Exploring the Frontier of 3D Bioprinting for Tendon Regeneration: A Review
Abstract
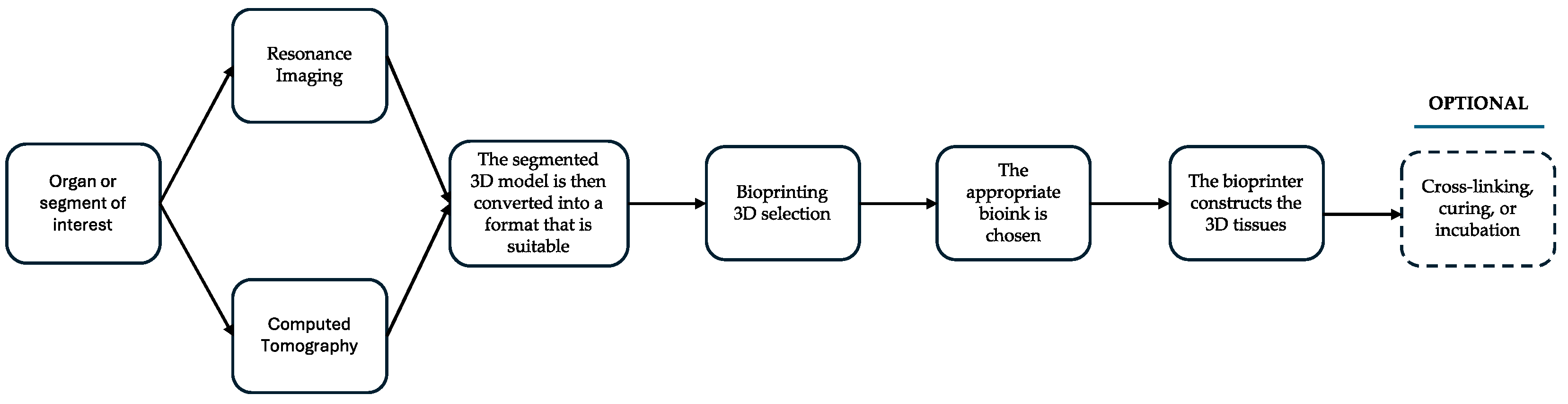
:1. Introduction
2. 3D Printing
2.1. Inkjet Bioprinting
2.2. Extrusion-Based Bioprinting
2.3. Stereolithography
2.4. Laser-Assisted Bioprinting
2.5. Fused Deposition Modeling
3. Bioinks
3.1. Composition and Properties
3.2. Natural and Synthetic Polymers
3.3. Functionalization and Bioactive Additives
3.4. Challenges and Future Directions of Bioinks
| Biomaterial | Properties | Applications in Tendon Bioprinting |
|---|---|---|
| Collagen [26,27,28] | Natural polymer, high biocompatibility, promotes cell adhesion and proliferation | Mimics native extracellular matrix (ECM), supports cellular alignment and growth, used in scaffold fabrication |
| Polylactic Acid (PLA) [19] | Biodegradable, good mechanical strength, tunable degradation rate | Used in FDM printing, provides structural support, and can be combined with other materials for enhanced properties |
| Polycaprolactone (PCL) [25] | Biodegradable, flexible, slow degradation rate | Provides long-term mechanical support, used in combination with bioactive molecules for enhanced regeneration |
| Gelatin Methacrylate (GelMA) [10] | Photocrosslinkable, good cell compatibility, adjustable mechanical properties | Used in Digital Light Processing (DLP) printing, supports cell encapsulation and tissue formation, and can be modified for improved properties |
| Silk Fibroin [10] | High tensile strength, biocompatible, promotes cell attachment | Used for creating mechanically robust scaffolds, supports tendon-like mechanical properties and tissue regeneration |
| Alginate [11] | Biocompatible, easy to process, forms hydrogels upon crosslinking | Used as a bioink component, provides a hydrated environment for cells, often combined with other materials for improved stability |
| Hyaluronic Acid [11,19] | Natural polymer promotes cell migration and proliferation, and hydrophilic | Enhances scaffold hydration and cell migration, used in combination with other materials for improved mechanical properties |
| Decellularized Extracellular Matrix (dECM) [11] | Contains native ECM components, promotes cell attachment and differentiation | Used to create bioactive scaffolds that closely mimic the native tendon environment, support tissue-specific regeneration |
4. Tendon 3D Bioprinting
4.1. Functional Properties of Healthy Tendons
4.2. Functional Properties of 3D Bioprinting Tendons
5. Challenges and Future Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Thorpe, C.T.; Screen, H.R.C. Tendon Structure and Composition. In Metabolic Influences on Risk for Tendon Disorders; Ackermann, P.W., Hart, D.A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 3–10. [Google Scholar]
- Benjamin, M.; Kaiser, E.; Milz, S. Structure-function relationships in tendons: A review. J. Anat. 2008, 212, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.-C.; Guo, Q.; Li, B. Tendon biomechanics and mechanobiology—A mini-review of basic concepts and recent advancements. J. Hand Ther. 2012, 25, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Nordin, M.; Frankel, V.H. Basic Biomechanics of the Musculoskeletal System, 4th ed.Lippincott Williams & Wilkins: Baltimore, MD, USA, 2012. [Google Scholar]
- Resch, H.; Breitfuss, H. Spontaneous tendon ruptures. Etiology, pathogenesis and therapy. Orthopade 1995, 24, 209–219. [Google Scholar] [PubMed]
- Snedeker, J.G.; Foolen, J. Tendon injury and repair—A perspective on the basic mechanisms of tendon disease and future clinical therapy. Acta Biomater. 2017, 63, 18–36. [Google Scholar] [CrossRef]
- França, R.; Winkler, J.; Hsu, H.H.; Rahimnejad, M.; Abdali, Z. Dental Biomaterials; 3D Printing — Additive Manufacturing of Dental BiomaterialsWorld Scientific Series: From Biomaterials Towards Medical Devices; World Scientific: Singapore, 2018; pp. 421–462. [Google Scholar]
- Bianchi, E.; Ruggeri, M.; Rossi, S.; Vigani, B.; Miele, D.; Bonferoni, M.C.; Sandri, G.; Ferrari, F. Innovative Strategies in Tendon Tissue Engineering. Pharmaceutics 2021, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.-C.; Nirmala, X. Application of Tendon Stem/Progenitor Cells and Platelet-Rich Plasma to Treat Tendon Injuries. Oper. Tech. Orthop. 2016, 26, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Alhaskawi, A.; Zhou, H.; Dong, Y.; Zou, X.; Ezzi, S.H.A.; Kota, V.G.; Abdulla, M.H.A.; Tu, T.; Alenikova, O.; Abdalbary, S.; et al. Advancements in 3D-printed artificial tendon. J. Biomed. Mater. Res. Part B Appl. Biomater. 2024, 112, e35364. [Google Scholar] [CrossRef]
- Gao, Q.; Zhao, H.M.; Yang, F.F.; Fu, J.-Z.; He, Y. Practical Laboratory Methods for 3D Bioprinting. In 3D Bioprinting for Reconstructive Surgery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 7–32. [Google Scholar]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D Bioprinting for Biomedical Devices and Tissue Engineering: A Review of Recent Trends and Advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef]
- Vanaei, S.; Parizi, M.S.; Vanaei, S.; Salemizadehparizi, F.; Vanaei, H.R. An Overview on Materials and Techniques in 3D Bioprinting Toward Biomedical Application. Eng. Regen. 2021, 2, 1–18. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Rezvaninejad, R.; Rezvaninejad, R.; França, R. Biomaterials in bone and mineralized tissue engineering using 3D printing and bioprinting technologies. Biomed. Phys. Eng. Express 2021, 7, 062001. [Google Scholar]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Dou, C.; Perez, V.; Qu, J.; Tsin, A.; Xu, B.; Li, J. A State-of-the-Art Review of Laser-Assisted Bioprinting and Its Future Research Trends. ChemBioEng Rev. 2021, 8, 517–534. [Google Scholar] [CrossRef]
- Sorkio, A.; Koch, L.; Koivusalo, L.; Deiwick, A.; Miettinen, S.; Chichkov, B.; Skottman, H. Human Stem Cell Based Corneal Tissue Mimicking Structures Using Laser-Assisted 3D Bioprinting and Functional Bioinks. Biomaterials 2018, 171, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D Bioprinting: An Overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef] [PubMed]
- Benwood, C.; Chrenek, J.; Kirsch, R.L.; Masri, N.Z.; Richards, H.; Teetzen, K.; Willerth, S.M. Natural Biomaterials and Their Use as Bioinks for Printing Tissues. Bioengineering 2021, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, T.G.; Manolesou, D.; Dimakakos, E.; Tsoucalas, G.; Vavuranakis, M.; Tousoulis, D. 3D Bioprinting Methods and Techniques: Applications on Artificial Blood Vessel Fabrication. Acta Cardiol. Sin. 2019, 35, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Screen, H.R.C.; Berk, D.E.; Kadler, K.E.; Ramirez, F.; Young, M.F. Tendon functional extracellular matrix. J. Orthop. Res. 2015, 33, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Maganaris, C.N.; Paul, J.P. In vivo human tendon mechanical properties. J. Physiol. 1999, 521 Pt 1, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Murrell, G.A.C. The basic science of tendinopathy. Clin. Orthop. Relat. Res. 2008, 466, 1528–1538. [Google Scholar] [CrossRef]
- Potyondy, T.; Uquillas, J.A.; Tebon, P.J.; Byambaa, B.; Hasan, A.; Tavafoghi, M.; Mary, H.; Aninwene, G.E.; Pountos, I.; Khademhosseini, A.; et al. Recent advances in 3D bioprinting of musculoskeletal tissues. Biofabrication 2021, 13, 022001. [Google Scholar] [CrossRef]
- Wu, Y.; Jyh, F.; YS, W.; Sun, J. Fabrication of 3D scaffolds via E-jet printing for tendon tissue repair. In Proceedings of the ASME 2015 International Manufacturing Science and Engineering Conference, Charlotte, NC, USA, 8–12 June 2015. [Google Scholar]
- Mozdzen, L.C.; Rodgers, R.; Banks, J.M.; Bailey, R.C.; Harley, B.A. Increasing the strength and bioactivity of collagen scaffolds using customizable arrays of 3D-printed polymer fibers. Acta Biomater. 2016, 33, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Kishore, V.; Uquillas, J.A.; Dubikovsky, A.; Alshehabat, M.A.; Snyder, P.W.; Breur, G.J.; Akkus, O. In vivo response to electrochemically aligned collagen bioscaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Uquillas, J.A.; Kishore, V.; Akkus, O. Genipin crosslinking elevates the strength of electrochemically aligned collagen to the level of tendons. J. Mech. Behav. Biomed. Mater. 2012, 15, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Viidik, A. The effect of training on the tensile strength of isolated rabbit tendons. Scand. J. Plast. Reconstr. Surg. 1967, 1, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.-H.; Lee, Y.-J.; Park, N.R.; Heo, S.C.; Hudson, D.M.; Fernandes, A.A.; Friday, C.S.; Hast, M.W.; Corr, D.T.; Keene, D.R.; et al. Characterization of TGFβ1-induced tendon-like structure in the scaffold-free three-dimensional tendon cell culture system. Sci. Rep. 2024, 14, 9495. [Google Scholar] [CrossRef] [PubMed]
- Skylar-Scott, M.A.; Uzel, S.G.M.; Nam, L.L.; Ahrens, J.H.; Truby, R.L.; Damaraju, S.; Lewis, J.A. Biomanufacturing of Organ-Specific Tissues with High Cellular Density and Embedded Vascular Channels. Sci. Adv. 2019, 5, 2459. [Google Scholar] [CrossRef] [PubMed]
- Grigoryan, B.; Paulsen, S.J.; Corbett, D.C.; Sazer, D.W.; Fortin, C.L.; Zaita, A.J.; Greenfield, P.T.; Calafat, N.J.; Gounley, J.P.; Ta, A.H.; et al. Multivascular Networks and Functional Intravascular Topologies within Biocompatible Hydrogels. Science 2019, 364, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lim, J.; Teoh, S.H. Review: Development of clinically relevant scaffolds for vascularised bone tissue engineering. Biotechnol. Adv. 2018, 36, 684–702. [Google Scholar]
- Visser, J.; Peters, B.; Burger, T.J.; Boomstra, J.; Dhert, W.J.A.; Melchels, F.P.W.; Malda, J. Biofabrication of multi-material anatomically shaped tissue constructs. Biofabrication 2015, 7, 035009. [Google Scholar]
- Koch, L.; Deiwick, A.; Schlie, S.; Michael, S.; Gruene, M.; Coger, V.; Zychlinski, D.; Schambach, A.; Reimers, K.; Vogt, P.M.; et al. Skin tissue generation by laser cell printing. Biotechnol. Bioeng. 2012, 109, 1855–1863. [Google Scholar] [CrossRef]
- Costa, P.F.; Vaquette, C.; Zhang, Q.; Reis, R.L.; Ivanovski, S.; Hutmacher, D.W. Advanced tissue engineering scaffold design for regeneration of the complex hierarchical periodontal structure. J. Clin. Periodontol. 2013, 41, 283–294. [Google Scholar] [CrossRef] [PubMed]
| Bioprinting Technique
and References | Example of Application | Advantages |
|---|---|---|
| Inkjet Bioprinting [23] | Creating cell-laden constructs for tendon repair by precisely depositing droplets of bioink containing tendon-derived cells and growth factors. | High resolution and precision, ability to print multiple cell types and bioactive molecules simultaneously, relatively low cost, and rapid printing speed. |
| Extrusion-Based Bioprinting [33] | Fabricating PCL scaffolds that support tenocyte proliferation and alignment, enhancing tendon regeneration. | Ability to print a wide range of biomaterials, high mechanical strength of printed constructs, suitability for creating large and complex structures, incorporation of cells and growth factors within the bioink. |
| Stereolithography (SLA) [34] | Creating high-resolution GelMA-based scaffolds with intricate microarchitectures that mimic the native tendon structure, promoting cell viability and alignment. | High resolution and precision, ability to create complex and detailed structures, suitability for printing photocrosslinkable hydrogels. |
| Laser-Assisted Bioprinting (LAB) [35] | Depositing cells and biomaterials with high precision to create constructs that promote tendon regeneration, such as patterning tenocytes and ECM components. | High precision and resolution, ability to print cells and biomaterials without direct contact, minimal thermal damage to cells, creation of highly detailed and organized tissue constructs. |
| Fused Deposition Modeling (FDM) [36] | Fabricating PCL scaffolds that mimic the mechanical properties of native tendons, supporting cell attachment, proliferation, and alignment. | High mechanical strength and stability of printed constructs, ability to print a wide range of thermoplastic materials, suitability for creating large and complex structures, relatively cost-effective and widely accessible. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosset, J.; Olaniyanu, E.; Stein, K.; Almeida, N.D.; França, R. Exploring the Frontier of 3D Bioprinting for Tendon Regeneration: A Review. Eng 2024, 5, 1838-1849. https://doi.org/10.3390/eng5030098
Rosset J, Olaniyanu E, Stein K, Almeida ND, França R. Exploring the Frontier of 3D Bioprinting for Tendon Regeneration: A Review. Eng. 2024; 5(3):1838-1849. https://doi.org/10.3390/eng5030098
Chicago/Turabian StyleRosset, Josée, Emmanuel Olaniyanu, Kevin Stein, Nátaly Domingues Almeida, and Rodrigo França. 2024. "Exploring the Frontier of 3D Bioprinting for Tendon Regeneration: A Review" Eng 5, no. 3: 1838-1849. https://doi.org/10.3390/eng5030098
APA StyleRosset, J., Olaniyanu, E., Stein, K., Almeida, N. D., & França, R. (2024). Exploring the Frontier of 3D Bioprinting for Tendon Regeneration: A Review. Eng, 5(3), 1838-1849. https://doi.org/10.3390/eng5030098







