Abstract
Anaerobic digestion is a biological process that transforms high-strength organic effluents into biogas with multiple benefits. However, concurrent with organics’ biological transformation, a liquid phase with a high solid content is also derived from this process. Valorizing this fraction is not an easy task if an agronomic application cannot be considered as a suitable option. The thermal valorization of this fraction allows for energy extraction but also gives rise to additional capital investment and increases the energy demand of the global process. In addition, the thermal treatment of digestate has to deal with a mineralized material. The changes in organic matter due to anaerobic digestion were studied in the present manuscript, by evaluating the thermal behavior of samples, activation energy, and organic transformation using Fourier transform infrared (FTIR) spectroscopy. Digested samples of a mixture composed of manure and glycerin (5% v/v) were studied. The stabilization caused a dramatic decrease in aliphatic compounds, greatly increasing the mineral content of the sample. Results from differential scanning calorimetry (DSC) indicated an energy content of 11 kJ/g for the feed material and a reduction to 9.6 kJ/g for the long-term stabilized sample. The activation energy of the feed was 249.5 kJ/mol, whereas this value was reduced to 70–80 kJ/mol for digested samples. If the valorization route selected for digestates is thermal conversion, the lower energy content and more complex structure of these materials (higher content of lignin and protein-type compounds) must be carefully evaluated.
1. Introduction
Anaerobic digestion is a biological process that has several benefits when considering the conversion of organic effluents into biogas and its capacity to recycle nutrients. Biogas is a gaseous stream mainly composed of methane and carbon dioxide, which can serve directly as a fuel in multiple energy production systems or upgraded to reach a quality similar to that of natural gas [1]. However, along with organics’ biological degradation, a liquid slurry is also derived from this process, containing microbial biomass, undigested components, and a high proportion of minerals. The resulting slurry is known as digestate. Traditionally, application to crops is the final disposal option, bringing environmental and agronomic benefits [2]. This practice allows for nutrient recycling and improves soil quality, as demonstrated by several authors [3,4,5]. Management methods must take into account soil nutrient conditions and regional environmental regulations to establish fertilization rates [6].
The characteristics of digestate mainly depend on the process performance (operating parameters) and intrinsic characteristics of the feed. A detailed review performed by Fernández-Domínguez et al. [7] aggregated information regarding digestate characterization and factors affecting digestion outcomes. In a subsequent work, the same authors provided insight regarding the chemical structure of digestate based on inoculum type, digestion time, and substrate composition, reporting significant differences in digestates based on these parameters [8]. The biological anaerobic process occurs in a series of reactions where hydrolysis of complex substrates is the first stage, solubilizing particles and making them accessible to the microflora. Conversion into organic acids and subsequent degradation into methane follow a diversity of microbial pathways [9,10], ending with biogas as the product of interest and digestate as a residual by-product. Several digestion configurations have been proposed, including centralized and decentralized centers, partial decentralization by performing the initial digestion stage in small units and centralizing the subsequent digestion phase, or centralizing only the valorization of biogas after the initial digestion stage [11,12,13,14]. Regardless of the configuration option selected, a practical solution for the final disposal of digestate is necessary.
The biological degradation of organic materials starts with the preferential conversion of readily degradable compounds (polysaccharides) and the accumulation of complex components with longer degradation time, thus leading to the build-up of recalcitrant structures in the anaerobic slurry [15,16]. Therefore, the quality of the organic material suffers changes during the biological conversion, increasing mineralization and accumulating recalcitrant molecules. Any modification in reactor operating parameters will affect digestate characteristics, thus influencing its energetic content and subsequent thermal valorization.
The deployment of digestion plants has given rise to a secondary problem associated with the great amount of digestate requiring a final disposal option. If land spreading is not feasible due to regulation constraints, thermal valorization such as pyrolysis, gasification, and incineration become suitable alternatives [17,18,19,20]. Other new technological options involve the production of proteins or the use of digestate as a carbon or nutrient source in different biological processes, as it may be the production of ethanol, polyhydroxyalkanoates, biopesticides, and microalgae cultivation, among others [21,22,23,24]. However, these processes are in an incipient state of research; thus, practical application is yet to be found. When considering digestate as a raw material, the application of the final product and the simultaneous avoidance of cross-contamination are relevant aspects. Digestates are usually derived from manures. Therefore, the risk of creating a public health problem associated with the presence of viruses and bacteria should be carefully examined. The use and valorization of digestates need to be performed safely and in compliance with environmental, health, and legal requirements [25].
Research activities currently focus on integrating digestion by-products to increase the cycling of nutrients in compliance with circular economy principles [26,27]. However, the energy demand of the value chain should also be carefully evaluated. Otherwise, the increase in energy requirements may offset any benefit associated with the cycling of materials. In the case of the thermal treatment of digestates, the energy demand associated with the drying stage may be excessive, making the whole process unfeasible. Composting and bio-drying are two ways to reduce water content and improve the stability of organics [28]. Although considered a low-energy-demanding process compared to thermal drying, the requirement of an extensive area and the careful control of emissions may act as a disincentive for this type of installation.
Solar dryers are a type of drying equipment characterized by a low energy demand (20–30 kWh/t sludge [29]). These drying methods have not been as widely installed as should have been expected, based on all the benefits associated with their performance, mainly because of the need for an extensive area for installing greenhouses where halls of sludge drying beds will be placed. The drying time is already high in summer periods, where high drying efficiencies and pathogen abatement are attained. However, during the winter period, drying time is further increased along with a decrease in drying efficiency, thus limiting the application of the technology to regions with high solar radiation or needing the aid of a boiler to supply additional heat [30,31].
Other aspects requiring careful analysis are the changes suffered by the organic material during anaerobic stabilization and, therefore, their implications in a subsequent thermal treatment. Therefore, thermal valorization of digestates must confront two main problems: the energy associated with dewatering and drying and the handling of the mineralized material. The present manuscript aimed to analyze changes experienced by the digestate during an extended stabilization phase and the implications of the mineralization attained when considering the conversion of the anaerobic slurry by thermal processing. The novelty of the present work is based on establishing an integration link between operational conditions associated with anaerobic digestion and those of thermal valorization of the digested slurry when thermal treatment is the selected option for attaining energy and nutrient recovery from waste streams.
2. Materials and Methods
Swine manure was obtained from a farm located near León (Spain). The inoculum used was digested sludge from the wastewater treatment plant of León. Collection point and conservation were carried out as described in Fierro et al. [32]. Residual glycerin was used to boost biogas production at a 5% volumetric ratio. This ratio was selected based on previous work performed by the authors [33,34]. The glycerin was obtained from a local biodiesel plant located in San Cristobal de Entreviñas (Zamora, Spain). The characteristics of the inoculum and substrate are shown in Table 1.

Table 1.
Characteristics of materials used in the digestion process.
The co-digestion of swine manure and residual glycerin was carried out in continuously stirred tank reactors with a working volume of 3 L, operating at 34 ± 1 °C. The hydraulic retention time (HRT) was set constant at 30 days based on previous experimental work carried out by the authors [34]. Initially, the reactor was fed at a low load during the first 30 days, considering this period the acclimation stage. Subsequently, the desired HRT was established, and the system worked at a constant load of 2.4 g VS/L day for the remaining period (70 days). The cessation of feeding was considered the beginning of the stabilization period; thus, a zero value was given to this day. Only total gas production and solid evolution were registered. Process parameters were followed for a 300-day period under room conditions (temperatures between 20 and 22 °C). Reactor content was transferred to amber glass storage bottles.
Analytical Techniques
Total solid (TS), volatile solid (VS), ammonium, and chemical oxygen demand (COD) measurements were carried out following standard methods [35]. pH was measured using a Crison pH meter (Mettler Toledo, Barcelona, España). The feeding sample (feed) and three digested samples were analyzed: one collected right at the end of the working period (dig_0d), then after 30 days of stabilization (dig_30d), and at the end of the period (dig_300d).
Thermal analysis was carried out using the methodology described by González et al. [36] using a TA Instruments model Q600 (TA Instruments, New Catle, DE, USA). A total of 5 mg of sample was used and submitted under 100 mL/min of air flow. The heating ramp was 10 °C/min until reaching 700 °C. The mass loss (thermogravimetric curves, TG), derivative thermogravimetric curves (DTG), and differential scanning calorimetry (DSC) were obtained from this analysis. Two replicates were analyzed, and mean values were used for representing the thermal profiles. The thermal profiles obtained from DTG curves were deconvoluted using OriginPro 2015 Software. The multipeak function was used for this analysis using the Gauss approach and visual selection of approximate peak location. Estimation of activation energy was carried out using the methodology described by Gómez et al. [37], applying the linearization method proposed by Dodampola et al. [38]. Activation energy (Ea) was estimated for every single peak previously deconvoluted, and the global Ea value was calculated as the weighted average, using normalized area values expressed as percentage.
Fourier transform infrared (FTIR) spectra were recorded based on the methodology described by Arenas et al. [39]. An FTIR Thermo Scientific Nicolet iS5 (Thermo Fisher Scientific, Waltham, MA, USA) (ID7 ATR accessory, a monolithic diamond ATR crystal with high efficiency) spectrophotometer was used (4000–650 cm−1 range at a rate of 0.5 cm/s). Sixteen scans were collected with 0.482 cm−1 spacing. Each spectrum was averaged and corrected against ambient air as the background. Hierarchical cluster analysis (HCA) and principal component analysis (PCA) were used for evaluating FTIR spectra using OriginPro 2015 software (OriginLab, Northampton, MA, USA).
3. Results and Discussion
3.1. Digestion and Stabilization Period
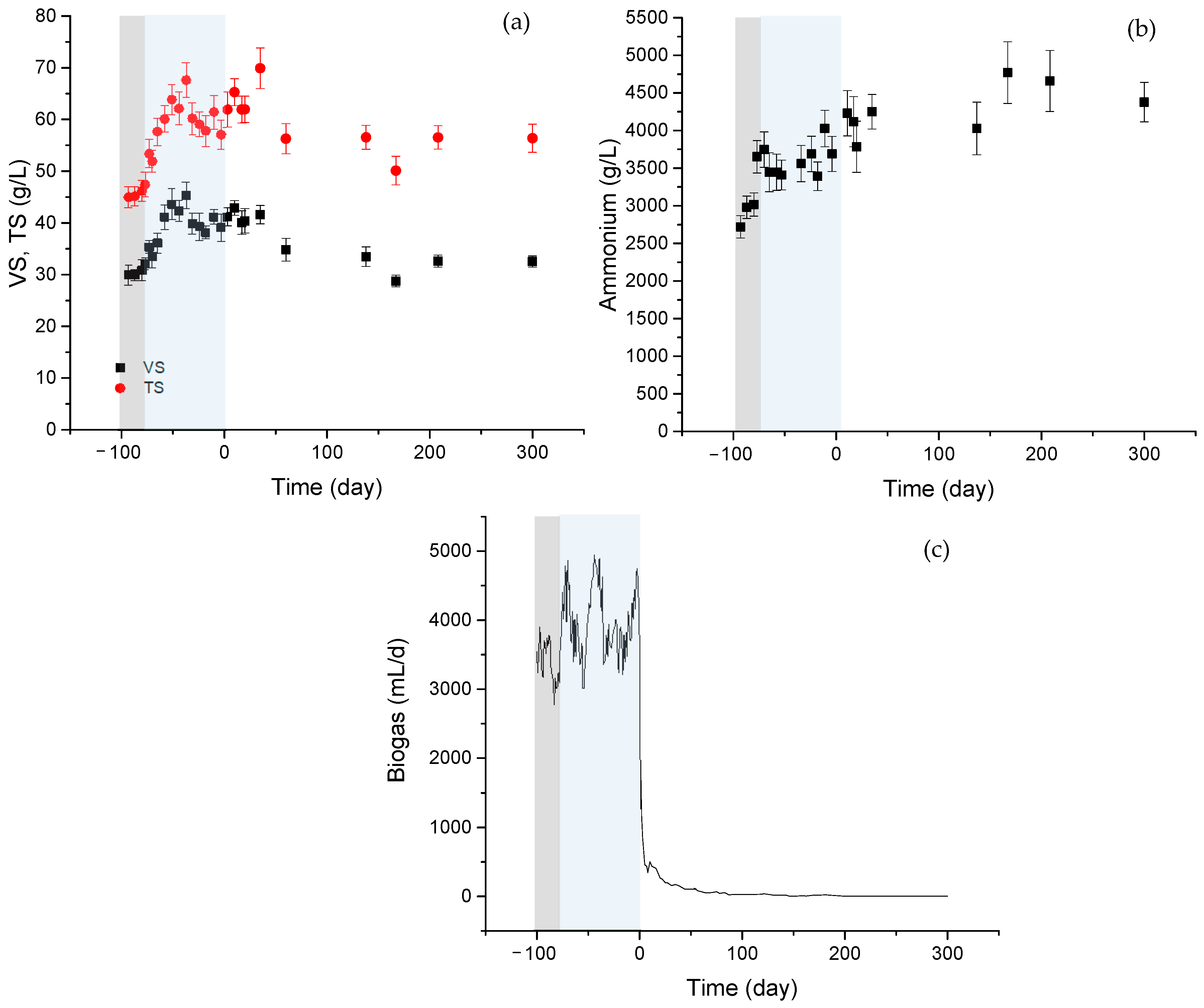
Figure 1 shows the results derived from the reactor performance. The startup of the reactor (gray region) and the working period (blue region) at an HRT of 30 days and 34 °C of temperature are also represented in the graph. A zero value was assigned to the cessation of reactor feeding.
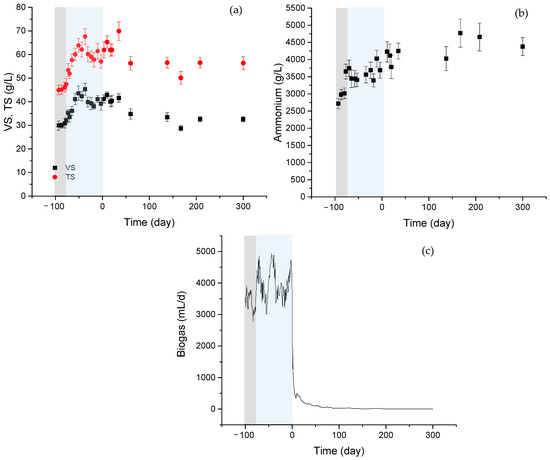
Figure 1.
(a) Evolution of VS. solids (black squares), TS solids (red circles), (b) ammonium concentration in digester liqueur, and (c) biogas production. Gray: acclimation period, Blue: working period. Stabilization period corresponds to days 0 to 300.
Figure 1a shows the evolution of VS and TS solids during the acclimation (gray region) and working period (blue region), along with the subsequent stabilization period under room temperature conditions. There was an initial increase in the solid content of the reactor. The acclimation period was characterized by a gradual increase in the organic load by increasing the daily volume fed to the reactor. A gradual increase in the ammonium concentration was also observed, which was associated with the degradation of proteins (see Figure 1b). A steady behavior was considered to be reached after a period equivalent to about 40–45 days (about 1.5 times HRT).
The specific biogas production was 547.2 ± 68.4 mL gas/g VS, a value estimated from biogas data derived from the working period (blue region in Figure 1c). The subsequent stabilization region was carried out at a different temperature. The digested material was transferred from the reactor (operating at 34 °C) to the storage bottle to be kept at room temperature. The disruption in operating conditions and transfer to a different container was the cause for the clear decrease in biogas production observed when initiating the stabilization period.
The stabilization of digestate caused a reduction in the solid content, which is observed in Figure 1c, reaching a final value of 32.5 g VS/L. The period corresponding to digestate stabilization was characterized by low gas production, with an increase in ammonium values after removing the feeding procedure, although no significant changes were observed in the solid content.
3.2. Thermal Analysis
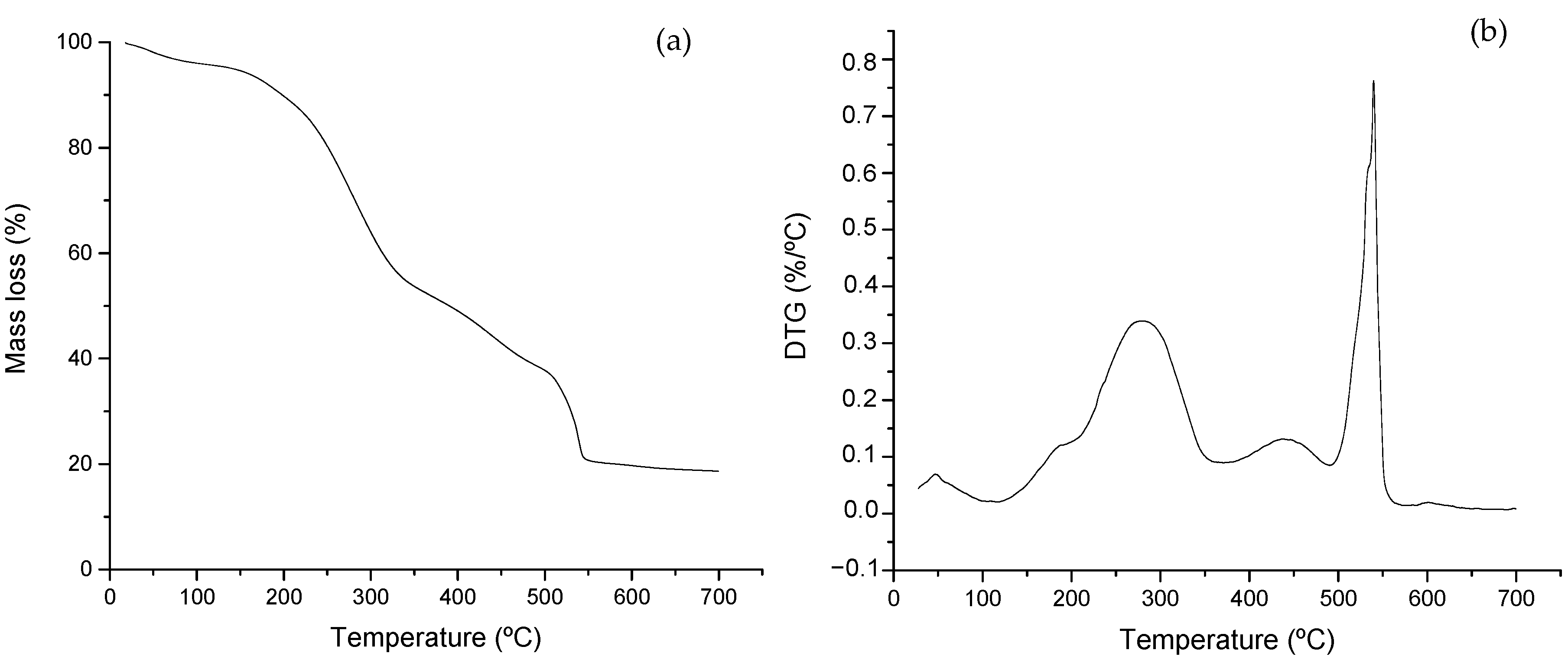
Figure 2 shows the thermal profiles derived from the analysis of the feeding material. Figure 2a shows the TG curve representing the mass loss experienced by the sample, and Figure 2b represents the derivative of the TG curve (DTG) with respect to temperature. The mixture of swine manure and glycerin shows two characteristics zones where thermal degradation of highly biodegradable material commonly takes place. In a previous work [36], the authors studied the thermal profiles of swine manure and glycerin as single substrates. In the present case, the interest was to focus on the evolution of organic materials when submitted to long-term degradation.
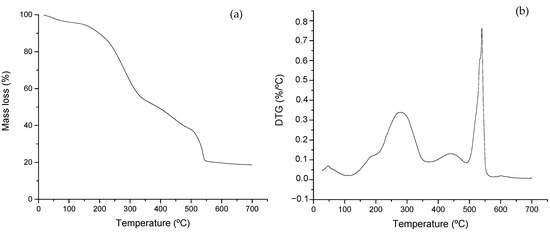
Figure 2.
Thermal profiles of the feeding mixture (glycerin and swine manure): (a) TG expressed as percentage (%) and (b) DTG curves obtained from the feed sample.
The degradation of labile compounds takes place first, with mass loss peaking at around 300 °C (see Figure 2b). The presence of glycerin can be observed by the early initiation of mass loss peaking around 200 °C [36,40]. This is associated with a severe mass decline observed in Figure 2a and a shoulder peak observed in Figure 2b. The degradation of cellulose and hemicellulose then takes place, along with simple lipids and amino acids [37,41], followed by a partial pyrolysis process, usually due to mass-transfer limitations during the analysis. The inflection point at around 300 °C in Figure 2a and the peaks at about 300 and 400 °C are associated with this phenomenon. The second main process takes place around 550 °C, evidencing the presence of lignin-type complex components along with the combustion of previous material partially degraded at a lower temperature [42,43]. Thermal analysis has been widely applied for characterizing biomass, particularly manure of different origins [16,44], due to the short time required for the analysis and the easiness of sample preparation and result interpretation.
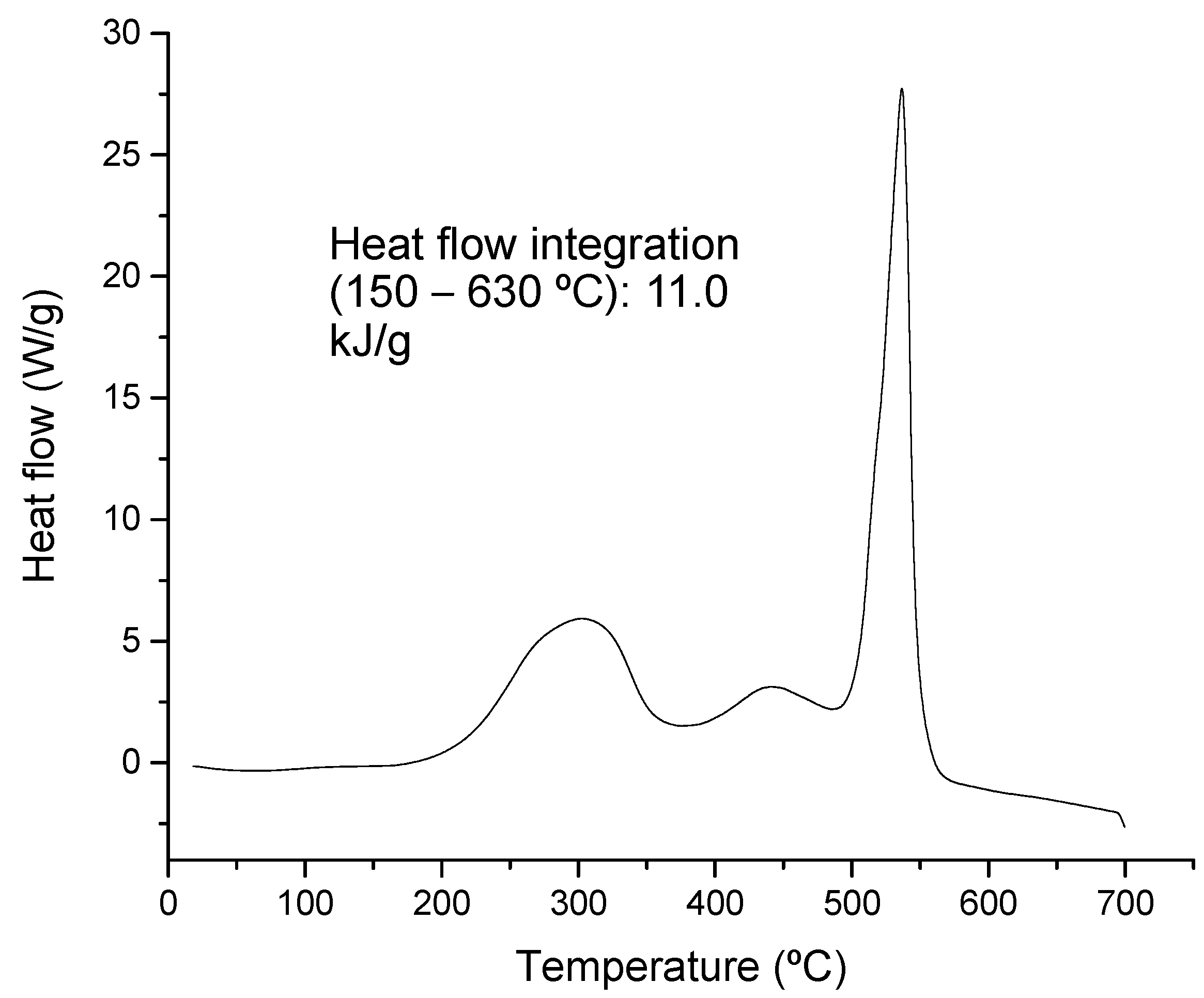
The thermal degradation process is associated with a release of energy when the sample shows an increment in temperature. In the present case, although an endothermic degradation characterizes glycerin, the mixture analyzed shows exothermic peaks during the evaluated temperature range. Figure 3 shows the DSC profile obtained, where a distinctive peak releasing a high amount of energy is identified at around 550 °C. This peak was associated with lignin-type materials, which were previously observed in the DTG curve (Figure 2a).
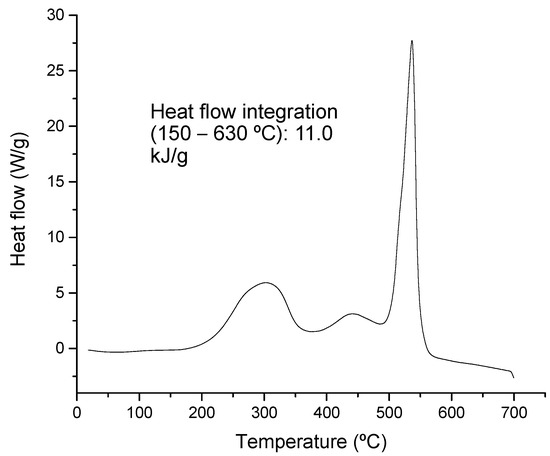
Figure 3.
DSC profile obtained from the feeding sample (mixture of swine manure and glycerin).
The profile resembles those analyzed under oxidizing conditions for waste biomass with high lignocellulosic content [45]. The atmosphere (inert or oxidizing conditions) under which the analysis is performed affects the structure of the profile because conversion and minimum activation energy are highly affected by the presence or lack of oxygen [46]. However, kinetic analysis can be performed in either case, although a careful comparison of results must be made, keeping in mind experimental conditions.
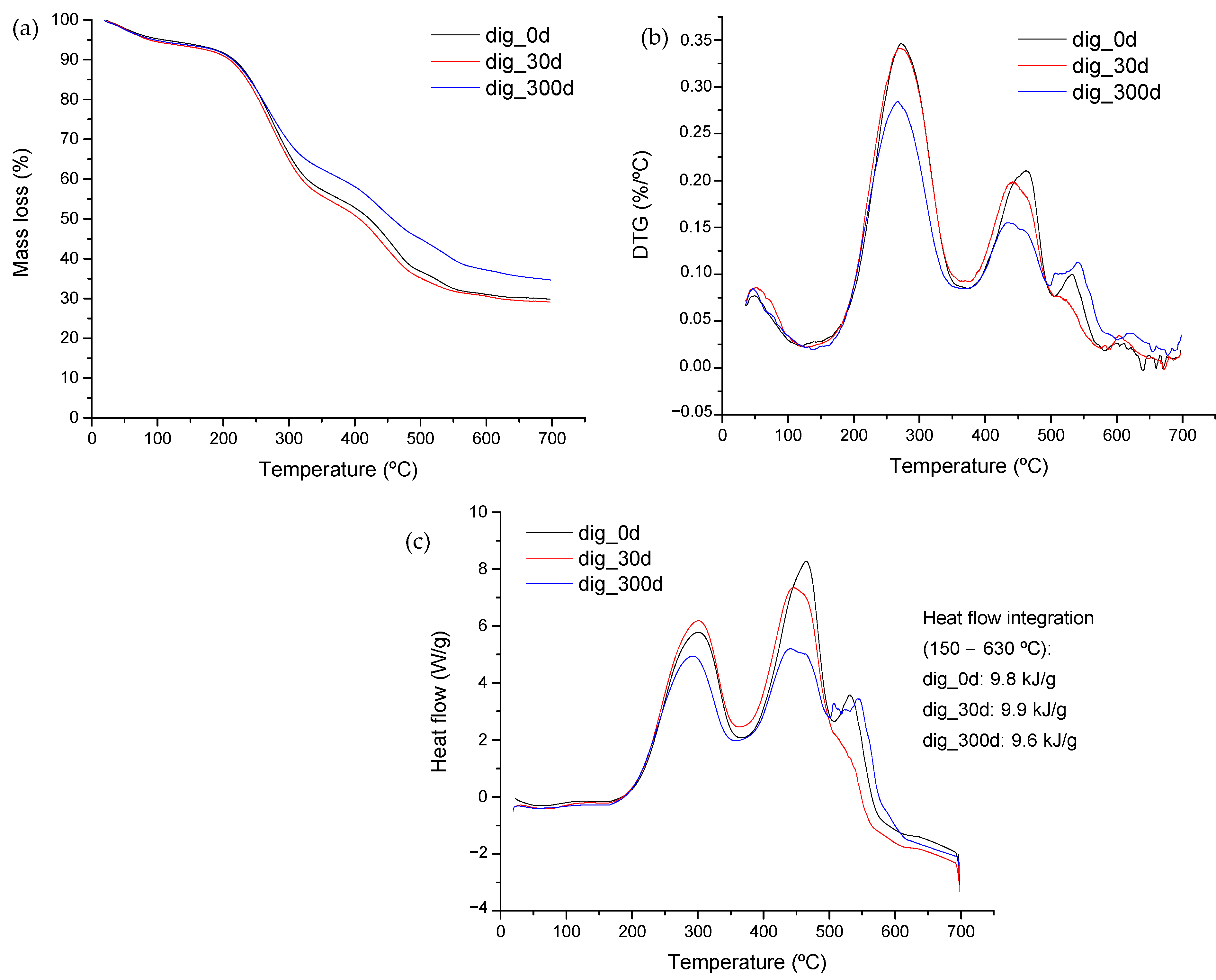
Figure 4 shows the results from the stabilization period. Mass-loss curves and their corresponding derivative are represented (Figure 4a,b). Significant changes were observed, although the analysis of solid content did not reveal any relevant difference. A high mineralization was observed at the end of the stabilization period which was discernible by the higher amount remaining once thermal degradation finished. Digested samples were taken right at the end of the digestion, a month later, and then almost a year later. Therefore, samples closer in time indeed are expected to show similarities as does occur in the TG and DTG profiles (as well as the DSC profiles) shown in Figure 4. The long-term stabilization allows for organics’ transformation, reducing the content of simple molecules and causing a concentration of those with complex structures, which are recalcitrant to the microflora.
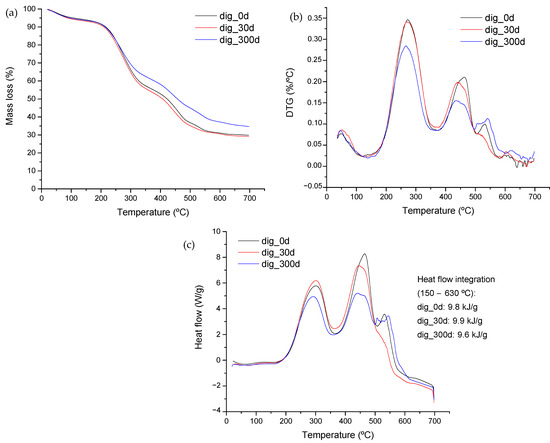
Figure 4.
(a) TG and (b) DTG curves from stabilized samples of swine manure and glycerin mixture. (c) DSC curves derived from digested samples at different stabilization times. Sample dig_0d was obtained from the digester at the end of the experiment. Sample dig_30d was obtained after 30 days of stabilization under room temperature conditions and sample dig_300d, after 300 days of stabilization.
The mass remaining at the end of the thermal analysis accounts for 18.6% in the case of the feed. Digestion increased this value, and further stabilization raised this amount to 34% (data from Figure 4a). This feature was previously observed by Martínez et al. [47], but in that case, analyzing the thermal behavior of digestate derived from fruit and vegetable wastes (fourth-range wastes).
The profile of the different peaks represented in the DTG curve (Figure 4b) indicates changes in the amount of material suffering degradation at each temperature range. Digestion transforms organic matter into biogas, concentrating mineral components in digestate and accumulating those of difficult degradation, therefore the greater size of the peak at 550 °C in the stabilized sample at day 300. The energy associated with each degradation stage can be observed in Figure 4c, where the DSC profiles are represented. Samples showed a greater intensity of the peak located at around 450 °C, with this behavior being an indication of the higher energy content of these complex materials.
The stabilization resulted in a significant decrease in the amount of organic components along with changes in their structure, demonstrating that the degradation of these structures by microbial microflora takes longer, as it is observed by the decrease in intensity of the 450 °C peak. The decrease in the energy content of the sample was also evidenced from the experiment. The fresh material showed a value of 11 kJ/g, whereas any of the digested samples reported values below 10 kJ/g (See Figure 4c). Therefore, if thermal valorization is intended, the thermal process will have to deal with a higher mineral-content material with lower energy, thus increasing fouling and tar-formation problems during thermal treatment.
3.3. Estimation of Activation Energy
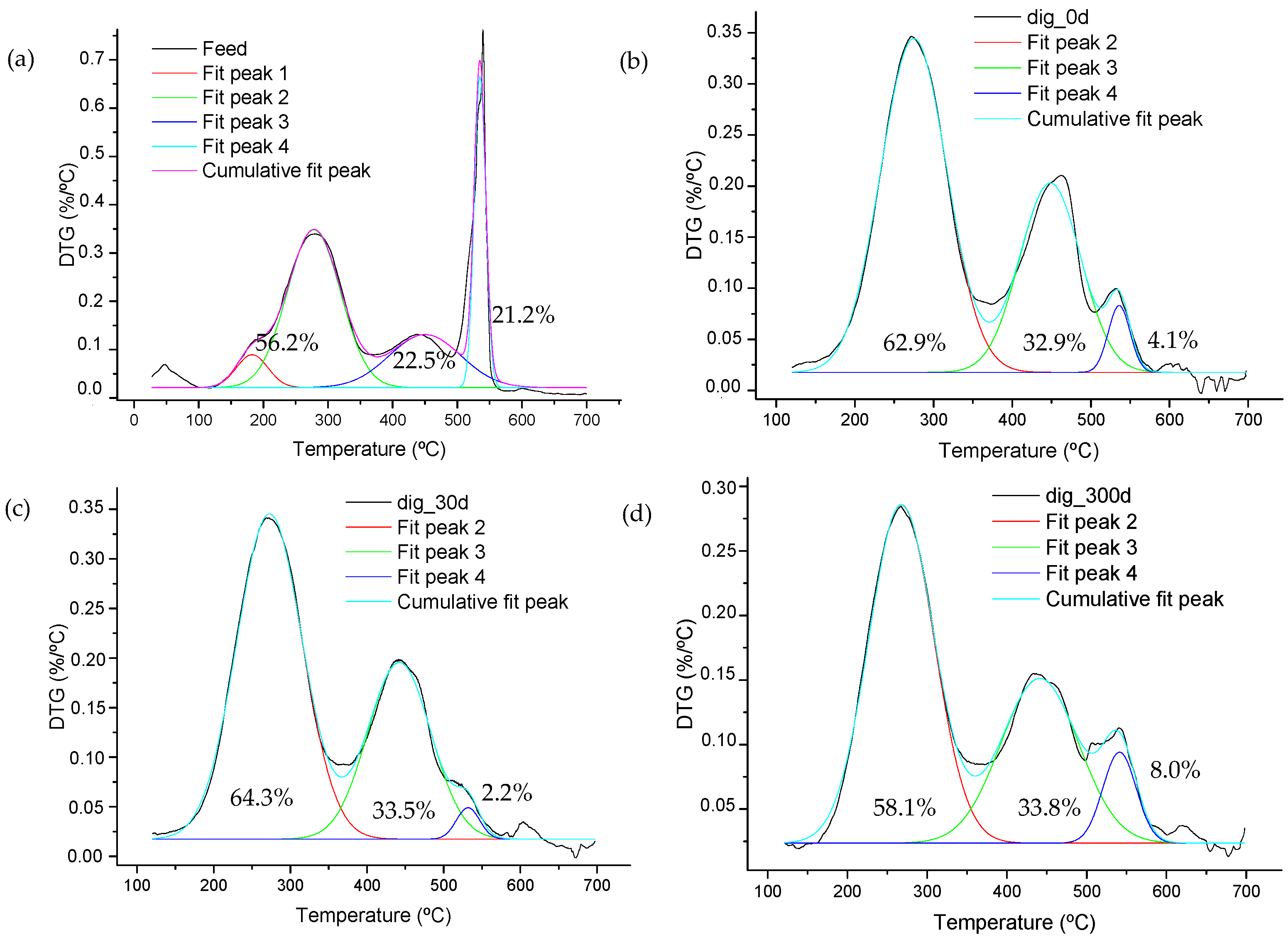
The transformation process can be separated into individual stages by deconvoluting peaks observed visually in the profile (see Figure 5). In the case of the sample representing the feed, a small peak associated with labile organics can be inferred, which was denoted as peak 1 (see Figure 5a). The remaining peaks had a recurrent presence in digested samples, with significant modifications in intensity and size.
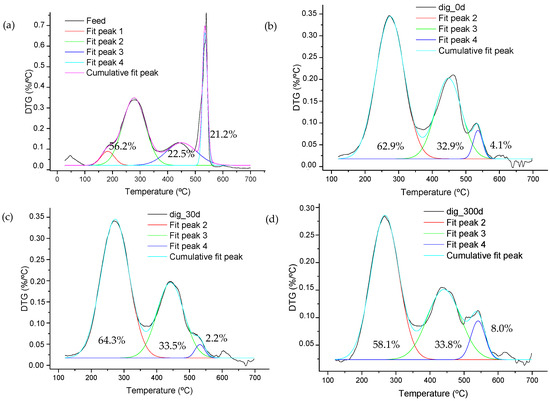
Figure 5.
Deconvolution procedure applied to DTG curves obtained from the (a) feeding material (Feed), (b) digested at day 0 (dig_0d), and (c) stabilized at days 30 (dig_30d) and (d) 300 (dig_300d). Percentages correspond to values derived after normalizing the DTG curves, representing the material loss associated with each peak.
The main striking difference is the almost complete removal of the 550 °C peak and the increase in one located at 450 °C, as observed in Figure 5b. Stabilized samples obtained after 30 and 300 days of storage have a similar profile to the digested sample obtained just at the end of digestion. However, these stabilized samples showed changes in the high-temperature region (Figure 5c,d), with the one derived at the end of the process showing a significant increase in aromatic components, a fact that is observed from the increase in the signal at 550 °C.
Figure 5 also shows the normalized values for the area of each deconvoluted peak. In the case of the feed, the percentage of labile compounds is the sum of both peaks (denoted as peak 1 and peak 2) found in the temperature range of 150–350 °C.
There were no apparent changes in the proportion of labile compounds, but regarding the combustion stage taking place at around 440–450 °C, the feed sample showed that 22.5% of organics contained in the sample react in this region, whereas on average, digested samples experience a conversion of 33.4% of organics in this same region. The peak at high temperature observed in the feeding sample was greatly degraded, resulting in a small peak being discernible in digested samples, which increases to 8.0% in the stabilized sample at day 300 (Figure 5d), probably as a result of being composed of microbial complex compounds and recalcitrant aromatic structures. The stabilization procedure also gave rise to a mass-loss signal located at temperatures above 600 °C which was associated with the formation of carbonates during the process.
The activation energy (Ea) was estimated based on the previous deconvolution procedure. Table 2 reports the values derived from single peaks and weighted averages estimated for the sample. There is a significant decrease in the average value of Ea for the digested samples. The results are in the range of those reported by different authors when evaluating manures. Under an inert atmosphere, Chen et al. [48] reported a value of 123.8–126 kJ/mol for fresh cattle manure. Similar results were also reported by Yuan et al. [49] when evaluating different isoconversional methods, with an Ea value ranging from 170.3 to 195.5 kJ/mol (average values of Ea obtained at different conversion stages), and slightly higher values were reported by Gu et al. [44] (200–232 kJ/mol). Sharara and Sadaka [50] studied swine manure samples of different origins, reporting lower values, in the range of 98–116 kJ/mol, also under inert atmospheres. Estimations of Ea derived from oxidizing atmospheres are usually lower than those derived from pyrolysis [51,52], thus explaining the lower values derived from the present study.

Table 2.
Activation energy (Ea) and peak location (peak maximum location, Pmax) obtained from the different samples using the deconvolution procedure.
Fernandez-Lopez et al. [53] reported that fresh and digested swine manure samples showed similar Ea values, thus indicating a negligible effect of anaerobic digestion. However, different authors have described changes in the thermal profiles, where a reduction in labile compounds and increased structural complexity were demonstrated using different spectroscopic techniques [8,11,50]. The results obtained in this study align with those of Otero et al. [54], who also reported a decrease in Ea after digesting cattle manure.
3.4. Fourier Transform Infrared (FTIR) Spectroscopy
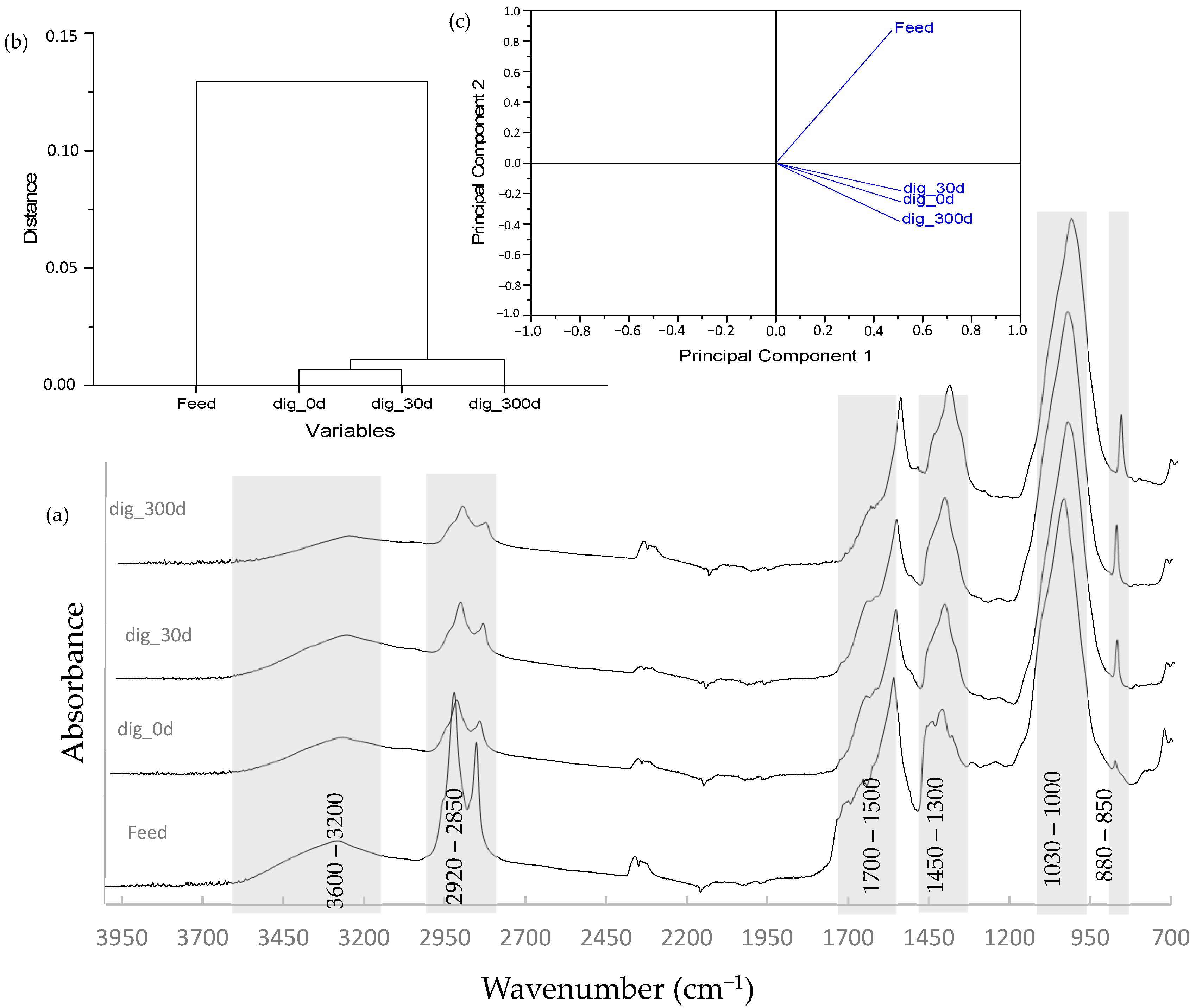
Spectroscopic analysis aids in understanding changes in organic material suffered during microbial degradation. This technique has provided useful information when evaluating the composting of lignocellulosic material and wastes [55,56,57] and studying the digestion process of different substrates [8,58,59,60]. Figure 6a shows the spectra obtained from the feed and digested samples.
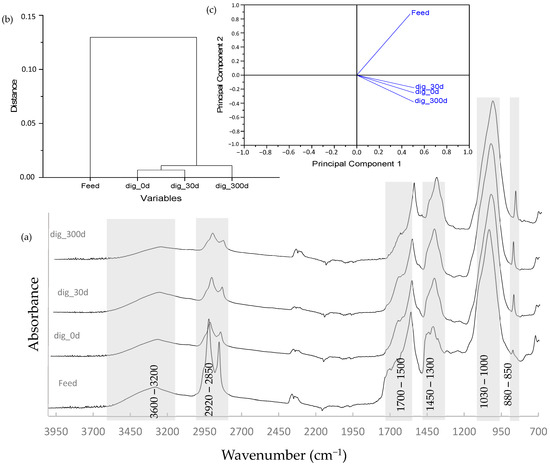
Figure 6.
(a) FTIR spectra obtained from the feed and digested samples. Results from the (b) dendrogram and (c) PCA are also shown.
The digestion process was characterized by the degradation of simple aliphatic structures, as observed from the intensity in signals of the two peaks located at the 2900–2800 cm−1 range, and the accumulation of aromatic and lignin-type structures, which is evident from the peaks located at 1560 and 1400 cm−1. A similar trend was reported by Provenzano et al. [61] and Fernández et al. [62], indicating the recurrent presence of a signal at around 1420 cm−1 representing microbial material. HCA and PCA indicated a strong correlation between all digested samples (see Figure 6b,c), clearly differentiating from the feed, finding a greater distance in the correlation between the feed and the sample stabilized at 300 days.
Table 3 shows the identification of FTIR signals. Changes experienced by the organic material are associated with the thermal behavior previously evaluated. A decrease in the aliphatic fraction is observed here by a decrease in signals ascribed to the region of 2950–2820 cm−1 and those located at approximately 1440–1410 cm−1. This decrease in aliphatic content coincided with results from the thermal analysis by the lower magnitude of the mass-loss peak observed in the DTG curves at the low-temperature range (150–350 °C). Aromatization of the stabilized samples is observed by the clear peak around 1600–1520 cm−1, explained by the accumulation of lignin-type materials. Increased mineralization of the digested samples is also observed in the FTIR spectra by the higher intensity associated with the signal at around 880–870 cm−1 [55].

Table 3.
Identification of the main signals found in FTIR spectra derived from the feed sample (swine manure and glycerin mixture) and digested samples (at different stabilization periods).
4. Conclusions
Clear changes in the structure of organic matter were observed during the extended stabilization of swine manure by anaerobic digestion. The amount of organic material decreased with time, along with the aliphatic nature of the sample. In contrast, an increase in carbonates was discernible. An accumulation of recalcitrant structures was observed from FTIR spectra. The thermal valorization of digestion must consider changes in the thermal behavior of the organic material. Therefore, any technology considered for treating digestates (pyrolysis, gasification, hydrothermal carbonization, among others) must take into account the different nature of these raw materials and the effect the high mineral content would have in tar formation and the presence of polycyclic aromatic hydrocarbons. The integration of anaerobic digestion and thermal valorization of digestates should consider the energy demand associated with digestate drying and its thermal characteristics. A critical assessment of the two parameters is necessary, along with evaluating their effect on the global energy balance of the process. Future research will consider these aspects.
Author Contributions
Conceptualization, X.G. and R.G.G.; methodology, R.G.G.; software, D.C.-P.; validation, X.G. and R.G.G.; formal analysis, X.G.; investigation, S.G.-R.; resources, X.G.; data curation, S.G.-R.; writing—original draft preparation, D.C.-P.; writing—review and editing, S.G.-R.; visualization, X.G.; supervision, X.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors wish to thank the support of the company Explotación Porcina Integral, S.A. (Exporinsa) and the collaboration of the personnel working in the Wastewater Treatment Plant of the City of León.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- González, R.; García-Cascallana, J.; Gómez, X. Energetic valorization of biogas. A comparison between centralized and decentralized approach. Renew. Energy 2023, 215, 119013. [Google Scholar] [CrossRef]
- Crolla, A.; Kinsley, C.; Pattey, E. Land application of digestate. In The Biogas Handbook; Wellinger, A., Murphy, J., Baxter, D., Eds.; Woodhead Publishing Series in Energy; Woodhead Publishing: Cambridge, UK, 2013; pp. 302–325. [Google Scholar] [CrossRef]
- Panuccio, M.R.; Romeo, F.; Mallamaci, C.; Muscolo, A. Digestate application on two different soils: Agricultural benefit and risk. Waste Biomass Valorization 2021, 12, 4341–4353. [Google Scholar] [CrossRef]
- Slepetiene, A.; Kochiieru, M.; Jurgutis, L.; Mankeviciene, A.; Skersiene, A.; Belova, O. The Effect of Anaerobic Digestate on the Soil Organic Carbon and Humified Carbon Fractions in Different Land-Use Systems in Lithuania. Land 2022, 11, 133. [Google Scholar] [CrossRef]
- García-López, A.M.; Delgado, A.; Anjos, O.; Horta, C. Digestate Not Only Affects Nutrient Availability but Also Soil Quality Indicators. Agronomy 2023, 13, 1308. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Y.; Wang, S.; Wang, Z.; Liu, Y.; Hu, Z.; Zhan, X. Environmental sustainability assessment of pig manure mono-and co-digestion and dynamic land application of the digestate. Renew. Sustain. Energy Rev. 2021, 137, 110476. [Google Scholar] [CrossRef]
- Fernández-Domínguez, D.; Guilayn, F.; Patureau, D.; Jimenez, J. Characterising the stability of the organic matter during anaerobic digestion: A selective review on the major spectroscopic techniques. Rev. Environ. Sci. Bio. 2022, 21, 691–726. [Google Scholar] [CrossRef]
- Fernández-Domínguez, D.; Patureau, D.; Jimenez, J. Impact of Substrate Biodegradability on the Identification of Endogenous Compounds During Anaerobic Digestion. Waste Biomass Valorization 2024, 15, 885–901. [Google Scholar] [CrossRef]
- González, J.; Sánchez, M.E.; Gómez, X. Enhancing Anaerobic Digestion: The Effect of Carbon Conductive Materials. C 2018, 4, 59. [Google Scholar] [CrossRef]
- González, R.; Peña, D.C.; Gómez, X. Anaerobic Co-Digestion of Wastes: Reviewing Current Status and Approaches for Enhancing Biogas Production. Appl. Sci. 2022, 12, 8884. [Google Scholar] [CrossRef]
- Gadaleta, G.; De Gisi, S.; Notarnicola, M. Feasibility Analysis on the Adoption of De-centralized Anaerobic Co-Digestion for the Treatment of Municipal Organic Waste with Energy Recovery in Urban Districts of Metropolitan Areas. Int. J. Environ. Res. Public Health 2021, 18, 1820. [Google Scholar] [CrossRef]
- Ellacuriaga, M.; González, R.; Gómez, X. Is Decentralized Anaerobic Digestion a Solution? Analyzing Biogas Production and Residential Energy Demand. Eng 2022, 3, 662–676. [Google Scholar] [CrossRef]
- González, R.; García-Cascallana, J.; Gutiérrez-Bravo, J.; Gómez, X. Decentralized Bio-gas Production in Urban Areas: Studying the Feasibility of Using High-Efficiency Engines. Eng 2023, 4, 2204–2225. [Google Scholar] [CrossRef]
- Myers, G.M.; Andersen, D.S.; Martens, B.J.; Raman, D.R. Cost Assessment of Centralizing Swine Manure and Corn Stover Co-Digestion Systems. Energies 2023, 16, 4315. [Google Scholar] [CrossRef]
- Cavallo, O.; de la Rosa, J.M.; González-Pérez, J.A.; Knicker, H.; Pezzolla, D.; Gigliotti, G.; Provenzano, M.R. Molecular characterization of digestates from solid-state anaerobic digestion of pig slurry and straw using analytical pyrolysis. J. Anal. Appl. Pyrol. 2018, 134, 73–82. [Google Scholar] [CrossRef]
- Gómez, X.; Blanco, D.; Lobato, A.; Calleja, A.; Martínez-Núñez, F.; Martin-Villacorta, J. Digestion of cattle manure under mesophilic and thermophilic conditions: Characterization of organic matter applying thermal analysis and 1 H NMR. Biodegradation 2011, 22, 623–635. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Ficara, E.; Aboulkas, A.; Barakat, A.; Carrère, H. New opportunities for agricultural digestate valorization: Current situation and perspectives. Energy Environ. Sci. 2015, 8, 2600–2621. [Google Scholar] [CrossRef]
- González-Arias, J.; Gil, M.V.; Fernández, R.Á.; Martínez, E.J.; Fernández, C.; Papaharalabos, G.; Gómez, X. Integrating anaerobic digestion and pyrolysis for treating digestates derived from sewage sludge and fat wastes. Environ. Sci. Pollut. Res. 2020, 27, 32603–32614. [Google Scholar] [CrossRef]
- Kubonova, L.; Janakova, I.; Malikova, P.; Drabinova, S.; Dej, M.; Smelik, R.; Skalny, P.; Heviankova, S. Evaluation of Waste Blends with Sewage Sludge as a Potential Material Input for Pyrolysis. Appl. Sci. 2021, 11, 1610. [Google Scholar] [CrossRef]
- Untoria, S.; Rouboa, A.; Monteiro, E. Hydrogen-Rich Syngas Production from Gasification of Sewage Sludge: Catalonia Case. Energies 2024, 17, 1492. [Google Scholar] [CrossRef]
- Zhuang, L.; Zhou, S.; Wang, Y.; Liu, Z.; Xu, R. Cost-effective production of Bacillus thuringiensis biopesticides by solid-state fermentation using wastewater sludge: Effects of heavy metals. Bioresour. Technol. 2011, 102, 4820–4826. [Google Scholar] [CrossRef]
- Pastor-Bueis, R.; Mulas, R.; Gómez, X.; González-Andrés, F. Innovative liquid formulation of digestates for producing a biofertilizer based on Bacillus siamensis: Field testing on sweet pepper. J. Plant Nutr. Soil Sci. 2017, 180, 748–758. [Google Scholar] [CrossRef]
- Battista, F.; Frison, N.; Pavan, P.; Cavinato, C.; Gottardo, M.; Fatone, F.; Eusebi, A.L.; Majone, M.; Zeppilli, M.; Valentino, F.; et al. Food wastes and sewage sludge as feedstock for an urban biorefinery producing biofuels and added-value bioproducts. J. Chem. Technol. Biotechnol. 2020, 95, 328–338. [Google Scholar] [CrossRef]
- Wang, W.; Chang, J.S.; Lee, D.J. Anaerobic digestate valorization beyond agricultural application: Current status and prospects. Bioresour. Technol. 2023, 373, 128742. [Google Scholar] [CrossRef]
- Lamolinara, B.; Pérez-Martínez, A.; Guardado-Yordi, E.; Fiallos, C.G.; Diéguez-Santana, K.; Ruiz-Mercado, G.J. Anaerobic digestate management, environmental impacts, and techno-economic challenges. Waste Manag. 2022, 140, 14–30. [Google Scholar] [CrossRef]
- Loizia, P.; Neofytou, N.; Zorpas, A.A. The concept of circular economy strategy in food waste management for the optimization of energy production through anaerobic digestion. Environ. Sci. Pollut. Res. 2019, 26, 14766–14773. [Google Scholar] [CrossRef] [PubMed]
- Ellacuriaga, M.; García-Cascallana, J.; Gómez, X. Biogas Production from Organic Wastes: Integrating Concepts of Circular Economy. Fuels 2021, 2, 144–167. [Google Scholar] [CrossRef]
- Lu, J.; Xu, S. Post-treatment of food waste digestate towards land application: A review. J. Clean. Prod. 2021, 303, 127033. [Google Scholar] [CrossRef]
- Radetic, B. Solar Sludge Drying. In Handbook of Water and Used Water Purification; Lahnsteiner, J., Ed.; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Mathioudakis, V.L.; Kapagiannidis, A.G.; Athanasoulia, E.; Diamantis, V.I.; Melidis, P.; Aivasidis, A. Extended dewatering of sewage sludge in solar drying plants. Desalination 2009, 248, 733–739. [Google Scholar] [CrossRef]
- An-nori, A.; Ezzariai, A.; El Mejahed, K.; El Fels, L.; El Gharous, M.; Hafidi, M. Solar drying as an eco-friendly Technology for sewage sludge stabilization: Assessment of micropollutant behavior, pathogen removal, and agronomic value. Front. Environ. Sci. 2022, 10, 814590. [Google Scholar] [CrossRef]
- Fierro, J.; Martínez, E.J.; Rosas, J.G.; Blanco, D.; Gómez, X. Anaerobic codigestion of poultry manure and sewage sludge under solid-phase configuration. Environ. Prog. Sustain. 2014, 33, 866–872. [Google Scholar] [CrossRef]
- Lobato, A.; Cuetos, M.J.; Gómez, X.; Morán, A. Improvement of biogas production by co-digestion of swine manure and residual glycerine. Biofuels 2010, 1, 59–68. [Google Scholar] [CrossRef]
- Fierro, J.; Martinez, E.J.; Rosas, J.G.; Fernández, R.A.; López, R.; Gomez, X. Co-Digestion of swine manure and crude glycerine: Increasing glycerine ratio results in preferential degradation of labile compounds. Water Air Soil Pollut. 2016, 227, 78. [Google Scholar] [CrossRef]
- American Public Health Association (APHA); American Water Works Association (AWWA); Water Environmental Federation (WEF). Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- González, R.; Smith, R.; Blanco, D.; Fierro, J.; Gómez, X. Application of thermal analysis for evaluating the effect of glycerine addition on the digestion of swine manure. J. Therm. Anal. Calorim. 2019, 135, 2277–2286. [Google Scholar] [CrossRef]
- Gómez, X.; Bernal, M.P.; Zárate, P.P.; Álvarez-Robles, M.J.; González, R.; Clemente, R. Thermal evaluation of plant biomass from the phytostabilisation of soils contaminated by potentially toxic elements. Chemosphere 2023, 342, 140116. [Google Scholar] [CrossRef]
- Dodampola, R.; Amarasingha, S.; Attygalle, D.; Weragoda, S. Improved method to extract kinetic parameters from thermograms. Mater. Today Proc. 2020, 23, 2–7. [Google Scholar] [CrossRef]
- Arenas, C.B.; Meredith, W.; Snape, C.E.; Gómez, X.; González, J.F.; Martinez, E.J. Effect of char addition on anaerobic digestion of animal by-products: Evaluating biogas production and process performance. Environ. Sci. Pollut. Res. 2020, 27, 24387–24399. [Google Scholar] [CrossRef]
- Gómez-Siurana, A.; Marcilla, A.; Beltrán, M.; Berenguer, D.; Martínez-Castellanos, I.; Catalá, L.; Menargues, S. TGA/FTIR study of the MCM-41-catalytic pyrolysis of tobacco and tobacco–glycerol mixtures. Thermochim. Acta 2014, 587, 24–32. [Google Scholar] [CrossRef]
- Martín-Mata, J.; Lahoz-Ramos, C.; Bustamante, M.A.; Marhuenda-Egea, F.C.; Moral, R.; Santos, A.; Sáez, J.A.; Bernal, M.P. Thermal and spectroscopic analysis of organic matter degradation and humification during composting of pig slurry in different scenarios. Environ. Sci. Pollut. Res. 2016, 23, 17357–17369. [Google Scholar] [CrossRef]
- Otero, M.; Sanchez, M.E.; Gómez, X.; Morán, A. Thermogravimetric analysis of biowastes during combustion. Waste Manag. 2010, 30, 1183–1187. [Google Scholar] [CrossRef]
- Pu, X.; Wei, M.; Chen, X.; Wang, L.; Deng, L. Thermal Decomposition Characteristics and Kinetic Analysis of Chicken Manure in Various Atmospheres. Agriculture 2022, 12, 607. [Google Scholar] [CrossRef]
- Gu, J.; Chong, C.T.; Mong, G.R.; Ng, J.-H.; Chong, W.W.F. Determination of Pyrolysis and Kinetics Characteristics of Chicken Manure Using Thermogravimetric Analysis Coupled with Particle Swarm Optimization. Energies 2023, 16, 1919. [Google Scholar] [CrossRef]
- da Silva, D.R.; Crespi, M.S.; Crnkovic, P.C.; Ribeiro, C.A. Pyrolysis, combustion and oxy-combustion studies of sugarcane industry wastes and its blends. J. Therm. Anal. Calorim. 2015, 121, 309–318. [Google Scholar] [CrossRef]
- Ashraf, M.; Ramzan, N.; Khan, R.U.; Durrani, A.K. Analysis of mixed cattle manure: Kinetics and thermodynamic comparison of pyrolysis and combustion processes. Case Stud. Therm. Eng. 2021, 26, 101078. [Google Scholar] [CrossRef]
- Martínez, E.J.; González, R.; Ellacuriaga, M.; Gómez, X. Valorization of Fourth-Range Wastes: Evaluating Pyrolytic Behavior of Fresh and Digested Wastes. Fermentation 2022, 8, 744. [Google Scholar] [CrossRef]
- Chen, G.; He, S.; Cheng, Z.; Guan, Y.; Yan, B.; Ma, W.; Leung, D.Y. Comparison of kinetic analysis methods in thermal decomposition of cattle manure by themogravimetric analysis. Bioresour. Technol. 2017, 243, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; He, T.; Cao, H.; Yuan, Q. Cattle manure pyrolysis process: Kinetic and thermodynamic analysis with isoconversional methods. Renew. Energy 2017, 107, 489–496. [Google Scholar] [CrossRef]
- Sharara, M.; Sadaka, S. Thermogravimetric analysis of swine manure solids obtained from farrowing, and growing-finishing farms. J. Sustain. Bioenergy Syst. 2014, 4, 75–86. [Google Scholar] [CrossRef]
- Fernandez, A.; Palacios, C.; Echegaray, M.; Mazza, G.; Rodriguez, R. Pyrolysis and combustion of regional agro-industrial wastes: Thermal behavior and kinetic parameters comparison. Combust. Sci. Technol. 2018, 190, 114–135. [Google Scholar] [CrossRef]
- Wu, W.; Mei, Y.; Zhang, L.; Liu, R.; Cai, J. Kinetics and reaction chemistry of pyrolysis and combustion of tobacco waste. Fuel 2015, 156, 71–80. [Google Scholar] [CrossRef]
- Fernandez-Lopez, M.; Pedrosa-Castro, G.J.; Valverde, J.L.; Sanchez-Silva, L. Kinetic analysis of manure pyrolysis and combustion processes. Waste Manag. 2016, 58, 230–240. [Google Scholar] [CrossRef]
- Otero, M.; Lobato, A.; Cuetos, M.J.; Sánchez, M.E.; Gómez, X. Digestion of cattle manure: Thermogravimetric kinetic analysis for the evaluation of organic matter conversion. Bioresour. Technol. 2011, 102, 3404–3410. [Google Scholar] [CrossRef]
- Carballo, T.; Gil, M.V.; Gómez, X.; González-Andrés, F.; Morán, A. Characterization of different compost extracts using Fourier-transform infrared spectroscopy (FTIR) and thermal analysis. Biodegradation 2008, 19, 815–830. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, X.; Liu, Y.; Li, H.; Liu, H. Effect of microbiological inoculants DN-1 on lignocellulose degradation during co-composting of cattle manure with rice straw monitored by FTIR and SEM. Environ. Prog. Sustain. 2016, 35, 345–351. [Google Scholar] [CrossRef]
- Sokač Cvetnić, T.; Krog, K.; Benković, M.; Jurina, T.; Valinger, D.; Radojčić Redovniković, I.; Gajdoš Kljusurić, J.; Jurinjak Tušek, A. Application of Near-Infrared Spectroscopy for Monitoring and/or Control of Composting Processes. Appl. Sci. 2023, 13, 6419. [Google Scholar] [CrossRef]
- Cuetos, M.J.; Morán, A.; Otero, M.; Gómez, X. Anaerobic co-digestion of poultry blood with OFMSW: FTIR and TG–DTG study of process stabilization. Environ. Technol. 2009, 30, 571–582. [Google Scholar] [CrossRef]
- Rodríguez-Abalde, Á.; Gómez, X.; Blanco, D.; Cuetos, M.J.; Fernández, B.; Flotats, X. Study of thermal pre-treatment on anaerobic digestion of slaughterhouse waste by TGA-MS and FTIR spectroscopy. Waste Manag. Res. 2013, 31, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Domínguez, D.; Patureau, D.; Houot, S.; Sertillanges, N.; Zennaro, B.; Jimenez, J. Prediction of organic matter accessibility and complexity in anaerobic digestates. Waste Manag. 2021, 136, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, M.R.; Malerba, A.D.; Pezzolla, D.; Gigliotti, G. Chemical and spectroscopic characterization of organic matter during the anaerobic digestion and successive composting of pig slurry. Waste Manag. 2014, 34, 653–660. [Google Scholar] [CrossRef]
- Fernández, C.; Cuetos, M.J.; Martínez, E.J.; Gómez, X. Thermophilic anaerobic digestion of cheese whey: Coupling H2 and CH4 production. Biomass Bioenergy 2015, 81, 55–62. [Google Scholar] [CrossRef]
- Mao, J.; Fang, X.; Schmidt-Rohr, K.; Carmo, A.M.; Hundal, L.S.; Thompson, M.L. Molecular-scale heterogeneity of humic acid in particle-size fractions of two Iowa soils. Geoderma 2007, 140, 17–29. [Google Scholar] [CrossRef]
- Amir, S.; Jouraiphy, A.; Meddich, A.; El Gharous, M.; Winterton, P.; Hafidi, M. Structural study of humic acids during composting of activated sludge-green waste: Elemental analysis, FTIR and 13C NMR. J. Hazard. Mater. 2010, 177, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Cuetos, M.J.; Gómez, X.; Otero, M.; Morán, A. Anaerobic digestion of solid slaughterhouse waste: Study of biological stabilization by Fourier Transform infrared spectroscopy and thermogravimetry combined with mass spectrometry. Biodegradation 2010, 21, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, Y.; Gao, H.J.; Mao, J.; Chu, W.; Thompson, M.L. Biochemical stabilization of soil organic matter in straw-amended, anaerobic and aerobic soils. Sci. Total Environ. 2018, 625, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Martínez, E.J.; Fierro, J.; Sánchez, M.E.; Gómez, X. Anaerobic co-digestion of FOG and sewage sludge: Study of the process by Fourier transform infrared spectroscopy. Int. Biodeterior. Biodegrad. 2012, 75, 1–6. [Google Scholar] [CrossRef]
- Hafidi, M.; Amir, S.; Revel, J.C. Structural characterization of olive mill waster-water after aerobic digestion using elemental analysis, FTIR and 13C NMR. Process Biochem. 2005, 40, 2615–2622. [Google Scholar] [CrossRef]
- Smidt, E.; Parravicini, V. Effect of sewage sludge treatment and additional aerobic post-stabilization revealed by infrared spectroscopy and multivariate data analysis. Bioresour. Technol. 2009, 100, 1775–1780. [Google Scholar] [CrossRef] [PubMed]
- Martínez, E.J.; Gil, M.V.; Fernandez, C.; Rosas, J.G.; Gómez, X. Anaerobic codigestion of sludge: Addition of butcher’s fat waste as a cosubstrate for increasing biogas production. PLoS ONE 2016, 11, e0153139. [Google Scholar] [CrossRef]
- Mehta, N.; Gaëtan, J.; Giura, P.; Azaïs, T.; Benzerara, K. Detection of biogenic amorphous calcium carbonate (ACC) formed by bacteria using FTIR spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 278, 121262. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).