A Case Report of Radiation-Induced Morphea Treated with Completion Mastectomy and Delayed Closure
Abstract
:1. Introduction
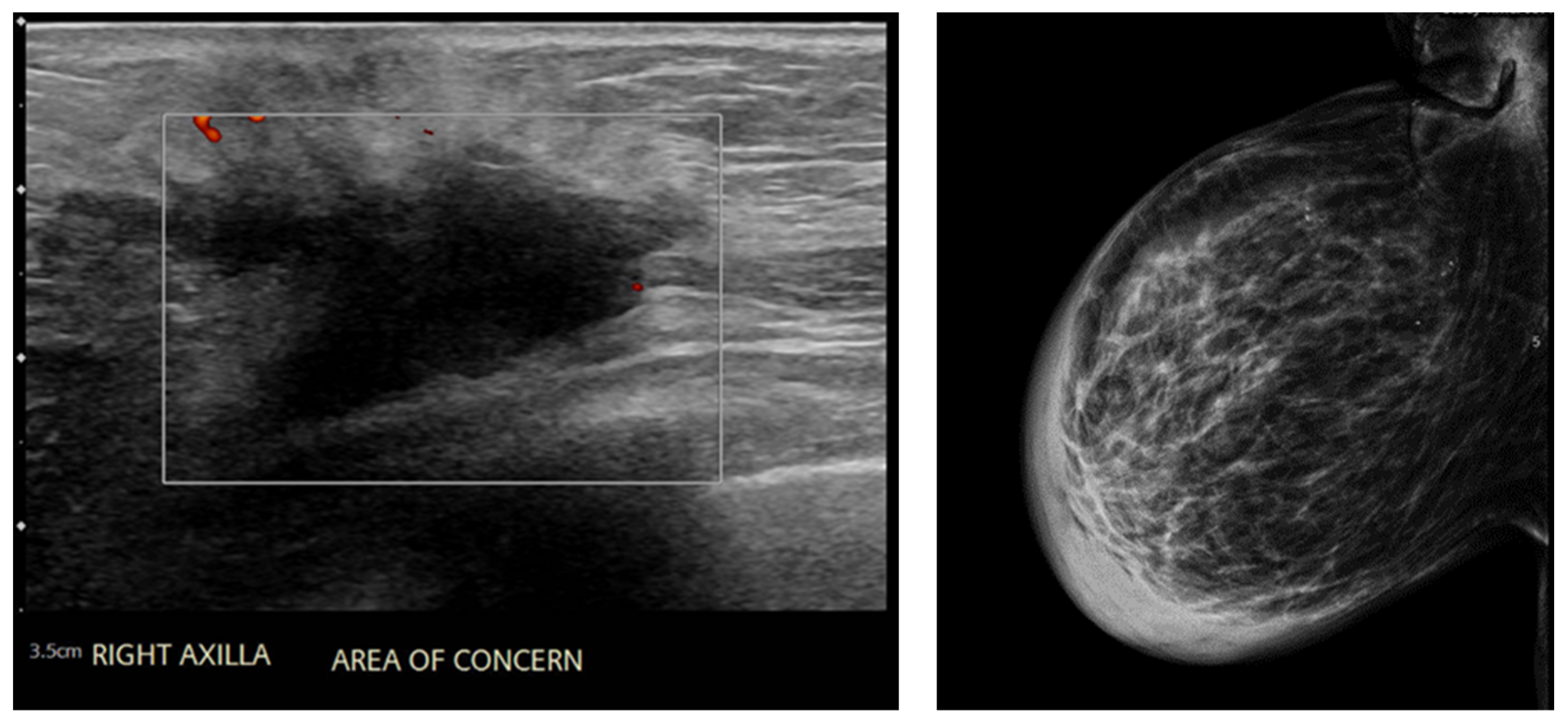
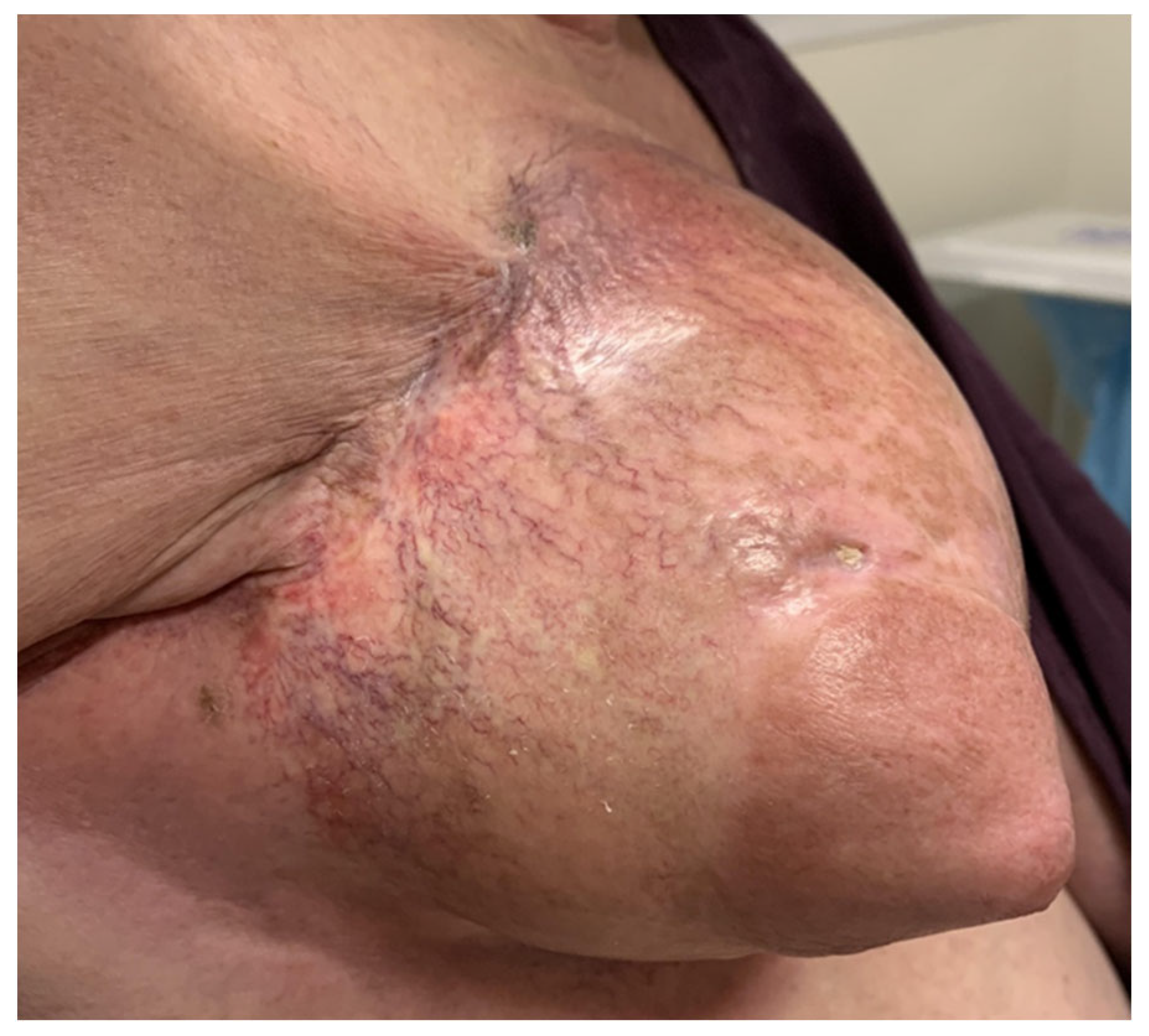
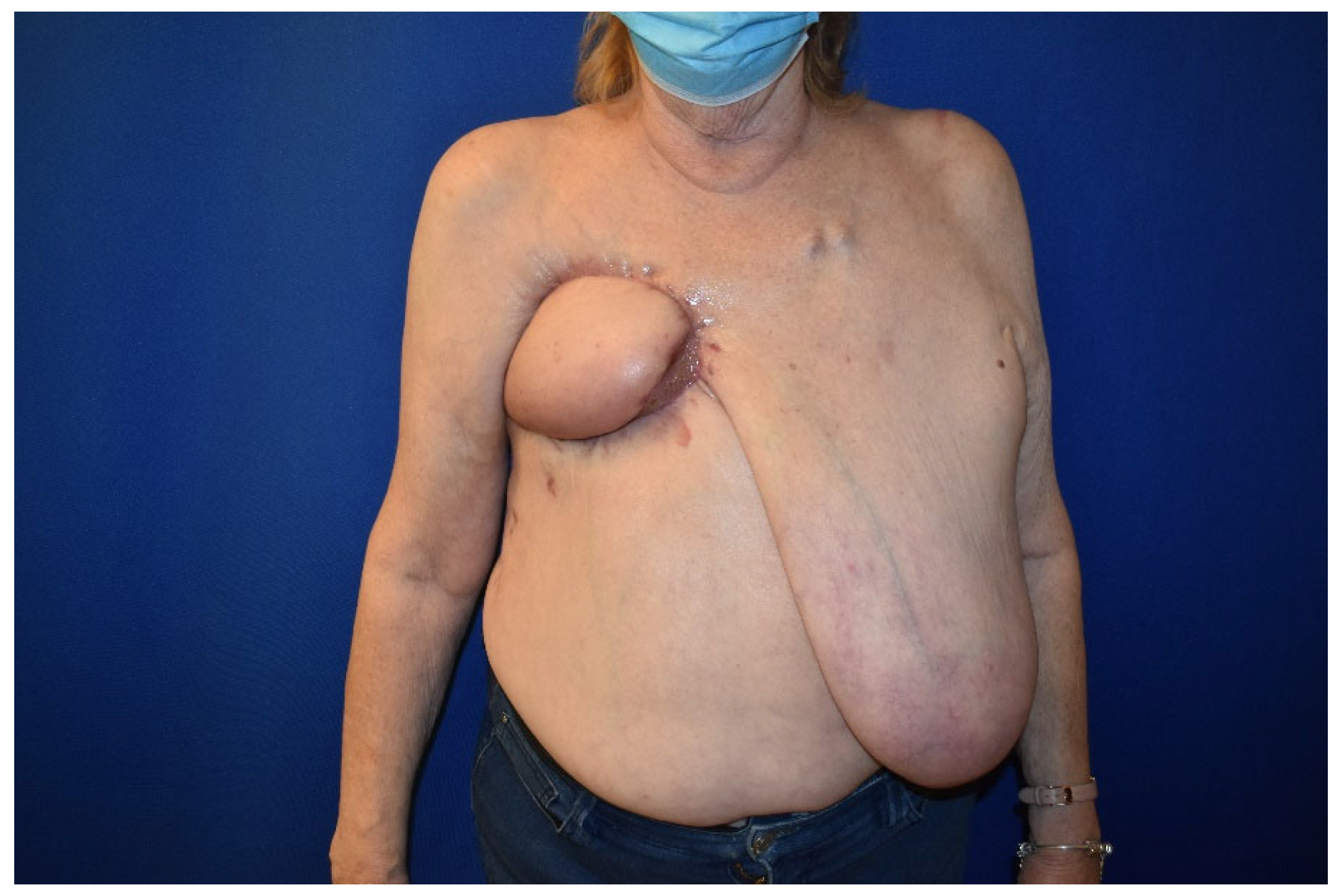
2. Case Presentation
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bielsa Marsol, I. Update on the classification and treatment of localized scleroderma. Actas Dermosifiliogr. 2013, 104, 654–666. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.S.; Nelson, A.M.; Su, W.P. Classification of morphea (localized scleroderma). Mayo Clin. Proc. 1995, 70, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Kushi, J.; Csuka, M.E. Generalized morphea after breast cancer radiation therapy. Case Rep. Rheumatol. 2011, 2011, 951948. [Google Scholar] [CrossRef] [PubMed]
- Colver, G.B.; Rodger, A.; Mortimer, P.S.; Savin, J.A.; Neill, S.M.; Hunter, J.A.A. Post-irradiation morphoea. Br. J. Dermatol. 1989, 120, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Ramseier, J.Y.; Ferreira, M.N.; Leventhal, J.S. Dermatologic toxicities associated with radiation therapy in women with breast cancer. Int. J. Womens Dermatol. 2020, 6, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Martini, G.; Ramanan, A.V.; Falcini, F.; Girschick, H.; Goldsmith, D.P.; Zulian, F. Successful treatment of severe or methotrexate-resistant juvenile localized scleroderma with mycophenolate mofetil. Rheumatology 2009, 48, 1410–1413. [Google Scholar] [CrossRef] [PubMed]
- Finnegan, P.; Kiely, L.; Gallagher, C.; Mhaolcatha, S.N.; Feeley, L.; Fitzgibbon, J.; White, J.; Bourke, J.; Murphy, L.A. Radiation-induced morphea of the breast-A case series. Skin Health Dis. 2023, 3, e148. [Google Scholar] [CrossRef] [PubMed]
- Dancey, A.L.; Waters, R.A. Morphea of the breast. Two case reports and discussion of the literature. J. Plast. Reconstr. Aesthet. Surg. 2006, 59, 1114–1117. [Google Scholar] [CrossRef] [PubMed]
- Olsen, M.A.; Lefta, M.; Dietz, J.R.; Brandt, K.E.; Aft, R.; Matthews, R.; Mayfield, J.; Fraser, V.J. Risk factors for surgical site infection after major breast operation. J. Am. Coll. Surg. 2008, 207, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Hosseini Hooshiar, M.; Badkoobeh, A.; Kolahdouz, S.; Tadayonfard, A.; Mozaffari, A.; Nasiri, K.; Salari, S.; Safaralizadeh, R.; Yasamineh, S. The potential use of nanozymes as an antibacterial agents in oral infection, periodontitis, and peri-implantitis. J. Nanobiotechnol. 2024, 22, 207. [Google Scholar] [CrossRef] [PubMed]
- Muzammil, K.; Hooshiar, M.H.; Varmazyar, S.; Omar, T.M.; Karim, M.M.; Aadi, S.; Kalavi, S.; Yasamineh, S. Potential use of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibition and prevention method in viral infection. Microb. Cell Factories 2024, 23, 90. [Google Scholar] [CrossRef] [PubMed]
- Yari, A.; Fasih, P.; Ghotbi, N.; Badkoobeh, A.; Goodarzi, A.; Hosseini Hooshiar, M. Do Platelet-Rich Concentrates Improve the Adverse Sequelae of Impacted Mandibular Third Molar Removal? J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2024, 82, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Liang, M.; Lei, Q.; Li, G.; Wu, S. The Current Status of Photodynamic Therapy in Cancer Treatment. Cancers 2023, 15, 585. [Google Scholar] [CrossRef] [PubMed]
- Amirjani, A.; Shokrani, P.; Sharif, S.A.; Moheb, H.; Ahmadi, H.; Ahmadiani, Z.S.; Paroushi, M.S. Plasmon-enhanced nano-photosensitizers: Game-changers in photodynamic therapy of cancers. J. Mater. Chem. 2023, 11, 3537–3566. [Google Scholar] [CrossRef] [PubMed]
- Partl, R.; Regitnig, P.; Tauber, G.; Pötscher, M.; Bjelic-Radisic, V.; Kapp, K.S. Radiation-induced morphea-a rare but severe late effect of adjuvant breast irradiation: Case report and review of the literature. Strahlenther. Und Onkol. 2018, 194, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.; Rheaume, D.; Barnes, P.; Tremaine, R.; Reardon, M. Postirradiation morphea: An underrecognized complication of treatment for breast cancer. Hum. Pathol. 2008, 39, 1680–1688. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, N.; Naeem, Z.; Bui, D.; Bakoulis, A. A Case Report of Radiation-Induced Morphea Treated with Completion Mastectomy and Delayed Closure. Surgeries 2024, 5, 758-763. https://doi.org/10.3390/surgeries5030060
Singh N, Naeem Z, Bui D, Bakoulis A. A Case Report of Radiation-Induced Morphea Treated with Completion Mastectomy and Delayed Closure. Surgeries. 2024; 5(3):758-763. https://doi.org/10.3390/surgeries5030060
Chicago/Turabian StyleSingh, Niharika, Zaina Naeem, Duc Bui, and Anastasia Bakoulis. 2024. "A Case Report of Radiation-Induced Morphea Treated with Completion Mastectomy and Delayed Closure" Surgeries 5, no. 3: 758-763. https://doi.org/10.3390/surgeries5030060





