The Effect of Superabsorbent Polymers on Mechanical Characteristics and Cracking Susceptibility of Alkali-Activated Mortars Containing Ground Granulated Blast-Furnace Slag and Copper Slag
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
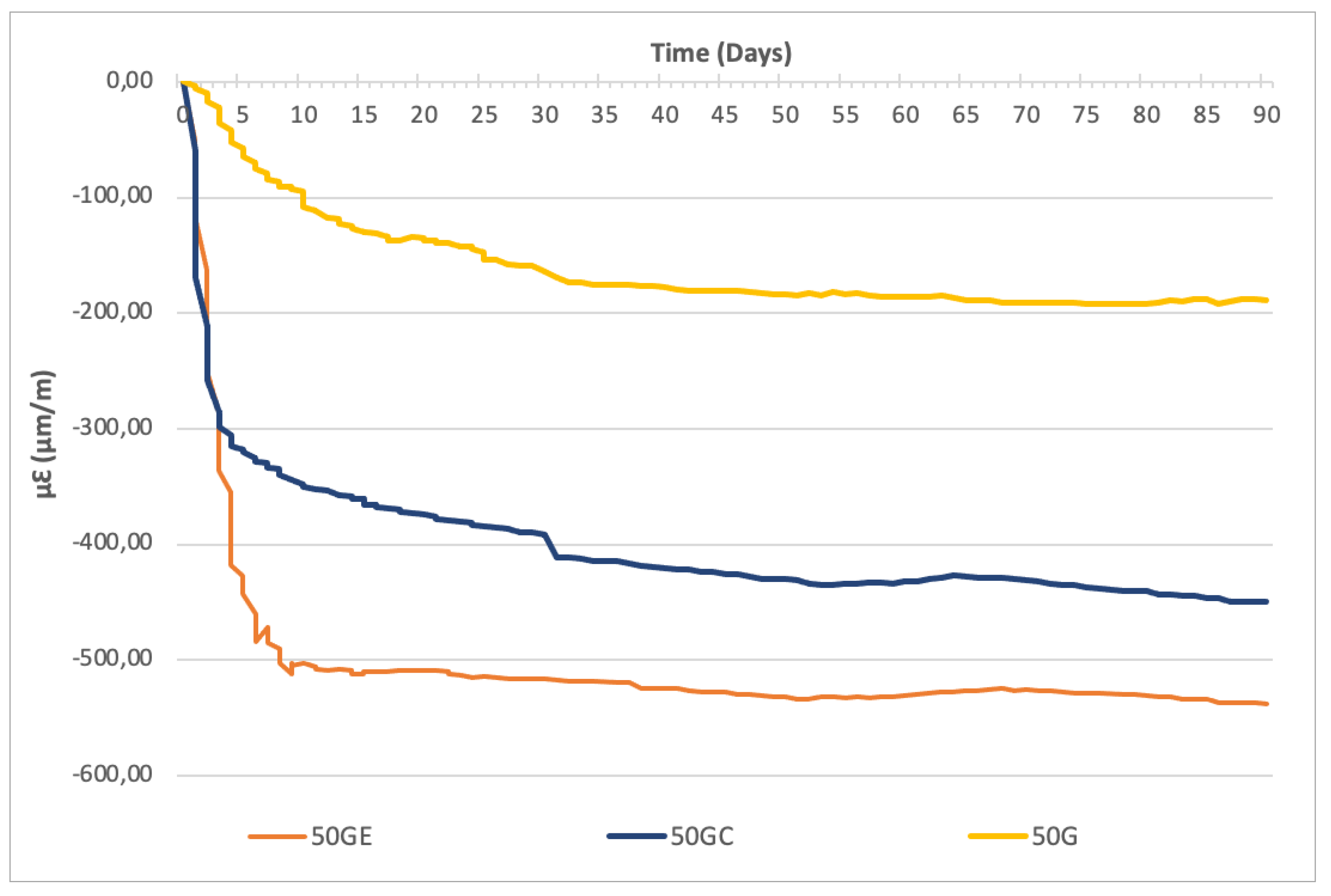
- Rate of reaction: SAP increases kinetics of alkali-activated GGBS-CS reactions. The water retention by SAP results in a higher alkali concentration available in the solution to activate the precursors, speeding up reactions. The capacity to maintain this effect depends on the type of polymer. SAP C (with lower WAC) is able to accelerate reactions up to approximately 3 days, while SAP E (higher WAC) can contribute up to 7 days. After this period of time, SAP collapses and reactions seem to follow at the same pace as the reference sample (without SAP).
- Degree of reaction: The effect of SAP depends on the type of precursor, especially in the capacity to form silicates in the presence of CS. In this case, SAP E, with higher absorption and smaller, well-distributed particles in the mix, leads to higher extension of reaction. In turn, the bigger particles and lower water absorption of SAP C can leave behind larger pores (after SAP collapsing), allowing more room for any volume change and reduction in AS of mortars. For samples with low or no CS content, the degree of reaction is less affected because GGBS (with higher reactivity) is rapidly activated by the alkali solution. At 90 days, the level of AS (which indicates the extension of this effect) is comparable to all GGBS samples (with or without SAP).
4. Conclusions
- (a)
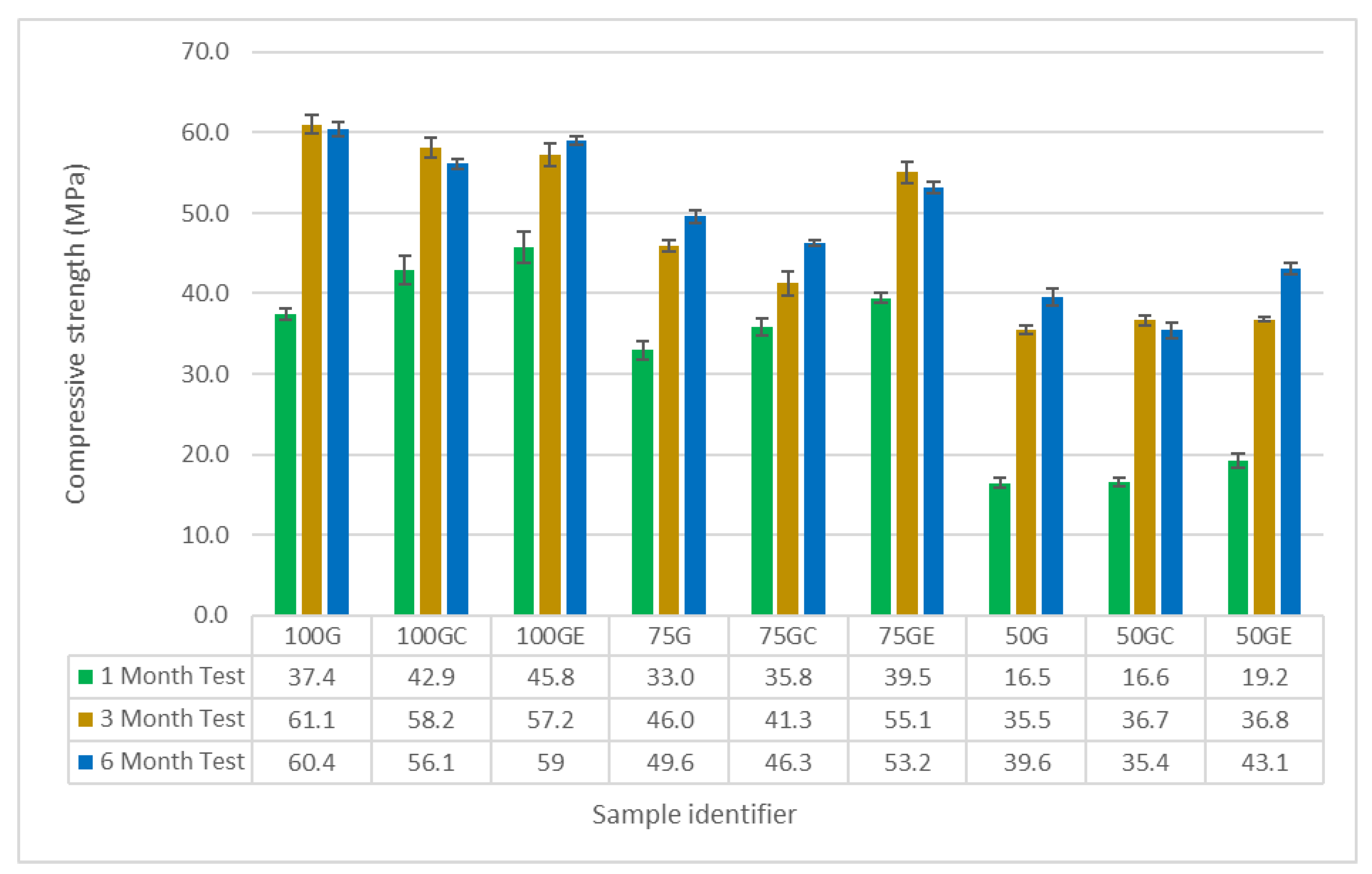
- Copper slag (CS) has lower reactivity than ground granulated blast-furnace slag (GGBS), even in a sodium silicate-activated system. CS acts as an aggregate when no water control takes place. This results in prolonged setting time, lower autogenous shrinkage, and lower compressive strength;
- (b)
- Water absorption capacity (WAC) of superabsorbent polymers (SAP) leads to increased pH values of systems due to lower dilution of alkali ions in aqueous solution. SAP with finer particles absorbs faster and more water from alkali-activated GGBS-CS solutions. However, this reduces workability of mortars up to approximately 10%, for mixes with the same ratio of fluid volume/precursor;
- (c)
- SAP increases the rate of activated GGBS-CS reactions observed in higher slopes of autogenous shrinkage curves. Depending on the type of polymer, a higher alkali concentration of SAP solutions speeds up early age reactions up to 7 days. After this period, SAP collapses, and the pace of reaction follows as for the reference sample;
- (d)
- SAP may also increase the degree of reactions, depending on the type of polymer and type of precursor. In the presence of CS, SAP, with higher absorption and smaller particles well-distributed in the mix, leads to a higher extension of reaction. For samples with high GGBS content (above 75%), the degree of reaction is less affected by SAP because of higher slag reactivity, which is rapidly activated by the alkali solution. At 90 days, the level of autogenous shrinkage is comparable to all GGBS mortars (with or without SAP);
- (e)
- The effect of faster and extended reaction of activated GGBS-CS mortars by SAP reflects in higher compressive strength. SAP with lower WAC and larger particles may lead to decreased autogenous shrinkage by providing more room for volume changes after its collapsing. However, it may lead to the reduction in compressive strength by 10% at 6 months, especially at high CS contents. On the contrary, finer SAP with higher WAC has higher ability to increase compressive strength of GGBS-CS mortars, achieving values 9% greater than the reference sample at 6 months.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UN Environment; Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-efficient cements: Potential economically viable solutions for a low CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Habert, G.; Miller, S.A.; Provis, J.L.; Favier, A.; Horvath, A.; Scrivener, K.L. Environmental impacts and decarbonization strategies in the cement and concrete industries. Nat. Rev. Earth Environ. 2020, 1, 559–573. [Google Scholar]
- Song, Q.; Guo, M.Z.; Ling, T.C. A review of elevated-temperature properties of alternative binders: Supplementary cementitious materials and alkali-activated materials. Constr. Build. Mater. 2022, 341, 127894. [Google Scholar] [CrossRef]
- Scrivener, K.; Martirena, F.; Bishnoi, S.; Maity, S. Calcined clay limestone cements (LC3). Cem. Concr. Res. 2018, 114, 49–56. [Google Scholar] [CrossRef]
- Singh, G.V.P.B.; Subramaniam, V.L.K. Production and characterization of low energy Portland composite cement from post-industrial waste. J. Clean. Prod. 2019, 239, 118024. [Google Scholar] [CrossRef]
- John, M.V.; Quattrone, M.; Abrāo, P.C.R.A.; Cardoso, F.A. Rethinking cement standards: Opportunities for a better future. Cem. Concr. Res. 2019, 124, 105832. [Google Scholar] [CrossRef]
- Umar, T.; Tahir, A.; Egbu, C.; Honnurvali, M.S.; Saidani, M.; Al-Bayati, A. Developing A Sustainable Concrete Using Ceramic Waste Powder. In Proceedings of the 11th International Conference on Construction in the 21st Century, London, UK, 9–10 September 2019. [Google Scholar]
- Sun, Y.; Wang, Z.H.; Park, D.J.; Kim, W.S.; Yan, S.R.; Lee, H.S. Analysis of the isothermal hydration heat of cement paste containing mechanically activated fly ash. Thermochim. Acta 2022, 715, 179273. [Google Scholar] [CrossRef]
- Rostami, R.; Klemm, A.J.; Almeida, F.C.R. Effect of superabsorbent polymers on microstructure and strength of blended cements mortars reinforced by polymeric fibre. Cement 2022, 9, 100041. [Google Scholar]
- Fallah-Valukolaee, S.; Mousavi, R.; Arjomandi, A.; Nematzadeh, M.; Kazemi, M. A comparative study of mechanical properties and life cycle assessment of high-strength concrete containing silica fume and nanosilica as a partial cement replacement. Structures 2022, 46, 838–851. [Google Scholar] [CrossRef]
- Umar, T.; Yousaf, M.; Akbar, M.; Abbas, N.; Hussain, Z.; Ansari, W.S. An experimental study on non-destructive evaluation of the mechanical characteristics of a sustainable concrete incorporating industrial waste. Materials 2022, 15, 7346. [Google Scholar] [CrossRef]
- Zia, A.; Pu, Z.; Holly, I.; Umar, T.; Tariq, M.A.U.R.; Sufian, M. A comprehensive review of incorporating steel fibers of waste tires in cement composites and its applications. Materials 2022, 15, 7420. [Google Scholar]
- Sampaio, D.O.A.; Tashima, M.M.; Costa, D.; Quinteiro, P.; Dias, A.C.; Alasaki, J.L. Evalutaion of the environmental performance of rice husk ash and tire rubber residues incorporated in concrete slabs. Constr. Build. Mater. 2022, 357, 129332. [Google Scholar]
- Almeida, F.C.R.; Sales, A.; Moretti, J.P.; Mendes, P.C.D. Use of sugarcane bagasse ash sand (SBAS) as corrosion retardant for reinforced Portland slag cement concrete. Constr. Build. Mater. 2022, 226, 72–82. [Google Scholar] [CrossRef]
- Duarte, M.S.; Almada, B.S.; dos Santos, W.J.; Bessa, S.A.L.; Bezerra, A.C.D.; Aguilar, M.T.P. Influence of mechanical treatment and magnetic separation on the performance of iron ore tailings as supplementary cementitious material. J. Build. Eng. 2022, 59, 105099. [Google Scholar] [CrossRef]
- Hemkemeier, T.A.; Almeida, F.C.R.; Sales, A.; Klemm, A.J. Corrosion monitoring by open circuit potential in steel reinforcements embedded in cementitious composites with industrial wastes. Case Stud. Constr. Mater. 2022, 16, e01042. [Google Scholar] [CrossRef]
- Lan, W.; Wu, A.; Yu, P. Development of a new controlled low strength filling material from the activation of copper slag: Influencing factors and mechanism analysis. J. Clean. Prod. 2020, 246, 119060. [Google Scholar]
- Feng, Y.; Yang, Q.; Chen, Q.; Kero, J.; Andersson, A.; Ahmed, H.; Engström, F.; Samuelsson, C. Characterization and evaluation of the pozzolanic activity of granulated copper slag modified with CaO. J. Clean. Prod. 2019, 232, 1112–1120. [Google Scholar] [CrossRef]
- Song, S.; Sohn, D.; Jennings, H.M.; Mason, T.O. Hydration of alkali-activated ground granulated blast furnace slag. J. Mater. Sci. 2000, 35, 249–257. [Google Scholar] [CrossRef]
- Siddique, R.; Bennacer, R. Use of iron and steel industry by product (GGBS) in cement paste and mortar. Resour. Conserv. Recycl. 2012, 69, 29–34. [Google Scholar] [CrossRef]
- Sasui, S.; Kim, G.; Koyama, T.; Chansomsak, S. Strength and Microstructure of Class-C Fly Ash and GGBS Blend Geopolymer Activated in NaOH & NaOH + Na2SiO3. Materials 2020, 13, 59. [Google Scholar]
- Siddique, R.; Khan, M.I. Supplementary Cementing Materials, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 121–170. [Google Scholar]
- Gorai, B.; Jana, R.K. Characteristics and utilisation of copper slag—A review. Resour. Conserv. Recycl. 2003, 39, 299–313. [Google Scholar] [CrossRef]
- Sithole, N.T.; Mashifana, T. Geosynthesis of building and construction materials through alkaline activation of ground blast furnace slag. Constr. Build. Mater. 2020, 264, 120712. [Google Scholar] [CrossRef]
- Chen, Q.; Tao, Y.; Feng, Q.; Zhang, Q.; Liu, Y. Utilisation of modified copper slag by Na2SO4 and CaO for unclassified lead/zinc mine tailings based cemented paste backfill. J. Environ. Manag. 2021, 290, 112608. [Google Scholar] [CrossRef] [PubMed]
- Phoo-ngernkham, T.; Maegawa, A.; Mishima, N.; Hatanaka, S.; Chindaprasirt, P. Effects of sodium hydroxide and sodium silicate solutions on compressive and shear bond strengths on FA-GBFS geopolymer. Constr. Build. Mater. 2015, 91, 1–8. [Google Scholar]
- Dang, J.; Zhao, J.; Du, Z. Effect of Superabsorbent Polymers on the Properties of Concrete. Polymers 2017, 9, 672. [Google Scholar]
- Lyu, Z.; Guo, Y.; Chen, Z.; Shen, A.; Qin, X.; Yang, J.; Zhao, M.; Wang, Z. Research on shrinkage development and fracture properties of internal curing pavement concrete based on humidity compensation. Constr. Build. Mater. 2019, 209, 417–431. [Google Scholar] [CrossRef]
- Hasholt, M.T.; Jensen, O.M. Chloride migration in concrete with superabsorbent polymers. Cem. Concr. Compos. 2015, 55, 290–297. [Google Scholar]
- Rostami, R.; Klemm, A.J.; Almeida, F.C.R. Reduction of shrinkage by Superabsorbent polymers (SAP) in fibre reinforced mortars. Constr. Build. Mater. 2021, 288, 123109. [Google Scholar] [CrossRef]
- Mechtcherine, V.; Wyrzkowski, M.; Schoro, C.; Snoeck, D.; Lura, P.; De Belie, N.; Mignon, A.; Van Vlierberghe, S.; Klemm, A.J.; Almeida, F.C.R.; et al. Application of superabsorbent polymers (SAP) in concrete construction- update of RILEM state-of-the-art report. Mater. Struct. 2021, 54, 80. [Google Scholar] [CrossRef]
- Oh, S.; Choi, Y.C. Superabsorbent polymers as internal curing agents in alkali activated slag mortars. Constr. Build. Mater. 2018, 159, 1–8. [Google Scholar] [CrossRef]
- Song, C.; Choi, Y.C.; Choi, S. Effect of internal curing by superabsorbent polymers—Internal relative humidity and autogenous shrinkage of alkali-activated slag mortars. Constr. Build. Mater. 2016, 123, 198–206. [Google Scholar] [CrossRef]
- Li, Z.; Wyrzykowski, M.; Dong, H.; Granja, J.; Azenha, M.; Lura, P.; Ye, G. Internal curing by superabsorbent polymers in alkali-activated slag. Cem. Concr. Res. 2020, 135, 106123. [Google Scholar] [CrossRef]
- Wang, P.; Chen, H.; Chen, P.; Pan, J.; Xu, Y.; Wang, H.; Shen, W.; Cao, K. Effect of internal curing by super absorbent polymer on the autogenous shrinkage of alkali-activated slag mortars. Materials 2020, 13, 4318. [Google Scholar] [CrossRef]
- Mechtcherine, V.; Snoeck, D.; Schröfl, C.; De Belie, N.; Klemm, A.J.; Ichimiya, K.; Moon, J.; Wyrzykowski, M.; Lura, P.; Toropovs, N. Testing superabsorbent polymer (SAP) sorption properties prior to implementation in concrete: 642 results of a RILEM Round-Robin Test. Mater. Struct. 2018, 51, 28. [Google Scholar] [CrossRef]
- Snoeck, D.; Schroefl, C.; Mechtcherine, V. Recommendation of RILEM TC 260-RSC: Testing sorption by superabsorbent polymers (SAP) prior to implementation in cement-based materials. Mater. Struct. 2018, 51, 116. [Google Scholar] [CrossRef]
- BS EN 1015-3:1999; Methods of Test for Mortar for Masonry. British Standards Institute: London, UK, 2007.
- BS EN 196-3:2016; Methods of Testing Cement. British Standards Institute: London, UK, 2016.
- ASTM C1698-09; Standard Test Method for Autogenous Strain of Cement Paste and Mortars. American Society for Testing and Materials: West Conshohocken, PA, USA, 2009.
- Wyrzykowski, M.; Zhangli, H.; Ghourchian, S.; Scrivener, K.; Lura, P. Corrugated tube protocol for autogenous shrinkage measurements: Review and statistical assessment. Mater. Struct. 2016, 50, 57. [Google Scholar]
- BS EN 1015-11:2019; Methods of Test for Mortar for Masonry. British Standards Institute: London, UK, 2019.
- Almeida, F.C.R.; Klemm, A.J. Effect of GGBS on Water Absorption Capacity and Stability of Superabsorbent Polymers Partially Crosslinked with Alkalis. J. Mater. Civ. Eng. 2018, 30, 12. [Google Scholar]
- Almeida, F.C.R.; Klemm, A.J. Efficiency of internal curing by superabsorbent polymers (SAP) in PC-GGBS mortars. Cem. Concr. Compos. 2018, 88, 41–55. [Google Scholar] [CrossRef]
- Rostami, R.; Klemm, A.J.; Almeida, F.C.R. The effect of SCMs in blended cements on sorption characteristics of Superabsorbent Polymers. Materials 2021, 14, 1609. [Google Scholar] [CrossRef]
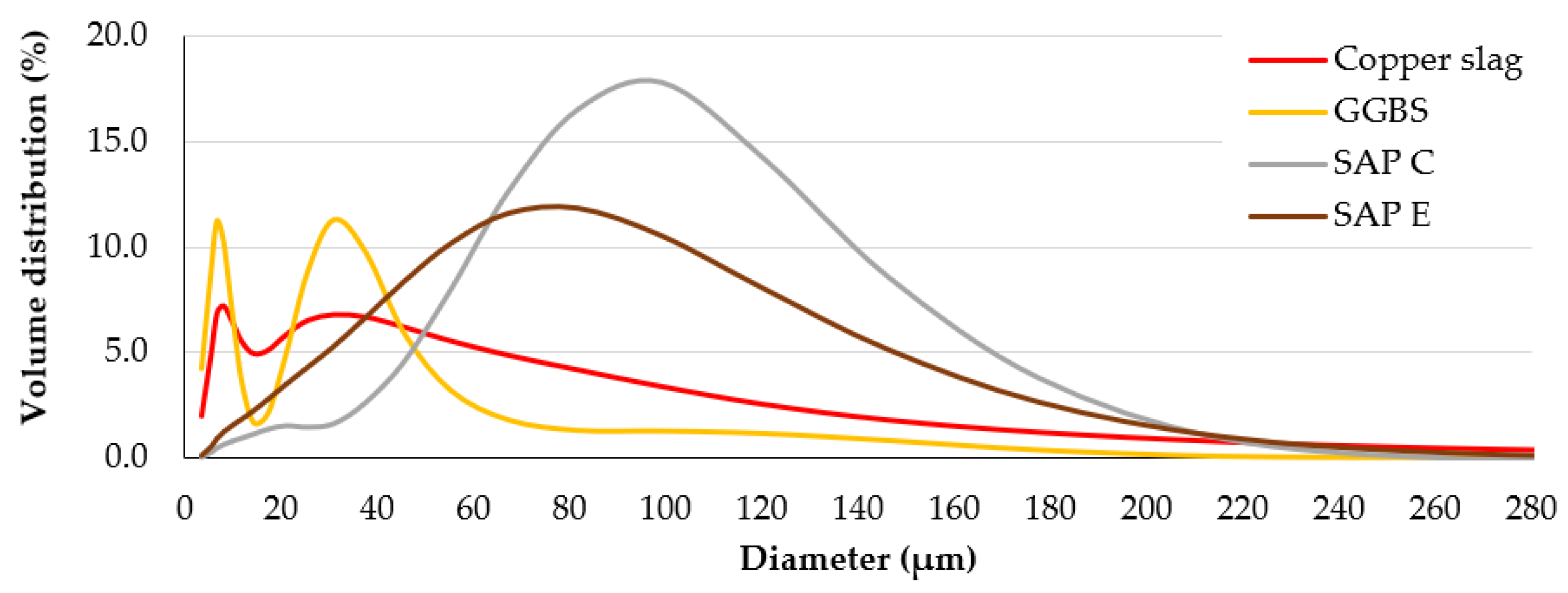
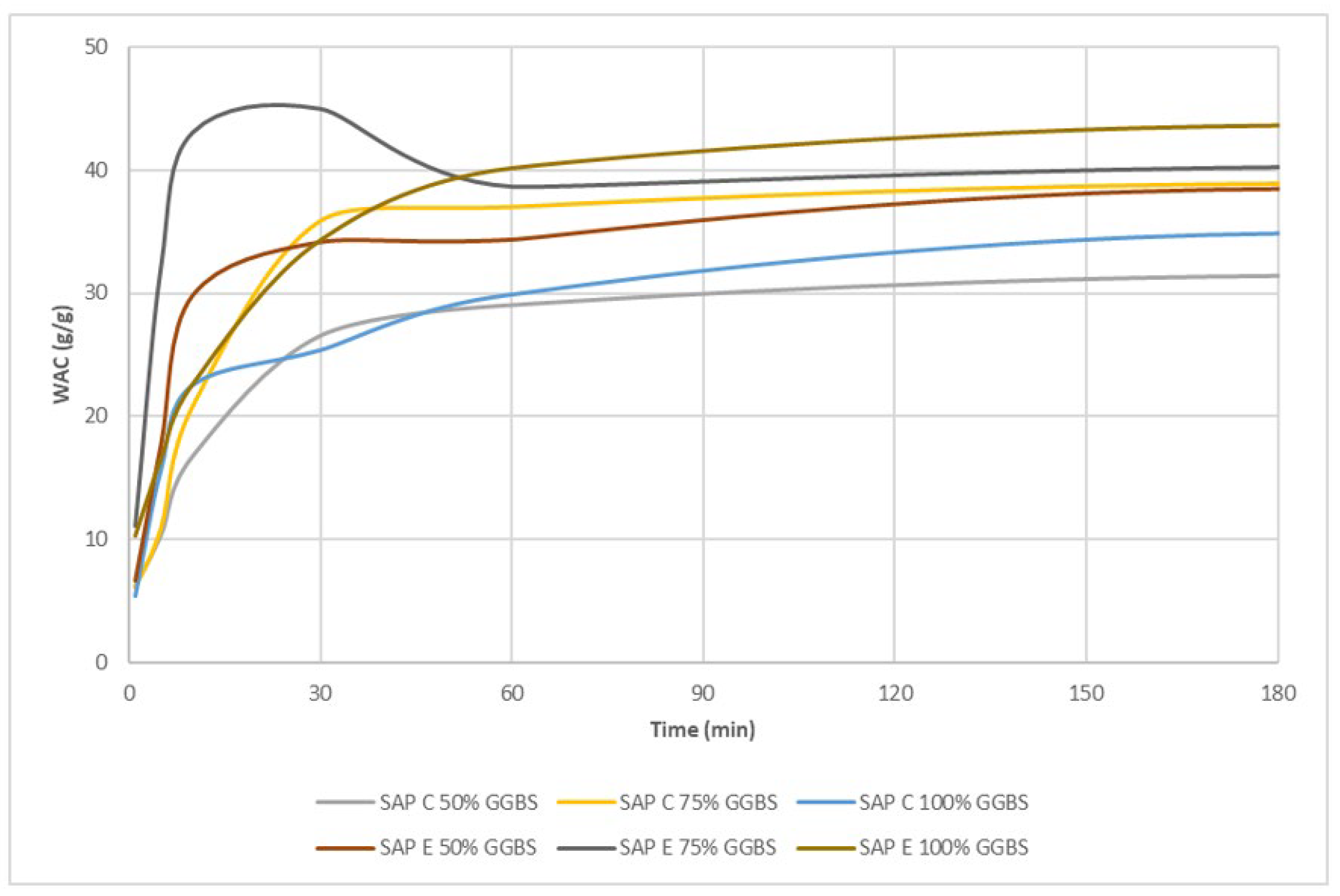


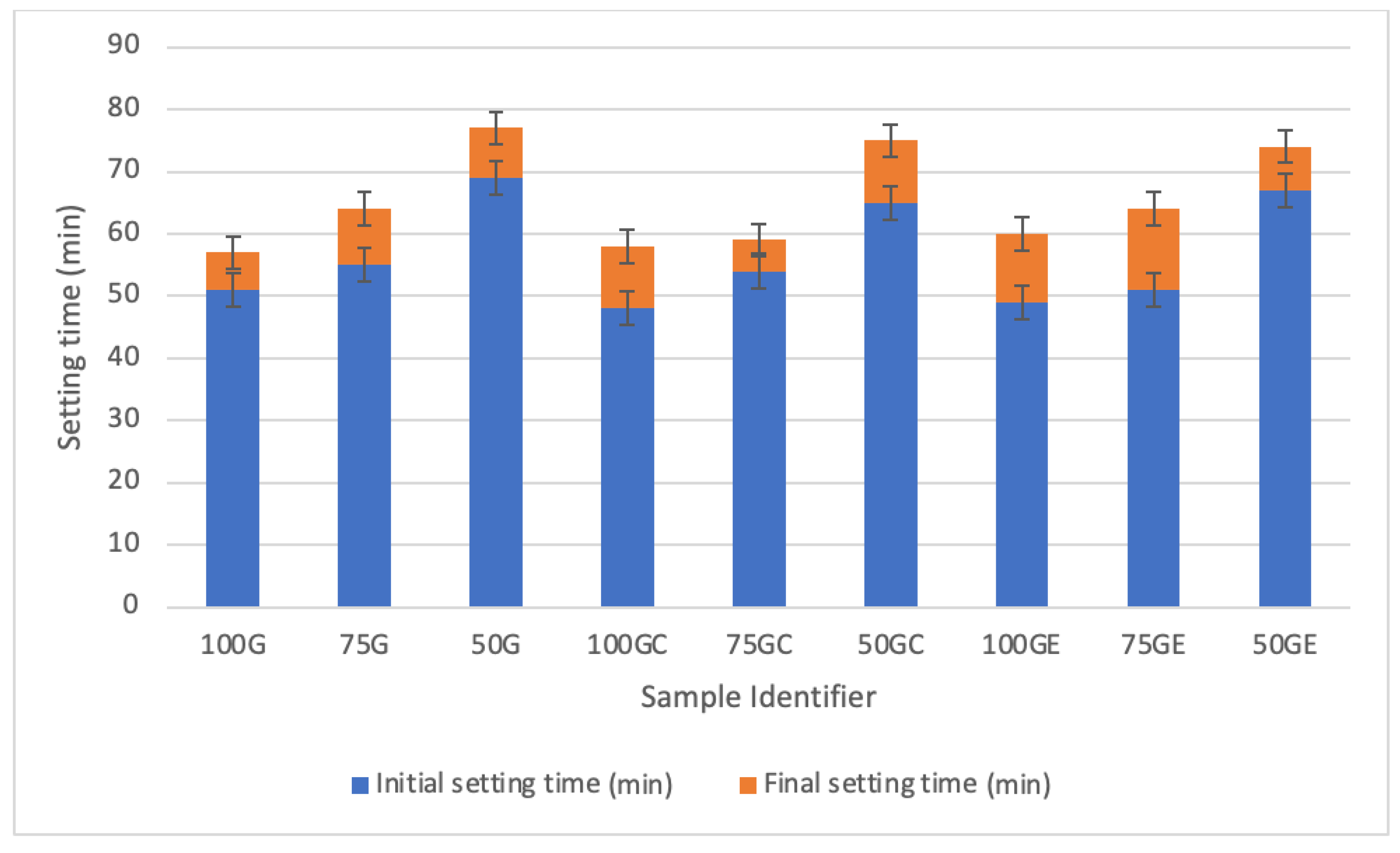

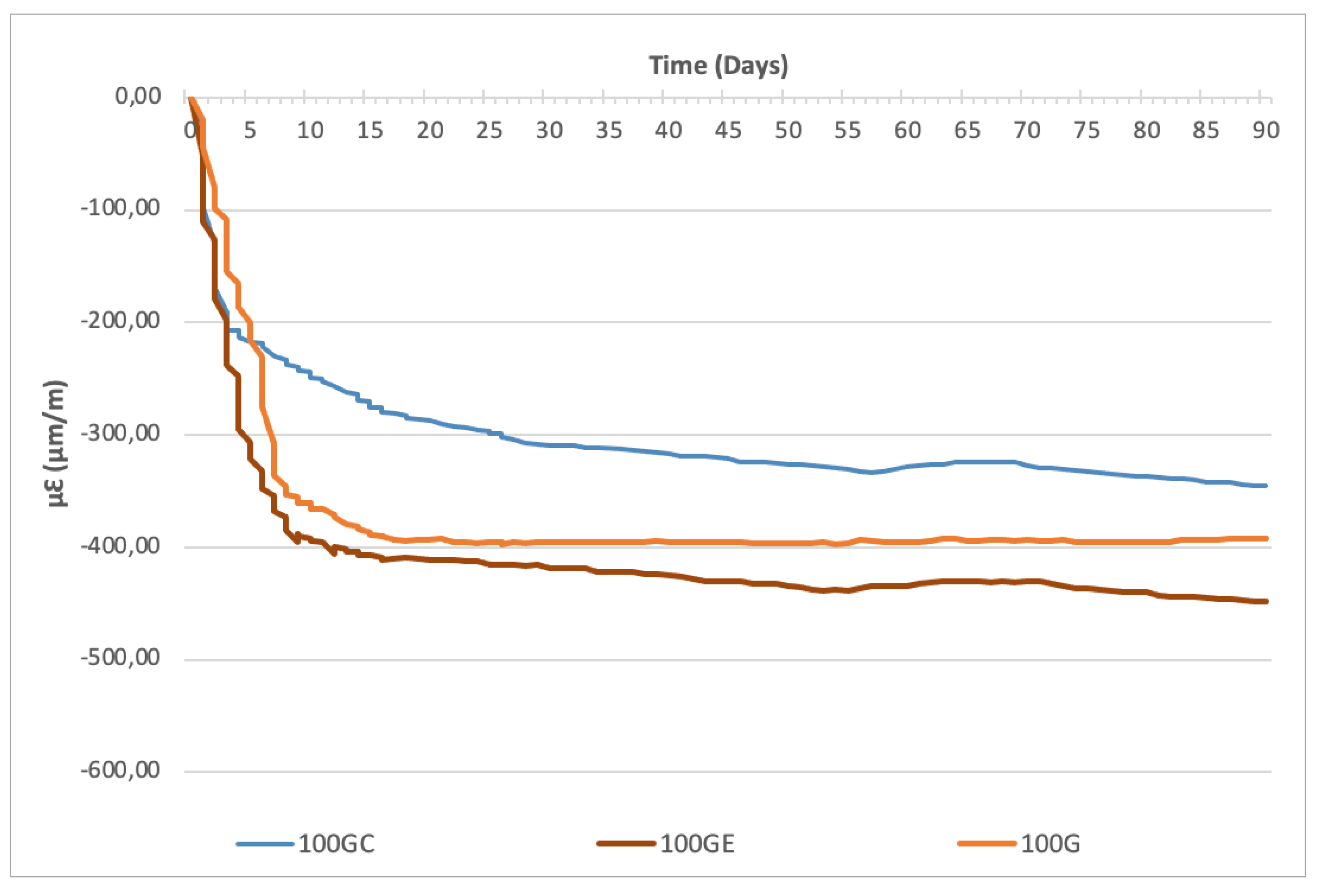

| Element | GGBS (%) | CS (%) |
|---|---|---|
| CaO | 40.04 | 3.90 |
| SiO2 | 36.01 | 37.30 |
| Al2O3 | 10.03 | 1.68 |
| Fe2O3 | 0.50 | 38.80 |
| MgO | 8.01 | 2.09 |
| SO3 | 0.20 | - |
| K2O | 0.70 | - |
| Na2O | 0.41 | - |
| Cr | - | 0.22 |
| Cu | - | 0.70 |
| Zn | - | 0.78 |
| Sample | Sand (g) | GGBS (g) | CS (g) | Water (g) | Sodium Silicate (g) | SAP C (g) | SAP E (g) |
|---|---|---|---|---|---|---|---|
| 100G | 200 | 100 | 0 | 33.34 | 36.51 | 0 | 0 |
| 75G | 200 | 75 | 25 | 33.34 | 36.51 | 0 | 0 |
| 50G | 200 | 50 | 50 | 33.34 | 36.51 | 0 | 0 |
| 100GC | 200 | 100 | 0 | 33.34 | 36.51 | 0.25 | 0 |
| 75GC | 200 | 75 | 25 | 33.34 | 36.51 | 0.25 | 0 |
| 50GC | 200 | 50 | 50 | 33.34 | 36.51 | 0.25 | 0 |
| 100GE | 200 | 100 | 0 | 33.34 | 36.51 | 0 | 0.25 |
| 75GE | 200 | 75 | 25 | 33.34 | 36.51 | 0 | 0.25 |
| 50GE | 200 | 50 | 50 | 33.34 | 36.51 | 0 | 0.25 |
| SAP C Measurements | |||||||
|---|---|---|---|---|---|---|---|
| Time | 100% GGBS | 75% GGBS | 50% GGBS | ||||
| Minutes | Hours | SAP C | Ref | SAP C | Ref | SAP C | Ref |
| 180 | 3 | 12.29 | 12.47 | 12.62 | 12.82 | 12.44 | 12.67 |
| 1440 | 24 | 12.31 | 12.17 | 12.65 | 12.57 | 12.50 | 12.42 |
| SAP E Measurements | |||||||
| Time | 100% GGBS | 75% GGBS | 50% GGBS | ||||
| Minutes | Hours | SAP E | Ref | SAP E | Ref | SAP E | Ref |
| 180 | 3 | 12.31 | 12.44 | 12.73 | 12.86 | 12.62 | 12.71 |
| 1440 | 24 | 12.33 | 12.26 | 12.67 | 12.53 | 12.52 | 12.44 |
| Sample | CS Content | Flow (mm) | Flow Reduction by CS Addition |
|---|---|---|---|
| 100G | 0% | 168 | - |
| 50G | 25% | 159 | 5.4% |
| 75G | 50% | 129 | 23.2% |
| Sample | Type of SAP | Flow (mm) | Flow Reduction by SAP |
|---|---|---|---|
| 100G | - | 168 | - |
| 100GC | SAP C | 153 | 8.9% |
| 100GE | SAP E | 156 | 9.6% |
| 75G | - | 159 | - |
| 75GC | SAP C | 148 | 6.9% |
| 75GE | SAP E | 141 | 11.3% |
| 50G | - | 129 | - |
| 50GC | SAP C | 125 | 3.1% |
| 50GE | SAP E | 122 | 5.4% |
| GGBS Content | CS Content | SAP C | SAP E |
|---|---|---|---|
| 100% | 0% | −7.1% | −2.3% |
| 50% | 25% | −6.7% | +7.3% |
| 75% | 50% | −10.6% | +8.8% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
MacLennan, S.; Almeida, F.C.R.; Klemm, A.J. The Effect of Superabsorbent Polymers on Mechanical Characteristics and Cracking Susceptibility of Alkali-Activated Mortars Containing Ground Granulated Blast-Furnace Slag and Copper Slag. CivilEng 2022, 3, 1077-1090. https://doi.org/10.3390/civileng3040061
MacLennan S, Almeida FCR, Klemm AJ. The Effect of Superabsorbent Polymers on Mechanical Characteristics and Cracking Susceptibility of Alkali-Activated Mortars Containing Ground Granulated Blast-Furnace Slag and Copper Slag. CivilEng. 2022; 3(4):1077-1090. https://doi.org/10.3390/civileng3040061
Chicago/Turabian StyleMacLennan, Stewart, Fernando C. R. Almeida, and Agnieszka J. Klemm. 2022. "The Effect of Superabsorbent Polymers on Mechanical Characteristics and Cracking Susceptibility of Alkali-Activated Mortars Containing Ground Granulated Blast-Furnace Slag and Copper Slag" CivilEng 3, no. 4: 1077-1090. https://doi.org/10.3390/civileng3040061
APA StyleMacLennan, S., Almeida, F. C. R., & Klemm, A. J. (2022). The Effect of Superabsorbent Polymers on Mechanical Characteristics and Cracking Susceptibility of Alkali-Activated Mortars Containing Ground Granulated Blast-Furnace Slag and Copper Slag. CivilEng, 3(4), 1077-1090. https://doi.org/10.3390/civileng3040061









