| Unfavorable mRS discharge (3–6) | 16.338 (−1076.4–1109.1) | 0.976 | 1.070 (0.171, 1.969) | 0.020 | 0.221 (−0.451, 0.893) | 0.520 |
| Baseline mRS | −0.079 (−0.872, 0.714) | 0.845 | −0.231 (−0.833, 0.372) | 0.453 | 0.299 (−0.180, 0.778) | 0.222 |
| Admission mRS | 1.775 (0.939, 2.611) | <0.001 | 0.844 (0.317, 1.371) | 0.002 | −0.009 (−0.443, 0.424) | 0.966 |
| ICH score | 1.345 (0.756, 1.934) | <0.001 | 0.682 (0.211, 1.154) | 0.005 | −0.260 (−0.680, 0.161) | 0.227 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilotra, K.; Basem, J.; Janssen, M.; Swarna, S.; Mani, R.; Ren, B.; Dashti, R. Stress-Induced Hyperglycemia Predicts Poor Outcomes in Primary Intracerebral Hemorrhage Patients. NeuroSci 2025, 6, 12. https://doi.org/10.3390/neurosci6010012
Gilotra K, Basem J, Janssen M, Swarna S, Mani R, Ren B, Dashti R. Stress-Induced Hyperglycemia Predicts Poor Outcomes in Primary Intracerebral Hemorrhage Patients. NeuroSci. 2025; 6(1):12. https://doi.org/10.3390/neurosci6010012
Chicago/Turabian StyleGilotra, Kevin, Jade Basem, Melissa Janssen, Sujith Swarna, Racheed Mani, Benny Ren, and Reza Dashti. 2025. "Stress-Induced Hyperglycemia Predicts Poor Outcomes in Primary Intracerebral Hemorrhage Patients" NeuroSci 6, no. 1: 12. https://doi.org/10.3390/neurosci6010012
APA StyleGilotra, K., Basem, J., Janssen, M., Swarna, S., Mani, R., Ren, B., & Dashti, R. (2025). Stress-Induced Hyperglycemia Predicts Poor Outcomes in Primary Intracerebral Hemorrhage Patients. NeuroSci, 6(1), 12. https://doi.org/10.3390/neurosci6010012






