Decoupling Alpha Desynchronization from Neural Resource Use: Evidence from Cognitive Load Modulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Cognitive Tasks
2.3. EEG Recording and Analysis
2.4. Phase Analysis for Evoked and Induced Activity
2.5. Statistical Analysis
2.5.1. Behavioral Responses
2.5.2. ERP Analysis
2.5.3. Alpha Band Analysis
3. Results
3.1. Behavioral Data
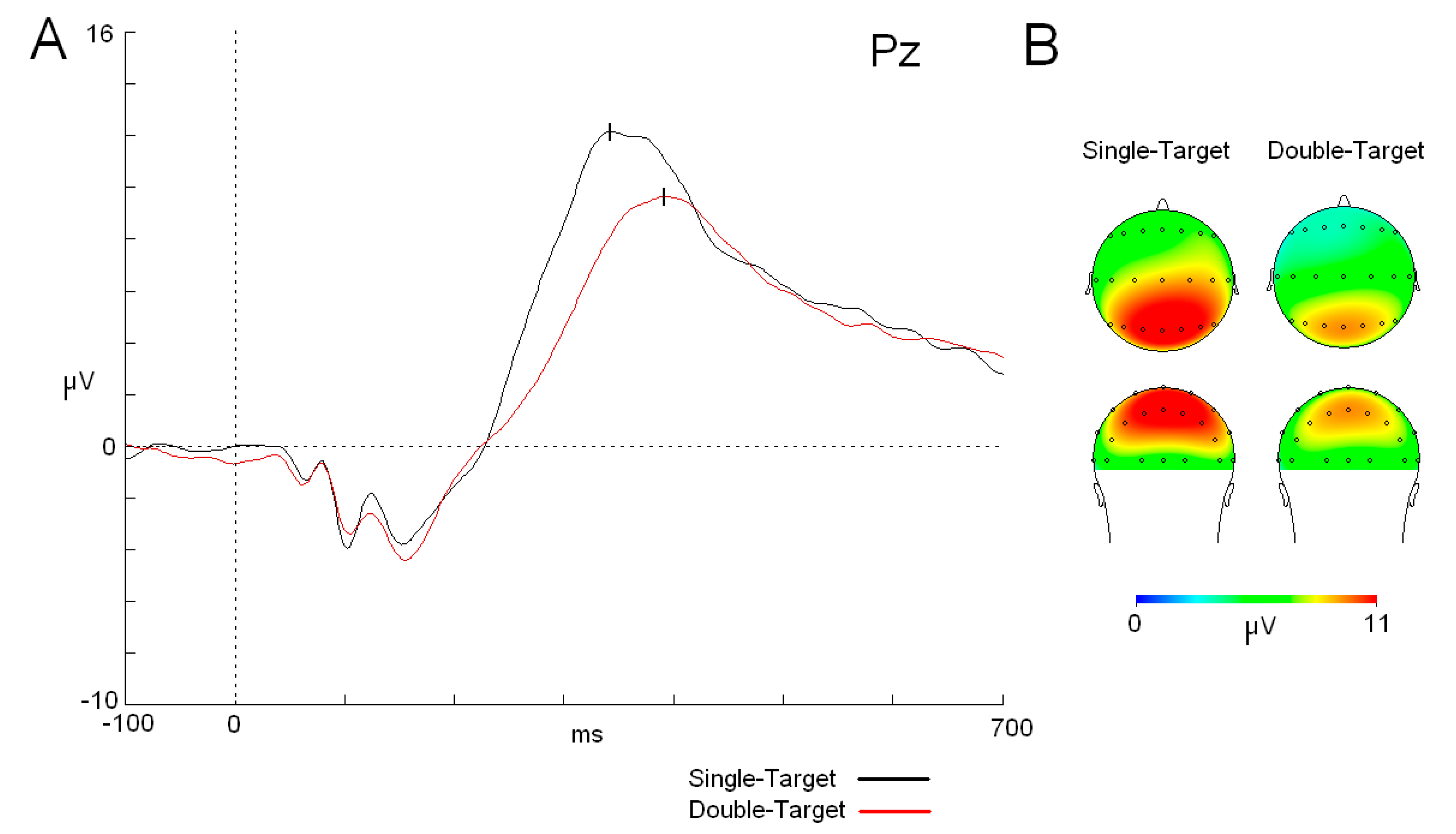
3.2. ERP
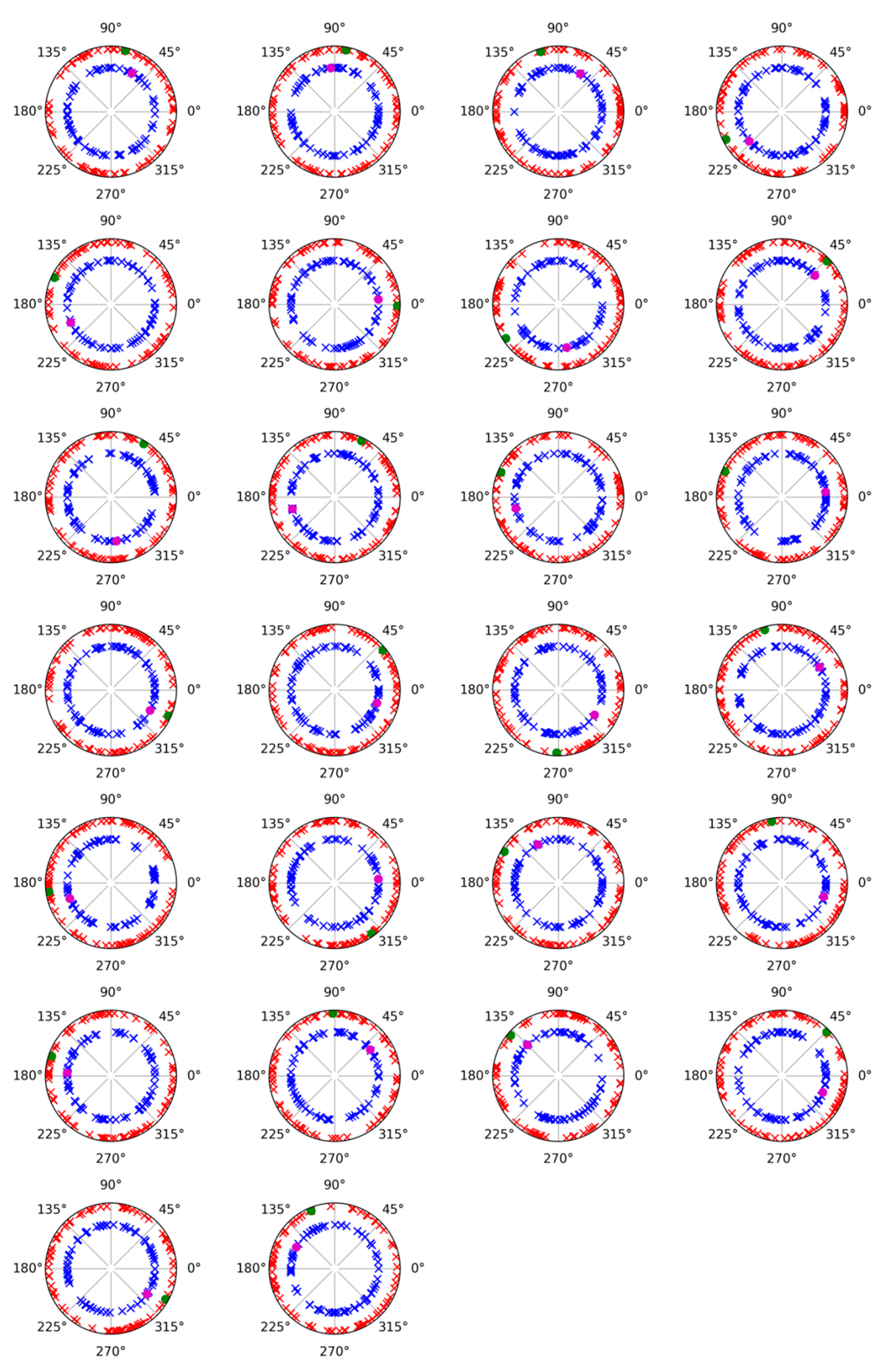
3.3. Alpha Band
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polich, J. Task difficulty, probability, and inter-stimulus interval as determinants of P300 from auditory stimuli. Electroencephalogr. Clin. Neurophysiol. 1987, 68, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Kok, A. On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology 2001, 38, 557–577. [Google Scholar] [CrossRef] [PubMed]
- Mussini, E.; Berchicci, M.; Bianco, V.; Perri, R.L.; Quinzi, F.; Di Russo, F. Effect of task complexity on motor and cognitive preparatory brain activities. Int. J. Psychophysiol. 2021, 159, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, P.D.; Falkenstein, M. Effects of task complexity on ERP components in Go/Nogo tasks. Int. J. Psychophysiol. 2013, 87, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Rueda-Delgado, L.M.; Solesio-Jofre, E.; Mantini, D.; Dupont, P.; Daffertshofer, A.; Swinnen, S.P. Coordinative task difficulty and behavioural errors are associated with increased long-range beta band synchronization. Neuroimage 2017, 146, 883–893. [Google Scholar] [CrossRef]
- Bianco, V.; Berchicci, M.; Livio Perri, R.; Quinzi, F.; Mussini, E.; Spinelli, D.; Di Russo, F. Preparatory ERPs in visual, auditory, and somatosensory discriminative motor tasks. Psychophysiology 2020, 57, e13687. [Google Scholar] [CrossRef]
- Di Russo, F.; Berchicci, M.; Bianco, V.; Perri, R.L.; Pitzalis, S.; Quinzi, F.; Spinelli, D. Normative event-related potentials from sensory and cognitive tasks reveal occipital and frontal activities prior and following visual events. Neuroimage 2019, 196, 173–187. [Google Scholar] [CrossRef]
- Mussini, E.; Berchicci, M.; Bianco, V.; Perri, R.L.; Quinzi, F.; Di Russo, F. The Role of Task Complexity on Frontal Event-related Potentials and Evidence in Favour of the Epiphenomenal Interpretation of the Go/No-Go N2 Effect. Neuroscience 2020, 449, 1–8. [Google Scholar] [CrossRef]
- Boashash, B. Time-Frequency Signal Analysis and Processing: A Comprehensive Reference, 2nd ed.; Academic Press: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Cohen, M.X. Analyzing Neural Time Series Data: Theory and Practice, Illustrated ed.; MIT Press: London, UK, 2014. [Google Scholar]
- Pfurtscheller, G.; Neuper, C.; Mohl, W. Event-related desynchronization (ERD) during visual processing. Int. J. Psychophysiol. 1994, 16, 147–153. [Google Scholar] [CrossRef]
- Vázquez, M.; Vaquero, E.; Cardoso, M.J.; Gómez, C.M. Temporal spectral evolution of electroencephalogram. Methodology and applications. Rev. Neurol. 2001, 33, 525–530. [Google Scholar]
- Lehtelä, L.; Salmelin, R.; Hari, R. Evidence for reactive magnetic 10-Hz rhythm in the human auditory cortex. Neurosci. Lett. 1997, 222, 111–114. [Google Scholar] [CrossRef]
- Salmelin, R.; Hari, R. Spatiotemporal characteristics of sensorimotor neuromagnetic rhythms related to thumb movement. Neuroscience 1994, 60, 537–550. [Google Scholar] [CrossRef]
- Vázquez-Marrufo, M.; Vaquero, E.; Cardoso, M.J.; Gómez, C.M. Temporal evolution of α and β bands during visual spatial attention. Cogn. Brain Res. 2001, 12, 315–320. [Google Scholar] [CrossRef]
- Vázquez-Marrufo, M.; García-Valdecasas, M.; Caballero-Diaz, R.; Martin-Clemente, R.; Galvao-Carmona, A. Multiple evoked and induced alpha modulations in a visual attention task: Latency, amplitude and topographical profiles. PLoS ONE 2019, 14, e0223055. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Marrufo, M.; Caballero-Díaz, R.; Martín-Clemente, R.; Galvao-Carmona, A.; González-Rosa, J.J. Individual test-retest reliability of evoked and induced alpha activity in human EEG data. PLoS ONE 2020, 15, e0239612. [Google Scholar] [CrossRef]
- Ciesielski, K.T.; Hämäläinen, M.S.; Geller, D.A.; Wilhelm, S.; Goldsmith, T.E.; Ahlfors, S.P. Dissociation between MEG alpha modulation and performance accuracy on visual working memory task in obsessive compulsive disorder. Human. Brain Mapp. 2007, 28, 1401–1414. [Google Scholar] [CrossRef] [PubMed]
- Lasaponara, S.; Pinto, M.; Aiello, M.; Tomaiuolo, F.; Doricchi, F. The hemispheric distribution of α-band EEG activity during orienting of attention in patients with reduced awareness of the left side of space (Spatial Neglect). J. Neurosci. 2019, 39, 4332–4343. [Google Scholar] [CrossRef]
- Vázquez-Marrufo, M.; Sarrias-Arrabal, E.; Martin-Clemente, R.; Galvao-Carmona, A.; Navarro, G.; Izquierdo, G. Altered phase and nonphase EEG activity expose impaired maintenance of a spatial-object attentional focus in multiple sclerosis patients. Sci. Rep. 2020, 10, 20721. [Google Scholar] [CrossRef]
- Sarrias-Arrabal, E.; Eichau, S.; Galvao-Carmona, A.; Domínguez, E.; Izquierdo, G.; Vázquez-Marrufo, M. Deficits in early sensory and cognitive processing are related to phase and nonphase EEG activity in Multiple Sclerosis patients. Brain Sci. 2021, 11, 629. [Google Scholar] [CrossRef]
- Keune, P.M.; Hansen, S.; Weber, E.; Zapf, F.; Habich, J.; Muenssinger, J.; Wolf, S.; Schönenberg, M.; Oschmann, P. Exploring resting-state EEG brain oscillatory activity in relation to cognitive functioning in multiple sclerosis. Clin. Neurophysiol. 2017, 128, 1746–1754. [Google Scholar] [CrossRef]
- Kiiski, H.; Reilly, R.B.; Lonergan, R.; Kelly, S.; O’Brien, M.C.; Kinsella, K.; Bramham, J.; Burke, T.; Donnchadha, S.O.; Nolan, H.; et al. Only low frequency event-related EEG activity is compromised in multiple sclerosis: Insights from an independent component clustering analysis. PLoS ONE 2012, 7, e45536. [Google Scholar] [CrossRef] [PubMed]
- Gómez, C.M.; Vaquero, E.; López-Mendoza, D.; González-Rosa, J.J.; Vázquez-Marrufo, M. Reduction of EEG power during expectancy periods in human. Acta Neurobiol. Exp. 2004, 64, 143–151. [Google Scholar] [CrossRef]
- Klimesch, W.; Sauseng, P.; Hanslmayr, S. EEG alpha oscillations: The inhibition–timing hypothesis. Brain Res. Rev. 2007, 53, 63–88. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Hu, Y.; Mao, Y.; Babiloni, C. Widespread cortical α-ERD accompanying visual oddball target stimuli is frequency but non-modality specific. Behav. Brain Res. 2015, 295, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Maresch, H.; Schuy, S. Inter- and intrahemispheric differences in the peak frequency of rhythmic activity within the alpha band. Electroencephalogr. Clin. Neurophysiol. 1977, 42, 77–83. [Google Scholar] [CrossRef]
- Worden, M.S.; Foxe, J.J.; Wang, N.; Simpson, C.V. Anticipatory biasing of visuospatial attention indexed by retinotopically specific alpha band electroencephalography increases over occipital cortex. J. Neurosci. 2000, 20, 1–6. [Google Scholar] [CrossRef]
- Sarrias-Arrabal, E.; Berchicci, M.; Bianco, V.; Vázquez-Marrufo, M.; Perri, R.L.; Di Russo, F. Temporal spectral evolution of pre-stimulus brain activity in visual and visuomotor tasks. Cogn. Neurodyn. 2023, 17, 1433–1446. [Google Scholar] [CrossRef]
- American Electroencephalography Society. Guideline Thirteen. J. Clin. Neurophysiol. 1994, 11, 111–113. [Google Scholar] [CrossRef]
- Gratton, G.; Coles, M.G.; Donchin, E. A new method for off-line removal of ocular artifact. Electroencephalogr. Clin. Neurophysiol. 1983, 55, 468–484. [Google Scholar] [CrossRef]
- Keil, A.; Müller, M.M. Feature selection in the human brain: Electrophysiological correlates of sensory enhancement and feature integration. Brain Res. 2010, 1313, 172–184. [Google Scholar] [CrossRef]
- David, O.; Kilner, J.M.; Friston, K.J. Mechanisms of evoked and induced responses in MEG/EEG. NeuroImage 2006, 31, 1580–1591. [Google Scholar] [CrossRef] [PubMed]
- Truccolo, W.A.; Ding, M.; Knuth, K.H.; Nakamura, R.; Bressler, S.L. Trial-to-trial variability of cortical evoked responses: Implications for the analysis of functional connectivity. Clin. Neurophysiol. 2002, 113, 206–226. [Google Scholar] [CrossRef] [PubMed]
- Padrón, I.; Fernández-Rey, J.; Acuña, C.; Pardo-Vazquez, J.L. Representing the consequences of our actions trial by trial: Complex and flexible encoding of feedback valence and magnitude. Neuroscience 2016, 333, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Shitova, N.; Roelofs, A.; Coughler, C.; Schriefers, H. P3 event-related brain potential reflects allocation and use of central processing capacity in language production. Neuropsychologia 2017, 106, 138–145. [Google Scholar] [CrossRef]
- Jia, H.; Li, H.; Yu, D. The relationship between ERP components and EEG spatial complexity in a visual Go/Nogo task. J. Neurophysiol. 2017, 117, 275–283. [Google Scholar] [CrossRef]
- Nenna, F.; Do, C.T.; Protzak, J.; Gramann, K. Alteration of brain dynamics during dual-task overground walking. Eur. J. Neurosci. 2020, 54, 8158–8174. [Google Scholar] [CrossRef] [PubMed]
- Reiser, J.E.; Wascher, E.; Rinkenauer, G.; Arnau, S. Cognitive-motor interference in the wild: Assessing the effects of movement complexity on task switching using mobile EEG. Eur. J. Neurosci. 2020, 54, 8175–8195. [Google Scholar] [CrossRef]
- Verleger, R.; Hamann, L.M.; Asanowicz, D.; Śmigasiewicz, K. Testing the S-R link hypothesis of P3b: The oddball effect on S1-evoked P3 gets reduced by increased task relevance of S2. Biol. Psychol. 2015, 108, 25–35. [Google Scholar] [CrossRef]
- Vázquez-Marrufo, M.; González-Rosa, J.J.; Galvao-Carmona, A.; Hidalgo-Muñoz, A.; Borges, M.; Ruíz-Peña, J.L.; Izquierdo, G. Retest reliability of individual P3 topography assessed by high density electroencephalography. PLoS ONE 2013, 8, e62523. [Google Scholar] [CrossRef]
- Houshmand-Chatroudi, A.; Rostami, R.; Nasrabadi, A.M.; Yotsumoto, Y. Effect of inhibition indexed by auditory P300 on transmission of visual sensory information. PLoS ONE 2021, 16, e0247416. [Google Scholar] [CrossRef]
- Sabate, M.; Llanos, C.; Enriquez, E.; Gonzalez, B.; Rodriguez, M. Fast modulation of alpha activity during visual processing and motor control. Neuroscience 2011, 189, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Volosin, M.; Horváth, J. Task difficulty modulates voluntary attention allocation, but not distraction in an auditory distraction paradigm. Brain Res. 2019, 1727, 146565. [Google Scholar] [CrossRef] [PubMed]
- Ghani, U.; Signal, N.; Niazi, I.K.; Taylor, D. A novel approach to validate the efficacy of single task ERP paradigms to measure cognitive workload. Int. J. Psychophysiol. 2020, 158, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Kuehl, L.K.; Brandt, E.S.; Hahn, E.; Dettling, M.; Neuhaus, A.H. Exploring the time course of N170 repetition suppression: A preliminary study. Int. J. Psychophysiol. 2013, 87, 183–188. [Google Scholar] [CrossRef]
- Perri, R.L.; Berchicci, M.; Bianco, V.; Quinzi, F.; Spinelli, D.; Di Russo, F. Perceptual load in decision making: The role of anterior insula and visual areas. An ERP study. Neuropsychologia 2019, 129, 65–71. [Google Scholar] [CrossRef]
- Heinrichs-Graham, E.; Wilson, T.W. Coding complexity in the human motor circuit. Human. Brain Mapp. 2015, 36, 5155–5167. [Google Scholar] [CrossRef]
- Toledo, D.R.; Barela, J.A.; Manzano, G.M.; Kohn, A.F. Age-related differences in EEG beta activity during an assessment of ankle proprioception. Neurosci. Lett. 2016, 622, 1–5. [Google Scholar] [CrossRef]
- Visani, E.; Mariotti, C.; Nanetti, L.; Mongelli, A.; Castaldo, A.; Panzica, F.; Rossi-Sebastiano, D.; Nigri, A.; Grisoli, M.; Franceschetti, F.; et al. Cortical network dysfunction revealed by magnetoencephalography in carriers of spinocerebellar ataxia 1 or 2 mutation. Clin. Neurophysiol. 2020, 131, 1548–1555. [Google Scholar] [CrossRef]


| Behavioral (Mean ± Standard Deviation) | |||
|---|---|---|---|
| ST | DT | Wilcoxon Test (p-Value) | |
| Reaction Time | 384 ± 57.09 | 447 ± 73.10 | <0.001 |
| Accuracy | 99.55 ± 1.21 | 99.51 ± 1.33 | 0.678 |
| ERP | |||
| Latency (mean ± standard deviation) | |||
| ST | DT | t-Student (p-Value) | |
| P3 | 353 ± 44 | 405 ± 50 | <0.001 |
| Alpha | |||
| Latency (mean ± standard deviation) | |||
| ST | DT | t-Student (p-Value) | |
| Evoked | 146 ± 34 | 151 ± 28 | 1.000 |
| Induced | 168 ± 72 | 198 ± 95 | 0.197 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Marrufo, M.; Caballero-Díaz, R.; Sarrias-Arrabal, E.; Martín-Clemente, R. Decoupling Alpha Desynchronization from Neural Resource Use: Evidence from Cognitive Load Modulation. NeuroSci 2025, 6, 32. https://doi.org/10.3390/neurosci6020032
Vázquez-Marrufo M, Caballero-Díaz R, Sarrias-Arrabal E, Martín-Clemente R. Decoupling Alpha Desynchronization from Neural Resource Use: Evidence from Cognitive Load Modulation. NeuroSci. 2025; 6(2):32. https://doi.org/10.3390/neurosci6020032
Chicago/Turabian StyleVázquez-Marrufo, Manuel, Rocío Caballero-Díaz, Esteban Sarrias-Arrabal, and Rubén Martín-Clemente. 2025. "Decoupling Alpha Desynchronization from Neural Resource Use: Evidence from Cognitive Load Modulation" NeuroSci 6, no. 2: 32. https://doi.org/10.3390/neurosci6020032
APA StyleVázquez-Marrufo, M., Caballero-Díaz, R., Sarrias-Arrabal, E., & Martín-Clemente, R. (2025). Decoupling Alpha Desynchronization from Neural Resource Use: Evidence from Cognitive Load Modulation. NeuroSci, 6(2), 32. https://doi.org/10.3390/neurosci6020032






