Brain Tumor Reporting and Data System (BT-RADS) for the Surveillance of Adult-Type Diffuse Gliomas after Surgery
Abstract
:1. Introduction
2. Methods
3. BT-RADS Score
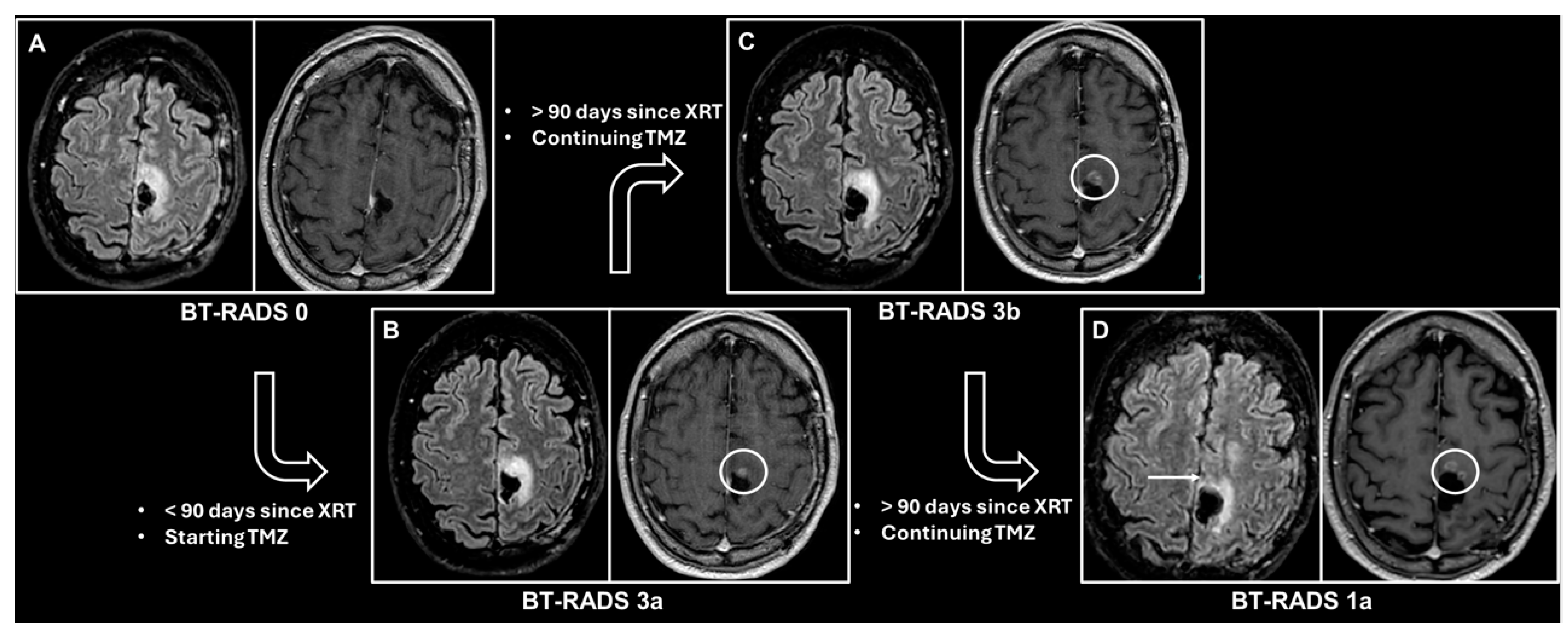
| Score | Imaging Findings | Management |
|---|---|---|
| 0 | New baseline, incomplete study, or otherwise unable to categorize. | Continued follow-up; no change. |
| 1 (improvement) | Reduction in enhancing component, FLAIR component, mass effect, or resolution of lesions. 1a—Reflects decreasing tumor burden and/or improving treatment effect. 1b—Effect from medications such as increasing steroids or initiating Avastin. | |
| 2 (stable) | No appreciable change. | |
| 3 (worsening) | Increase in enhancing component, FLAIR component, and/or mass effect. 3a (<90 days since radiation therapy)—Represents treatment effects, including radiation therapy and medications. 3b (>90 days since radiation therapy)—Represents an indeterminate mix of treatment effect and tumor worsening. 3c (<25% worsening in FLAIR and enhancement and first-time worse or new indeterminate lesion outside the expected high-dose radiation field)—Represents increasing tumor burden. | Decreased time interval of follow-up. Consider change in management in 3c. |
| 4 (definitely worsening) | Increase (>25% or <25% if progressively worsened over more than 1 study) in enhancing component, FLAIR component, mass effect, and/or new definitive lesion outside the expected high-dose radiation field. Worsening of imaging findings highly suspicious for tumor progression. | Change in management. |
4. The BT-RADS’s Diagnostic and Prognostic Value
5. The BT-RADS’s Reliability
6. The BT-RADS’s Report Quality and Acceptance by Physicians
7. Future Perspectives
8. A Summary of the BT-RADS’s Pros and Cons
Author Contributions
Funding
Conflicts of Interest
References
- Miller, K.D.; Ostrom, Q.T.; Kruchko, C.; Patil, N.; Tihan, T.; Cioffi, G.; Fuchs, H.E.; Waite, K.A.; Jemal, A.; Siegel, R.L.; et al. Brain and Other Central Nervous System Tumor Statistics, 2021. CA. Cancer J. Clin. 2021, 71, 381–406. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.; Hilario, A. Standardized Brain Tumor Reporting in the Multidisciplinary Spotlight: Pros of the BT-RADS. Eur. Radiol. 2024; Epub ahead of print. [Google Scholar] [CrossRef]
- Weinberg, B.D.; Gore, A.; Shu, H.-K.G.; Olson, J.J.; Duszak, R.; Voloschin, A.D.; Hoch, M.J. Management-Based Structured Reporting of Posttreatment Glioma Response with the Brain Tumor Reporting and Data System. J. Am. Coll. Radiol. JACR 2018, 15, 767–771. [Google Scholar] [CrossRef]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO Guidelines on the Diagnosis and Treatment of Diffuse Gliomas of Adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef]
- Ebaid, N.Y.; Ahmed, R.N.; Assy, M.M.; Amin, M.I.; Alaa Eldin, A.M.; Alsowey, A.M.; Abdelhay, R.M. Diagnostic Validity and Reliability of BT-RADS in the Management of Recurrent High-Grade Glioma. J. Neuroradiol. 2024, 51, 101190. [Google Scholar] [CrossRef]
- Parillo, M.; Vertulli, D.; Vaccarino, F.; Mallio, C.A.; Beomonte Zobel, B.; Quattrocchi, C.C. The Sensitivity of MIPs of 3D Contrast-Enhanced VIBE T1-Weighted Imaging for the Detection of Small Brain Metastases (≤5 Mm) on 1.5 Tesla MRI. Neuroradiol. J. 2024, 19714009241260802, Epub ahead of print. [Google Scholar] [CrossRef]
- Parillo, M.; Vertulli, D.; Mallio, C.A.; Quattrocchi, C.C. Imaging Findings in Carcinomatous Encephalitis Secondary to Malignant Melanoma. Egypt. J. Neurol. Psychiatry Neurosurg. 2023, 59, 76. [Google Scholar] [CrossRef]
- Parillo, M.; Vaccarino, F.; Quattrocchi, C.C. Imaging Findings in a Case of Leptomeningeal Myelomatosis, a Rare but Critical Central Nervous System Complication of Multiple Myeloma. Neuroradiol. J. 2023, 36, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, K.H.; Guha, A.; Thakur, M.; Mahajan, A.; Bhole, P.; Gupta, T. External Validation of the Brain Tumour Reporting and Data System (BT-RADS) in the Multidisciplinary Managementof Post-Treatment Gliomas. Pol. J. Radiol. 2024, 89, e148–e155. [Google Scholar] [CrossRef]
- Macdonald, D.R.; Cascino, T.L.; Schold, S.C.; Cairncross, J.G. Response Criteria for Phase II Studies of Supratentorial Malignant Glioma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1990, 8, 1277–1280. [Google Scholar] [CrossRef]
- Wen, P.Y.; Macdonald, D.R.; Reardon, D.A.; Cloughesy, T.F.; Sorensen, A.G.; Galanis, E.; Degroot, J.; Wick, W.; Gilbert, M.R.; Lassman, A.B.; et al. Updated Response Assessment Criteria for High-Grade Gliomas: Response Assessment in Neuro-Oncology Working Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 1963–1972. [Google Scholar] [CrossRef]
- Ramakrishnan, D.; von Reppert, M.; Krycia, M.; Sala, M.; Mueller, S.; Aneja, S.; Nabavizadeh, A.; Galldiks, N.; Lohmann, P.; Raji, C.; et al. Evolution and Implementation of Radiographic Response Criteria in Neuro-Oncology. Neuro-Oncol. Adv. 2023, 5, vdad118. [Google Scholar] [CrossRef]
- Parillo, M.; Mallio, C.A.; Van der Molen, A.J.; Rovira, À.; Dekkers, I.A.; Karst, U.; Stroomberg, G.; Clement, O.; Gianolio, E.; Nederveen, A.J.; et al. The Role of Gadolinium-Based Contrast Agents in Magnetic Resonance Imaging Structured Reporting and Data Systems (RADS). Magn. Reson. Mater. Phys. Biol. Med. 2024, 37, 15–25. [Google Scholar] [CrossRef]
- Parillo, M.; van der Molen, A.J.; Asbach, P.; Elsholtz, F.H.J.; Laghi, A.; Ronot, M.; Wu, J.S.; Mallio, C.A.; Quattrocchi, C.C. The Role of Iodinated Contrast Media in Computed Tomography Structured Reporting and Data Systems (RADS): A Narrative Review. Quant. Imaging Med. Surg. 2023, 13, 7621–7631. [Google Scholar] [CrossRef]
- BT-RADS Flowchart. Available online: https://btrads.com/wp-content/uploads/2018/05/BT-RADS-flow-chart-2018_02_01.pdf (accessed on 5 July 2024).
- Kim, S.; Hoch, M.J.; Cooper, M.E.; Gore, A.; Weinberg, B.D. Using a Website to Teach a Structured Reporting System, the Brain Tumor Reporting and Data System. Curr. Probl. Diagn. Radiol. 2021, 50, 356–361. [Google Scholar] [CrossRef]
- BT-RADS Template. Available online: https://btrads.com/wp-content/uploads/2019/11/BT-RADS-followup-template-v1_03_short-sample.pdf (accessed on 29 August 2024).
- Brain Tumor Reporting and Data System (BT-RADS). Available online: https://btrads.com/ (accessed on 5 July 2024).
- Almalki, Y.E.; Basha, M.A.A.; Metwally, M.I.; Zeed, N.A.; Nada, M.G.; Alduraibi, S.K.; Morsy, A.A.; Balata, R.; Al Attar, A.Z.; Amer, M.M.; et al. Validating Brain Tumor Reporting and Data System (BT-RADS) as a Diagnostic Tool for Glioma Follow-Up after Surgery. Biomedicines 2024, 12, 887. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Hoch, M.J.; Peng, L.; Somasundaram, A.; Chen, Z.; Weinberg, B.D. A Brain Tumor Reporting and Data System to Optimize Imaging Surveillance and Prognostication in High-Grade Gliomas. J. Neuroimaging Off. J. Am. Soc. Neuroimaging 2022, 32, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Abidi, S.A.; Hoch, M.J.; Hu, R.; Sadigh, G.; Voloschin, A.; Olson, J.J.; Shu, H.-K.G.; Neill, S.G.; Weinberg, B.D. Using Brain Tumor MRI Structured Reporting to Quantify the Impact of Imaging on Brain Tumor Boards. Tomography 2023, 9, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Parillo, M.; Mallio, C.A.; Pileri, M.; Dirawe, D.; Romano, A.; Bozzao, A.; Weinberg, B.; Quattrocchi, C.C. Interrater Reliability of Brain Tumor Reporting and Data System (BT-RADS) in the Follow up of Adult Primary Brain Tumors: A Single Institution Experience in Italy. Quant. Imaging Med. Surg. 2023, 13, 7423–7431. [Google Scholar] [CrossRef]
- Essien, M.; Cooper, M.E.; Gore, A.; Min, T.L.; Risk, B.B.; Sadigh, G.; Hu, R.; Hoch, M.J.; Weinberg, B.D. Interrater Agreement of BT-RADS for Evaluation of Follow-Up MRI in Treated Primary Brain Tumor Patients. AJNR Am. J. Neuroradiol. 2024; Epub ahead of print. [Google Scholar] [CrossRef]
- Gore, A.; Hoch, M.J.; Shu, H.-K.G.; Olson, J.J.; Voloschin, A.D.; Weinberg, B.D. Institutional Implementation of a Structured Reporting System: Our Experience with the Brain Tumor Reporting and Data System. Acad. Radiol. 2019, 26, 974–980. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Weinberg, B.D.; Hu, R.; Saindane, A.; Mullins, M.; Allen, J.; Hoch, M.J. Quantitative Improvement in Brain Tumor MRI Through Structured Reporting (BT-RADS). Acad. Radiol. 2020, 27, 780–784. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Y.; Wu, X.; Pan, Y.; Zhou, D.; Zhang, H.; Chen, Y.; Zhao, J.; Mo, Z.; Huang, B. Adding DSC PWI and DWI to BT-RADS Can Help Identify Postoperative Recurrence in Patients with High-Grade Gliomas. J. Neurooncol. 2020, 146, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Metwally, M.I.; Hafez, F.F.M.; Ibrahim, S.A.; Morsy, A.A.; Zeed, N.A. The Value of Adding DWI and FLAIR Signal Changes in the Resection Cavity on the Diagnostic Performance of BT-RADS Category 3 for Tumor Progression Prediction in Post-Treated Glioma Patients: A Prospective Pilot Study. Egypt. J. Radiol. Nucl. Med. 2023, 54, 52. [Google Scholar] [CrossRef]
- Ramesh, K.; Gurbani, S.S.; Mellon, E.A.; Huang, V.; Goryawala, M.; Barker, P.B.; Kleinberg, L.; Shu, H.-K.G.; Shim, H.; Weinberg, B.D. The Longitudinal Imaging Tracker (BrICS-LIT):A Cloud Platform for Monitoring Treatment Response in Glioblastoma Patients. Tomography 2020, 6, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Weinberg, B.D.; Gore, A.; Banerjee, I. A Scalable Natural Language Processing for Inferring BT-RADS Categorization from Unstructured Brain Magnetic Resonance Reports. J. Digit. Imaging 2020, 33, 1393–1400. [Google Scholar] [CrossRef]
- Weinberg, B.D.; Hoch, M.J. Brain Tumor-Radiology and Data System (BT-RADS)-an Imperfect System but a Worthwhile Start. Eur. Radiol. 2024; Epub ahead of print. [Google Scholar] [CrossRef]
- Martín-Noguerol, T.; Cabrera-Zubizarreta, A.; Luna, A. Standardized Reporting Systems for (Which?) Brain Tumors from in the Dark: Cons of the BT-RADS. Eur. Radiol. 2024; Epub ahead of print. [Google Scholar] [CrossRef]
- Rao, B.; Ikuta, I.; Mahajan, A.; Karam, A.A.; Zohrabian, V.M. Brain Tumor Reporting and Data System: A Pictorial Review. Neurographics 2021, 11, 175–185. [Google Scholar] [CrossRef]

| Investigators | Study Design | Number of MRIs | Main Findings |
|---|---|---|---|
| Ebaid et al. [5] | Prospective | 81 |
|
| Trivedi et al. [9] | Retrospective | 100 |
|
| Kim et al. [16] | Survey | - |
|
| Almalki et al. [19] | Prospective | 322 |
|
| Kim et al. [20] | Retrospective | 538 |
|
| Abidi et al. [21] | Prospective | 212 |
|
| Parillo et al. [22] | Retrospective | 147 |
|
| Essien et al. [23] | Retrospective | 103 |
|
| Gore et al. [24] | Survey | - |
|
| Zhang et al. [25] | Retrospective | - |
|
| Yang et al. [26] | Retrospective | 91 |
|
| Metwally et al. [27] | Prospective | 27 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parillo, M.; Quattrocchi, C.C. Brain Tumor Reporting and Data System (BT-RADS) for the Surveillance of Adult-Type Diffuse Gliomas after Surgery. Surgeries 2024, 5, 764-773. https://doi.org/10.3390/surgeries5030061
Parillo M, Quattrocchi CC. Brain Tumor Reporting and Data System (BT-RADS) for the Surveillance of Adult-Type Diffuse Gliomas after Surgery. Surgeries. 2024; 5(3):764-773. https://doi.org/10.3390/surgeries5030061
Chicago/Turabian StyleParillo, Marco, and Carlo Cosimo Quattrocchi. 2024. "Brain Tumor Reporting and Data System (BT-RADS) for the Surveillance of Adult-Type Diffuse Gliomas after Surgery" Surgeries 5, no. 3: 764-773. https://doi.org/10.3390/surgeries5030061






