Abstract
The skin is crucial for homeostasis and body defense, requiring quick healing to maintain internal balance. Initially used for bone repair, calcium hydroxyapatite (HAp) is now being studied for soft tissue engineering. This literature review investigated HAp’s role in tissue repair through searches on PubMed, Scopus (Elsevier), Science Direct, Springer Link, and Google Scholar databases without time restrictions, using keywords “hydroxyapatite AND skin AND wound” and “hydroxyapatite AND skin repair”. Inclusion criteria encompassed in vivo studies in humans and animals, English publications, full access, and sufficient data on HAp’s role in tissue repair. Exclusions included duplicates, unrelated articles, editor letters, reviews, comments, conference abstracts, dissertations, and theses. Out of the 472 articles initially identified, 139 met the inclusion criteria, with 21 focusing on HAp for tissue repair. Findings indicate that HAp and nano-HAp in skin regeneration are promising, especially when combined with other biomaterials, offering antimicrobial and anti-inflammatory benefits and stimulating angiogenesis. This suggests their potential application in dermatology, surgery, and dentistry, extending HAp’s versatility from hard tissues to enhancing critical properties for soft tissue repair and accelerating healing.
1. Introduction
As the largest and most important organ of the human body, the skin plays a fundamental role in maintaining homeostasis, preventing microbial and chemical invasion and serving as the body’s first line of defense against injuries, infections, and dehydration [1,2,3]. When the skin loses its integrity, the body’s natural response is to initiate a complex healing process characterized by three main stages: inflammation, proliferation, and remodeling.
In the initial inflammation stage, an inflammatory response is triggered, evidenced by up to five signs: redness, heat, rubor, edema, and functional limitation. This stage is responsible for clearing debris and pathogens in the area and preparing the site for the next phase. Soon after, the proliferation stage begins, where the body starts repairing the damaged tissue through the formation of new blood vessels (angiogenesis) and specialized cells like fibroblasts and endothelial cells, promoting the creation of granulation tissue to fill the wound. The proper functioning of this stage is crucial for restoring skin integrity. Finally, remodeling, the final phase of the process, involves the reorganization of collagen, maturation of the newly formed tissue, and deposition of the extracellular matrix (ECM) [3,4], followed by a cascade of events that lead to tissue repair. Throughout this cascade, any interruption or complication can lead to persistent infections, undesirable scars, and even complete failure of wound healing [5].
When damaged, the skin loses its protective function, facilitating the invasion of microorganisms and leading to severe infections [6,7,8]. Various conditions, such as diabetes, burns, and senile diseases, can cause significant skin defects [9,10,11,12]. Prolonged inflammation, with continuous secretion of pro-inflammatory mediators, impairs and delays healing [13,14]. Therefore, the repair and regeneration of soft tissues are growing areas of study due to their importance for human survival [11,13].
In tissue engineering, the need for the use of natural and synthetic substitutes that meet criteria of biocompatibility, biodegradability, and adequate mechanical strength has emerged [14,15,16,17]. Calcium hydroxyapatite is a bioactive ceramic based on calcium phosphate [18] that fits these criteria to become a good tissue substitute, as it helps in the attachment, proliferation, and differentiation of cells [19]. Additionally, it releases calcium and reduces wound size by inducing blood clotting, as calcium ions are mediators of wound healing and tissue regeneration [20].
Calcium hydroxyapatite nanoparticles have been shown to be excellent nanomaterials due to their modern properties of bioactivity, non-toxicity, and biocompatibility [21]. Moreover, hydroxyapatite is non-toxic and has seamless structures that promote accelerated cell proliferation, spreading, rapid bioabsorption, and regeneration in a short period of time [2]. There are several studies on its medical use in various fields, such as cartilage regeneration, as a coating for implants due to its hard tissue structure, and mainly for bone repair [22].
It is believed that the calcium particles released by hydroxyapatite trigger an inflammatory response already in the initial stage of healing, accelerating wound healing and promoting the migration and proliferation of fibroblasts and keratinocytes, the second stage of the wound regeneration cascade, essential for the formation of new epithelial tissue [19]. Additionally, HAp can modulate the inflammatory response, reducing pro-inflammatory cytokines and creating an environment conducive to tissue regeneration [23]. Recent studies show that HAp can also stimulate angiogenesis, providing nutrients and oxygen to damaged areas [24]. Therefore, through all these mechanisms, calcium hydroxyapatite can significantly accelerate skin wound healing.
Furthermore, when used in orofacial harmonization, calcium hydroxyapatite promotes the regeneration of collagen I and III, elastin, and proteoglycans, in addition to stimulating the formation of new tissue and vasculature. Clinically, this translates into firmer, brighter, more flexible, elastic, and hydrated skin with fewer wrinkles [25]. These effects can result in a younger and more aesthetic appearance, without leaving scar marks or resulting in thinner scars after complete healing. The authors of this study aim to fill any gaps that may exist in the available literature on the use of calcium hydroxyapatite and its role in skin tissue repair, presenting evidence, practical aspects, and applications of this substance. It is hoped that the knowledge gained from this literature review will contribute to the use of this material in skin healing.
2. Materials and Methods
We accessed and selected manuscripts from five databases, PubMed, Scopus (Elsevier), Science Direct, Springer Link, and Google Scholar, without temporal restrictions regarding the year of publication and using the following keywords: “hydroxyapatite AND skin AND wound”, “hydroxyapatite AND skin repair”, and “hydroxyapatite and/or burn wound treatment”. Through the intersection of these keywords, we conducted a detailed analysis of the results, considering the title and abstract of each scientific article as important criteria for selection. Each manuscript was analyzed to ensure the relevance of both the title and abstract. Subsequently, the manuscripts were classified as included or not included according to the established eligibility criteria. In selecting studies for a detailed analysis, two independent reviewers evaluated the manuscripts, considering the selection criteria to minimize bias.
The inclusion criteria consisted of studies conducted in humans and animals, in vivo/in vitro studies, and publications in English that allowed access to the full text and provided sufficient data to understand the role of calcium hydroxyapatite in tissue repair. We excluded duplicate articles, those not directly related to the objective of this review, cases where calcium hydroxyapatite was not used for tissue repair or was not the focus of the study, as well as articles, letters to the editor, reviews, commentaries, conference abstracts, dissertations, and theses from repositories.
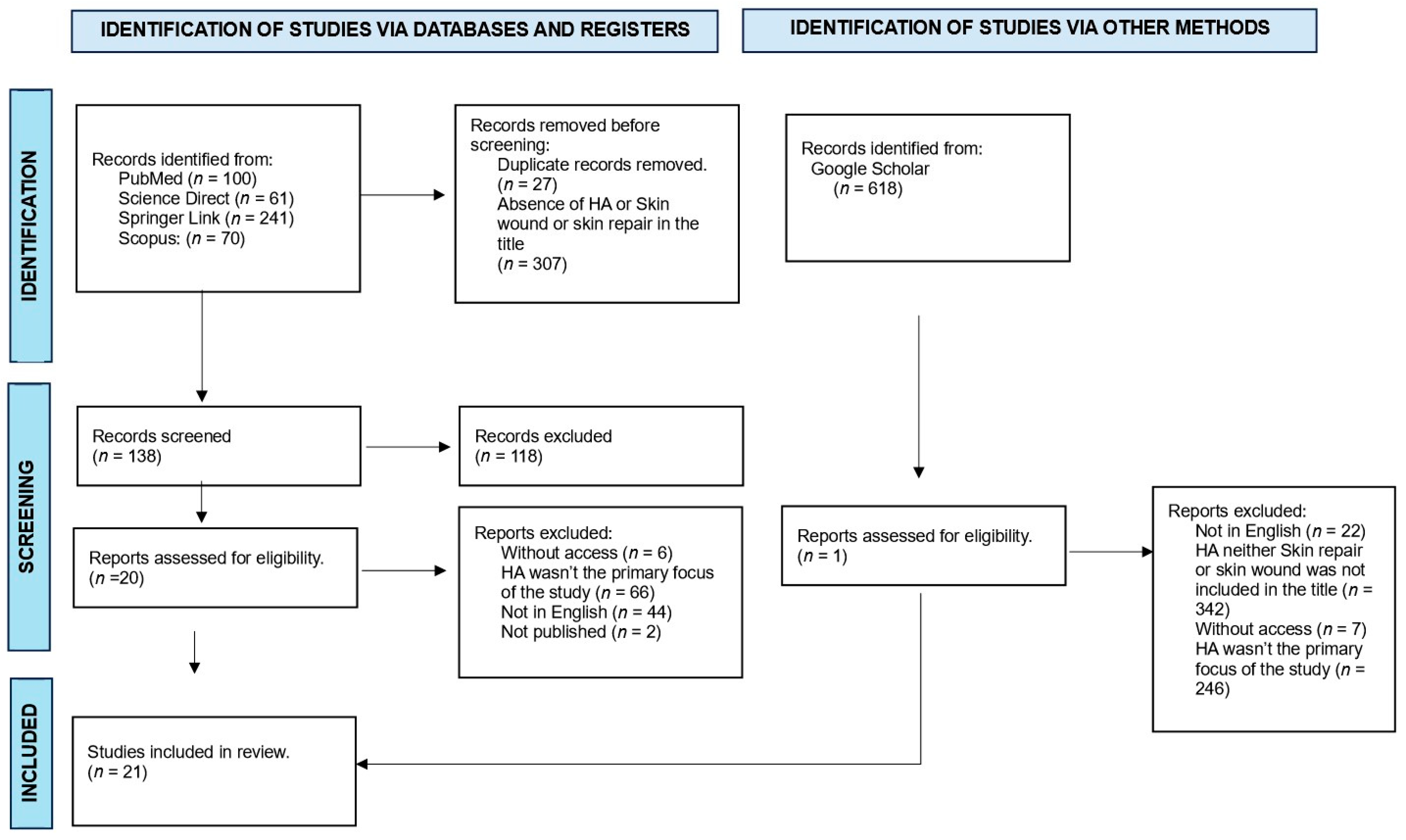
Studies with titles and abstracts related to the chosen topic were initially selected according to the focus of this review: the use of calcium hydroxyapatite in the tissue repair process. The next step involved excluding duplicate articles in the consulted databases and removing studies that did not meet the eligibility criteria through a careful reading of the texts. Special attention was given to the methodology used in the study, ensuring that the procedures were effectively related to the proposed theme. The article selection scheme is presented in Figure 1.
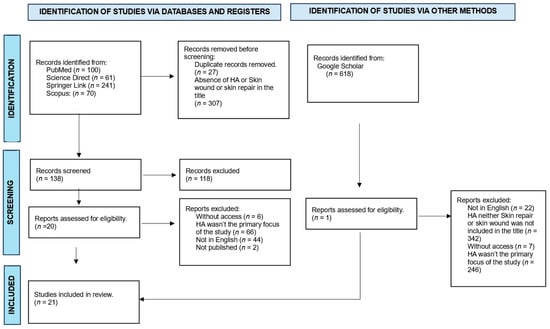
Figure 1.
Flowchart of article selection for detailed analysis.
3. Results
We identified 472 articles in the literature on calcium hydroxyapatite (HA). After applying the inclusion and exclusion criteria outlined in this paper, 139 articles were retained in the context of the role of hydroxyapatite in regeneration, from which we selected 21 studies that specifically addressed the use of the calcium hydroxyapatite compound as a material directly to achieve tissue repair. The results of this detailed analysis are presented in a systematic manner below (Table 1).

Table 1.
Articles selected by detailed analysis.
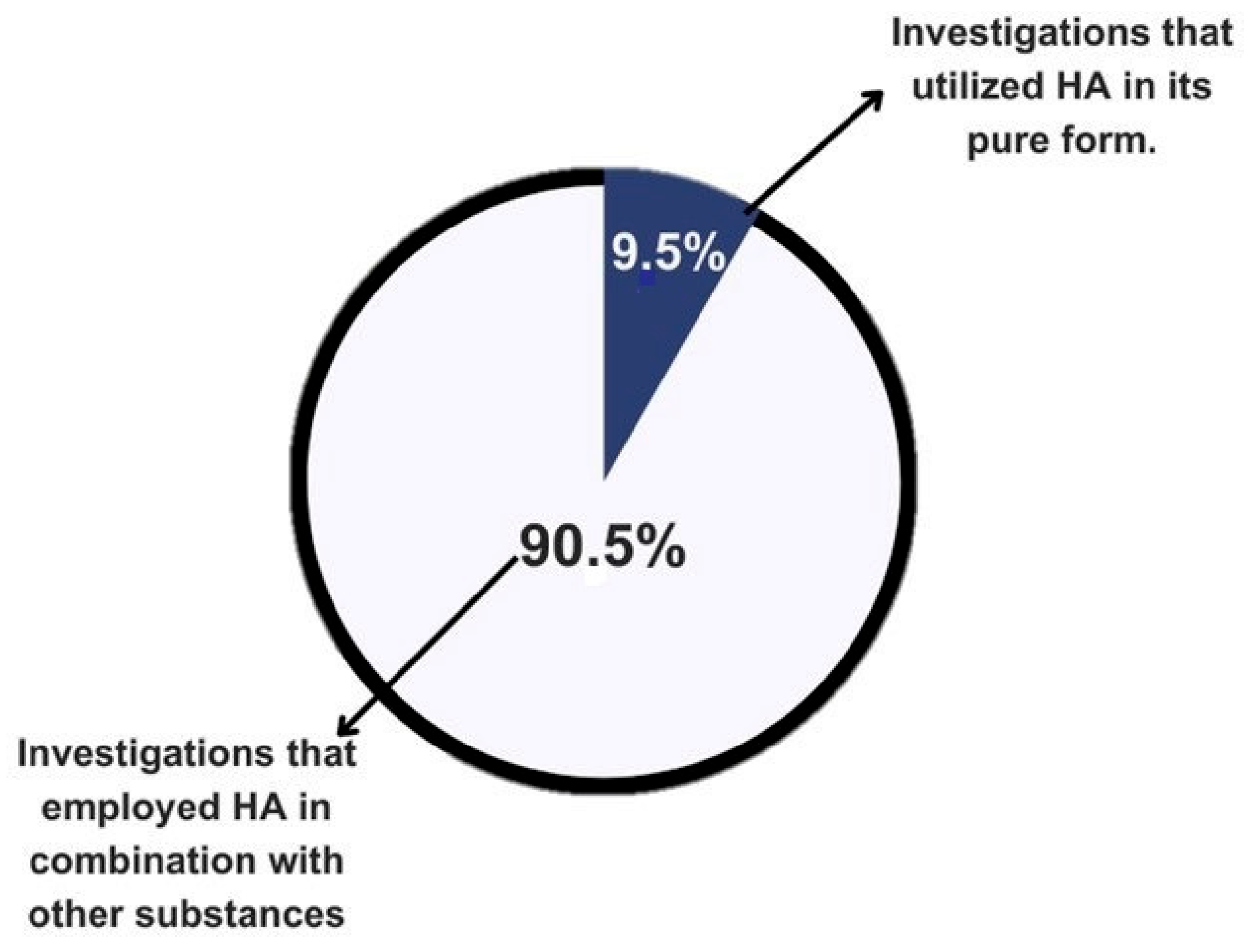
A total of 21 articles were presented in the results table. Of these, 90.5% of the authors used hydroxyapatite in combination with other materials, while only 9.5% used it in its pure form. This approach aims to provide greater clarity and understanding of the findings in the literature (Figure 2).
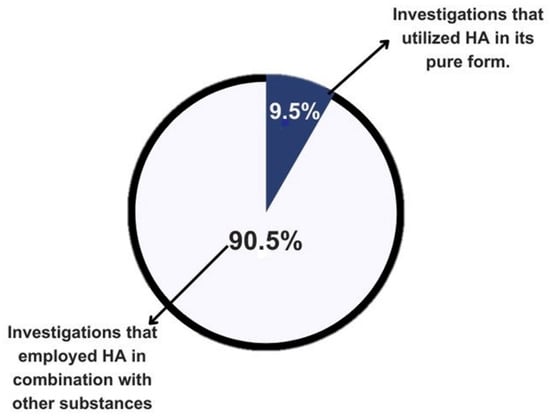
Figure 2.
Graphical representation of the literature on the use of calcium hydroxyapatite by purity.
4. Discussion
Considering the growing demand in recent decades for the integration of advanced technologies with the manufacturing of biomaterials and cell culture systems, we have observed a remarkable increase in the development of biomimetic tissues [17]. However, some authors point out that despite scientific advancements, the complexity of manufacturing processes and the costs involved in developing these technologies still represent significant challenges, resulting in a considerable gap in the implementation of these technologies in clinical practice [27,31,42]. The new advancements aim to create immunomodulatory biomaterials, with evaluations that encompass in vivo models and prioritize in vitro models whenever possible [16].
One of the greatest challenges of this new technological era has been the skin, the body’s main barrier, which is frequently exposed to various potential hazards. Its recovery is essential to perform its protective functions, both extrinsic and intrinsic, including vitamin D synthesis, immune defense, and excretion, among others [23,43,44]. According to Dam et al., 2023 [45], effective skin regeneration remains one of the biggest challenges due to its complex structure and the need to replicate its diverse functions. Several sectors have directed considerable resources towards the modulation of synthetic skin (in vitro) to mitigate the ongoing legal and ethical issues associated with product testing on skin and the use of animals, achieving gradual results but not highly utilized [16].
Current research on the application of calcium hydroxyapatite (HAp) in skin wound healing reveals a series of benefits, such as increased cell proliferation and promotion of neovascularization. Systematic studies indicate that HAp can stimulate collagen production, cell proliferation, and angiogenesis, which are crucial for skin regeneration [46]. However, there is still a significant lack of long-term studies evaluating the efficacy and safety of these materials in large-scale clinical trials that address existing methodological limitations and expand the understanding of long-term effects, thus ensuring a safe and effective transition to clinical practice [46,47].
This investigation consisted of conducting a comprehensive review of scientific articles indexed in databases, according to established descriptors and criteria for inclusion and exclusion of articles. The main objective was to explore the existing literature on the use of calcium hydroxyapatite and its contribution to the tissue repair process in the skin, presenting evidence, practical considerations, and possible applications of this substance. Among the various benefits of HAp highlighted in this study, its effectiveness in stimulating wound healing through mechanical, antibacterial, and regenerative properties via induced neovascularization stands out [48].
There is a preference for calcium hydroxyapatite (HAp) nanoparticles due to their high surface-to-volume ratio, which theoretically improves the interaction of this material with cells and tissues, promoting more efficient regeneration [45]. However, this type of ratio can also increase the chemical reactivity of the nanoparticles, potentially causing undesirable cytotoxic effects at certain concentrations [47]. Kawai et al., 2011 [27], report that the nanoparticles have the potential to release calcium ions in the acidic environments found in wounds in a controlled manner. Furthermore, it is important to note that the size of HAp nanoparticles can reach up to 100 nm, facilitating rapid and deep penetration into tissues, ensuring uniform and sustained distribution of therapeutic agents throughout the wound [49]. Despite the potential for controlled release, there are still significant technical challenges related to the safe amount of release, especially in smaller nanoparticles (<50 nm), and it is necessary to ensure that it supports the healing tissue for the required time without causing adverse inflammatory responses [47]. It is found that research addressing the inflammatory response, biocompatibility, and controlled distribution of calcium hydroxyapatite nanoparticles is necessary to ensure complete safety in their clinical application.
The origin of HAp can be natural, found mainly in bones, representing a significant percentage of their composition, or synthetic, a compound of calcium phosphate (Ca10(PO4)6(OH)2) similar to an important component present in bone tissue and commonly used as artificial bone [28], which induces osseointegration due to its biocompatibility properties [50]. However, there are two factors that need to be investigated in depth: the origin and the synthesis method, as these could significantly influence the performance of the material [51,52].
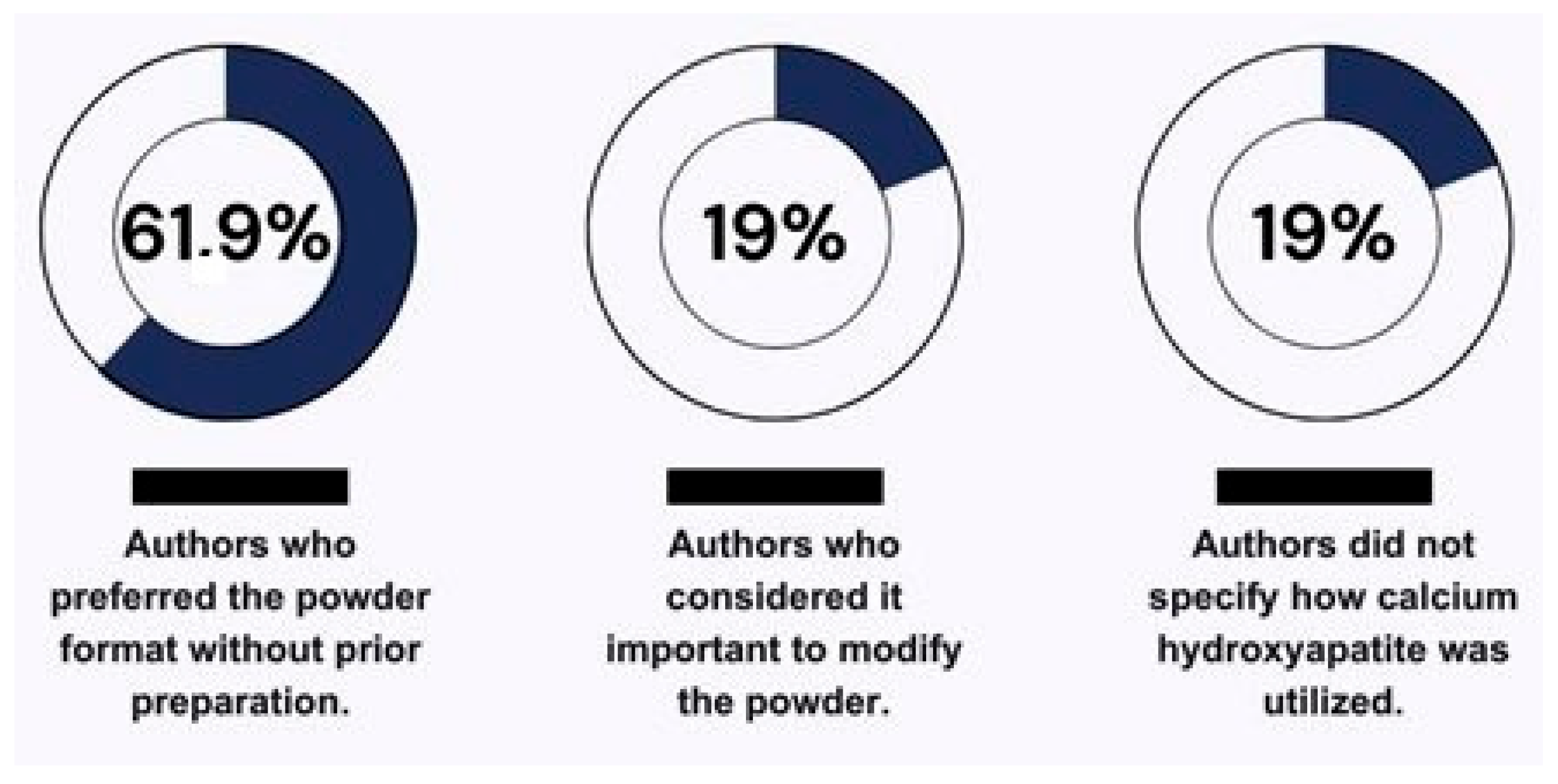
According to the findings of this review, the most commonly used form of HA in the presented literature was powder, without prior preparation, with 61.9% of the authors preferring its use in this format [2,19,26,30,32,34,35,36]. Meanwhile, 19% of the authors considered it important to modify the powder or prepare it before the experiment to facilitate handling of the material according to the application of each study [12,28,29] (Figure 3). This divergence in preparation leads to a lack of standardization in study methods, which could hinder future studies when making direct comparisons of the results [53].
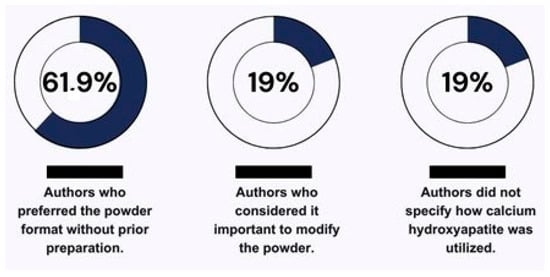
Figure 3.
Illustration of the varied utilization of calcium hydroxyapatite by the authors.
During the thorough analysis of the articles presented in Table 1, it was observed that out of the 21 articles, 20 utilized the nanoparticle form of calcium hydroxyapatite. Of these, only five articles described the exact dimensions [2,12,29,40,41]. It is important to emphasize, as mentioned in the previous paragraphs, that knowing the exact size of the HAp nanoparticles is crucial to enable other researchers to optimize their properties for specific applications. Addressing these challenges is essential for effective implementation in clinical practice, ensuring maximum therapeutic efficacy in future studies.
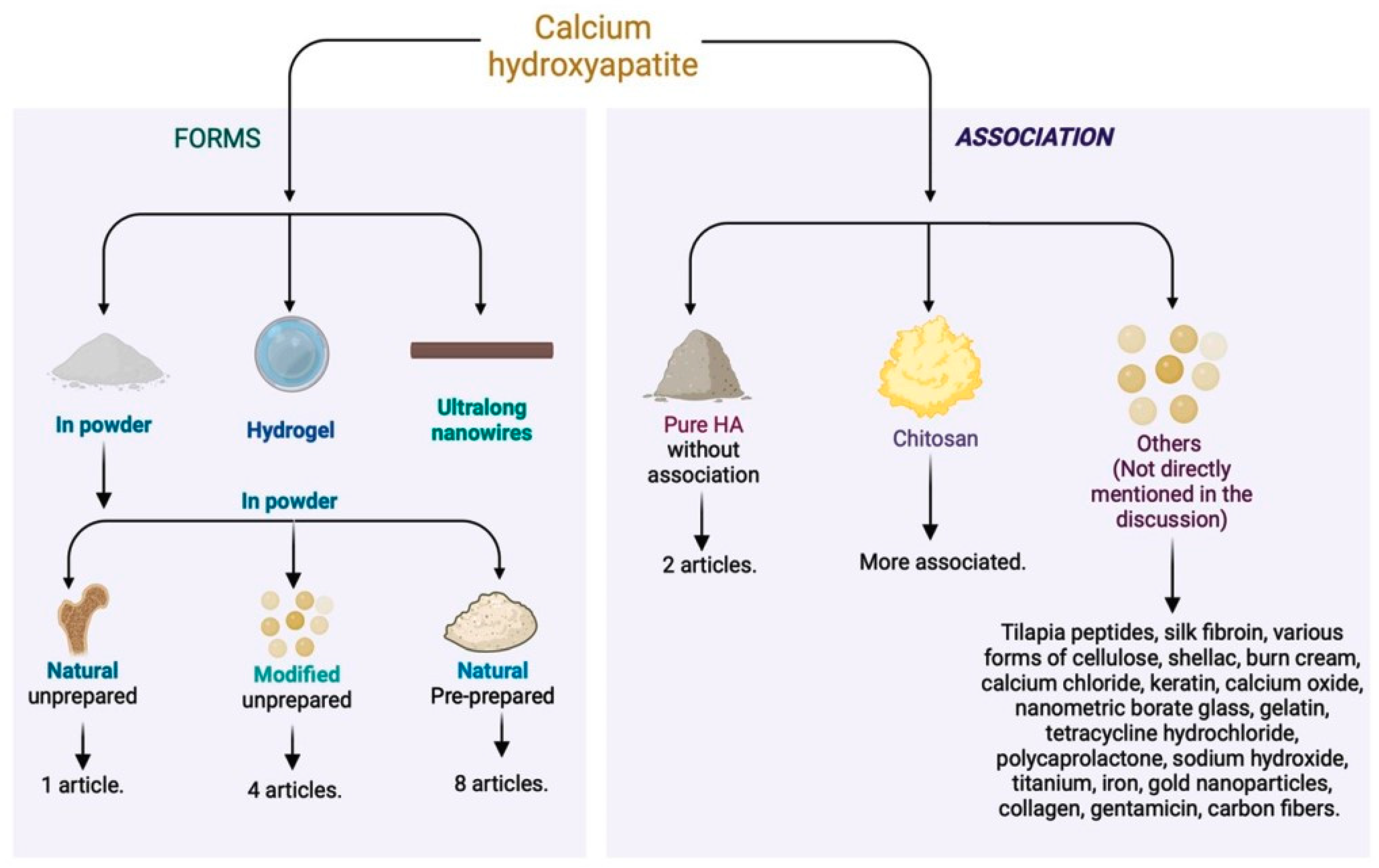
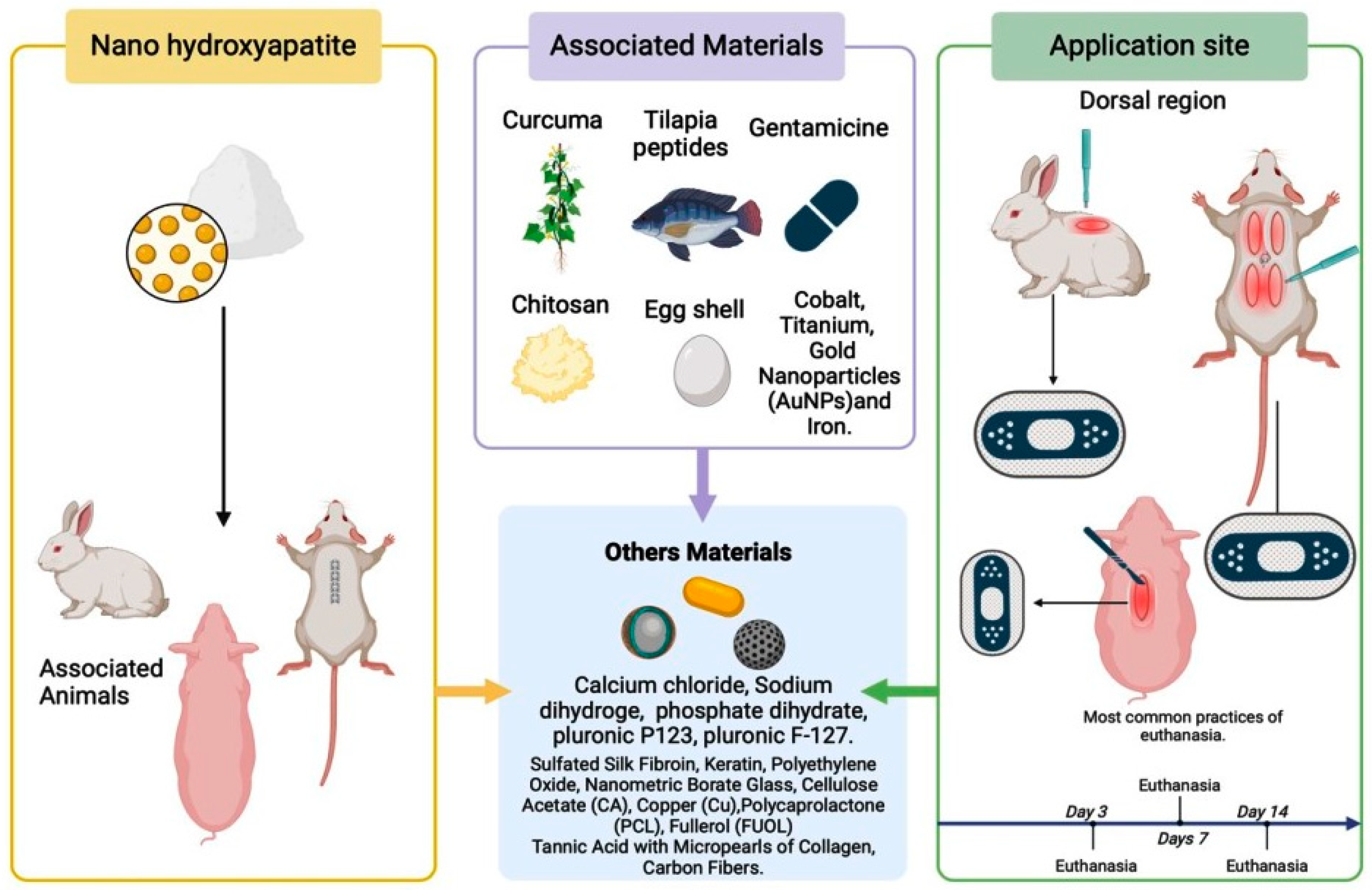
Regarding the associated material, most of the articles used calcium hydroxyapatite in conjunction with other materials. Only two articles [26,27] used it in its pure form. The most employed compound with calcium hydroxyapatite was chitosan [2,12,19,32,35,36], a polymer that stimulates wound healing and improves hemostasis by promoting collagen synthesis by fibroblasts (Figure 4). This substance, with anti-hemorrhagic and antimicrobial properties [54,55,56], increases the stability of the growth factor in grafts. The combination of chitosan and hydroxyapatite appears promising but requires more long-term clinical studies to validate its efficacy and safety [57] (Figure 4).
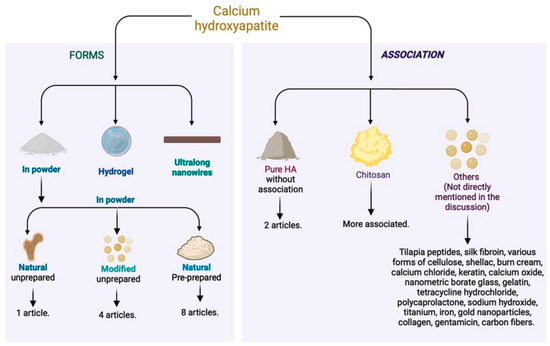
Figure 4.
Different forms of calcium hydroxyapatite used in the literature and associated materials for skin tissue regeneration.
The combination of these materials results in antibacterial efficacy and cytocompatibility, accelerating the healing of burns and other skin wounds. In addition to the use of hydroxyapatite, combinations were explored to enhance the action of chitosan. In one study, more promising results were obtained by combining chitosan, hydroxyapatite nanorods, and titanium (Ti) [35]. Another approach involved the incorporation of nano-hydroxyapatite and tetracycline hydrochloride into the polyelectrolyte complex nanofibers of chitosan [34], providing prolonged drug release effects.
Two experimental studies highlighted the promising potential of calcium hydroxyapatite in different wound healing approaches. In the first study, conducted on rabbits, the combination of calcium hydroxyapatite in two concentrations (0.5% and 1.0%) with chitosan, tilapia peptide, and burn ointment (MEBO) demonstrated ideal antibacterial efficacy and cytocompatibility to accelerate the healing process in burns [2]. However, it is crucial to evaluate the applicability of these results in humans through rigorous clinical trials [39]. In the second study, the research focused on the use of pure calcium hydroxyapatite combined with gentamicin to evaluate the feasibility of combining the two materials in diabetic foot ulcers. This study was conducted on rats, and it was observed that collagen matrices containing hydroxyapatite successfully demonstrated cell adhesion and proliferation, allowing a remarkable improvement in skin repair [39]. These results suggest significant potential for the treatment of chronic conditions, but translation to clinical practice requires further validation [58].
The creation of a flexible biopaper, by combining ultralong calcium hydroxyapatite nanowires with carbon fibers, enabled wound healing with mechanical properties through the continuous release of calcium ions and promotion of angiogenesis [11].
Other authors decided to investigate the inorganic hemostatic efficacy of ultralong calcium hydroxyapatite (HA) nanowires through an aerogel. The experiment was conducted on the skin of rats, and the results confirmed the promotion of wound healing, attributed to the water absorption capacity present in the blood, thus concentrating blood platelets [34]. Of the 21 articles listed in the table, calcium hydroxyapatite was used in 76.19% of them in various forms and combinations for the preparation of dressings. The remaining 23.81% of the studies adopted this substance in different approaches, including skin regeneration through intraincisional application [19] and injectable hydrogel [39].
The literature review opened a window of possibilities regarding the application sites and the preference for specific animal models, leading to a study of the various contexts in which these animals were chosen and used. The studies predominantly used rats as experimental models [19,33,34,38,40] and mice [27,29,30]. These rodent species are preferred due to their genetic similarity to humans, availability, and cost-effectiveness [58]. However, extrapolating the results from animal models to humans should be performed with caution, considering the significant physiological differences [59].
Most authors standardized their histopathological analysis periods, with evaluations commonly conducted at 2 days [30,34], 3 days [3], 5 days [19], and 7 days [38,40], highlighting a uniformity in approaches. Another study chose a different schedule, combining hours and days [25], facilitating a deeper understanding of the results. Some researchers selected the histopathological analysis periods based on the specific objectives of their studies [2,12,28,29,42]. Despite the different methodologies for histopathological evaluations, the results were similar among the studies (Figure 5).
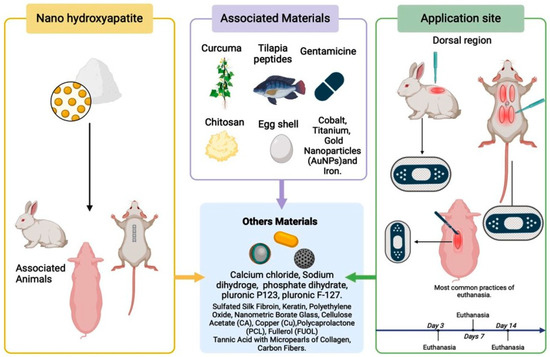
Figure 5.
Representation of the components associated with hydroxyapatite, highlighting its use in animals in their respective locations.
The use of calcium hydroxyapatite is not limited to tissue repair in healthy skin. One study addresses the use of hydroxyapatite (HA) as a therapeutic agent for skin repair in diabetic patients with chronic ulcers [39]. Considering this work as part of our investigation highlights its importance as a promising perspective for future research focused on the specific evaluation of lesions associated with or exacerbated by diabetes, resulting in chronic ulcers. Subsequent research is recommended to explore the therapeutic potential of calcium hydroxyapatite in the treatment of these lesions resulting from the diabetic condition.
5. Conclusions
In conclusion, the findings of this review emphasize the crucial role of calcium hydroxyapatite in skin regeneration and wound healing. While its benefits are well documented in bone regeneration and as a filling material, there is a clear need to explore its effectiveness in skin regeneration further. This is particularly important in medical fields like dermatology and surgery, where treating skin wounds is a top priority. Moreover, the urgency to speed up healing processes, especially in patients with conditions like diabetes, underscores the importance of more research and practical use of calcium hydroxyapatite. Future studies should focus on understanding its potential in its pure form, without other materials, to firmly establish its benefits in skin repair.
The promising results outlined here set the stage for integrating nano-composite biomaterials based on calcium hydroxyapatite into regenerative medicine, offering new possibilities for tissue engineering and wound management. Ultimately, nano hydroxyapatite emerges as a versatile and effective option, poised to transform the field of wound healing and regenerative medicine.
Author Contributions
Conceptualization, P.T.E.C., C.P.C.d.S., D.V.B. and R.L.B.; Methodology, P.T.E.C., C.P.C.d.S., T.M.C., L.P.V.A. and R.L.B.; Formal Analysis, P.T.E.C., C.P.C.d.S., T.M.C. and L.P.V.A.; Investigation, P.T.E.C., C.P.C.d.S., D.V.B. and R.L.B.; Data Curation, P.T.E.C., C.P.C.d.S., T.M.C. and L.P.V.A.; Writing—Original Draft Preparation, P.T.E.C. and C.P.C.d.S.; Writing—Review and Editing, D.V.B. and R.L.B.; Visualization, P.T.E.C., C.P.C.d.S., T.M.C., L.P.V.A. and D.V.B.; Supervision, R.L.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Harris-Tryon, T.A.; Grice, E.A. Microbiota and maintenance of skin barrier function. Science 2022, 27, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Qianqian, O.; Songzhi, K.; Yongmei, H.; Xianghong, J.; Sidong, L.; Puwang, L.; Hui, L. Preparation of Nano-Hydroxyapatite/Chitosan/Tilapia Skin Peptides Hydrogels and Its Burn Wound Treatment. Int. J. Biol. Macromol. 2021, 181, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Reinke, J.M.; Sorg, H. Wound Repair and Regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Broussard, K.C.; Powers, J.G. Wound Dressings: Selecting the Most Appropriate Type. Am. J. Clin. Dermatol. 2013, 14, 449–459. [Google Scholar] [CrossRef] [PubMed]
- El Ayadi, A.; Jaw, J.W.; Prasai, A. Current Approaches Targeting the Wound Healing Phases to Attenuate Fibrosis and Scarring. Int. J. Mol. Sci. 2020, 21, 1105. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Renick, P.; Senkowsky, J.; Nair, A.; Tang, L. Diagnostics for Wound Infections. Adv. Wound Care 2021, 10, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Murphree, R.W. Impairments in Skin Integrity. Nurs. Clin. N. Am. 2017, 52, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, M.; Vigani, B.; Bianchi, E.; Valentino, C.; Totaro Fila, C.; Boselli, C.; Icaro Cornaglia, A.; Viseras, C.; Rossi, S.; Sandri, G. Hydroxyapatite-Doped Microspheres in Chronic Wound Regeneration. J. Drug Deliv. Sci. Technol. 2023, 86, 104758. [Google Scholar] [CrossRef]
- Beyene, R.T.; Derryberry, S.L.; Barbul, A. The Effect of Comorbidities on Wound Healing. Surg. Clin. N. Am. 2020, 100, 695–705. [Google Scholar] [CrossRef]
- Dos Santos Malavazzi, T.C.; Fernandes, K.P.S.; Lopez, T.C.C.; Rodrigues, M.F.S.D.; Horliana, A.C.R.T.; Bussadori, S.K.; Mesquita-Ferrari, R.A. Effects of the Invasive and Non-Invasive Systemic Photobiomodulation Using Low-Level Laser in Experimental Models: A Systematic Review. Lasers Med. Sci. 2023, 38, 137. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Hao, L.S.; Ning, B.B.; Zhu, Y.K.; Guan, J.B.; Ren, H.W.; Yu, H.P.; Zhu, Y.J.; Duan, J.L. Biopaper Based on Ultralong Hydroxyapatite Nanowires and Cellulose Fibers Promotes Skin Wound Healing by Inducing Angiogenesis. Coatings 2022, 12, 479. [Google Scholar] [CrossRef]
- Peifen, M.; Mengyun, L.; Jinglong, H.; Danqian, L.; Yan, T.; Liwei, X.; Han, Z.; Jianlong, D.; Lingyan, L.; Guanghui, Z.; et al. New Skin Tissue Engineering Scaffold with Sulfated Silk Fibroin/Chitosan/Hydroxyapatite and Its Application. Biochem. Biophys. Res. Commun. 2023, 640, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Singh, R.K.; Kim, H.W.; Baino, F. “Hard” Ceramics for “Soft” Tissue Engineering: Paradox or Opportunity? Acta Biomater. 2020, 115, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Buchaim, R.L.; Goissis, G.; Andreo, J.C.; Roque, D.D.; Roque, J.S.; Buchaim, D.V.; Rodrigues, A.D.C. Biocompatibility of anioniccollagen matrices and its influence on the orientation of cellular growth. Braz. Dent. Sci. 2007, 10, 12–20. [Google Scholar] [CrossRef]
- Debels, H.; Hamdi, M.; Abberton, K.; Morrison, W. Dermal Matrices and Bioengineered Skin Substitutes: A Critical Review of Current Options. Plast. Reconstr. Surg. Glob. Open 2015, 3, e284. [Google Scholar] [CrossRef] [PubMed]
- Savoji, H.; Godau, B.; Hassani, M.S.; Akbari, M. Skin Tissue Substitutes and Biomaterial Risk Assessment and Testing. Front. Bioeng. Biotechnol. 2018, 6, 86. [Google Scholar] [CrossRef] [PubMed]
- Vig, K.; Chaudhari, A.; Tripathi, S.; Dixit, S.; Sahu, R.; Pillai, S.; Dennis, V.A.; Singh, S.R. Advances in Skin Regeneration Using Tissue Engineering. Int. J. Mol. Sci. 2017, 18, 789. [Google Scholar] [CrossRef] [PubMed]
- de Moraes, R.; Plepis, A.M.d.G.; Martins, V.d.C.A.; Garcia, C.F.; Galdeano, E.A.; Maia, F.L.M.; Machado, E.G.; Munhoz, M.d.A.e.S.; Buchaim, D.V.; Fernandes, V.A.R.; et al. Viability of Collagen Matrix Grafts Associated with Nanohydroxyapatite and Elastin in Bone Repair in the Experimental Condition of Ovariectomy. Int. J. Mol. Sci. 2023, 24, 15727. [Google Scholar] [CrossRef]
- Derakhshi, M.; Naseri, M.; Vafaeipour, Z.; Malaekeh-Nikouei, B.; Jafarian, A.H.; Ansari, L. Enhanced Wound-Healing Efficacy of Electrospun Mesoporous Hydroxyapatite Nanoparticle-Loaded Chitosan Nanofiber Developed Using Pluronic F127. Int. J. Biol. Macromol. 2023, 240, 124427. [Google Scholar] [CrossRef] [PubMed]
- Lalehzar, S.S.; Talebi, A.; Fesharaki, M. Evaluation of the Effectiveness of Nano-Hydroxyapatite Particles in Wound Healing in an Animal Study. Bio Med. 2023, 15, 536. [Google Scholar] [CrossRef]
- Namasivayam, S.K.R.; Venkatachalam, G.; Bharani, R.S.A. Noteworthy Enhancement of Wound-Healing Activity of Triphala Biomass Metabolite-Loaded Hydroxyapatite Nanocomposite. Appl. Nanosci. 2021, 11, 1511–1530. [Google Scholar] [CrossRef]
- Sutha, M.; Sowndarya, K.; Chandran, M.; Yuvaraj, D.; Bharathiraja, B.; Praveen Kumar, R. Synthesis of Value Added Biomimetic Material of Hydroxyapatite Using Aqueous Calcareous Fish Wastes. In Energy, Environment, and Sustainability; Springer Nature: Berlin/Heidelberg, Germany, 2018; pp. 59–64. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, M. Skin Barrier Function and the Microbiome. Int. J. Mol. Sci. 2022, 23, 13071. [Google Scholar] [CrossRef]
- Zhang, S.; He, T.; Zhao, F.; Tan, Q.; Li, D.; Wang, Q.; Xiao, Y.; Zhang, X. Development of a multifunctional nano-hydroxyapatite platform (nHEA) for advanced treatment of severely infected full-thickness skin wounds. Acta Biomater. 2024, 181, 440–452. [Google Scholar] [CrossRef]
- Aguilera, S.; McCarthy, A.; Khalifian, S.; Lorenc, Z.P.; Goldie, K.; Chernoff, W.G. The Role of Calcium Hydroxylapatite (Radiesse) as a Regenerative Aesthetic Treatment: A Narrative Review. Aesthetic Surg. J. 2023, 43, 1063–1090. [Google Scholar] [CrossRef] [PubMed]
- Majeed, A.A.; Al Naimi, R.A. Role of Hydroxyapatite in Healing of Experimentally Induced Cutaneous Wound in Rabbits. J. Vet. Sci. 2012, 5, 74–81. [Google Scholar]
- Kawai, K.; Larson, B.J.; Ishise, H.; Carre, A.L.; Nishimoto, S.; Longaker, M.; Lorenz, H.P. Calcium-Based Nanoparticles Accelerate Skin Wound Healing. PLoS ONE 2011, 6, e27106. [Google Scholar] [CrossRef]
- Lamkhao, S.; Tandorn, S.; Thavornyutikarn, P.; Chokethawai, K.; Rujijanagul, G.; Thongkorn, K.; Jarupoom, P.; Randorn, C. Synergistic Amalgamation of Shellac with Self-Antibacterial Hydroxyapatite and Carboxymethyl Cellulose: An Interactive Wound Dressing for Ensuring Safety and Efficacy in Preliminary in Vivo Studies. Int. J. Biol. Macromol. 2023, 253, 126809. [Google Scholar] [CrossRef]
- Fan, J.; Lei, T.; Yu, M.; Wang, Y.; Cao, F.; Yang, Q.; Tian, F.; Liu, Y. Keratin/PEO/Hydroxyapatite Nanofiber Membrane with Improved Mechanical Property for Potential Burn Dressing Application. Fibers Polym. 2020, 21, 366–375. [Google Scholar] [CrossRef]
- Chen, R.; Li, Q.; Zhang, Q.; Xu, S.; Han, J.; Huang, P.; Yu, Z.; Jia, D.; Liu, J.; Jia, H.; et al. Nanosized HCA-Coated Borate Bioactive Glass with Improved Wound Healing Effects on Rodent Model. Chem. Eng. J. 2021, 426, 130299. [Google Scholar] [CrossRef]
- Elsayed, M.T.; Hassan, A.A.; Abdelaal, S.A.; Taher, M.M.; Ahmed, M.K.; Shoueir, K.R. Morphological, Antibacterial, and Cell Attachment of Cellulose Acetate Nanofibers Containing Modified Hydroxyapatite for Wound Healing Utilizations. J. Mater. Res. Technol. 2020, 9, 13927–13936. [Google Scholar] [CrossRef]
- Wang, J.; Cai, N.; Chan, V.; Zeng, H.; Shi, H.; Xue, Y.; Yu, F. Antimicrobial Hydroxyapatite Reinforced-Polyelectrolyte Complex Nanofibers with Long-Term Controlled Release Activity for Potential Wound Dressing Application. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 624, 126722. [Google Scholar] [CrossRef]
- Zheng, Y.; Ma, W.; Yang, Z.; Zhang, H.; Ma, J.; Li, T.; Niu, H.; Zhou, Y.; Yao, Q.; Chang, J.; et al. An Ultralong Hydroxyapatite Nanowire Aerogel for Rapid Hemostasis and Wound Healing. Chem. Eng. J. 2022, 430, 132912. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, D. In Vitro and in Vivo Evaluations of Nanofibrous Nanocomposite Based on Carboxymethyl Cellulose/Polycaprolactone/Cobalt-Doped Hydroxyapatite as the Wound Dressing Materials. Arab. J. Chem. 2022, 15, 104270. [Google Scholar] [CrossRef]
- Han, J.; Qi, J.; Du, J.; Zhang, G. Fabrication of Chitosan Hydrogel Incorporated with Ti-Doped Hydroxyapatite for Efficient Healing and Care of Joint Wound. Mater. Lett. 2020, 278, 128415. [Google Scholar] [CrossRef]
- Cunha, C.S.; Castro, P.J.; Sousa, S.C.; Pullar, R.C.; Tobaldi, D.M.; Piccirillo, C.; Pintado, M.M. Films of Chitosan and Natural Modified Hydroxyapatite as Effective UV-Protecting, Biocompatible and Antibacterial Wound Dressings. Int. J. Biol. Macromol. 2020, 159, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Grzeczkowicz, A.; Drabik, M.; Lipko, A.; Bącal, P.; Kwiatkowska, A.; Kazimierczak, B.; Granicka, L.H. A Composite Membrane System with Gold Nanoparticles, Hydroxyapatite, and Fullerenol for Dual Interaction for Biomedical Purposes. Membranes 2021, 11, 565. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bai, X.; Ren, C.; Ma, Y.; Liu, Y. Construction of Injectable Collagen-Microgel/Tannic Acid/Nano-Hydroxyapatite Granular Hydrogel and Evaluation of Its Potential in Wound Healing. J. Bioact. Compat. Polym. 2023, 38, 325–339. [Google Scholar] [CrossRef]
- Hutting, K.H.; de Stegge, W.B.A.; van Netten, J.J.; Cate, W.A.T.; Smeets, L.; Welten, G.M.J.M.; Scharn, D.M.; de Vries, J.-P.P.M.; van Baal, J.G. Surgical Treatment of Diabetic Foot Ulcers Complicated by Osteomyelitis with Gentamicin-loaded Calcium Sulphate-hydroxyapatite Biocomposite. J. Clin. Med. 2021, 10, 371. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, N.; Sousa, A.; Cunha-Reis, C.; Oliveira, A.L.; Granja, P.L.; Monteiro, F.J.; Sousa, S.R. New Prospects in Skin Regeneration and Repair Using Nanophased Hydroxyapatite Embedded in Collagen Nanofibers. Nanomedicine 2021, 33, 102353. [Google Scholar] [CrossRef] [PubMed]
- Mehedi Hasan, M.; Nuruzzaman Khan, M.; Haque, P.; Rahman, M.M. Novel Alginate-Di-Aldehyde Cross-Linked Gelatin/Nano-Hydroxyapatite Bioscaffolds for Soft Tissue Regeneration. Int. J. Biol. Macromol. 2018, 117, 1110–1117. [Google Scholar] [CrossRef]
- Okabayashi, R.; Nakamura, M.; Okabayashi, T.; Tanaka, Y.; Nagai, A.; Yamashita, K. Efficacy of Polarized Hydroxyapatite and Silk Fibroin Composite Dressing Gel on Epidermal Recovery from Full-Thickness Skin Wounds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 90B, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Bergqvist, C.; Ezzedine, K. Vitamin D and the Skin: What Should a Dermatologist Know? G. Ital. Dermatol. E Venereol. 2019, 154, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qu, L.; Mijakovic, I.; Wei, Y. Advances in the Human Skin Microbiota and Its Roles in Cutaneous Diseases. Microb. Cell Fact. 2022, 21, 176. [Google Scholar] [CrossRef]
- Dam, P.; Celik, M.; Ustun, M.; Saha, S.; Saha, C.; Kacar, E.A.; Kugu, S.; Karagulle, E.N.; Tasoglu, S.; Buyukserin, F.; et al. Wound healing strategies based on nanoparticles incorporated in hydrogel wound patches. RSC Adv. 2023, 13, 21345–21364. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Meçani, R.; Niehot, C.D.; Phillips, T.; Kolb, J.; Daughtry, H.; Muka, T. Skin regeneration-related mechanisms of Calcium Hydroxylapatite (CaHA): A systematic review. Front. Med. 2023, 10, 1195934. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ng, S.; Heng, B.C.; Guo, J.; Ma, L.; Tan, T.T.; Ng, K.W.; Loo, S.C. Cytotoxicity of hydroxyapatite nanoparticles is shape and cell dependent. Arch Toxic 2013, 87, 1037–1052. [Google Scholar] [CrossRef] [PubMed]
- Soccol, A.T.; Bettega, S.; Noronha, L.; Sass, S.; Soccol, V.T.; Scholz, M.R.; Mocellin, M. Defect Repair in Rat mandible with Hydroxyapatite Cement to Small Intestine Submucosa. Braz. J. Otorhinolaryngol. 2006, 72, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Lara-Ochoa, S.; Ortega-Lara, W.; GuerreroBeltrán, C.E. Hydroxyapatite Nanoparticles in Drug Delivery: Physicochemistry and Applications. Pharmaceutics 2021, 13, 1642. [Google Scholar] [CrossRef]
- Costa, A.C.F.M.; Lima, M.G.; Lima, L.H.M.A.; Cordeiro, V.V.; Viana, K.M.S.; Souza, C.V.; Lira, H.L. Hidroxiapatita: Obtenção, Caracterização e Aplicações. Rev. Eletrônica Mater. Process. 2009, 4, 3. [Google Scholar]
- Kumar, R.; Mohanty, S. Hydroxyapatite: A Versatile Bioceramic for Tissue Engineering. J. Inorg. Organomet. Polym. Mater. 2022, 32, 4461–4477. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, Z.; Li, W.; Fan, Y.; Li, Z.; Wei, J. Hydroxyapatite Based Materials for Bone Tissue Engineering: A Brief and Comprehensive Introduction. Crystals 2021, 11, 149. [Google Scholar] [CrossRef]
- Finisie, M.R.; Josué, A.; Fávere, V.T.; Laranjeira, M.C.M. Synthesis of Calcium-Phosphate and Chitosan Bioceramics for Bone Regeneration. An. Acad. Bras. Ciências 2001, 73, 4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, X.; Wang, S.; Ding, C.; Zhao, Y.; Zhang, S.; Sun, S.; Zhang, L.; Ma, S.; Ding, Q.; Liu, W. Polyvinylpyrrolidone/Chitosan-Loaded Dihydromyricetin-Based Nanofiber Membrane Promotes Diabetic Wound Healing by Anti-Inflammatory and Regulating Autophagy-Associated Protein Expression. Int. J. Biol. Macromol. 2024, 259, 29160. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Chitins and Chitosans for the Repair of Wounded Skin, Nerve, Cartilage and Bone. Carbohydr. Polym. 2009, 76, 167–182. [Google Scholar] [CrossRef]
- Praneeth, Y.; Komal Devgon, I.; Sachan RS, K.; Rana, A.; Karnwal, A.; Kumar, A. Chitosan in Wound Healing: A Mini Review on Ethical Perspective on Sustainable and Biomedical Biomaterials. Regen. Eng Transl. Med. 2023, 2023, 1–30. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Ma, Y.; Jiang, Y. Calcium Phosphate-Based Nanomaterials: Preparation, Multifunction, and Application for Bone Tissue Engineering. Molecules 2023, 28, 4790. [Google Scholar] [CrossRef] [PubMed]
- Szpirer, C. Rat Models of Human Diseases and Related Phenotypes: A Systematic Inventory of the Causative Genes. J. Biomed. Sci. 2020, 27, 84. [Google Scholar] [CrossRef] [PubMed]
- Baumans, V. The Aspects of the Use of Rodents in Experimental Research. In Rodent Model as Tools in Ethical Biomedical Research; Springer: Cham, Switzerland, 2016; pp. 7–12. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).