Advances in Instrumentation and Implant Technology for Spine Oncology: A Focus on Carbon Fiber Technologies
Abstract
1. Introduction
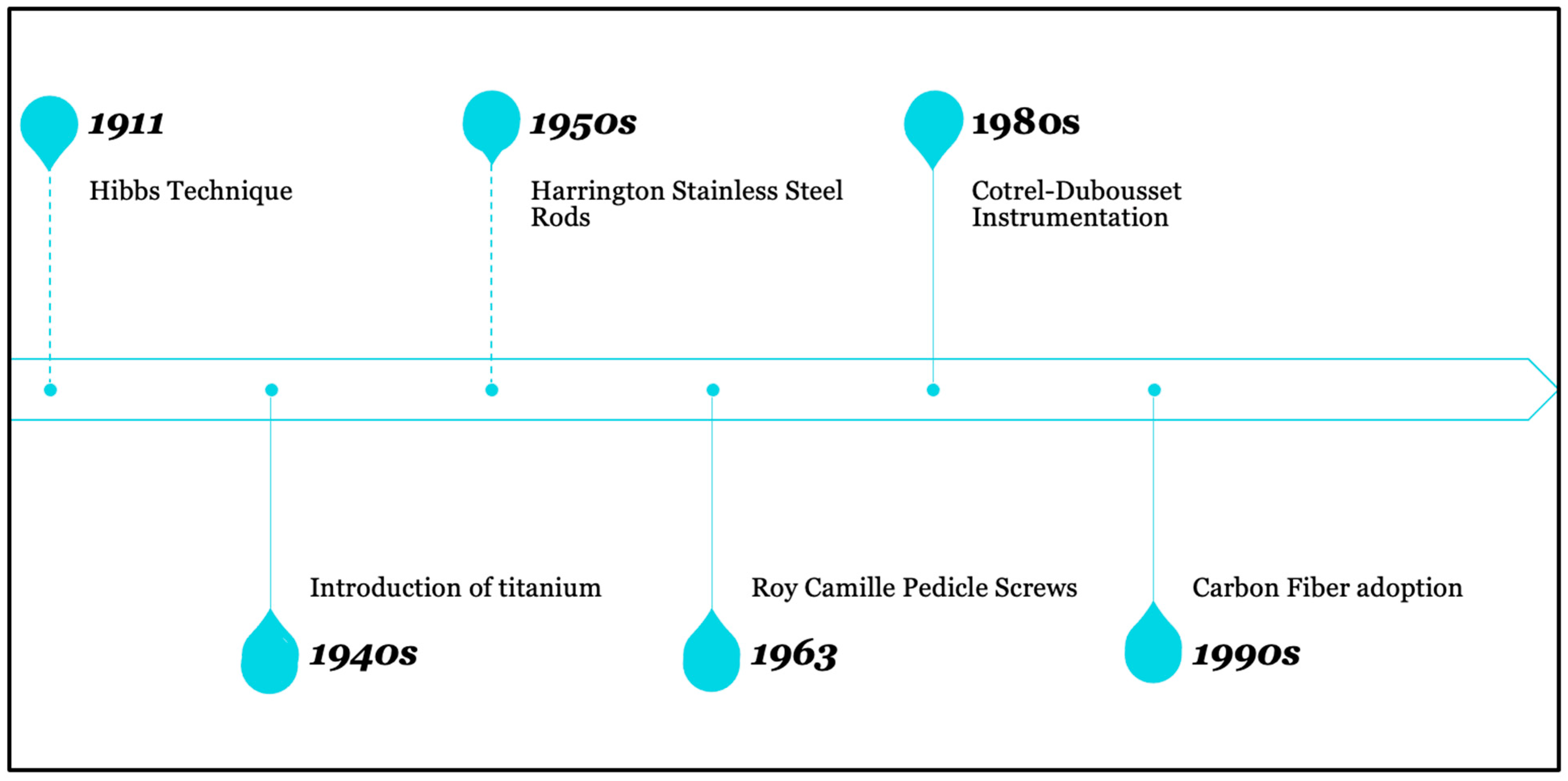
2. Historical Overview of Instrumentation in Spine Surgery
3. Recent Advances in Instrumentation for Spine Oncology
4. Introduction to Implant Technologies
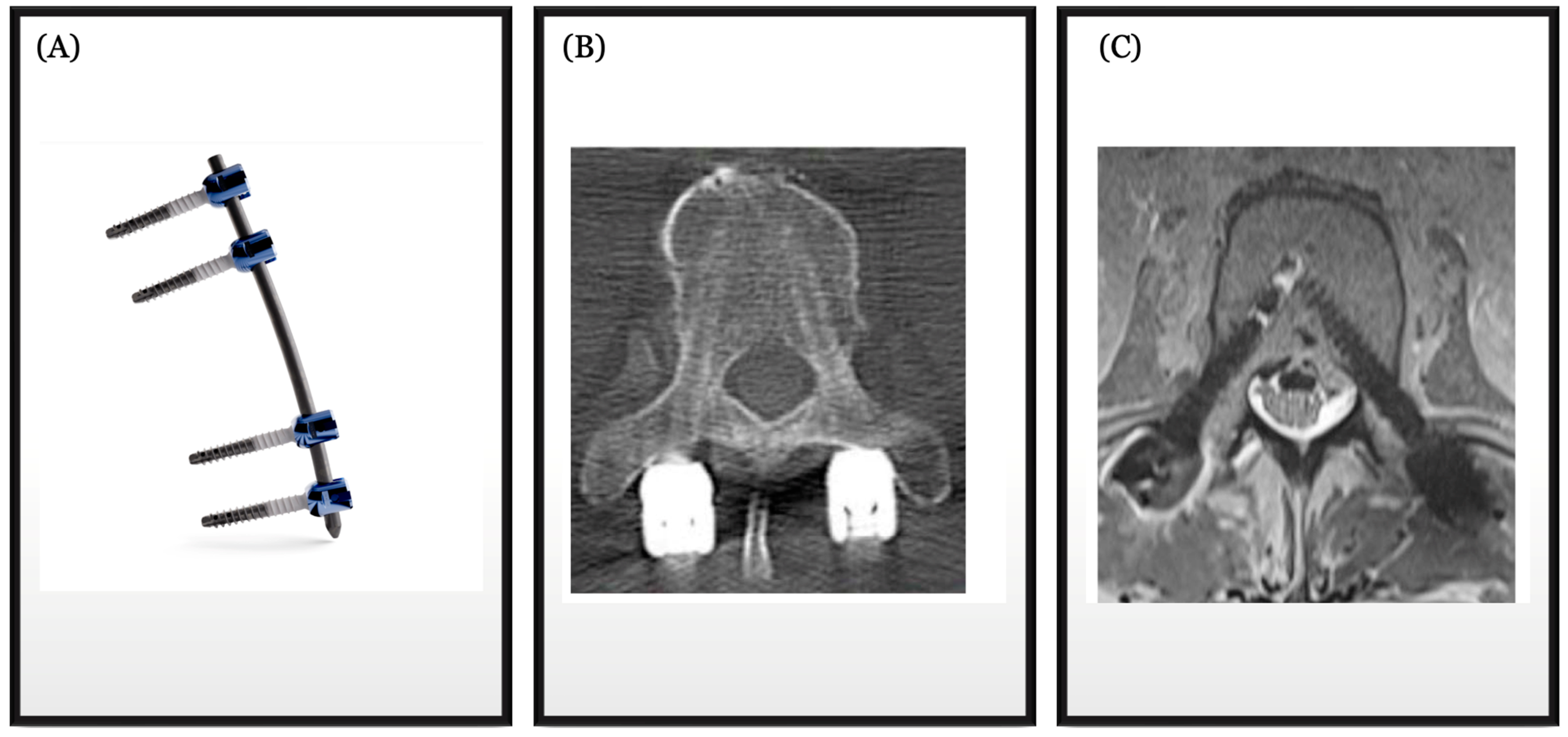
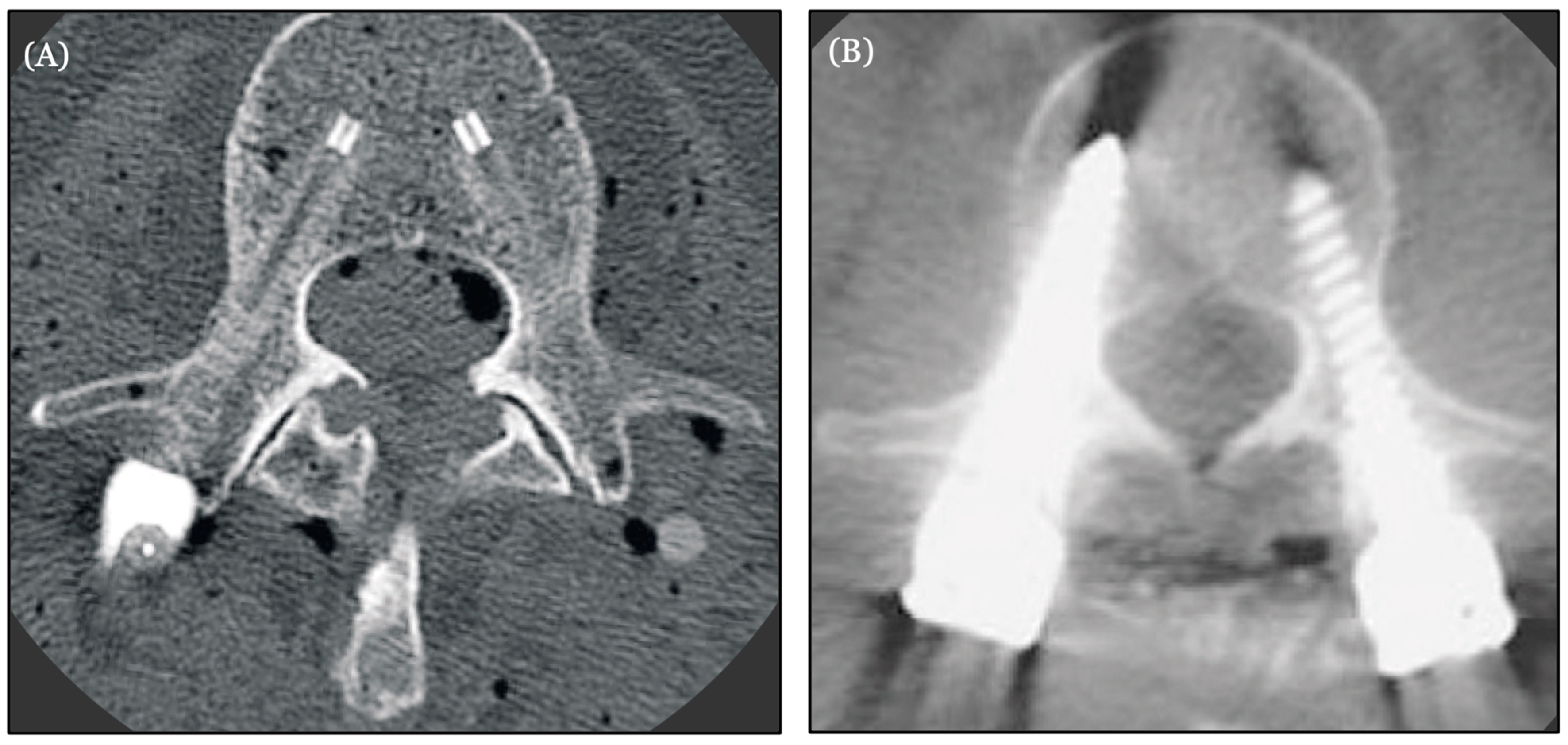
5. Carbon Fiber in Spine Oncology
6. New Techniques and Potential Horizons in Spine Oncology
7. Integration of Carbon Fiber Technology with Advanced Techniques
8. Patient-Centered Outcomes and Carbon Fiber Implants
9. The Future of Spine Oncology Instrumentation and Implants
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Neal, M.T.; Richards, A.E.; Curley, K.L.; Patel, N.P.; Ashman, J.B.; Vora, S.A.; Kalani, M.A. Carbon fiber-reinforced PEEK instrumentation in the spinal oncology population: A retrospective series demonstrating technique, feasibility, and clinical outcomes. Neurosurg. Focus 2021, 50, E13. [Google Scholar] [CrossRef] [PubMed]
- Charest-Morin, R.; Fisher, C.G.; Sahgal, A.; Boriani, S.; Gokaslan, Z.L.; Lazary, A.; Reynolds, J.; Bettegowda, C.; Rhines, L.D.; Dea, N. Primary Bone Tumor of the Spine-An Evolving Field: What a General Spine Surgeon Should Know. Glob. Spine J. 2019, 9 (Suppl. S1), 108S–116S. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Missenard, G.; Bouthors, C.; Fadel, E.; Court, C. Surgical strategies for primary malignant tumors of the thoracic and lumbar spine. Orthop. Traumatol. Surg. Res. 2020, 106, S53–S62. [Google Scholar] [CrossRef] [PubMed]
- Harel, R.; Doron, O.; Knoller, N. Minimally Invasive Spine Metastatic Tumor Resection and Stabilization: New Technology Yield Improved Outcome. Biomed. Res. Int. 2015, 2015, 948373. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shiber, M.; Kimchi, G.; Knoller, N.; Harel, R. The Evolution of Minimally Invasive Spine Tumor Resection and Stabilization: From K-Wires to Navigated One-Step Screws. J. Clin. Med. 2023, 12, 536. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petersen, R. Carbon Fiber Biocompatibility for Implants. Fibers 2016, 4, 1. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tedesco, G.; Gasbarrini, A.; Bandiera, S.; Ghermandi, R.; Boriani, S. Composite PEEK/Carbon fiber implants can increase the effectiveness of radiotherapy in the management of spine tumors. J. Spine Surg. 2017, 3, 323–329, Erratum in J. Spine Surg. 2018, 4, 167. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Warburton, A.; Girdler, S.J.; Mikhail, C.M.; Ahn, A.; Cho, S.K. Biomaterials in Spinal Implants: A Review. Neurospine 2020, 17, 101–110. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Virk, S.; Qureshi, S.; Sandhu, H. History of Spinal Fusion: Where We Came from and Where We Are Going. HSS J. 2020, 16, 137–142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cho, S.K.; Kim, Y.J. History of spinal deformity surgery part I: The Pre-modern Era. Korean J. Spine 2011, 8, 1–8. [Google Scholar] [CrossRef]
- Miller, D.J.; Vitale, M.G. Dr. Russell A. Hibbs: Pioneer of Spinal Fusion. Spine 2015, 40, 1311–1313. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.K.; Brayton, A.; Chua, V.B.; Luerssen, T.G.; Jea, A. The lasting legacy of Paul Randall Harrington to pediatric spine surgery: Historical vignette. J. Neurosurg. Spine 2013, 18, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Roy-Camille, R.; Roy-Camille, M.; Demeulenaere, C. Ostéosynthèse du rachis dorsal, lombaire et lombo-sacré par plaques métalliques vissées dans les pédicules vertébraux et les apophyses articulaires [Osteosynthesis of dorsal, lumbar, and lumbosacral spine with metallic plates screwed into vertebral pedicles and articular apophyses]. Presse Med. 1970, 78, 1447–1448. (In French) [Google Scholar] [PubMed]
- Shufflebarger, H.L.; Clark, C.E. Cotrel-Dubousset instrumentation. Orthopedics 1988, 11, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- Costăchescu, B.; Niculescu, A.G.; Iliescu, B.F.; Dabija, M.G.; Grumezescu, A.M.; Rotariu, D. Current and Emerging Approaches for Spine Tumor Treatment. Int. J. Mol. Sci. 2022, 23, 15680. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumar, N.; Patel, R.; Wadhwa, A.C.; Kumar, A.; Milavec, H.M.; Sonawane, D.; Singh, G.; Benneker, L.M. Basic concepts in metal work failure after metastatic spine tumour surgery. Eur. Spine J. 2018, 27, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Pérez de la Torre, R.A.; Ramanathan, S.; Williams, A.L.; Perez-Cruet, M.J. Minimally-Invasive Assisted Robotic Spine Surgery (MARSS). Front. Surg. 2022, 9, 884247. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Móga, K.; Ferencz, A.; Haidegger, T. What Is Next in Computer-Assisted Spine Surgery? Advances in Image-Guided Robotics and Extended Reality. Robotics 2022, 12, 1. [Google Scholar] [CrossRef]
- Sayari, A.J.; Pardo, C.; Basques, B.A.; Colman, M.W. Review of robotic-assisted surgery: What the future looks like through a spine oncology lens. Ann. Transl. Med. 2019, 7, 224. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Porras, J.L.; Pennington, Z.; Hung, B.; Hersh, A.; Schilling, A.; Goodwin, C.R.; Sciubba, D.M. Radiotherapy and Surgical Advances in the Treatment of Metastatic Spine Tumors: A Narrative Review. World Neurosurg. 2021, 151, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Baron, H.; Martinez-del-Campo, E.; Crawford, N.R.; Theodore, N. Robotics in spinal surgery: The future is here. Concern 2016, 13, 49. [Google Scholar]
- Massaad, E.; Shankar, G.M.; Shin, J.H. Novel Applications of Spinal Navigation in Deformity and Oncology Surgery-Beyond Screw Placement. Oper. Neurosurg. 2021, 21 (Suppl. 1), S23–S38. [Google Scholar] [CrossRef] [PubMed]
- Barzilai, O.; Bilsky, M.H.; Laufer, I. The Role of Minimal Access Surgery in the Treatment of Spinal Metastatic Tumors. Glob. Spine J. 2020, 10 (Suppl. S2), 79S–87S. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dick, J.C.; Bourgeault, C.A. Notch sensitivity of titanium alloy, commercially pure titanium, and stainless steel spinal implants. Spine 2001, 26, 1668–1672. [Google Scholar] [CrossRef] [PubMed]
- Litak, J.; Szymoniuk, M.; Czyżewski, W.; Hoffman, Z.; Litak, J.; Sakwa, L.; Kamieniak, P. Metallic Implants Used in Lumbar Interbody Fusion. Materials 2022, 15, 3650. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mao, J.Z.; Fritz, A.G.; Lucas, J.P.; Khan, A.; Popoola, D.O.; Becker, A.B.; Adetunji, A.; Levy, B.R.; Agyei, J.O.; O’Connor, T.E.; et al. Assessment of Rod Material Types in Spine Surgery Outcomes: A Systematic Review. World Neurosurg. 2021, 146, e6–e13. [Google Scholar] [CrossRef] [PubMed]
- Raso, J.; Chi, J.; Labaran, L.A.; Frank, C.; Shen, F.H. Carbon fiber lumbopelvic reconstruction following sacral giant cell tumor resection: Illustrative case. J. Neurosurg. Case Lessons 2023, 5, CASE22555. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Han, S.; Hyun, S.J.; Kim, K.J.; Jahng, T.A.; Kim, H.J. Comparative Study Between Cobalt Chrome and Titanium Alloy Rods for Multilevel Spinal Fusion: Proximal Junctional Kyphosis More Frequently Occurred in Patients Having Cobalt Chrome Rods. World Neurosurg. 2017, 103, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Uri, O.; Folman, Y.; Laufer, G.; Behrbalk, E. A Novel Spine Fixation System Made Entirely of Carbon-Fiber-Reinforced PEEK Composite: An In Vitro Mechanical Evaluation. Adv. Orthop. 2020, 2020, 4796136. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, Z.; Liu, P.; Zhang, X.; Xin, J.; Wang, Y.; Zou, X.; Mei, X.; Zhang, S.; Zhang, S. Strategies to improve bioactive and antibacterial properties of polyetheretherketone (PEEK) for use as orthopedic implants. Mater. Today Bio 2022, 16, 100402. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yeung, C.M.; Bhashyam, A.R.; Patel, S.S.; Ortiz-Cruz, E.; Lozano-Calderón, S.A. Carbon Fiber Implants in Orthopaedic Oncology. J. Clin. Med. 2022, 11, 4959. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ha, S.W.; Kirch, M.; Birchler, F.; Eckert, K.L.; Mayer, J.; Wintermantel, E.; Sittig, C.; Pfund-Klingenfuss, I.; Textor, M.; Spencer, N.D.; et al. Surface activation of polyetheretherketone (PEEK) and formation of calcium phosphate coatings by precipitation. J. Mater. Sci. Mater. Med. 1997, 8, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Katzer, A.; Marquardt, H.; Westendorf, J.; Wening, J.V.; von Foerster, G. Polyetheretherketone--cytotoxicity and mutagenicity in vitro. Biomaterials 2002, 23, 1749–1759. [Google Scholar] [CrossRef] [PubMed]
- Jockisch, K.A.; Brown, S.A.; Bauer, T.W.; Merritt, K. Biological response to chopped-carbon-fiber-reinforced peek. J. Biomed. Mater. Res. 1992, 26, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Krätzig, T.; Mende, K.C.; Mohme, M.; Kniep, H.; Dreimann, M.; Stangenberg, M.; Westphal, M.; Gauer, T.; Eicker, S.O. Carbon fiber-reinforced PEEK versus titanium implants: An in vitro comparison of susceptibility artifacts in CT and MR imaging. Neurosurg. Rev. 2021, 44, 2163–2170. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Murthy, N.K.; Wolinsky, J.P. Utility of carbon fiber instrumentation in spinal oncology. Heliyon 2021, 7, e07766. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schwendner, M.; Ille, S.; Kirschke, J.S.; Bernhardt, D.; Combs, S.E.; Meyer, B.; Krieg, S.M. Clinical evaluation of vertebral body replacement of carbon fiber-reinforced polyetheretherketone in patients with tumor manifestation of the thoracic and lumbar spine. Acta Neurochir. 2023, 165, 897–904. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Müther, M.; Lüthge, S.; Gerwing, M.; Stummer, W.; Schwake, M. Management of Spinal Dumbbell Tumors via a Minimally Invasive Posterolateral Approach and Carbon Fiber-Reinforced Polyether Ether Ketone Instrumentation: Technical Note and Surgical Case Series. World Neurosurg. 2021, 151, 277–283.e1. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Ding, X.; Yin, L.; Zhai, H.; Liu, H.; Kassaee, A.; Hill-Kayser, C.; Lustig, R.A.; McDonough, J.; Both, S. The effects of titanium mesh on passive-scattering proton dose. Phys. Med. Biol. 2014, 59, N81–N89. [Google Scholar] [CrossRef]
- Verburg, J.M.; Seco, J. Dosimetric accuracy of proton therapy for chordoma patients with titanium implants. Med. Phys. 2013, 40, 071727. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Galloway, R.; Gikas, N.; Golomohammad, R.; Sherriff, J.; Czyz, M.S.r. Separation Surgery, Fixation With Carbon-Fiber Implants, and Stereotactic Body Radiotherapy for Oligometastatic Spinal Disease. Cureus 2022, 14, e31370. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghermandi, R.; Tosini, G.; Lorenzi, A.; Griffoni, C.; La Barbera, L.; Girolami, M.; Pipola, V.; Barbanti Brodano, G.; Bandiera, S.; Terzi, S.; et al. Carbon Fiber-Reinforced PolyEtherEtherKetone (CFR-PEEK) Instrumentation in Degenerative Disease of Lumbar Spine: A Pilot Study. Bioengineering 2023, 10, 872. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Avanzini, A.; Battini, D.; Petrogalli, C.; Pandini, S.; Donzella, G. Anisotropic behaviour of extruded short carbon fibre reinforced peek under static and fatigue loading. Appl. Compos. Mater. 2022, 29, 1041–1060. [Google Scholar] [CrossRef]
- Bruner, H.J.; Guan, Y.; Yoganandan, N.; Pintar, F.A.; Maiman, D.J.; Slivka, M.A. Biomechanics of polyaryletherketone rod composites and titanium rods for posterior lumbosacral instrumentation. Presented at the 2010 Joint Spine Section Meeting. Laboratory investigation. J. Neurosurg. Spine 2010, 13, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Oikonomidis, S.; Greven, J.; Bredow, J.; Eh, M.; Prescher, A.; Fischer, H.; Thüring, J.; Eysel, P.; Hildebrand, F.; Kobbe, P.; et al. Biomechanical effects of posterior pedicle screw-based instrumentation using titanium versus carbon fiber reinforced PEEK in an osteoporotic spine human cadaver model. Clin. Biomech. 2020, 80, 105153. [Google Scholar] [CrossRef] [PubMed]
- Lindtner, R.A.; Schmid, R.; Nydegger, T.; Konschake, M.; Schmoelz, W. Pedicle screw anchorage of carbon fiber-reinforced PEEK screws under cyclic loading. Eur. Spine J. 2018, 27, 1775–1784. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Zhang, H.A.L.; Yang, S.; Zhang, J.; Wang, H.; Zhou, Z.; Zhou, Y.; Zhao, J.; Jiang, Z. Enhanced bioactivity and osteogenic property of carbon fiber reinforced polyetheretherketone composites modified with amino groups. Colloids Surf. B Biointerfaces 2020, 193, 111098. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Yang, D.; Yang, C.; Spintzyk, S.; Scheideler, L.; Li, P.; Li, D.; Geis-Gerstorfer, J.; Rupp, F. Carbon Fiber Reinforced PEEK Composites Based on 3D-Printing Technology for Orthopedic and Dental Applications. J. Clin. Med. 2019, 8, 240. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, J.; Yu, W.; Shi, R.; Yang, S.; Zhang, J.; Han, X.; Zhou, Z.; Gao, W.; Li, Y.; Zhao, J. Osseointegration behavior of carbon fiber reinforced polyetheretherketone composites modified with amino groups: An in vivo study. J. Biomed. Mater. Res. B Appl. Biomater. 2023, 111, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Li, C.S.; Vannabouathong, C.; Sprague, S.; Bhandari, M. The Use of Carbon-Fiber-Reinforced (CFR) PEEK Material in Orthopedic Implants: A Systematic Review. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2015, 8, 33–45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boriani, S.; Tedesco, G.; Ming, L.; Ghermandi, R.; Amichetti, M.; Fossati, P.; Krengli, M.; Mavilla, L.; Gasbarrini, A. Carbon-fiber-reinforced PEEK fixation system in the treatment of spine tumors: A preliminary report. Eur. Spine J. 2018, 27, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Breckenridge, C.; de Almeida, R.; Haider, A.; Muir, M.; Bird, J.; North, R.; Rhines, L.; Tatsui, C. Carbon Fiber-Reinforced Polyetheretherketone Spinal Implants for Treatment of Spinal Tumors: Perceived Advantages and Limitations. Neurospine 2023, 20, 317–326. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carbon-Fiber International Collaboration Initiative Research Group. Complications of patients with bone tumors treated with carbon-fiber plates: An international multicenter study. Sci. Rep. 2022, 12, 18969. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Henzen, D.; Schmidhalter, D.; Guyerm, G.; Stenger-Weisser, A.; Ermiş, E.; Poel, R.; Deml, M.C.; Fix, M.K.; Manser, P.; Aebersold, D.M.; et al. Feasibility of postoperative spine stereotactic body radiation therapy in proximity of carbon and titanium hybrid implants using a robotic radiotherapy device. Radiat. Oncol. 2022, 17, 94. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hubertus, V.; Wessels, L.; Früh, A.; Tkatschenko, D.; Nulis, I.; Bohner, G.; Prinz, V.; Onken, J.; Czabanka, M.; Vajkoczy, P.; et al. Navigation accuracy and assessability of carbon fiber-reinforced PEEK instrumentation with multimodal intraoperative imaging in spinal oncology. Sci. Rep. 2022, 12, 15816. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Joerger, A.K.; Shiban, E.; Krieg, S.M.; Meyer, B. Carbon-fiber reinforced PEEK instrumentation for spondylodiscitis: A single center experience on safety and efficacy. Sci. Rep. 2021, 11, 2414. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oh, J.; Visco, Z.R.; Ojukwu, D.I.; Galgano, M.A. Applications of Carbon Fiber Instrumentation in Spinal Oncology: Recent Innovations in Spinal Instrumentation and 2-Dimensional Illustrative Operative Video. Oper. Neurosurg. 2023, 24, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Scharschmidt, T.J.; Ohnmeiss, D.D.; Lieberman, I.H. Robotic assisted surgeries for the treatment of spine tumors. Int. J. Spine Surg. 2015, 9, 1. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raysi Dehcordi, S.; Marzi, S.; Ricci, A.; Di Cola, F.; Galzio, R.J. Less invasive approaches for the treatment of cervical schwannomas: Our experience. Eur. Spine J. 2012, 21, 887–896. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meyer, B.; Thomé, C.; Vajkoczy, P.; Kehl, V.; Dodel, R.; Ringel, F.; DYNORFUSE Study Group. Lumbar dynamic pedicle-based stabilization versus fusion in degenerative disease: A multicenter, double-blind, prospective, randomized controlled trial. J. Neurosurg. Spine 2022, 37, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Lee, K.H.; Lee, C.S.; Jung, J.Y.; Park, J.H.; Kim, G.L.; Kim, K.T. Instrumented surgical treatment for metastatic spinal tumors: Is fusion necessary? J. Neurosurg. Spine 2019, 32, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, C.; Curran, J.; Benzel, E.C.; Potter, R.; Magge, S.N.; Harrington JFJr Coumans, J.V.; Ghogawala, Z. Radiographic predictors of delayed instability following decompression without fusion for degenerative grade I lumbar spondylolisthesis. J. Neurosurg. Spine 2013, 18, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.; Faris, P.; McIntosh, G.; Manners, S.; Abraham, E.; Bailey, C.S.; Paquet, J.; Cadotte, D.; Jacobs, W.B.; Rampersaud, Y.R.; et al. Decompression alone vs. decompression plus fusion for claudication secondary to lumbar spinal stenosis. Spine J. 2019, 19, 1633–1639. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, Y.; Zou, D.; Yuan, B.; Ke, H.Z.; Li, W. Therapeutics for enhancement of spinal fusion: A mini review. J. Orthop. Translat. 2021, 31, 73–79. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Elder, B.D.; Ishida, W.; Goodwin, C.R.; Bydon, A.; Gokaslan, Z.L.; Sciubba, D.M.; Wolinsky, J.P.; Witham, T.F. Bone graft options for spinal fusion following resection of spinal column tumors: Systematic review and meta-analysis. Neurosurg. Focus 2017, 42, E16. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.S.; Kou, T.D. Risk of cancer after lumbar fusion surgery with recombinant human bone morphogenic protein-2 (rh-BMP-2). Spine 2013, 38, 1862–1868. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mazur-Hart, D.J.; Yamamoto, E.A.; Yoo, J.; Orina, J.N. Bone morphogenetic protein and cancer in spinal fusion: A propensity score-matched analysis. J. Neurosurg. Spine 2023, 39, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Beschloss, A.M.; DiCindio, C.M.; Lombardi, J.S.; Shillingford, J.N.; Laratta, J.L.; Holderread, B.; Louie, P.; Pugely, A.J.; Sardar, Z.; Khalsa, A.S.; et al. The Rise and Fall of Bone Morphogenetic Protein 2 Throughout the United States. Clin. Spine Surg. 2022, 35, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Feler, J.; Sun, F.; Bajaj, A.; Hagan, M.; Kanekar, S.; Sullivan, P.L.Z.; Fridley, J.S.; Gokaslan, Z.L. Complication Avoidance in Surgical Management of Vertebral Column Tumors. Curr. Oncol. 2022, 29, 1442–1454. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Glennie, R.A.; Rampersaud, Y.R.; Boriani, S.; Reynolds, J.J.; Williams, R.; Gokaslan, Z.L.; Schmidt, M.H.; Varga, P.P.; Fisher, C.G. A Systematic Review With Consensus Expert Opinion of Best Reconstructive Techniques After Osseous En Bloc Spinal Column Tumor Resection. Spine 2016, 41 (Suppl. 20), S205–S211. [Google Scholar] [CrossRef] [PubMed]
- Safari, M.; Khoshnevisan, A. An overview of the role of cancer stem cells in spine tumors with a special focus on chordoma. World J. Stem Cells 2014, 6, 53–64. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pour-Rashidi, A.; Mohammadi, E.; Rezaei, N.; Hanaei, S. Stem Cells and Targeted Gene Therapy in Brain and Spinal Cord Tumors. Adv. Exp. Med. Biol. 2023, 1394, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Grady, C.; Melnick, K.; Porche, K.; Dastmalchi, F.; Hoh, D.J.; Rahman, M.; Ghiaseddin, A. Glioma Immunotherapy: Advances and Challenges for Spinal Cord Gliomas. Neurospine 2022, 19, 13–29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, J.; Hu, L.; Bok, S.; Yallowitz, A.R.; Cung, M.; McCormick, J.; Zheng, L.J.; Debnath, S.; Niu, Y.; Tan, A.Y.; et al. A vertebral skeletal stem cell lineage driving metastasis. Nature 2023, 621, 602–609. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kang, K.T.; Koh, Y.G.; Son, J.; Yeom, J.S.; Park, J.H.; Kim, H.J. Biomechanical evaluation of pedicle screw fixation system in spinal adjacent levels using polyetheretherketone, carbon-fiber-reinforced polyetheretherketone, and traditional titanium as rod materials. Compos. Part B Eng. 2017, 130, 248–256. [Google Scholar] [CrossRef]
- Brantigan, J.W.; Steffee, A.D.; Geiger, J.M. A carbon fiber implant to aid interbody lumbar fusion: Mechanical testing. Spine 1991, 16 (Suppl. S6), S277–S282. [Google Scholar] [CrossRef] [PubMed]
- Disch, A.C.; Luzzati, A.; Melcher, I.; Schaser, K.D.; Feraboli, F.; Schmoelz, W. Three-dimensional stiffness in a thoracolumbar en-bloc spondylectomy model: A biomechanical in vitro study. Clin. Biomech. 2007, 22, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Disch, A.C.; Schaser, K.D.; Melcher, I.; Luzzati, A.; Feraboli, F.; Schmoelz, W. En bloc spondylectomy reconstructions in a biomechanical in-vitro study. Eur. Spine J. 2008, 17, 715–725. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cawley, D.T.; Alzakri, A.; Fujishiro, T.; Kieser, D.C.; Tavalaro, C.; Boissiere, L.; Obeid, I.; Pointillart, V.; Vital, J.M.; Gille, O. Carbon-fibre cage reconstruction in anterior cervical corpectomy for multilevel cervical spondylosis: Mid-term outcomes. J. Spine Surg. 2019, 5, 251–258. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Y.; Chen, D.; Guo, Y.; Wang, X.; Lu, X.; He, Z.; Yuan, W. Subsidence of titanium mesh cage: A study based on 300 cases. J. Spinal Disord. Tech. 2008, 21, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Quan, Z.; Zhao, Z.; Luo, X.; Tang, K.; Li, J.; Zhou, X.; Jiang, D. Evaluation of anterior cervical reconstruction with titanium mesh cages versus nano-hydroxyapatite/polyamide66 cages after 1- or 2-level corpectomy for multilevel cervical spondylotic myelopathy: A retrospective study of 117 patients. PLoS ONE 2014, 9, e96265. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rousseau, M.A.; Lazennec, J.Y.; Saillant, G. Circumferential arthrodesis using PEEK cages at the lumbar spine. J. Spinal Disord. Tech. 2007, 20, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.H.; Gasbarrini, A.; Lui, D.F.; Reynolds, J.; Capua, J.; Boriani, S. Integrated Custom Composite Polyetheretherketone/Carbon fiber (PEEK/CF) Vertebral Body Replacement (VBR) in the Treatment of Bone Tumors of the Spine: A Preliminary Report From a Multicenter Study. Spine 2022, 47, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Pugely, A.J.; Lindsay, C.P.; Hall, J.; Orness, M.E.; Toossi, N.; Bucklen, B. Are modular pedicle screws associated with a high complication rate following posterior spinal fixation? J. Spine Surg. 2023, 9, 133–138. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hsieh, J.Y.; Chuang, S.M.; Chen, C.S.; Wang, J.H.; Chen, P.Q.; Huang, Y.Y. Novel Modular Spine Blocks Affect the Lumbar Spine on Finite Element Analysis. Spine Surg. Relat. Res. 2022, 6, 533–539. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perez-Roman, R.J.; Boddu, J.V.; Bashti, M.; Bryant, J.P.; Amadasu, E.; Gyedu, J.S.; Wang, M.Y. The Use of Carbon Fiber-Reinforced Instrumentation in Patients with Spinal Oncologic Tumors: A Systematic Review of Literature and Future Directions. World Neurosurg. 2023, 173, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.L.; Rumley, J.; Griffith, M.; Agochukwu, U.; DeVine, J. Correlating Psychological Comorbidities and Outcomes After Spine Surgery. Glob. Spine J. 2020, 10, 929–939. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Heer, E.W.; Ten Have, M.; van Marwijk, H.W.J.; Dekker, J.; de Graaf, R.; Beekman, A.T.F.; van der Feltz-Cornelis, C.M. Pain as a risk factor for common mental disorders. Results from the Netherlands Mental Health Survey and Incidence Study-2: A longitudinal, population-based study. Pain 2018, 159, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Denton, H.; Dodds, M.; Delaney, F.; Kavanagh, E. Use of Carbon Fibre Implants in Metastatic Spinal Surgery. Ir. Med. J. 2021, 114, P473. [Google Scholar]
- Khan, H.A.; Ber, R.; Neifert, S.N.; Kurland, D.B.; Laufer, I.; Kondziolka, D.; Chhabra, A.; Frempong-Boadu, A.K.; Lau, D. Carbon fiber-reinforced PEEK spinal implants for primary and metastatic spine tumors: A systematic review on implant complications and radiotherapy benefits. J. Neurosurg. Spine 2023, 39, 534–547. [Google Scholar] [CrossRef] [PubMed]
- Massaad, E.; Fatima, N.; Hadzipasic, M.; Alvarez-Breckenridge, C.; Shankar, G.M.; Shin, J.H. Predictive Analytics in Spine Oncology Research: First Steps, Limitations, and Future Directions. Neurospine 2019, 16, 669–677. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hopkins, B.S.; Mazmudar, A.; Driscoll, C.; Svet, M.; Goergen, J.; Kelsten, M.; Shlobin, N.A.; Kesavabhotla, K.; Smith, Z.A.; Dahdaleh, N.S. Using artificial intelligence (AI) to predict postoperative surgical site infection: A retrospective cohort of 4046 posterior spinal fusions. Clin. Neurol. Neurosurg. 2020, 192, 105718. [Google Scholar] [CrossRef] [PubMed]
- Benzakour, A.; Altsitzioglou, P.; Lemée, J.M.; Ahmad, A.; Mavrogenis, A.F.; Benzakour, T. Artificial intelligence in spine surgery. Int. Orthop. 2023, 47, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Barzilai, O.; Martin, A.; Reiner, A.S.; Laufer, I.; Schmitt, A.; Bilsky, M.H. Clinical reliability of genomic data obtained from spinal metastatic tumor samples. Neuro Oncol. 2022, 24, 1090–1100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ljungqvist, O.; Scott, M.; Fearon, K.C. Enhanced Recovery After Surgery: A Review. JAMA Surg. 2017, 152, 292–298. [Google Scholar] [CrossRef]
- Altman, A.D.; Helpman, L.; McGee, J.; Samouëlian, V.; Auclair, M.H.; Brar, H.; Nelson, G.S.; Society of Gynecologic Oncology of Canada’s Communities of Practice in ERAS and Venous Thromboembolism. Enhanced recovery after surgery: Implementing a new standard of surgical care. CMAJ 2019, 191, E469–E475. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zaed, I.; Bossi, B.; Ganau, M.; Tinterri, B.; Giordano, M.; Chibbaro, S. Current state of benefits of Enhanced Recovery After Surgery (ERAS) in spinal surgeries: A systematic review of the literature. Neurochirurgie 2022, 68, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Debono, B.; Wainwright, T.W.; Wang, M.Y.; Sigmundsson, F.G.; Yang, M.M.H.; Smid-Nanninga, H.; Bonnal, A.; Le Huec, J.C.; Fawcett, W.J.; Ljungqvist, O.; et al. Consensus statement for perioperative care in lumbar spinal fusion: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Spine J. 2021, 21, 729–752. [Google Scholar] [CrossRef] [PubMed]
- Naftalovich, R.; Singal, A.; Iskander, A.J. Enhanced Recovery After Surgery (ERAS) protocols for spine surgery—Review of literature. Anaesthesiol. Intensive Ther. 2022, 54, 71–79. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bogani, G.; Sarpietro, G.; Ferrandina, G.; Gallotta, V.; DIDonato, V.; Ditto, A.; Pinelli, C.; Casarin, J.; Ghezzi, F.; Scambia, G.; et al. Enhanced recovery after surgery (ERAS) in gynecology oncology. Eur. J. Surg. Oncol. 2021, 47, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Engelman, D.T.; Ben Ali, W.; Williams, J.B.; Perrault, L.P.; Reddy, V.S.; Arora, R.C.; Roselli, E.E.; Khoynezhad, A.; Gerdisch, M.; Levy, J.H.; et al. Guidelines for Perioperative Care in Cardiac Surgery: Enhanced Recovery After Surgery Society Recommendations. JAMA Surg. 2019, 154, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Melloul, E.; Lassen, K.; Roulin, D.; Grass, F.; Perinel, J.; Adham, M.; Wellge, E.B.; Kunzler, F.; Besselink, M.G.; Asbun, H.; et al. Guidelines for Perioperative Care for Pancreatoduodenectomy: Enhanced Recovery after Surgery (ERAS) Recommendations 2019. World J. Surg. 2020, 44, 2056–2084. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, E.M. Multidisciplinary spine oncology care across the disease continuum. Neurooncol. Pract. 2020, 7 (Suppl. S1), i1–i4. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Conti, A.; Acker, G.; Kluge, A.; Loebel, F.; Kreimeier, A.; Budach, V.; Vajkoczy, P.; Ghetti, I.; Germano’, A.F.; Senger, C. Decision Making in Patients With Metastatic Spine. The Role of Minimally Invasive Treatment Modalities. Front. Oncol. 2019, 9, 915. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Carbon Fiber | Titanium | Steel | |
|---|---|---|---|
| Strength-to weight ratio | High tensile strength of 3.6 GPa and low density of about 1.8 g/cm3. This gives it exceptional strength-to-weight ratio | Density of about 4.51 g/cm3. Tensile strength of 240 Mpa. Good strength-weight-ratio | Tensile strength of ~250 Mpa. Density of about 8 g/cm3. Thus, worst strength-to-weight ratio of the three. |
| Energy Conductivity | Low thermal conductivity and not a conductor of electricity | Moderate thermal conductivity. Conductor of electricity | Moderate thermal conductivity. Conductor of electricity |
| Corrosion and Fatigue Resistance | Elastic modulus of 233 GPa. Good fatigue resistance, particularly with repetitive loading. Excellent corrosion resistant | Exceptional fatigue resistance. Elastic modulus of 120 GPa. Outstanding corrosion resistance | Good fatigue resistance; with elastic modulus of 200 GPa. Not corrosion resistant |
| Imaging Compatibility | Safe MRI magnetic field. Does not cause artifact on CT | Safe on MRI magnetic field. Some artifact on CT | Incompatible with MRI imaging. Significant artifact on CT |
| Title (Year) | Materials | Study/Tests | Conclusion | Overall Positive Outcome (Y/N) |
|---|---|---|---|---|
| Alvarez-Breckenridge et al. (2023) | Carbon fiber reinforced PEEK (CFR-PEEK) | Assessment of the perceived advantages of improved imaging quality, postradiation treatment planning and recurrent tumor detection in spinal tumor patients treated with CFR-PEEK | CFR-PEEK is safe and effective for spinal stabilization in both primary and metastatic tumors. Better postoperative radiating planning and local tumor recurrence detection were achieved as a result of improved postoperative imaging quality [52] | Y |
| Carbon-Fiber International Collaboration Initiative Research Group (2022) | Carbon Fiber plates | Postoperative complications in bone tumor patients treated with Carbon Fiber plates | The use of Carbon Fiber plates in post-tumor resection reconstructions is safe and showed seemingly low complication profile [53] | Y |
| Ghermandi et al. (2023) | Carbon fiber reinforced PEEK (CFR-PEEK) | Evaluation of Carbon Fiber-Reinforced PEEK in the treatment of degenerative lumbar spine disease | CFR-PEEK implants is safe and effective in the treatment of spinal degenerative diseases [42] | Y |
| Henzen et al. (2022) | Carbon fiber reinforced PEEK and Titanium (CFP-T) hybrid implant | Feasibility of postoperative stereotactic body radiation therapy (SBRT) | Dosimetry and delivery of postoperative SBRT is feasible in patients with CFP-T implants using a CyberKnife system [54] | Y |
| Hubertus et al. (2022) | Carbon fiber reinforced PEEK (CFR-PEEK) | Evaluation of the performance and precision of 3D intraoperative imaging and navigation systems in thoraco-lumbar instrumentation with CFR-PEEK pedicle screws | Navigation accuracy was considerably lower for CFR-PEEK pedicle screws than reported for titanium implants. CT may be the best imaging modality for CFR-PEEK instrumentation assessment [55] | N |
| Joerger et al. (2021) | Carbon fiber reinforced PEEK (CFR-PEEK) | Comparative assessment of pedicle screw loosening and relapse with CFR-PEEK vs titanium in spondylodiscitis patients | More CFR-PEEK pedicle screw loosening than with titanium likely due to stronger bacterial adhesion in spondylodiscitis patients. No difference in fusion rates [56] | N |
| Neal et al. (2021) | Carbon fiber reinforced PEEK (CFR-PEEK) | Feasibility and advantages of CFR-PEEK implants in both primary and secondary osseous spinal tumors | CFR-PEEK is comparable to titanium in functionality. Additionally, the imaging characteristics of CFR-PEEK implants result in enhanced safety and efficacy for postoperative radiation planning and surveillance [1] | Y |
| Oh et al. (2023) | Carbon fiber reinforced PEEK (CFR-PEEK) | Feasibility of CFR-PEEK to provide structural stability while allowing monitoring and treatment of spinal oncologic deformity | CFR-PEEK is safe, effective and may provide relatively more benefit than existing instrumentation for treatment of thoracolumbar posterior spinal pathology [57] | Y |
| Schwendner et al. (2023) | Carbon fiber reinforced PEEK (CFR-PEEK) | Assessment of the clinical and radiological outcomes in patients treated with CFR-PEEK dorsoventral instrumentation | The use of CFR-PEEK for vertebral body replacement in thoracic and lumbar spinal tumor patients offers an improved management option, particularly in terms of postoperative radiotherapy and MRI-based follow-up [37] | Y |
| Uri et al. (2020) | Carbon fiber reinforced PEEK (CFR-PEEK) | In vitro evaluation of Carbon Fiber-Reinforce PEEK | CFR-PEEK composite pedicle screw has superior fatigue properties than titanium-made implants of comparable mechanical properties. The fatigue resistance is similar to bone; it is radiolucent and results in artifact-free images on CT/MRI [29] | Y |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amadi, I.; Kabangu, J.-L.K.; Bhargav, A.G.; Ohiorhenuan, I.E. Advances in Instrumentation and Implant Technology for Spine Oncology: A Focus on Carbon Fiber Technologies. Surgeries 2024, 5, 499-516. https://doi.org/10.3390/surgeries5030041
Amadi I, Kabangu J-LK, Bhargav AG, Ohiorhenuan IE. Advances in Instrumentation and Implant Technology for Spine Oncology: A Focus on Carbon Fiber Technologies. Surgeries. 2024; 5(3):499-516. https://doi.org/10.3390/surgeries5030041
Chicago/Turabian StyleAmadi, Iheanyi, Jean-Luc K. Kabangu, Adip G. Bhargav, and Ifije E. Ohiorhenuan. 2024. "Advances in Instrumentation and Implant Technology for Spine Oncology: A Focus on Carbon Fiber Technologies" Surgeries 5, no. 3: 499-516. https://doi.org/10.3390/surgeries5030041
APA StyleAmadi, I., Kabangu, J.-L. K., Bhargav, A. G., & Ohiorhenuan, I. E. (2024). Advances in Instrumentation and Implant Technology for Spine Oncology: A Focus on Carbon Fiber Technologies. Surgeries, 5(3), 499-516. https://doi.org/10.3390/surgeries5030041







