Machine and Deep Learning Trends in EEG-Based Detection and Diagnosis of Alzheimer’s Disease: A Systematic Review
Abstract
1. Introduction
- •
- Artificial intelligence is a booming branch that offers an alternative to understanding diseases. However, it is susceptible to the input data and its processing. This work analyzes these critical points described in the state of the art.
- •
- No work has been carried out in the last ten years with this approach to analysis; before the application of artificial intelligence algorithms, their selection and classification levels focused on Alzheimer’s disease.
- •
- This review covers the analysis of EEG signal databases for use in AI, the demographic data of the patients that comprise them, and the data acquisition paradigms, resulting in a necessary tool for future research.
2. Materials and Methods
Search Strategy
- •
- TITLE-ABS-KEY ((mci OR (mild AND cognitive AND impairment) OR (amnestic AND mild AND cognitive AND impairment) OR Alzheimer) AND (eeg OR electroencephalography) AND (detection OR diagnosis OR classification OR diagnostic) AND ((deep AND learning) OR (machine AND learning)) AND (LIMIT-TO (DOCTYPE, “ar”)) AND (LIMIT-TO (PUBYEAR, 2023) OR LIMIT-TO (PUBYEAR, 2022) OR LIMIT-TO (PUBYEAR, 2021) OR LIMIT-TO (PUBYEAR, 2020) OR LIMIT-TO (PUBYEAR, 2019) OR LIMIT-TO (PUBYEAR, 2018) OR LIMIT-TO (PUBYEAR, 2017))).
- •
- Use of AD or MCI databases.
- •
- Use of EEG data.
- •
- Use of classification methods based on ML or DL algorithms.
- •
- The works presenting objective performance measures were included, which allows an accurate evaluation of ML and DL’s capacity to diagnose MCI and AD.
- •
- Study selection criteria: Only studies that met predefined inclusion criteria, which guaranteed the use of EEG, were included. These criteria included specifying the EEG acquisition methodology, using cohorts diagnosed with Alzheimer’s or MCI, and applying standardized machine learning or deep learning techniques.
- •
- Review of methodologies: The methodologies used in each study for the acquisition and processing of EEG data were reviewed in detail. This included the evaluation of recording parameters, experimental conditions, and preprocessing procedures, ensuring that they met the standards established in the scientific literature.
- •
- Peer review: All studies selected for review underwent a peer review process, ensuring the additional scrutiny of the validity and reliability of the data and methods used.
- •
- Transparency and reproducibility: Studies were considered that provided sufficient detail about their methods and data, allowing the reproducibility of the experiments. Transparency in the presentation of results and analysis methods was also an important criterion for inclusion.
3. Results
3.1. Traditional Alzheimer’s Diagnosis Techniques for Database Formation
3.2. Demographic Data of the Participants from the Databases
3.3. EEG Acquisition
3.3.1. Number of Electrodes in the Acquisition of EEG Signals
3.3.2. Analysis of Activities in Patients for Clinical Data Collection
3.3.3. Sampling Frequencies of EEG Signals
3.4. Filtering Methods for Signal Processing
3.5. Feature Extraction
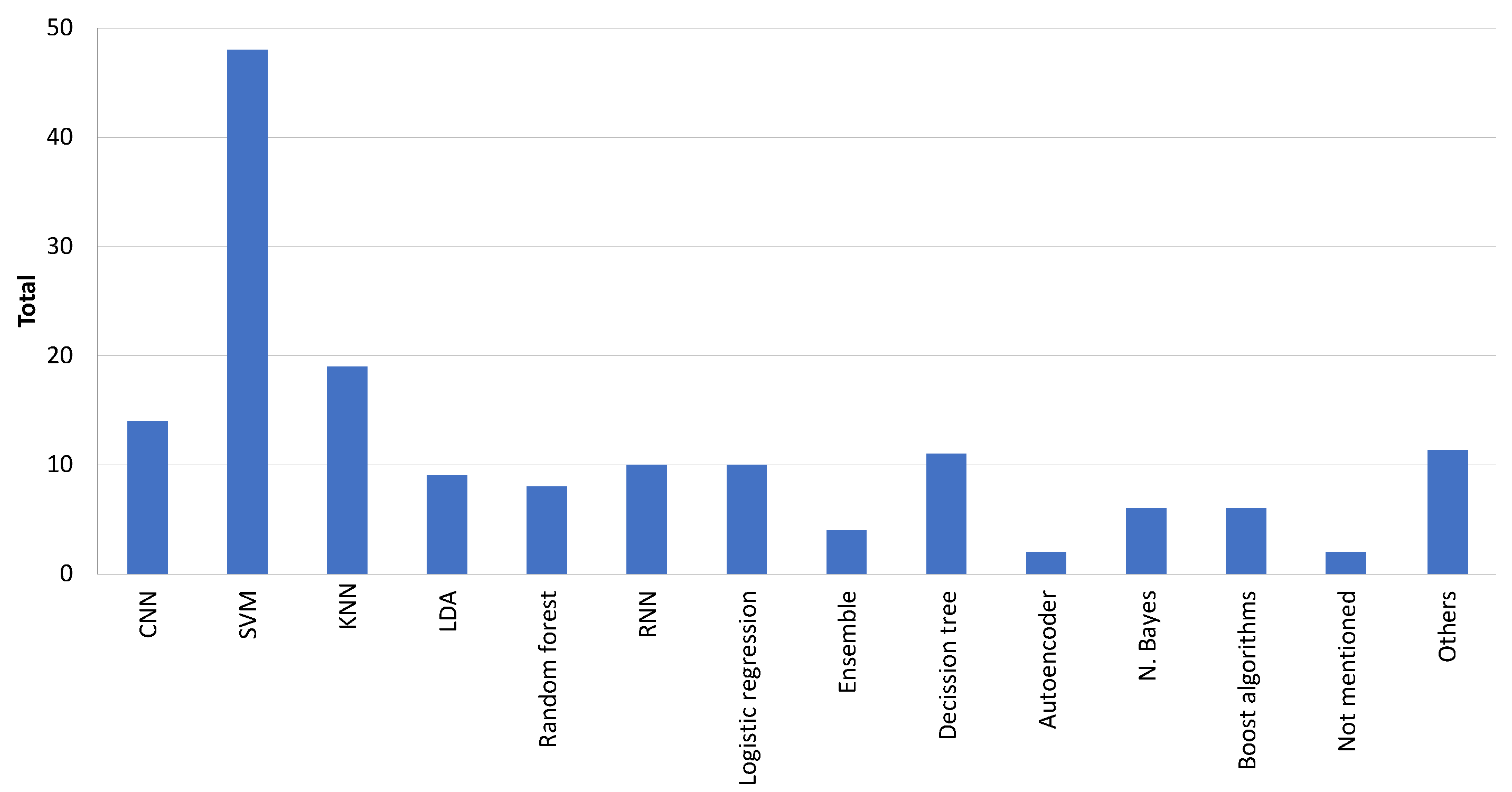
3.6. Classification Techniques Approach for Alzheimer Detection
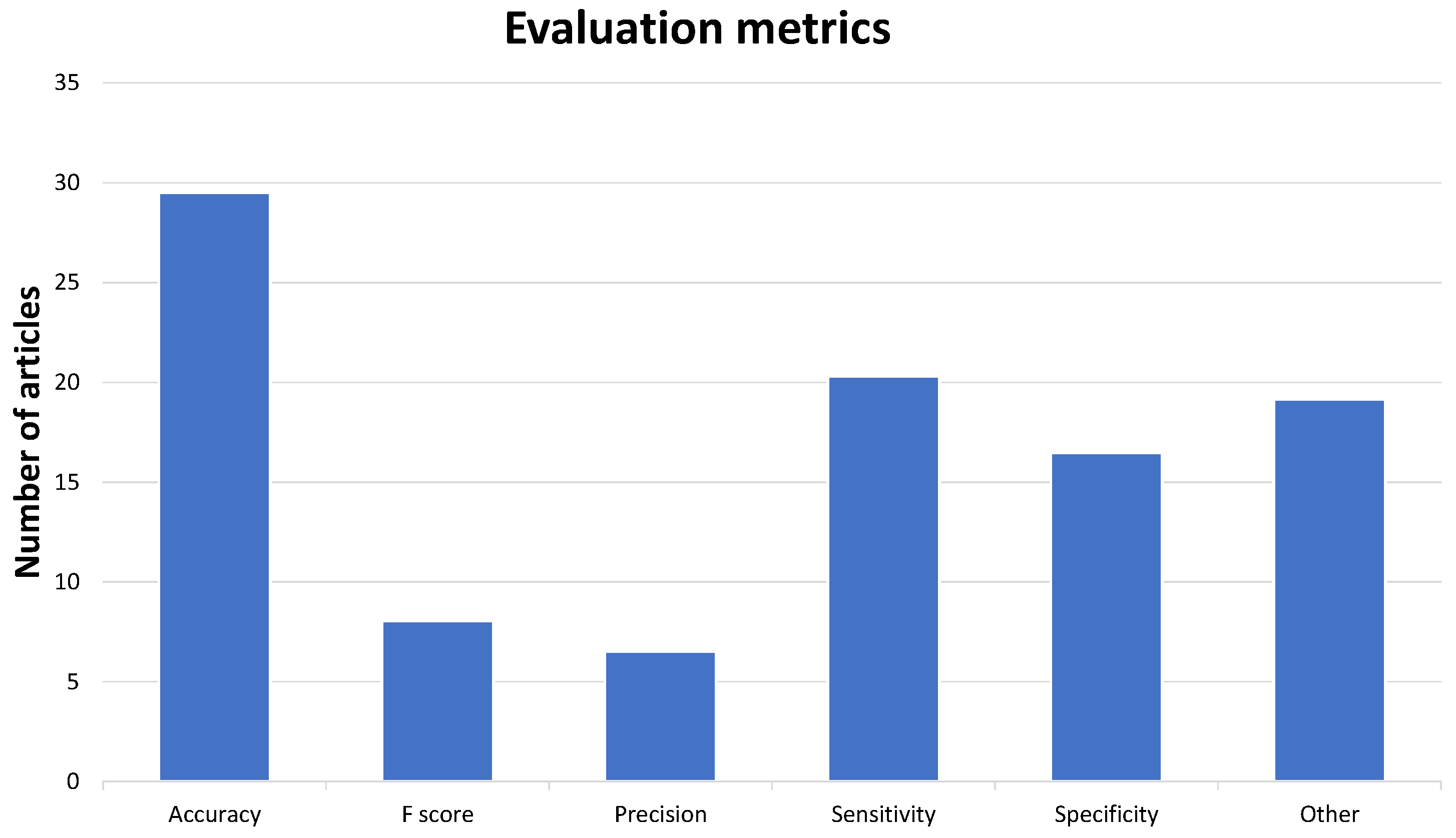
3.7. Evaluation Metrics
3.8. Results in the Classification Achieved
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dauwan, M.; Zande, J.J.; Dellen, E.; Sommer, I.E.; Scheltens, P.; Lemstra, A.W.; Stam, C.J. Random forest to differentiate dementia with Lewy bodies from Alzheimer’s disease. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2016, 4, 99–106. [Google Scholar] [CrossRef]
- Cao, J.; Zhao, Y.; Shan, X.; Blackburn, D.; Wei, J.; Erkoyuncu, J.A.; Chen, L.; Sarrigiannis, P.G. Ultra-high-resolution time-frequency analysis of EEG to characterise brain functional connectivity with the application in Alzheimer’s disease. J. Neural Eng. 2022, 19, 046034. [Google Scholar] [CrossRef]
- Sidulova, M.; Nehme, N.; Park, C.H. Towards Explainable Image Analysis for Alzheimer’s Disease and Mild Cognitive Impairment Diagnosis. In Proceedings of the 2021 IEEE Applied Imagery Pattern Recognition Workshop (AIPR), Washington, DC, USA, 12–14 October 2021. [Google Scholar] [CrossRef]
- Duan, F.; Huang, Z.; Sun, Z.; Zhang, Y.; Zhao, Q.; Cichocki, A.; Yang, Z.; Sole-Casals, J. Topological Network Analysis of Early Alzheimer’s Disease Based on Resting-State EEG. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 2164–2172. [Google Scholar] [CrossRef]
- Kashefpoor, M.; Rabbani, H.; Barekatain, M. Supervised dictionary learning of EEG signals for mild cognitive impairment diagnosis. Biomed. Signal Process. Control. 2019, 53, 101559. [Google Scholar] [CrossRef]
- McBride, J.C.; Zhao, X.; Munro, N.B.; Jicha, G.A.; Schmitt, F.A.; Kryscio, R.J.; Smith, C.D.; Jiang, Y. Sugihara causality analysis of scalp EEG for detection of early Alzheimer’s disease. NeuroImage Clin. 2015, 7, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Araújo, T.; Teixeira, J.P.; Rodrigues, P.M. Smart-Data-Driven System for Alzheimer Disease Detection through Electroencephalographic Signals. Bioengineering 2022, 9, 141. [Google Scholar] [CrossRef] [PubMed]
- Morabito, F.C.; Campolo, M.; Ieracitano, C.; Ebadi, J.M.; Bonanno, L.; Bramanti, A.; Desalvo, S.; Mammone, N.; Bramanti, P. Deep convolutional neural networks for classification of mild cognitive impaired and Alzheimer’s disease patients from scalp EEG recordings. In Proceedings of the 2016 IEEE 2nd International Forum on Research and Technologies for Society and Industry Leveraging a better tomorrow (RTSI), Bologna, Italy, 7–9 September 2016. [Google Scholar]
- Drage, R.; Escudero, J.; Parra, M.A.; Scally, B.; Anghinah, R.; Araujo, A.V.L.D.; Basile, L.F.; Abasolo, D. A novel deep learning approach using AlexNet for the classification of electroencephalograms in Alzheimer’s Disease and Mild Cognitive Impairment. In Proceedings of the 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Glasgow, UK, 11–15 July 2022. [Google Scholar]
- Nematzadeh, S.; Kiani, F.; Torkamanian-Afshar, M.; Aydin, N. Tuning hyperparameters of machine learning algorithms and deep neural networks using metaheuristics: A bioinformatics study on biomedical and biological cases. Comput. Biol. Chem. 2022, 97, 107619. [Google Scholar] [CrossRef] [PubMed]
- Grueso, S.; Viejo-Sobera, R. Machine learning methods for predicting progression from mild cognitive impairment to Alzheimer’s disease dementia: A systematic review. Alzheimer’s Res. Ther. 2021, 13, 162. [Google Scholar] [CrossRef] [PubMed]
- Farina, F.; Emek-Savaş, D.; Rueda-Delgado, L.; Boyle, R.; Kiiski, H.; Yener, G.; Whelan, R. A comparison of resting state EEG and structural MRI for classifying Alzheimer’s disease and mild cognitive impairment. NeuroImage 2020, 215, 116795. [Google Scholar] [CrossRef]
- Alvi, A.M.; Siuly, S.; Wang, H.; Wang, K.; Whittaker, F. A deep learning based framework for diagnosis of mild cognitive impairment. Knowl.-Based Syst. 2022, 248, 108815. [Google Scholar] [CrossRef]
- Alvi, A.M.; Siuly, S.; Wang, H. A Long Short-Term Memory Based Framework for Early Detection of Mild Cognitive Impairment From EEG Signals. IEEE Trans. Emerg. Top. Comput. Intell. 2023, 7, 375–388. [Google Scholar] [CrossRef]
- Miltiadous, A.; Tzimourta, K.D.; Giannakeas, N.; Tsipouras, M.G.; Afrantou, T.; Ioannidis, P.; Tzallas, A.T. Alzheimer’s Disease and Frontotemporal Dementia: A Robust Classification Method of EEG Signals and a Comparison of Validation Methods. Diagnostics 2021, 11, 1437. [Google Scholar] [CrossRef]
- Yin, J.; Cao, J.; Siuly, S.; Wang, H. An Integrated MCI Detection Framework Based on Spectral-temporal Analysis. Int. J. Autom. Comput. 2019, 16, 786–799. [Google Scholar] [CrossRef]
- Seifallahi, M.; Mehraban, A.H.; Galvin, J.E.; Ghoraani, B. Alzheimer’s Disease Detection Using Comprehensive Analysis of Timed Up and Go Test via Kinect V.2 Camera and Machine Learning. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 1589–1600. [Google Scholar] [CrossRef]
- Li, X.; Yang, C.; Xie, P.; Han, Y.; Su, R.; Li, Z.; Liu, Y. The diagnosis of amnestic mild cognitive impairment by combining the characteristics of brain functional network and support vector machine classifier. J. Neurosci. Methods 2021, 363, 109334. [Google Scholar] [CrossRef]
- Geng, D.; Wang, C.; Fu, Z.; Zhang, Y.; Yang, K.; An, H. Sleep EEG-Based Approach to Detect Mild Cognitive Impairment. Front. Aging Neurosci. 2022, 14, 865558. [Google Scholar] [CrossRef]
- San-Martin, R.; Johns, E.; Quispe Mamani, G.; Tavares, G.; Phillips, N.A.; Fraga, F.J. A method for diagnosis support of mild cognitive impairment through EEG rhythms source location during working memory tasks. Biomed. Signal Process. Control. 2021, 66, 102499. [Google Scholar] [CrossRef]
- Oltu, B.; Akşahin, M.F.; Kibaroğlu, S. A novel electroencephalography based approach for Alzheimer’s disease and mild cognitive impairment detection. Biomed. Signal Process. Control. 2021, 63, 102223. [Google Scholar] [CrossRef]
- Rutkowski, T.M.; Abe, M.S.; Komendzinski, T.; Otake-Matsuura, M. Older adult mild cognitive impairment prediction from multiscale entropy EEG patterns in reminiscent interior image working memory paradigm. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Virtual, 1–5 November 2021. [Google Scholar]
- Moguilner, S.; Birba, A.; Fittipaldi, S.; Gonzalez-Campo, C.; Tagliazucchi, E.; Reyes, P.; Matallana, D.; Parra, M.A.; Slachevsky, A.; Farías, G.; et al. Multi-feature computational framework for combined signatures of dementia in underrepresented settings. J. Neural Eng. 2022, 19, 046048. [Google Scholar] [CrossRef]
- Fan, C.C.; Xie, H.; Peng, L.; Yang, H.; Ni, Z.L.; Wang, G.; Zhou, Y.J.; Chen, S.; Fang, Z.; Huang, S.; et al. Group Feature Learning and Domain Adversarial Neural Network for aMCI Diagnosis System Based on EEG. In Proceedings of the 2021 IEEE International Conference on Robotics and Automation (ICRA), Xi’an, China, 30 May–5 June 2021; pp. 9340–9346. [Google Scholar] [CrossRef]
- Siuly, S.; Alcin, O.F.; Kabir, E.; Sengur, A.; Wang, H.; Zhang, Y.; Whittaker, F. A new framework for automatic detection of patients with mild cognitive impairment using resting-state EEG signals. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 1966–1976. [Google Scholar] [CrossRef]
- Durongbhan, P.; Zhao, Y.; Chen, L.; Zis, P.; De Marco, M.; Unwin, Z.C.; Venneri, A.; He, X.; Li, S.; Zhao, Y.; et al. A Dementia Classification Framework Using Frequency and Time-Frequency Features Based on EEG Signals. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, Y.; Durongbhan, P.; Chen, L.; Liu, J.; Billings, S.A.; Zis, P.; Unwin, Z.C.; De Marco, M.; Venneri, A.; et al. Imaging of Nonlinear and Dynamic Functional Brain Connectivity Based on EEG Recordings With the Application on the Diagnosis of Alzheimer’s Disease. IEEE Trans. Med. Imaging 2020, 39, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Trambaiolli, L.R.; Spolaôr, N.; Lorena, A.C.; Anghinah, R.; Sato, J.R. Feature selection before EEG classification supports the diagnosis of Alzheimer’s disease. Clin. Neurophysiol. 2017, 128, 2058–2067. [Google Scholar] [CrossRef] [PubMed]
- Nobukawa, S.; Yamanishi, T.; Kasakawa, S.; Nishimura, H.; Kikuchi, M.; Takahashi, T. Classification Methods Based on Complexity and Synchronization of Electroencephalography Signals in Alzheimer’s Disease. Front. Psychiatry 2020, 11, 255. [Google Scholar] [CrossRef] [PubMed]
- Fruehwirt, W.; Zhang, P.; Gerstgrasser, M.; Grossegger, D.; Schmidt, R.; Benke, T.; Dal-Bianco, P.; Ransmayr, G.; Weydemann, L.; Garn, H.; et al. Bayesian Gaussian Process Classification from Event-Related Brain Potentials in Alzheimer’s Disease. In Proceedings of the Artificial Intelligence in Medicine; ten Teije, A., Popow, C., Holmes, J.H., Sacchi, L., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 65–75. [Google Scholar] [CrossRef]
- Kanda, P.A.M.; Trambaiolli, L.R.; Lorena, A.C.; Fraga, F.J.; Basile, L.F.I.; Nitrini, R.; Anghinah, R. Clinician’s road map to wavelet EEG as an Alzheimer’s disease biomarker. Clin. EEG Neurosci. 2014, 45, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Wang, H. Early Alzheimer’s disease diagnosis based on EEG spectral images using deep learning. Neural Netw. 2019, 114, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Youssef, N.; Xiao, S.; Liu, M.; Lian, H.; Li, R.; Chen, X.; Zhang, W.; Zheng, X.; Li, Y.; Li, Y. Functional brain networks in mild cognitive impairment based on resting electroencephalography signals. Front. Comput. Neurosci. 2021, 15, 698386. [Google Scholar] [CrossRef]
- AlSharabi, K.; Bin Salamah, Y.; Abdurraqeeb, A.M.; Aljalal, M.; Alturki, F.A. EEG Signal Processing for Alzheimer’s Disorders Using Discrete Wavelet Transform and Machine Learning Approaches. IEEE Access 2022, 10, 89781–89797. [Google Scholar] [CrossRef]
- Zeng, H.; Fang, X.; Zhao, Y.; Wu, J.; Li, M.; Zheng, H.; Xu, F.; Pan, D.; Dai, G. EMCI: A novel EEG-based mental workload assessment index of mild cognitive impairment. IEEE Trans. Biomed. Circuits Syst. 2022, 16, 902–914. [Google Scholar] [CrossRef]
- Trinh, T.T.; Tsai, C.F.; Hsiao, Y.T.; Lee, C.Y.; Wu, C.T.; Liu, Y.H. Identifying Individuals With Mild Cognitive Impairment Using Working Memory-Induced Intra-Subject Variability of Resting-State EEGs. Front. Comput. Neurosci. 2021, 15, 700467. [Google Scholar] [CrossRef]
- Hsiao, Y.T.; Tsai, C.F.; Wu, C.T.; Trinh, T.T.; Lee, C.Y.; Liu, Y.H. MCI Detection Using Kernel Eigen-Relative-Power Features of EEG Signals. Actuators 2021, 10, 152. [Google Scholar] [CrossRef]
- Ieracitano, C.; Mammone, N.; Bramanti, A.; Hussain, A.; Morabito, F.C. A Convolutional Neural Network approach for classification of dementia stages based on 2D-spectral representation of EEG recordings. Neurocomputing 2019, 323, 96–107. [Google Scholar] [CrossRef]
- Ieracitano, C.; Mammone, N.; Hussain, A.; Morabito, F.C. A Convolutional Neural Network based self-learning approach for classifying neurodegenerative states from EEG signals in dementia. In Proceedings of the 2020 International Joint Conference on Neural Networks (IJCNN), Glasgow, UK, 19–24 July 2020. [Google Scholar]
- Capecci, E.; Morabito, F.C.; Campolo, M.; Mammone, N.; Labate, D.; Kasabov, N. A feasibility study of using the NeuCube spiking neural network architecture for modelling Alzheimer’s disease EEG data. In Advances in Neural Networks: Computational and Theoretical Issues; Smart Innovation, Systems and Technologies; Springer International Publishing: Cham, Switzerland, 2015; pp. 159–172. [Google Scholar]
- Ieracitano, C.; Mammone, N.; Bramanti, A.; Marino, S.; Hussain, A.; Morabito, F.C. A time-frequency based machine learning system for brain states classification via EEG signal processing. In Proceedings of the 2019 International Joint Conference on Neural Networks (IJCNN), Budapest, Hungary, 14–19 July 2019. [Google Scholar]
- You, Z.; Zeng, R.; Lan, X.; Ren, H.; You, Z.; Shi, X.; Zhao, S.; Guo, Y.; Jiang, X.; Hu, X. Alzheimer’s Disease Classification With a Cascade Neural Network. Front. Public Health 2020, 8, 584387. [Google Scholar] [CrossRef]
- Biagetti, G.; Crippa, P.; Falaschetti, L.; Luzzi, S.; Turchetti, C. Classification of Alzheimer’s Disease from EEG Signal Using Robust-PCA Feature Extraction. Procedia Comput. Sci. 2021, 192, 3114–3122. [Google Scholar] [CrossRef]
- Safi, M.S.; Safi, S.M.M. Early detection of Alzheimer’s disease from EEG signals using Hjorth parameters. Biomed. Signal Process. Control. 2021, 65, 102338. [Google Scholar] [CrossRef]
- Wei, J.; Xiao, W.; Zhang, S.; Wang, P. Mild cognitive impairment classification convolutional neural network with attention mechanism. In Proceedings of the 2020 IEEE 16th International Conference on Control & Automation (ICCA), Singapore, 9–11 October 2020. [Google Scholar]
- Bairagi, V. EEG signal analysis for early diagnosis of Alzheimer disease using spectral and wavelet based features. Int. J. Inf. Technol. 2018, 10, 403–412. [Google Scholar] [CrossRef]
- Chouvarda, I.; Mpaltadoros, L.; Boutziona, I.; Tsakonas, G.; Tsolaki, M.; Diamantaras, K. Exploring Classification in Open and Closed Eyes EEG Data for People with Cognitive Disorders. In Proceedings of the 15th International Joint Conference on Biomedical Engineering Systems and Technologies; SCITEPRESS—Science and Technology Publications: Setubal, Portugal, 2022. [Google Scholar] [CrossRef]
- Li, X.; Zhou, H.; Su, R.; Kang, J.; Sun, Y.; Yuan, Y.; Han, Y.; Chen, X.; Xie, P.; Wang, Y.; et al. A mild cognitive impairment diagnostic model based on IAAFT and BiLSTM. Biomed. Signal Process. Control. 2023, 80, 104349. [Google Scholar] [CrossRef]
- Song, Z.; Deng, B.; Wang, J.; Wang, R. Biomarkers for Alzheimer’s Disease Defined by a Novel Brain Functional Network Measure. IEEE Trans. Biomed. Eng. 2019, 66, 41–49. [Google Scholar] [CrossRef]
- Sibilano, E.; Brunetti, A.; Buongiorno, D.; Lassi, M.; Grippo, A.; Bessi, V.; Micera, S.; Mazzoni, A.; Bevilacqua, V. An attention-based deep learning approach for the classification of subjective cognitive decline and mild cognitive impairment using resting-state EEG. J. Neural Eng. 2023, 20, 016048. [Google Scholar] [CrossRef]
- Fan, M.; Yang, A.C.; Fuh, J.L.; Chou, C.A. Topological Pattern Recognition of Severe Alzheimer’s Disease via Regularized Supervised Learning of EEG Complexity. Front. Neurosci. 2018, 12, 685. [Google Scholar] [CrossRef]
- Ruiz-Gómez, S.; Gómez, C.; Poza, J.; Gutiérrez-Tobal, G.; Tola-Arribas, M.; Cano, M.; Hornero, R. Automated Multiclass Classification of Spontaneous EEG Activity in Alzheimer’s Disease and Mild Cognitive Impairment. Entropy 2018, 20, 35. [Google Scholar] [CrossRef]
- Jeong, T.; Park, U.; Kang, S.W. Novel quantitative electroencephalogram feature image adapted for deep learning: Verification through classification of Alzheimer’s disease dementia. Front. Neurosci. 2022, 16, 1033379. [Google Scholar] [CrossRef]
- Waser, M.; Benke, T.; Dal-Bianco, P.; Garn, H.; Mosbacher, J.A.; Ransmayr, G.; Schmidt, R.; Seiler, S.; Sorensen, H.B.D.; Jennum, P.J. Neuroimaging markers of global cognition in early Alzheimer’s disease: A magnetic resonance imaging–electroencephalography study. Brain and Behavior 2018, 9, e01197. [Google Scholar] [CrossRef]
- Rodrigues, P.M.; Bispo, B.C.; Garrett, C.; Alves, D.; Teixeira, J.P.; Freitas, D. Lacsogram: A new EEG tool to diagnose Alzheimer’s disease. IEEE J. Biomed. Health Inform. 2021, 25, 3384–3395. [Google Scholar] [CrossRef]
- Pirrone, D.; Weitschek, E.; Di Paolo, P.; De Salvo, S.; De Cola, M.C. EEG Signal Processing and Supervised Machine Learning to Early Diagnose Alzheimer’s Disease. Appl. Sci. 2022, 12, 5413. [Google Scholar] [CrossRef]
- Movahed, R.A.; Hamedani, N.E.; Sadredini, S.Z.; Rezaeian, M.-R. An Automated EEG-based mild cognitive impairment diagnosis framework using spectral and functional connectivity features. In Proceedings of the 2021 28th National and 6th International Iranian Conference on Biomedical Engineering (ICBME), Tehran, Iran, 25–26 November 2021; pp. 271–275. [Google Scholar] [CrossRef]
- Fiscon, G.; Weitschek, E.; De Cola, M.C.; Felici, G.; Bertolazzi, P. An integrated approach based on EEG signals processing combined with supervised methods to classify Alzheimer’s disease patients. In Proceedings of the 2018 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Madrid, Spain, 3–6 December 2018. [Google Scholar] [CrossRef]
- Movahed, R.A.; Rezaeian, M. Automatic diagnosis of mild cognitive impairment based on spectral, functional connectivity, and nonlinear EEG-based features. Comput. Math. Methods Med. 2022, 2022, 2014001. [Google Scholar] [CrossRef]
- Yu, H.; Lei, X.; Song, Z.; Liu, C.; Wang, J. Supervised Network-Based Fuzzy Learning of EEG Signals for Alzheimer’s Disease Identification. IEEE Trans. Fuzzy Syst. 2020, 28, 60–71. [Google Scholar] [CrossRef]
- Alessandrini, M.; Biagetti, G.; Crippa, P.; Falaschetti, L.; Luzzi, S.; Turchetti, C. EEG-Based Alzheimer’s Disease Recognition Using Robust-PCA and LSTM Recurrent Neural Network. Sensors 2022, 22, 3696. [Google Scholar] [CrossRef]
- Cai, L.; Wei, X.; Liu, J.; Zhu, L.; Wang, J.; Deng, B.; Yu, H.; Wang, R. Functional Integration and Segregation in Multiplex Brain Networks for Alzheimer’s Disease. Front. Neurosci. 2020, 14, 51. [Google Scholar] [CrossRef]
- Williams, P.; White, A.; Merino, R.B.; Hardin, S.; Mizelle, J.C.; Kim, S. Facial Recognition Task for the Classification of Mild Cognitive Impairment with Ensemble Sparse Classifier. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 2242–2245. [Google Scholar] [CrossRef]
- Sridhar, S.; Manian, V. EEG and Deep Learning Based Brain Cognitive Function Classification. Computers 2020, 9, 104. [Google Scholar] [CrossRef]
- Su, R.; Li, X.; Li, Z.; Han, Y.; Cui, W.; Xie, P.; Liu, Y. Constructing biomarker for early diagnosis of aMCI based on combination of multiscale fuzzy entropy and functional brain connectivity. Biomed. Signal Process. Control. 2021, 70, 103000. [Google Scholar] [CrossRef]
- Chedid, N.; Tabbal, J.; Kabbara, A.; Allouch, S.; Hassan, M. The development of an automated machine learning pipeline for the detection of Alzheimer’s Disease. Sci. Rep. 2022, 12, 18137. [Google Scholar] [CrossRef]
- Lee, K.; Choi, K.M.; Park, S.; Lee, S.H.; Im, C.H. Selection of the optimal channel configuration for implementing wearable EEG devices for the diagnosis of mild cognitive impairment. Alzheimer’s Res. Ther. 2022, 14, 170. [Google Scholar] [CrossRef]
- Timothy, L.T.; Krishna, B.M.; Nair, U. Recurrence quantification analysis of mci eeg under resting and visual memory task conditions. Biomed. Eng. Appl. Basis Commun. 2019, 31, 1950025. [Google Scholar] [CrossRef]
- Park, J.; Jang, S.; Gwak, J.; Kim, B.C.; Lee, J.J.; Choi, K.Y.; Lee, K.H.; Jun, S.C.; Jang, G.J.; Ahn, S. Individualized diagnosis of preclinical Alzheimer’s Disease using deep neural networks. Expert Syst. Appl. 2022, 210, 118511. [Google Scholar] [CrossRef]
- Vecchio, F.; Miraglia, F.; Alù, F.; Menna, M.; Judica, E.; Cotelli, M.; Rossini, P.M. Classification of Alzheimer’s Disease with Respect to Physiological Aging with Innovative EEG Biomarkers in a Machine Learning Implementation. J. Alzheimer’s Dis. 2020, 75, 1253–1261. [Google Scholar] [CrossRef]
- Kim, D.; Kim, K. Detection of early stage Alzheimer’s disease using EEG relative power with deep neural network. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 352–355. [Google Scholar]
- Cejnek, M.; Vysata, O.; Valis, M.; Bukovsky, I. Novelty detection-based approach for Alzheimer’s disease and mild cognitive impairment diagnosis from EEG. Med. Biol. Eng. Comput. 2021, 59, 2287–2296. [Google Scholar] [CrossRef]
- Sharma, N.; Kolekar, M.H.; Jha, K. Iterative Filtering Decomposition Based Early Dementia Diagnosis Using EEG With Cognitive Tests. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 1890–1898. [Google Scholar] [CrossRef]
- Han, S.H.; Pyun, J.M.; Yeo, S.; Kang, D.W.; Jeong, H.T.; Kang, S.W.; Kim, S.; Youn, Y.C. Differences between memory encoding and retrieval failure in mild cognitive impairment: Results from quantitative electroencephalography and magnetic resonance volumetry. Alzheimer’s Res. Ther. 2021, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Kolekar, M.; Jha, K.; Kumar, Y. EEG and Cognitive Biomarkers Based Mild Cognitive Impairment Diagnosis. IRBM 2019, 40, 113–121. [Google Scholar] [CrossRef]
- Houmani, N.; Dreyfus, G.; Vialatte, F.B. Epoch-based Entropy for Early Screening of Alzheimer’s Disease. Int. J. Neural Syst. 2015, 25, 1550032. [Google Scholar] [CrossRef]
- Shan, X.; Cao, J.; Huo, S.; Chen, L.; Sarrigiannis, P.G.; Zhao, Y. Spatial-temporal graph convolutional network for Alzheimer classification based on brain functional connectivity imaging of electroencephalogram. Hum. Brain Mapp. 2022, 43, 5194–5209. [Google Scholar] [CrossRef]
- Tavares, G.; San-Martin, R.; Ianof, J.N.; Anghinah, R.; Fraga, F.J. Improvement in the automatic classification of Alzheimer’s disease using EEG after feature selection. In Proceedings of the 2019 IEEE International Conference on Systems, Man and Cybernetics (SMC), Bari, Italy, 6–9 October 2019. [Google Scholar] [CrossRef]
- Li, F.; Matsumori, S.; Egawa, N.; Yoshimoto, S.; Yamashiro, K.; Mizutani, H.; Uchida, N.; Kokuryu, A.; Kuzuya, A.; Kojima, R.; et al. Predictive diagnostic approach to dementia and dementia subtypes using wireless and mobile electroencephalography: A pilot study. Bioelectricity 2022, 4, 3–11. [Google Scholar] [CrossRef]
- Grässler, B.; Herold, F.; Dordevic, M.; Gujar, T.A.; Darius, S.; Böckelmann, I.; Müller, N.G.; Hökelmann, A. Multimodal measurement approach to identify individuals with mild cognitive impairment: Study protocol for a cross-sectional trial. BMJ Open 2021, 11, e046879. [Google Scholar] [CrossRef]
- Chai, J.; Wu, R.; Li, A.; Xue, C.; Qiang, Y.; Zhao, J.; Zhao, Q.; Yang, Q. Classification of mild cognitive impairment based on handwriting dynamics and qEEG. Comput. Biol. Med. 2023, 152, 106418. [Google Scholar] [CrossRef]
- Simpraga, S.; Alvarez-Jimenez, R.; Mansvelder, H.D.; van Gerven, J.M.A.; Groeneveld, G.J.; Poil, S.S.; Linkenkaer-Hansen, K. EEG machine learning for accurate detection of cholinergic intervention and Alzheimer’s disease. Sci. Rep. 2017, 7, 5775. [Google Scholar] [CrossRef]
- Ding, Y.; Chu, Y.; Liu, M.; Ling, Z.; Wang, S.; Li, X.; Li, Y. Fully automated discrimination of Alzheimer’s disease using resting-state electroencephalography signals. Quant. Imaging Med. Surg. 2022, 12, 1063–1078. [Google Scholar] [CrossRef]
- Schumacher, J.; Thomas, A.J.; Peraza, L.R.; Firbank, M.; Cromarty, R.; Hamilton, C.A.; Donaghy, P.C.; O’Brien, J.T.; Taylor, J.P. EEG alpha reactivity and cholinergic system integrity in Lewy body dementia and Alzheimer’s disease. Alzheimer’s Res. Ther. 2020, 12, 46. [Google Scholar] [CrossRef]
- Zorick, T.; Landers, J.; Leuchter, A.; Mandelkern, M.A. EEG multifractal analysis correlates with cognitive testing scores and clinical staging in mild cognitive impairment. J. Clin. Neurosci. 2020, 76, 195–200. [Google Scholar] [CrossRef]
- Houmani, N.; Vialatte, F.; Gallego-Jutglà, E.; Dreyfus, G.; Nguyen-Michel, V.H.; Mariani, J.; Kinugawa, K. Diagnosis of Alzheimer’s disease with Electroencephalography in a differential framework. PLoS ONE 2018, 13, e0193607. [Google Scholar] [CrossRef] [PubMed]
- Fiscon, G.; Weitschek, E.; Felici, G.; Bertolazzi, P.; De Salvo, S.; Bramanti, P.; De Cola, M.C. Alzheimer’s disease patients classification through EEG signals processing. In Proceedings of the 2014 IEEE Symposium on Computational Intelligence and Data Mining (CIDM), Orlando, FL, USA, 9–12 December 2014; pp. 105–112. [Google Scholar]
- Perez-Valero, E.; Morillas, C.; Lopez-Gordo, M.A.; Carrera-Muñoz, I.; López-Alcalde, S.; Vílchez-Carrillo, R.M. An Automated Approach for the Detection of Alzheimer’s Disease From Resting State Electroencephalography. Front. Neuroinformatics 2022, 16, 924547. [Google Scholar] [CrossRef] [PubMed]
- Jesus, B., Jr.; Cassani, R.; McGeown, W.J.; Cecchi, M.; Fadem, K.C.; Falk, T.H. Multimodal prediction of Alzheimer’s disease severity level based on resting-state EEG and structural MRI. Front. Hum. Neurosci. 2021, 15, 700627. [Google Scholar] [CrossRef] [PubMed]
- Fouladi, S.; Safaei, A.A.; Mammone, N.; Ghaderi, F.; Ebadi, M.J. Efficient deep neural networks for classification of Alzheimer’s disease and mild cognitive impairment from scalp EEG recordings. Cognit. Comput. 2022, 14, 1247–1268. [Google Scholar] [CrossRef]
- Crook-Rumsey, M.; Howard, C.J.; Doborjeh, Z.; Doborjeh, M.; Ramos, J.I.E.; Kasabov, N.; Sumich, A. Spatiotemporal EEG Dynamics of Prospective Memory in Ageing and Mild Cognitive Impairment. Cogn. Comput. 2022, 15, 1273–1299. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, H.; Zhu, L.; Ren, H.; Dang, G.; Su, X.; Lan, X.; Jiang, X.; Zhang, X.; Feng, J.; et al. Classification of Cognitive Impairment and healthy controls based on Transcranial Magnetic Stimulation Evoked Potentials. Front. Aging Neurosci. 2021, 13, 804384. [Google Scholar] [CrossRef] [PubMed]
- Amini, M.; Pedram, M.M.; Moradi, A.; Ouchani, M. Diagnosis of Alzheimer’s Disease by Time-Dependent Power Spectrum Descriptors and Convolutional Neural Network Using EEG Signal. Comput. Math. Methods Med. 2021, 2021, 5511922. [Google Scholar] [CrossRef] [PubMed]
- Cecere, C.; Corrado, C.; Polikar, R. Diagnostic utility of EEG based biomarkers for Alzheimer’s disease. In Proceedings of the 2014 40th Annual Northeast Bioengineering Conference (NEBEC), Boston, MA, USA, 25–27 April 2014; pp. 1–2. [Google Scholar]
- Cicalese, P.A.; Li, R.; Ahmadi, M.B.; Wang, C.; Francis, J.T.; Selvaraj, S.; Schulz, P.E.; Zhang, Y. An EEG-fNIRS hybridization technique in the four-class classification of alzheimer’s disease. J. Neurosci. Methods 2020, 336, 108618. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhang, J.; Li, C.; Yu, Z.; Yan, Z.; Jiang, J. Development of a Machine Learning Model to Discriminate Mild Cognitive Impairment Subjects from Normal Controls in Community Screening. Brain Sci. 2022, 12, 1149. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Yan, Z.; Sheng, C.; Wang, M.; Guan, Q.; Yu, Z.; Han, Y.; Jiang, J. A Novel Detection Tool for Mild Cognitive Impairment Patients Based on Eye Movement and Electroencephalogram. J. Alzheimer’s Dis. 2019, 72, 389–399. [Google Scholar] [CrossRef]
- Jervis, B.W.; Bigan, C.; Jervis, M.; Besleaga, M. New-onset Alzheimer’s disease and normal subjects 100% differentiated by P300. Am. J. Alzheimer’s Dis. Other Dement. 2019, 34, 308–313. [Google Scholar] [CrossRef]
- Laskaris, N.; Tarnanas, I.; Tsolaki, M.; Vlaikidis, N.; Karlovasitou, A. Improved detection of amnestic MCI by means of discriminative vector quantization of single-trial cognitive ERP responses. J. Neurosci. Methods 2013, 212, 344–354. [Google Scholar] [CrossRef]
- Klepl, D.; He, F.; Wu, M.; Blackburn, D.J.; Sarrigiannis, P. EEG-based graph neural network classification of Alzheimer’s disease: An empirical evaluation of functional connectivity methods. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 2651–2660. [Google Scholar] [CrossRef]
- Amezquita-Sanchez, J.P.; Mammone, N.; Morabito, F.C.; Marino, S.; Adeli, H. A novel methodology for automated differential diagnosis of mild cognitive impairment and the Alzheimer’s disease using EEG signals. J. Neurosci. Methods 2019, 322, 88–95. [Google Scholar] [CrossRef]
- Perez-Valero, E.; Lopez-Gordo, M.Á.; Gutiérrez, C.M.; Carrera-Muñoz, I.; Vílchez-Carrillo, R.M. A self-driven approach for multi-class discrimination in Alzheimer’s disease based on wearable EEG. Comput. Methods Programs Biomed. 2022, 220, 106841. [Google Scholar] [CrossRef]
- Mitsukura, Y.; Sumali, B.; Watanabe, H.; Ikaga, T.; Nishimura, T. Frontotemporal EEG as potential biomarker for early MCI: A case-control study. BMC Psychiatry 2022, 22, 289. [Google Scholar] [CrossRef]
- Dimitriadis, S.I.; Laskaris, N.A.; Bitzidou, M.P.; Tarnanas, I.; Tsolaki, M.N. A novel biomarker of amnestic MCI based on dynamic cross-frequency coupling patterns during cognitive brain responses. Front. Neurosci. 2015, 9, 350. [Google Scholar] [CrossRef]
- Kulkarni, N. EEG signal analysis for mild Alzheimer’s disease diagnosis by means of spectral- and complexity-based features and machine learning techniques. In Proceedings of the 2nd International Conference on Data Engineering and Communication Technology; Advances in Intelligent Systems and Computing. Springer: Singapore, 2019; pp. 395–403. [Google Scholar]
- Cassani, R.; Falk, T. Alzheimer’s Disease Diagnosis and Severity Level Detection Based on Electroencephalography Modulation Spectral “Patch” Features. IEEE J. Biomed. Health Informatics 2019, 24, 1982–1993. [Google Scholar] [CrossRef]
- Komolovaitė, D.; Maskeliūnas, R.; Damaševičius, R. Deep convolutional neural network-based visual stimuli classification using electroencephalography signals of healthy and Alzheimer’s disease subjects. Life 2022, 12, 374. [Google Scholar] [CrossRef]
- Jovic, A. 1. Feature selection in biomedical signal classification process and current software implementations. In Intelligent Decision Support Systems; Borra, S., Dey, N., Bhattacharyya, S., Bouhlel, M.S., Eds.; De Gruyter: Berlin Germany; Boston, MA, USA, 2019; pp. 1–30. [Google Scholar]
- Ieracitano, C.; Mammone, N.; Hussain, A.; Morabito, F.C. A novel multi-modal machine learning based approach for automatic classification of EEG recordings in dementia. Neural Netw. 2020, 123, 176–190. [Google Scholar] [CrossRef]
- Adeli, H.; Ghosh-Dastidar, S.; Dadmehr, N. Alzheimer’s disease and models of computation: Imaging, classification, and neural models. J. Alzheimer’s Dis. 2005, 7, 187–199. [Google Scholar] [CrossRef]
- Parmar, S.K.; Ramwala, O.A.; Paunwala, C.N. Performance evaluation of svm with non-linear kernels for eeg-based dyslexia detection. In Proceedings of the 2021 IEEE 9th Region 10 Humanitarian Technology Conference (R10-HTC), Bangalore, India, 30 September–2 October 2021; pp. 1–6. [Google Scholar]
- Roy, A.M. Adaptive transfer learning-based multiscale feature fused deep convolutional neural network for EEG MI multiclassification in brain–computer interface. Eng. Appl. Artif. Intell. 2022, 116, 105347. [Google Scholar] [CrossRef]
- Aviles, M.; Alvarez-Alvarado, J.M.; Robles-Ocampo, J.B.; Sevilla-Camacho, P.Y.; Rodríguez-Reséndiz, J. Optimizing RNNs for EMG signal classification: A novel strategy using Grey Wolf Optimization. Bioengineering 2024, 11, 77. [Google Scholar] [CrossRef] [PubMed]
- Höller, Y.; Bathke, A.C.; Uhl, A.; Strobl, N.; Lang, A.; Bergmann, J.; Nardone, R.; Rossini, F.; Zauner, H.; Kirschner, M.; et al. Combining SPECT and Quantitative EEG Analysis for the Automated Differential Diagnosis of Disorders with Amnestic Symptoms. Front. Aging Neurosci. 2017, 9, 290. [Google Scholar] [CrossRef] [PubMed]
- Klepl, D.; He, F.; Wu, M.; Blackburn, D.J.; Sarrigiannis, P.G. Adaptive Gated Graph Convolutional Network for Explainable Diagnosis of Alzheimer’s Disease using EEG Data. IEEE Trans. Neural Syst. Rehabil. Eng. 2023, 31, 3978–3987. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Li, H.; Zhou, G.; Gu, X.; Xue, J. Discriminant Subspace Low-Rank Representation Algorithm for Electroencephalography-Based Alzheimer’s Disease Recognition. Front. Aging Neurosci. 2022, 14, 943436. [Google Scholar] [CrossRef]
- Gelbard-Sagiv, H.; Pardo, S.; Getter, N.; Guendelman, M.; Benninger, F.; Kraus, D.; Shriki, O.; Ben-Sasson, S. Optimizing electrode configurations for wearable EEG seizure detection using machine learning. Sensors 2023, 23, 5805. [Google Scholar] [CrossRef] [PubMed]
- Westover, M.B.; Gururangan, K.; Markert, M.S.; Blond, B.N.; Lai, S.; Benard, S.; Bickel, S.; Hirsch, L.J.; Parvizi, J. Diagnostic value of electroencephalography with ten electrodes in critically ill patients. Neurocrit. Care 2020, 33, 479–490. [Google Scholar] [CrossRef]
- Wang, R.; He, Q.; Shi, L.; Che, Y.; Xu, H.; Song, C. Automatic detection of Alzheimer’s disease from EEG signals using an improved AFS–GA hybrid algorithm. Cogn. Neurodyn. 2024. [Google Scholar] [CrossRef]

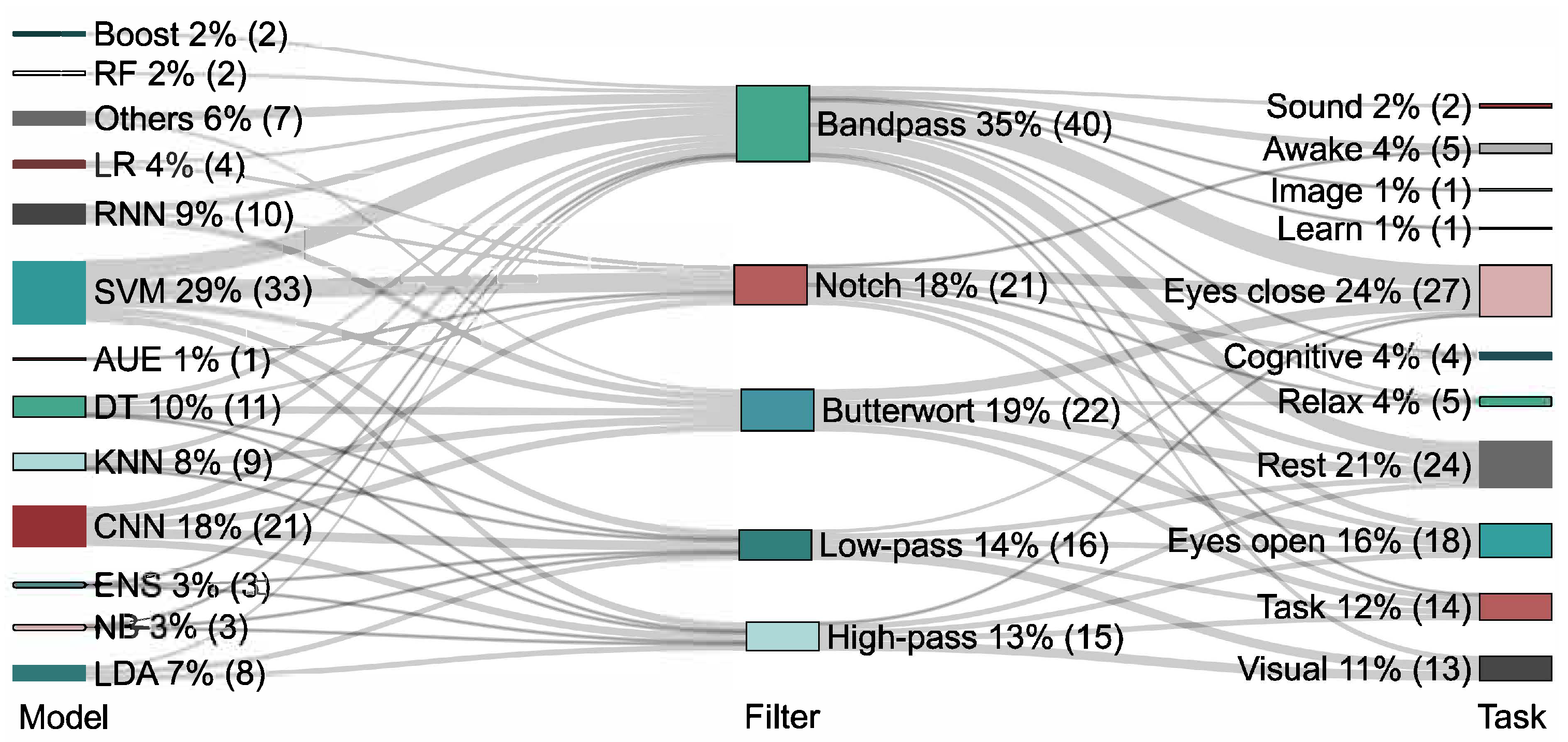
| Ref. | Electrode Configuration |
|---|---|
| [4,5,7,8,12,13,16,51,52,53,54,55,56,57,58,59] | 19 |
| [29,49,60,61,62,63,64,65,65] | 16 |
| [6,20,35,66,67,68,69,70,71] | 32 |
| [3,72,73,74,75,76] | 21 |
| [42,50] | 64 |
| [2,23,77,78] | 128 |
| [36,37,46,79,80,81,82,83] | Less common configurations |
| Ref. | Acquisition Paradigm |
|---|---|
| [5,16,18,21,22,24,25,28,29,32,33,38,57,58,59,70,72,82,83,86,87] | Closed eyes |
| [2,6,12,27,35,42,47,54,71,73,74,75,77,79,88,89,90] | Open eyes |
| [20,26,33,80,91,92] | Responses to stimuli and cognitive tasks |
| [19,41,93] | Sleep |
| [48,49,64] | Physical activity |
| Ref. | Sampling Frequency |
|---|---|
| [3,5,7,13,16,28,30,40,51,54,55,56,57,58,59,63,70,72,73,75,76,87,88,93,94] | 256 Hz |
| [6,12,20,32,36,37,47,69,71,92,95,96,97] | 500 Hz |
| [8,18,23,49,56,58,60,62,65,66,87,93,98,99] | 1024 Hz |
| [45,48,67,77] | 1 kHz |
| [4,23,61,72,76,90,100] | 128 Hz |
| [91,92] | 5 kHz |
| [26,27,78,79,100] | Frequencies *** |
| Ref. | Cutoff Frequency |
|---|---|
| [15,16,23,25,36,37,38,39,40,41,44,48,49,51,57,60,67,101] | 0.5 Hz high pass |
| [7,9,20,22,52,53,55,56,63,65,69,81,86,92,102,103,103] | 1 Hz high pass |
| [8,12,19,33,34,50,83,99,104] | 0.1 Hz high pass |
| [4,8,16,19,22,25,35,38,40,41,46,56,57,59,60,63,66,79,86,90,96,97,101,103,105] | 30 Hz low pass |
| [7,15,18,23,48,49,50,53,55,65,70,80,81,89,95,102,106] | 45 Hz low pass |
| Ref. | Filter |
|---|---|
| [3,4,6,12,14,15,17,26,34,36,46,47,52,62,70,78,91,97,102] | Butterworth |
| [9,20,33,35,48,59,75,81,83,95,106,107] | ICA |
| [44,78] | Chebyshev |
| [28,34] | Elliptical |
| [31,45] | Wavelet |
| [4,8,16,19,22,25,35,38,40,41,46,59,60,63,66,79,86,97,101,103] | FIR |
| Ref. | Classification Technique |
|---|---|
| [2,4,6,7,13,15,16,17,18,20,25,28,29,31,34,36,37,41,44,46,49,54,55,56,57,59,62,67,68,70,73,75,78,80,81,83,86,88,89,92,96,103,105,106,109,114] | SVM |
| [7,13,15,25,26,27,34,43,44,46,56,57,59,73,89,94,99,104,105] | KNN |
| [3,7,8,32,38,39,42,45,69,79,90,93,107] | CNN |
| [7,15,21,34,55,56,58,59,73,78,87] | DT |
| [24,34,40,50,52,55,61,64,71,91] | RNN |
| [12,22,41,51,66,78,82,83,97,109] | Linear regression |
| [1,15,23,33,34,47,78,81,89] | RF |
| [57,78,81] | Boost |
| [34,35,36,37,44,52,76,83,95] | Latent Dirichlet allocation |
| [7,15,34,59,83,98] | Bayesian |
| [7,63,73,83] | Ensemble |
| [41,109] | Autoencoders |
| [77,100,115] | GNN |
| [5,60,65,72,101,102,116] | Other works |
| Reference | N° Volunteers | Classification Model | Filtering Range (Hz) | Performance |
|---|---|---|---|---|
| [55] | 11 healthy, 8 MCI, 19 AD | SVM with radial kernel, multilayer perceptron (MLP) and DT | 1–40 | DT 94.88% SVM 95.10% MLP 95.55% |
| [70] | 120 healthy and 175 EA | SVM | 0.2–47 | 95% |
| [64] | 28 healthy and 7 MCI | Bidirectional LSTM | 3–30 | 91.93% |
| [45] | 15 healthy and 16 MCI | CNN | 8–30 | 79.66% |
| [13] | 16 healthy and 11 MCI | GRU | 0–32 | 96.91% |
| [14] | 16 healthy and 11 MCI | LSTM | 0.5–50 | 96.41% |
| [18] | 21 healthy and 28 MCI | SVM with Gaussian kernel | 0–40 | 86.6% |
| [89] | 89 EA | SVM with linear and Gaussian kernels, RF and KNN | 0.5–45 | RMSE of 1.682 between predicted and actual MMSE values when measuring disease progression |
| [75] | 13 healthy, 16 MCI, 15 EA | SVM with Gaussian kernel | 0.5–65 | 88% |
| [46] | 50 healthy and 50 EA | SVM and KNN | 0.5–30 | 94% |
| [107] | Synthetic EEG signals were generated from 8 healthy patients and 1 using EA generative adversarial networks and variational autoencoders | EEGNet, DeepConvNet, and EEGNet SSVEP | 4–40 | 50.2% |
| [73] | 15 healthy, 16 MCI and 16 EA | KNN | Iterative filtering | 92% |
| [78] | 17 healthy and 19 AD | Logistic regression, SVM, RF, extra trees, DT, stochastic gradient descent, Ada boosting, and gradient boosting | 0.4–115 Hz | 95.6% |
| [27] | 20 healthy and 20 EA | KNN | 2–680 | 90% |
| [8] | 23 healthy, 56 MCI and 63 AD | CNN | 0.1–30 | 80% |
| [61] | 15 healthy and 20 EA | LSTM | - | 97.9% |
| [81] | 39 healthy and 40 MCI | SVM with Gaussian kernel, RF and Xgboost | 1–45 | XGboost 87.34% SVM 93.7% RF 84.81% |
| [19] | 20 healthy and 20 EA | Cubic SVM and bidirectional GRU (Bi-GRU) | 0.1–30 | Cubic SVM 90.51% Bi-GRU 93.46% |
| [7] | 11 healthy, 8 MCI and 19 EA | CNN, ensemble, KNN, SVM, naive Bayes, discriminant analysis and DT | 1–40 | 57% |
| [102] | 9 healthy, 6 MCI and 11 EA | MLP | 1–45 | 82.5% |
| [100] | 20 healthy and 20 AD | GNN | 0.1–51 | 92% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aviles, M.; Sánchez-Reyes, L.M.; Álvarez-Alvarado, J.M.; Rodríguez-Reséndiz, J. Machine and Deep Learning Trends in EEG-Based Detection and Diagnosis of Alzheimer’s Disease: A Systematic Review. Eng 2024, 5, 1464-1484. https://doi.org/10.3390/eng5030078
Aviles M, Sánchez-Reyes LM, Álvarez-Alvarado JM, Rodríguez-Reséndiz J. Machine and Deep Learning Trends in EEG-Based Detection and Diagnosis of Alzheimer’s Disease: A Systematic Review. Eng. 2024; 5(3):1464-1484. https://doi.org/10.3390/eng5030078
Chicago/Turabian StyleAviles, Marcos, Luz María Sánchez-Reyes, José Manuel Álvarez-Alvarado, and Juvenal Rodríguez-Reséndiz. 2024. "Machine and Deep Learning Trends in EEG-Based Detection and Diagnosis of Alzheimer’s Disease: A Systematic Review" Eng 5, no. 3: 1464-1484. https://doi.org/10.3390/eng5030078
APA StyleAviles, M., Sánchez-Reyes, L. M., Álvarez-Alvarado, J. M., & Rodríguez-Reséndiz, J. (2024). Machine and Deep Learning Trends in EEG-Based Detection and Diagnosis of Alzheimer’s Disease: A Systematic Review. Eng, 5(3), 1464-1484. https://doi.org/10.3390/eng5030078









