Efficient Reduction of Carbon Tetrachloride in an Electrochemical Reactor with a Three-Dimensional Electrode
Abstract
1. Introduction
2. Materials and Methods
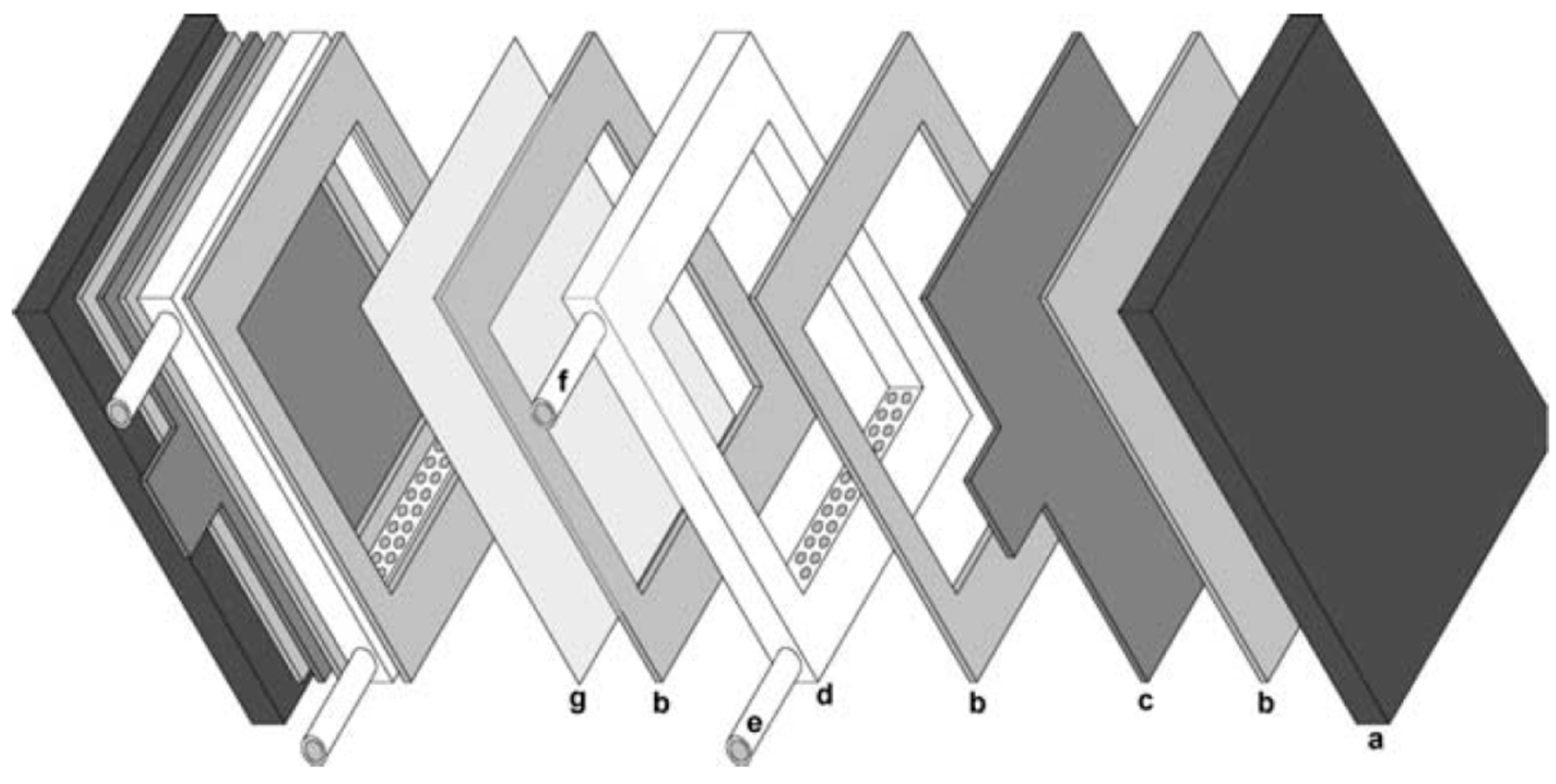
2.1. Electrochemical Reactor
2.2. Mass Transport Characterization Experiments
2.3. Electrolysis of Carbon Tetrachloride with a Three-Dimensional Electrode
3. Results
3.1. Mass Transport Caracterization
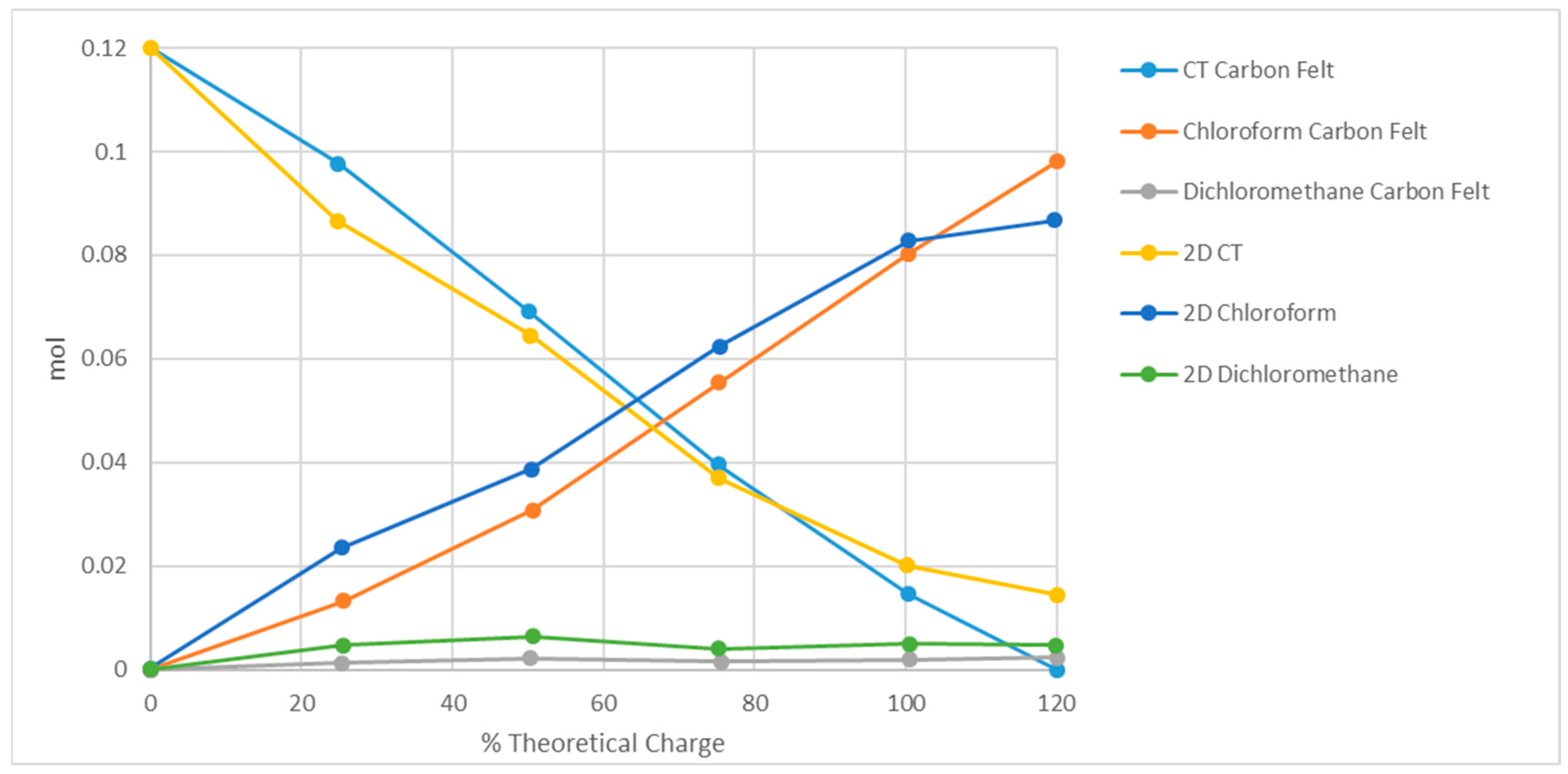
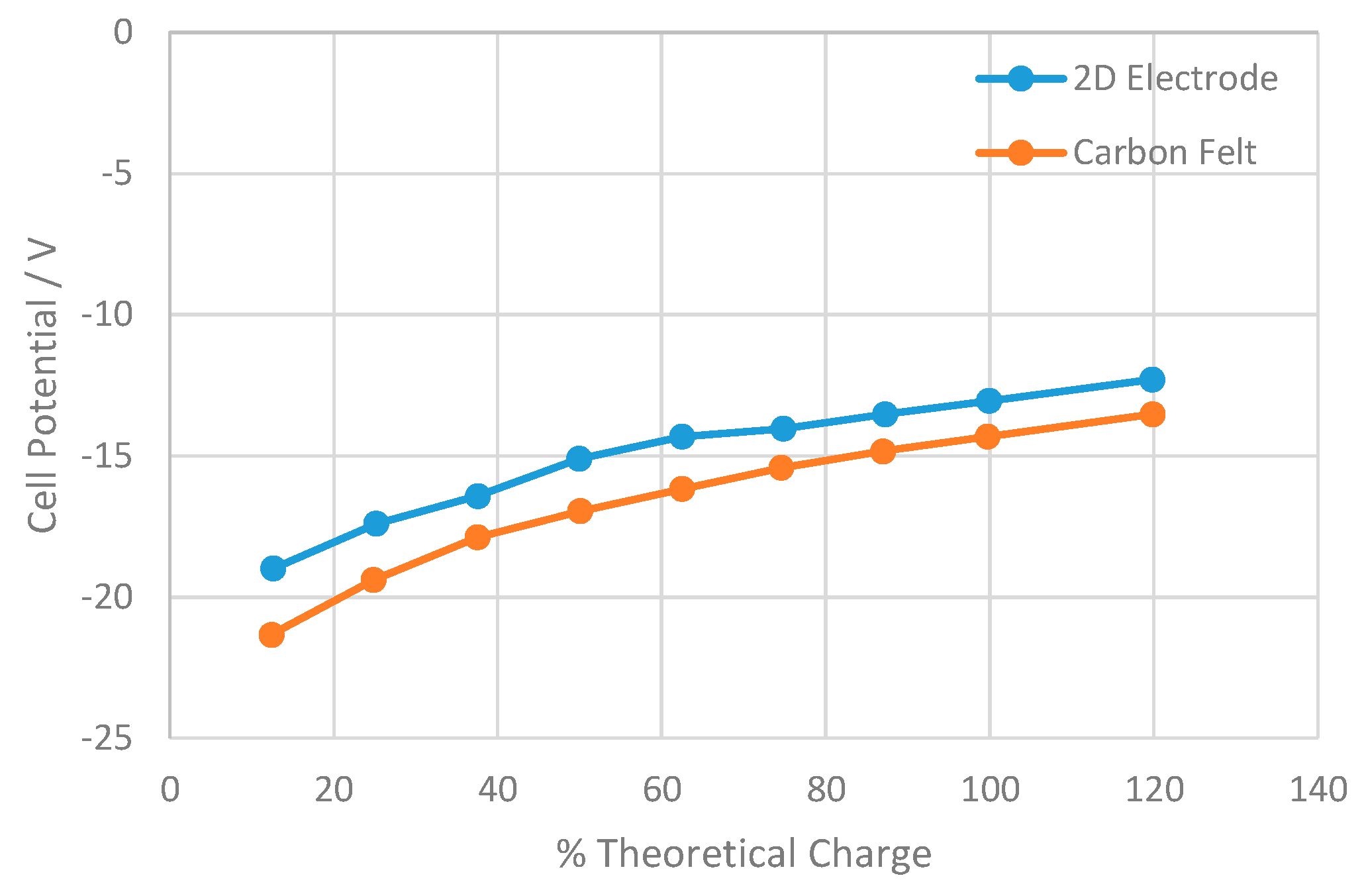
3.2. Electrolysis of Carbon Tetrachloride
4. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, B.; Debut, A. Green Chemistry—New Perspectives; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar] [CrossRef]
- Rathi, P.; Nausheen, S. Nisha Green chemistry and technology for sustainable development. Int. J. Sci. Res. Arch. 2023, 8, 161–165. [Google Scholar] [CrossRef]
- Matthews, M.A. Green electrochemistry. Examples and challenges. Pure Appl. Chem. 2001, 73, 1305–1308. [Google Scholar] [CrossRef]
- United Nations. Montreal Protocol on Substances that Deplete the Ozone Layer; United Nations Environment Progamme: Montreal, QC, Canada, 1987.
- Jiao, Y.; Qiu, C.; Huang, L.; Wu, K.; Ma, H.; Chen, S.; Ma, L.; Wu, D. Reductive dechlorination of carbon tetrachloride by zero-valent iron and related iron corrosion. Appl. Catal. B Environ. 2009, 91, 434–440. [Google Scholar] [CrossRef]
- Yo-Ping, W.; Yang-Soo, W. Pyrolysis of chloromethanes. Combust. Flame 2000, 122, 312–326. [Google Scholar]
- Zhu, X.; Zhou, L.; Li, Y.; Han, B.; Feng, Q. Rapid Degradation of Carbon Tetrachloride by Microscale Ag/Fe Bimetallic Particles. Int. J. Environ. Res. Public Health 2021, 18, 2124. [Google Scholar] [CrossRef] [PubMed]
- Abdelbassit, M.S.; Popoola, S.A.; Saleh, T.A.; Abdallah, H.H.; Al-Saadi, A.A.; Alhooshani, K.R. DFT and Kinetic Evaluation of Chloromethane Removal Using Cost-Effective Activated Carbon. Arab. J. Sci. Eng. 2020, 45, 4705–4716. [Google Scholar] [CrossRef]
- Meng, F.; Ma, Y.; Wang, Y. Degradation of carbon tetrachloride using ultrasound-assisted nanoscaled zero-valent iron particles@sulfur/nitrogen dual-doped reduced graphene oxide composite: Kinetics, activation energy, effects of reaction conditions and degradation mechanism. Appl. Organometal. Chem. 2019, 33, e5014. [Google Scholar] [CrossRef]
- Liu, Z.; Arnold, R.G.; Betterton, E.A.; Festa, K.D. Electrolytic Reduction of Low Molecular Weight Chlorinated Aliphatic Compounds: Structural and Thermodynamic Effects on Process Kinetics. Environ. Sci. Eng. 2000, 34, 804–811. [Google Scholar] [CrossRef]
- Xu, W.-Y.; Gao, T.-Y. Dechlorination of carbon tetrachloride by the catalyzed Fe-Cu process. J. Environ. Sci. 2007, 19, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Farrell, J. Reductive Dechlorination of Trichloroethene and Carbon Tetrachloride Using Iron and Palladized-Iron Cathodes. Environ. Sci. Technol. 2000, 34, 173–179. [Google Scholar] [CrossRef]
- Wang, J.; Farrell, J. Feasibility study for reductive destruction of carbon tetrachloride using bare and polymer coated nickel electrodes. J. Appl. Electrochem. 2005, 35, 243–248. [Google Scholar] [CrossRef]
- Polcaro, A.; Palmas, S.; Renoldi, F.; Mascia, M. Three-dimensional electrodes for the electrochemical combustion of organic pollutants. Electrochimica Acta 2000, 46, 389–394. [Google Scholar] [CrossRef]
- Yu, D.; Pei, Y.; Ji, Z.; He, X.; Yao, Z. A review on the landfill leachate treatment technologies and application prospects of three-dimensional electrode technology. Chemosphere 2022, 291, 132895. [Google Scholar] [CrossRef] [PubMed]
- Le, T.X.H.; Bechelany, M.; Cretin, M. Carbon felt based-electrodes for energy and environmental applications: A review. Carbon 2017, 122, 564–591. [Google Scholar] [CrossRef]
- Molina, V.; Montiel, V.; Domínguez, M.; Aldaz, A. Electrolytic synthesis of chloroform from carbon tetrachloride in mild conditions. Laboratory approach. Electrochem. Commun. 2003, 5, 246–252. [Google Scholar] [CrossRef]
- Lei, J.; Cai, Q.; Yang, Q.; Wang, Y. Removal of Carbon Tetrachloride by Enhanced Reduction in a Dual-Chamber Electrochemical Reactor. J. Environ. Eng. 2021, 147, 04021024. [Google Scholar] [CrossRef]




| v (cm s−1) | kmAe (s−1) | km (m s−1) | ||
|---|---|---|---|---|
| Piston Flow | Simple Bath | Piston Flow | Simple Bath | |
| 0.90 | 4.1 × 10−2 | 4.1 × 10−2 | 1.8 × 10−6 | 1.8 × 10−6 |
| 1.50 | 5.6 × 10−2 | 5.7 × 10−2 | 2.5 × 10−6 | 2.5 × 10−6 |
| 1.80 | 6.0 × 10−2 | 6.2 × 10−2 | 2.6 × 10−6 | 2.7 × 10−6 |
| 2.10 | 6.6 × 10−2 | 6.8 × 10−2 | 2.9 × 10−6 | 3.0 × 10−6 |
| 2.70 | 7.6 × 10−2 | 7.9 × 10−2 | 3.3 × 10−6 | 3.5 × 10−6 |
| Electrode | Efficiency CT (%) | Efficiency H2 (%) | CT Removed (%) | Initial Potential (V) | Final Potential (V) | Product Yield (%) | SP Chloroform | SP Dichloromethane |
|---|---|---|---|---|---|---|---|---|
| Planar | 73 | 27 | 86.9 | −21.3 | −13.6 | 93 | 94 | 6 |
| Carbon felt | 88 | 7.0 | 100 | −18.9 | −12.3 | 90 | 97 | 3 |
| Electrode | Energy Consumption for CT (kWh kg−1) | Energy Consumption for Chloroform (kWh kg−1) | CT Removal (kg m−2 day−1) | Chloroform Production (kg m−2 day−1) |
|---|---|---|---|---|
| Planar | 0.081 | 0.11 | 20.5 | 14.0 |
| Carbon felt | 0.059 | 0.090 | 25.3 | 16.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina, V.M.; Moreno-Toral, E.; Ramos-Carrillo, A. Efficient Reduction of Carbon Tetrachloride in an Electrochemical Reactor with a Three-Dimensional Electrode. Eng 2024, 5, 983-991. https://doi.org/10.3390/eng5020054
Molina VM, Moreno-Toral E, Ramos-Carrillo A. Efficient Reduction of Carbon Tetrachloride in an Electrochemical Reactor with a Three-Dimensional Electrode. Eng. 2024; 5(2):983-991. https://doi.org/10.3390/eng5020054
Chicago/Turabian StyleMolina, Víctor M., Esteban Moreno-Toral, and Antonio Ramos-Carrillo. 2024. "Efficient Reduction of Carbon Tetrachloride in an Electrochemical Reactor with a Three-Dimensional Electrode" Eng 5, no. 2: 983-991. https://doi.org/10.3390/eng5020054
APA StyleMolina, V. M., Moreno-Toral, E., & Ramos-Carrillo, A. (2024). Efficient Reduction of Carbon Tetrachloride in an Electrochemical Reactor with a Three-Dimensional Electrode. Eng, 5(2), 983-991. https://doi.org/10.3390/eng5020054







