Cog Threads for Transvaginal Prolapse Repair: Ex-Vivo Studies of a Novel Concept
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cog Threads
2.2. Animal Tissue
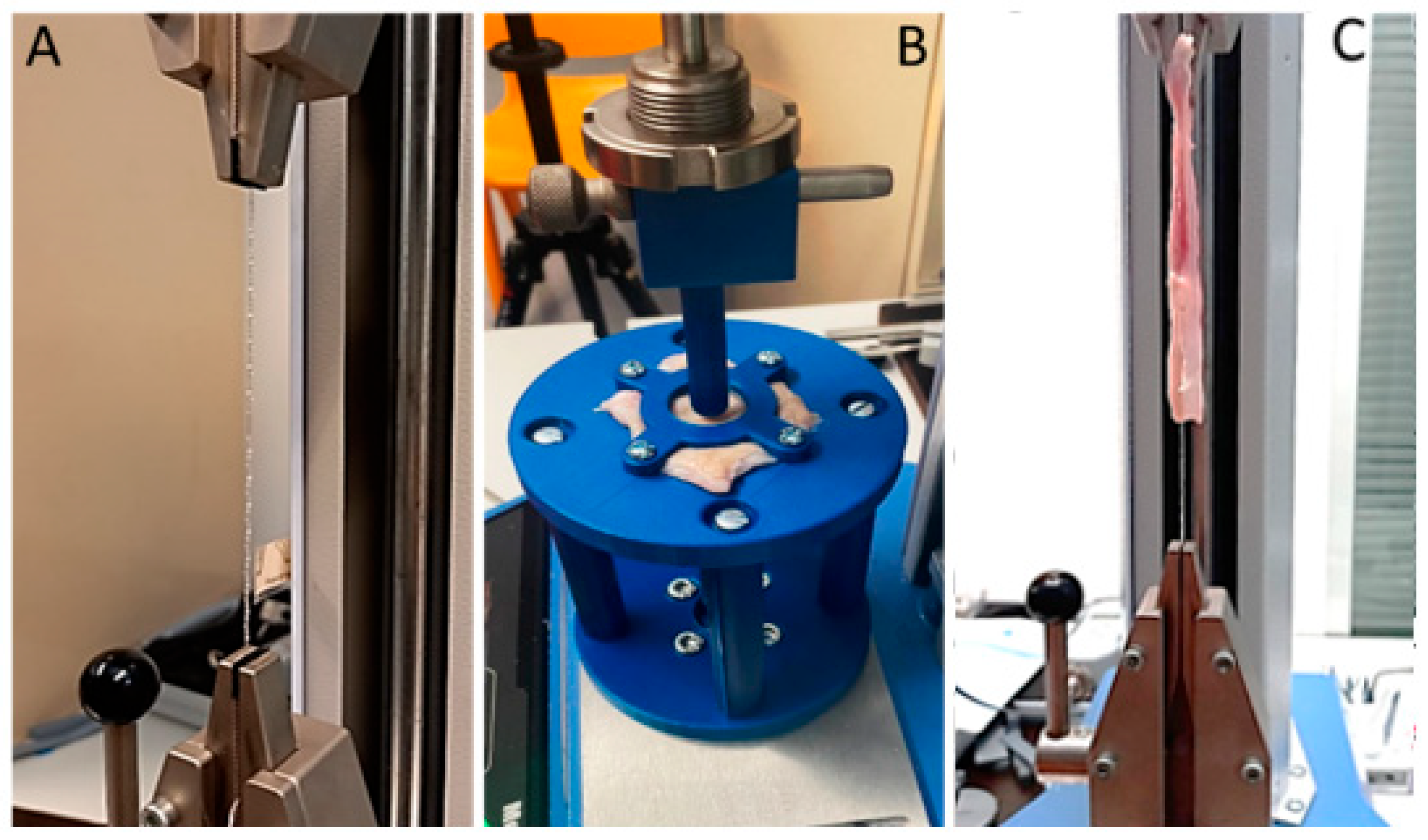
2.3. Ball Burst Test
2.4. Pull-Out Test
2.5. Statistical Analysis
3. Results
Cog Threads
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iglesia, C.B.; Smithling, K.R. Pelvic Organ Prolapse. Am. Fam. Physician 2017, 96, 179–185. [Google Scholar] [PubMed]
- Dieter, A.A.; Wilkins, M.F.; Wu, J.M. Epidemiological trends and future care needs for pelvic floor disorders. Curr. Opin. Obstet. Gynecol. 2015, 27, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Rada, M.P.; Jones, S.; Falconi, G. A systematic review and meta-synthesis of qualitative studies on pelvic organ prolapse for the development of core outcome sets. Neurourol. Urodyn. 2020, 39, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Maher, C.F.; Baessler, K.K.; Barber, M.D.; Cheong, C.; Consten, E.C.J.; Cooper, K.G. Surgical management of pelvic organ prolapse. Climacteric 2019, 22, 229–235. [Google Scholar] [CrossRef]
- Weintraub, A.Y.; Glinter, H.; Marcus-Braun, N. Narrative review of the epidemiology, diagnosis and pathophysiology of pelvic organ prolapse. Int. Braz. J. Urol. 2020, 46, 5–14. [Google Scholar] [CrossRef]
- Rogers, R.G.; Fashokun, T.B.; Brubaker, L.; Eckler, K. (Eds.) Pelvic Organ Prolapse in Women: Epidemiology, Risk Factors, Clinical Manifestations, and Management, UpToDate. 2020. Available online: https://www.uptodate.com/home/index.html (accessed on 21 April 2022).
- Rynkevic, R.; Martins, P.; Hympanova, L.; Almeida, H.; Fernandes, A.A.; Deprest, J. Biomechanical and morphological properties of the multiparous ovine vagina and effect of subsequent pregnancy. J. Biomech. 2017, 57, 94–102. [Google Scholar] [CrossRef]
- Hympanova, L.; Rynkevic, R.; Urbankova, I.; Blacher, S.; De Landsheere, L.; Mackova, K.; Krofta, L.; Deprest, J. Morphological and Functional Changes in the Vagina following Critical Lifespan Events in the Ewe. Gynecol. Obstet. Investig. 2019, 84, 360–368. [Google Scholar] [CrossRef]
- Rahn, D.D.; Ruff, M.D.; Brown, S.A.; Tibbals, H.; Word, R.A. Biomechanical properties of the vaginal wall: Effect of pregnancy, elastic fiber deficiency, and pelvic organ prolapse. Am. J. Obstet. Gynecol. 2008, 198, 590.e1–590.e6. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, D.; Szwarcensztein, K.; Mauskopf, J.A.; Slack, M.C. Rate, type, and cost of pelvic organ prolapse surgery in Germany, France, and England. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 144, 177–181. [Google Scholar] [CrossRef]
- Mascarenhas, T.; Ricon-Ferraz, A.; Nogueira, P.; Lopes, F.; Freitas, A. Pelvic organ prolapse surgical management in Portugal and FDA safety communication have an impact on vaginal mesh. Int. Urogynecol. J. 2015, 25, 113–122. [Google Scholar] [CrossRef]
- Bugge, C.; Adams, E.J.; Gopinath, D.; Reid, F. Pessaries (mechanical devices) for pelvic organ prolapse in women. Cochrane Database Syst. Rev. 2013, 11, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Geynisman-Tan, J.; Kenton, K. Surgical Updates in the Treatment of Pelvic Organ Prolapse. Rambam Maimonides Med. J. 2017, 8, e0017. [Google Scholar] [CrossRef] [PubMed]
- National Guideline Alliance (UK). Urinary incontinence and pelvic organ prolapse in women: Management. In NICE Guideline; National Institute for Health and Care Excellence: London, UK, 2019. [Google Scholar]
- Haylen, B.T.; Freeman, R.M.; Swift, S.E.; Cosson, M.; Davila, G.W.; Deprest, J.; Dwyer, P.L.; Fatton, B.; Kocjancic, E.; Lee, J.; et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint terminology and classification of the complications related directly to the insertion of prostheses (meshes, implants, tapes) and grafts in female pelvic flo. Neurourol. Urodyn. 2011, 30, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Abed, H.; Rahn, D.D.; Lowenstein, L.; Balk, E.M.; Clemons, J.L.; Rogers, G.; Systematic Review Group of the Society of Gynecologic Surgeons. Incidence and management of graft erosion, wound granulation, and dyspareunia following vaginal prolapse repair with graft materials: A systematic review. Int. Urogynecol. J. 2011, 22, 789–798. [Google Scholar] [CrossRef]
- Agrawal, A.; Avill, R. Mesh migration following repair of inguinal hernia: A case report and review of literature. Hernia 2006, 10, 79–82. [Google Scholar] [CrossRef]
- Food and Drug Administration. Serious Complications Associated with Transvaginal Placement of Surgical Mesh in Repair of Pelvic Organ Prolapse and Stress Urinary Incontinence; Public Health Notification; Food and Drug Administration FDA: Silver Spring, MD, USA, 2008. [Google Scholar]
- FDA. Urogynecologic Surgical Mesh Implants; U.S. Food and Drug Aministration: Silver Spring, MD, USA, 2019. Available online: https://www.fda.gov/medical-devices/implants-and-prosthetics/urogynecologic-surgical-mesh-implants (accessed on 18 July 2019).
- Myung, Y.; Jung, C. Mini-midface Lift Using Polydioxanone Cog Threads. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2920. [Google Scholar] [CrossRef]
- Wong, V.; Rafiq, N.; Kalyan, R. Hanging by a thread: Choosing the right thread for the right patient. J. Dermat. Cosmetol. 2017, 1, 86–88. [Google Scholar] [CrossRef] [Green Version]
- Dua, A.; Bhardwaj, B. A case report on use of cog threads and dermal fillers for facial-lifting in facioscapulohumeral muscular dystrophy. J. Cutan. Aesthet. Surg. 2019, 12, 52–55. [Google Scholar]
- Kalra, R. Use of barbed threads in facial rejuvenation. Indian J. Plast. Surg. 2008, 41, S93–S100. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.G.; Jung, J.; Hwang, S.; Park, C.O.; Hwang, S.; Jo, M.; Sin, M.H.; Kim, H.H.; Rhee, K.-J. Histological Evaluation of Bioresorbable Threads in Rats. Korean J. Clin. Lab. Sci. 2018, 50, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, J.; Goldman, R.H. Barbed Suture: A Review of the Obstetrics and Gynecology. Rev. Obstet. Gynecol. 2013, 6, 107–115. [Google Scholar] [PubMed]
- Hüsch, T.; Mager, R.; Ober, E.; Bentler, R.; Ulm, K.; Haferkamp, A. Quality of life in women of non-reproductive age with transvaginal mesh repair for pelvic organ prolapse: A cohort study. Int. J. Surg. 2016, 33, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Pahwa, R.; Goyal, A.; Bansal, P. Chronic Inflammation; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK493173/ (accessed on 21 April 2022).
- Hympánová, L.; Rynkevic, R.; Román, S.; da Cunha, M.G.M.; Mazza, E.; Zündel, M.; Urbánková, I.; Gallego, M.R.; Vange, J.; Callewaert, G.; et al. Assessment of Electrospun and Ultra-lightweight Polypropylene Meshes in the Sheep Model for Vaginal Surgery. Eur. Urol. Focus 2020, 6, 190–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenberg, J.A. The use of barbed sutures in obstetrics and gynecology. Rev. Obstet. Gynecol. 2010, 3, 82–91. [Google Scholar] [PubMed]
- Achtari, C.; Dwyer, P.L. Sexual function and pelvic floor disorders. Best Pract. Res. Clin. Obstet. Gynaecol. 2005, 19, 993–1008. [Google Scholar] [CrossRef] [PubMed]
- Ingle, N.P.; Cong, H.; King, M.W. Barbed Suture Technology; Woodhead Publishing Limited: Cambridge, UK, 2013. [Google Scholar]
- Kress, D. The history of barbed suture suspension: Applications, and visions for the future. In Simplified Facial Rejuvenation; Springer: Berlin/Heildeberg, Germany, 2008; pp. 247–256. [Google Scholar]
- Dällenbach, P. To mesh or not to mesh: A review of pelvic organ reconstructive surgery. Int. J. Womens Health 2015, 7, 331–343. [Google Scholar] [CrossRef] [Green Version]
- Gülbitti, H.A.; Colebunders, F.; Pirayesh, A.; Bertossi, D.; van der Lei, B. Thread-Lift Sutures: Still in the Lift? A Systematic Review of the Literature. Plast. Reconstr. Surg. 2018, 141, 341e–347e. [Google Scholar] [CrossRef]






| Tissue | Nr. of Specimens | Stiffness in Comfort Zone (N/mm) | Comfort Zone Length (mm) | Stiffness in Stress Zone (N/mm) | Ultimate Load (N) | Elongation at Ultimate Load (mm) |
|---|---|---|---|---|---|---|
| Vaginal wall | n = 5 | 0.16 ± 0.09 | 9.74 ± 0.43 | 14.26 ± 1.48 | 108.9 ± 9.76 | 17.60 ± 0.72 |
| Vaginal wall with cog threads | n = 5 | 1.01 ± 0.15 | 9.38 ± 1.32 | 21.75 ± 2.73 | 177.0 ± 5.42 | 16.32 ± 0.54 |
| P value | 0.0015 * | 0.8019 | 0.0426 * | 0.0003 * | 0.1915 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, C.; Martins, P.; Silva, E.; Hympanova, L.; Rynkevic, R. Cog Threads for Transvaginal Prolapse Repair: Ex-Vivo Studies of a Novel Concept. Surgeries 2022, 3, 101-110. https://doi.org/10.3390/surgeries3020012
Soares C, Martins P, Silva E, Hympanova L, Rynkevic R. Cog Threads for Transvaginal Prolapse Repair: Ex-Vivo Studies of a Novel Concept. Surgeries. 2022; 3(2):101-110. https://doi.org/10.3390/surgeries3020012
Chicago/Turabian StyleSoares, Catarina, Pedro Martins, Elisabete Silva, Lucie Hympanova, and Rita Rynkevic. 2022. "Cog Threads for Transvaginal Prolapse Repair: Ex-Vivo Studies of a Novel Concept" Surgeries 3, no. 2: 101-110. https://doi.org/10.3390/surgeries3020012
APA StyleSoares, C., Martins, P., Silva, E., Hympanova, L., & Rynkevic, R. (2022). Cog Threads for Transvaginal Prolapse Repair: Ex-Vivo Studies of a Novel Concept. Surgeries, 3(2), 101-110. https://doi.org/10.3390/surgeries3020012







