Microelectrode Studies of Tertiary Amines in Organic Solvents: Considering Triethanolamine to Estimate the Composition of Acetic Acid–Ethyl Acetate Mixtures
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
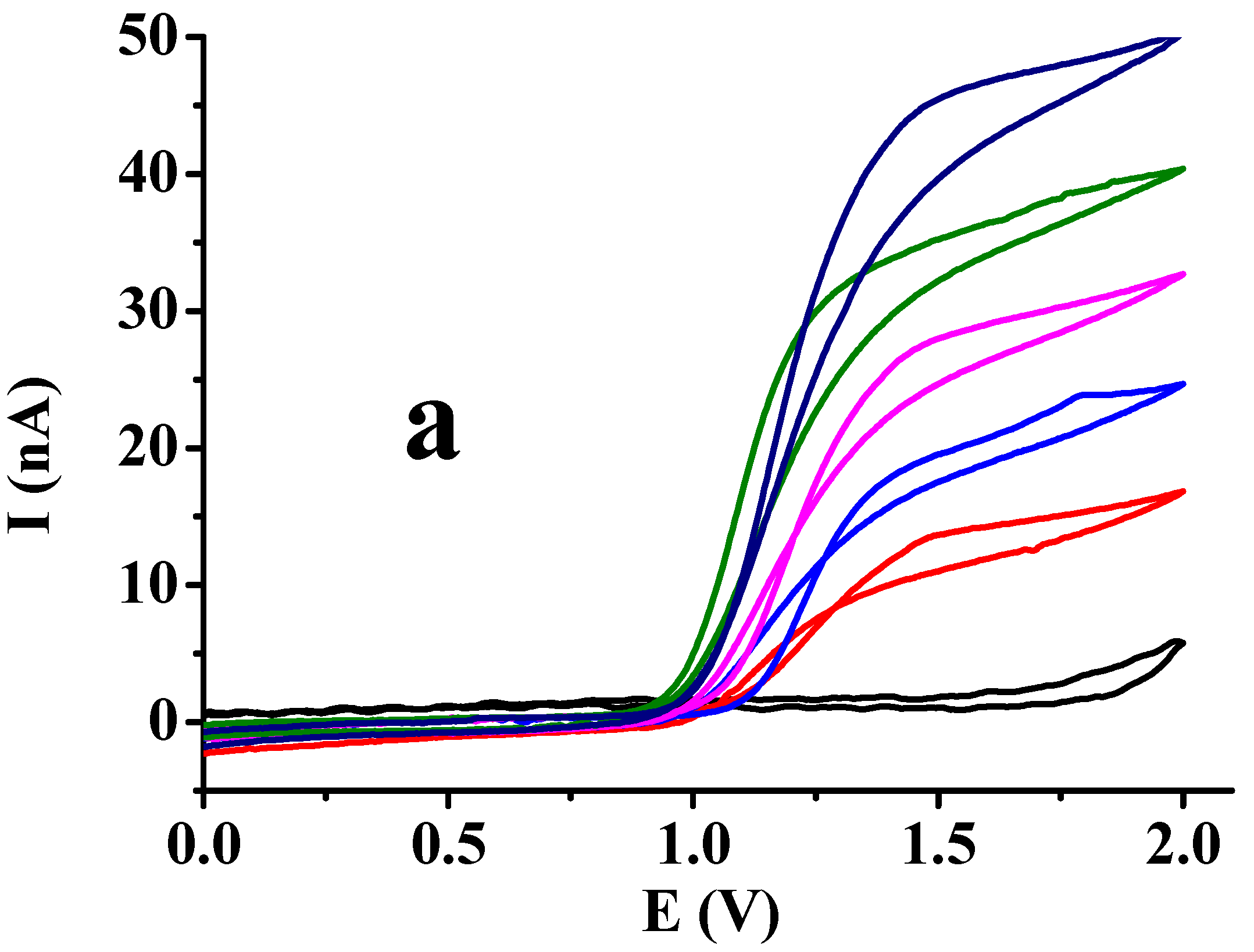
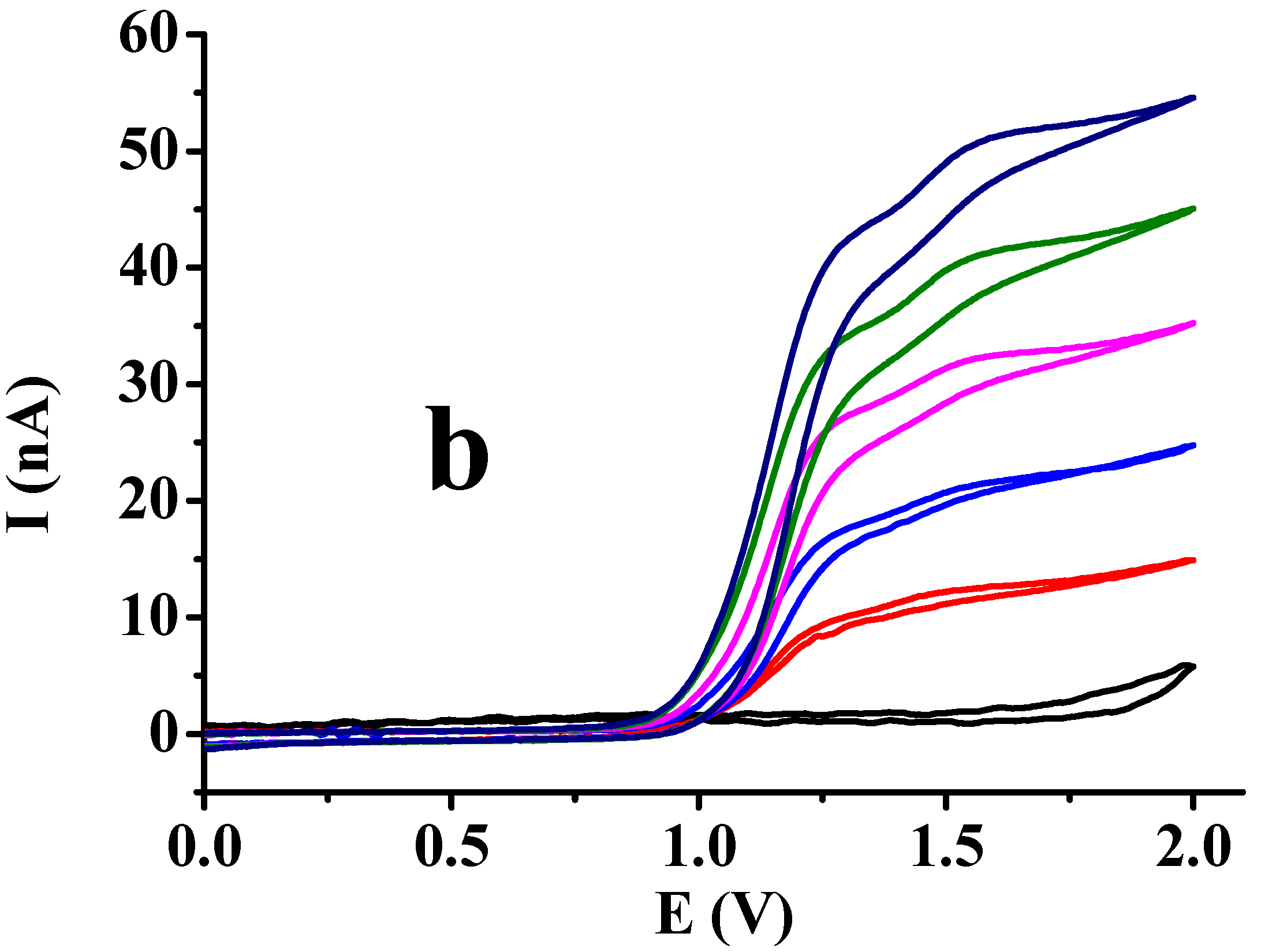
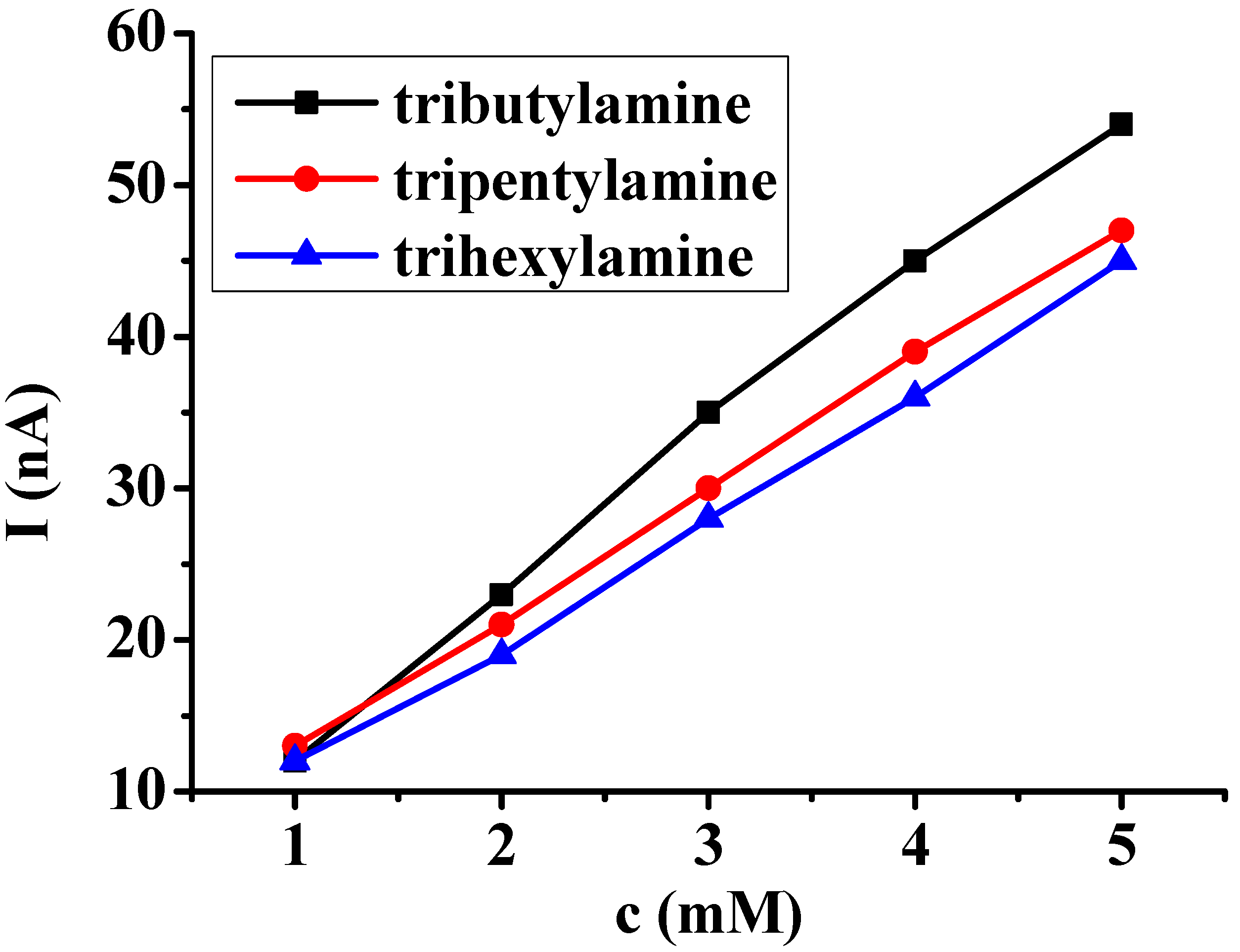
3.1. Studies of Different Organic Solvents
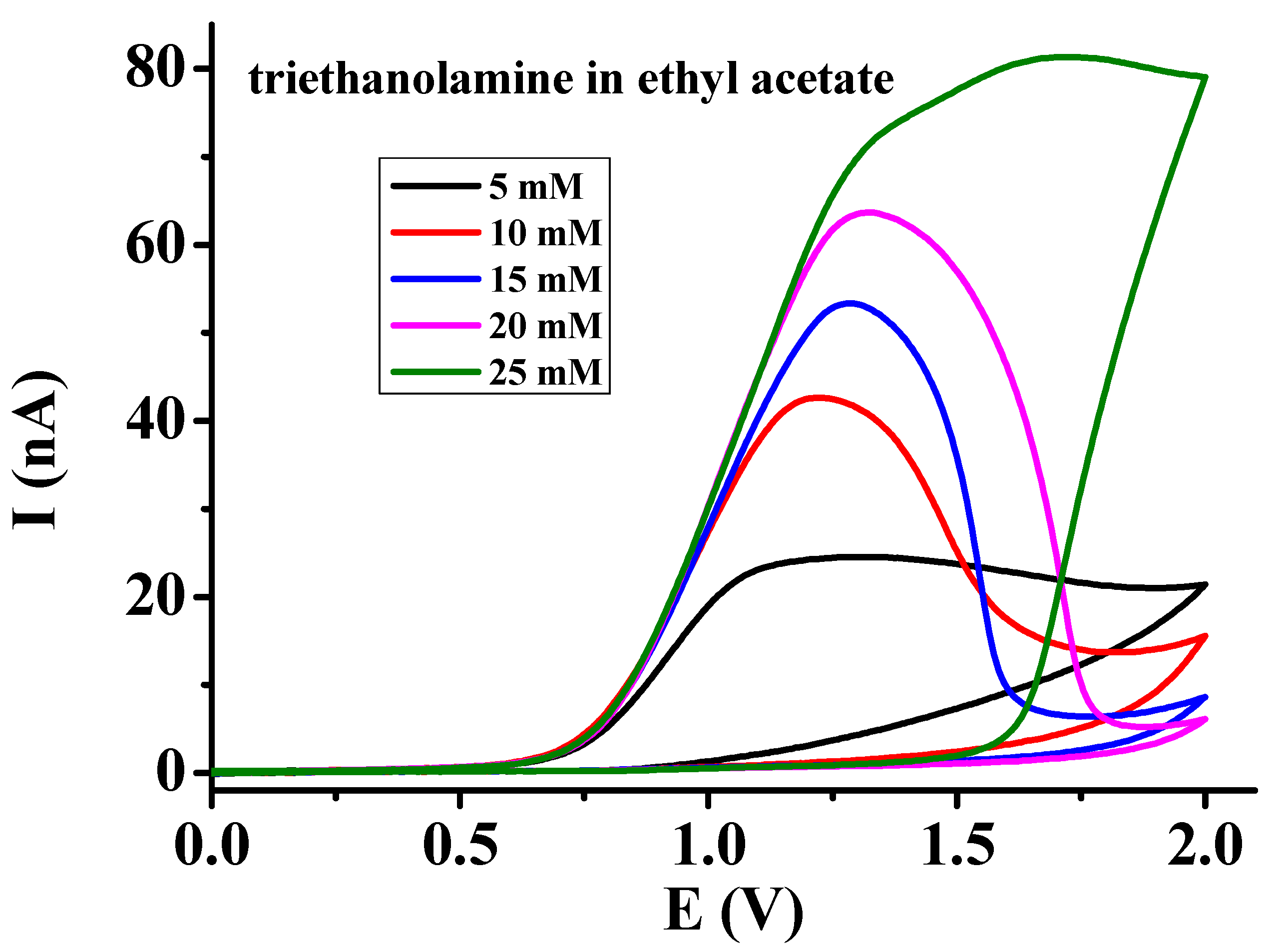
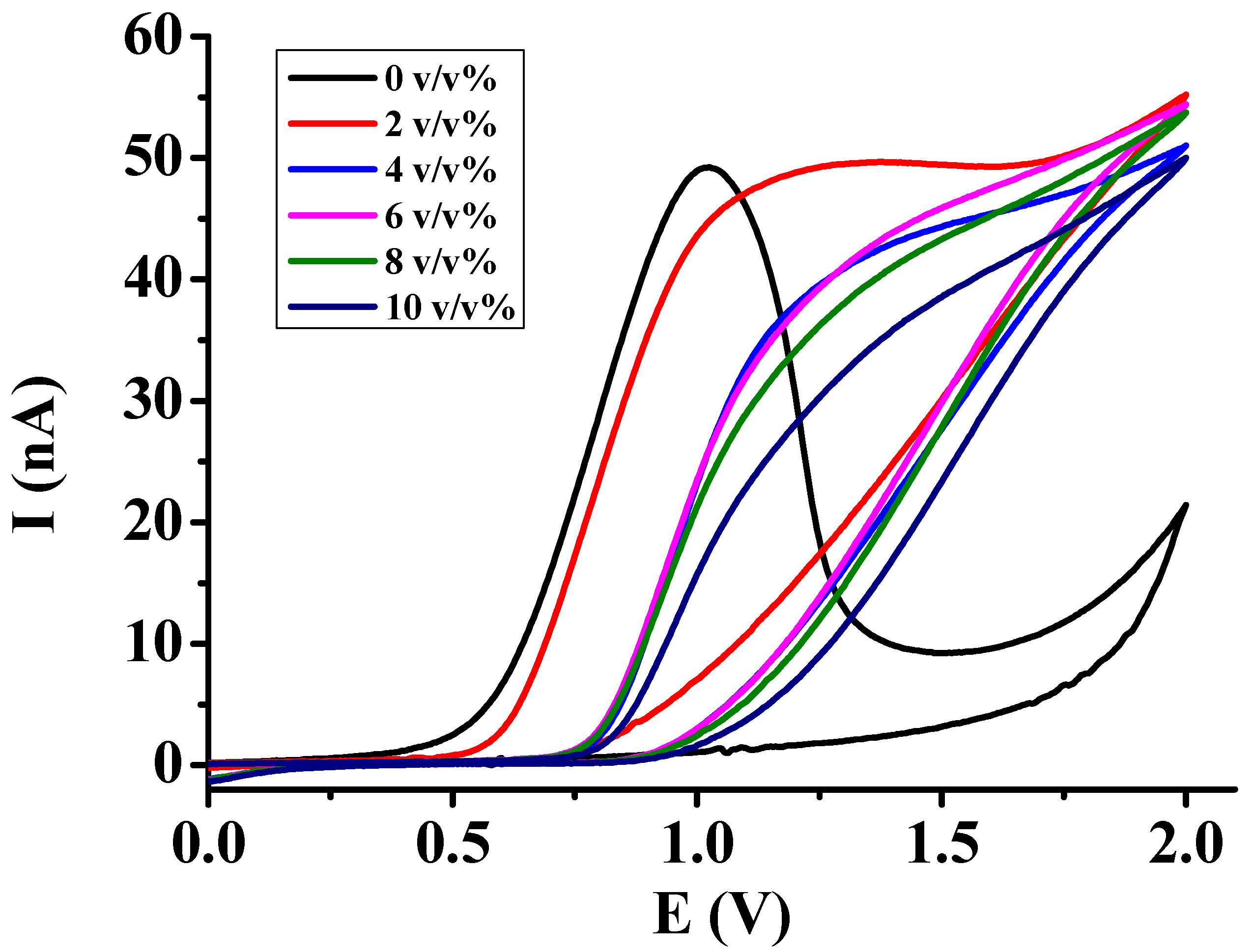
3.2. Investigations in Acetic Acid, Ethyl Acetate, and Their Binary Mixtures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Myland, J.C.; Oldham, K.B. General theory of steady-state voltammetry. J. Electroanal. Chem. 1993, 347, 49–91. [Google Scholar] [CrossRef]
- Oldham, K.B. Theory of steady-state voltammetry without supporting electrolyte. J. Electroanal. Chem. 1992, 337, 91–126. [Google Scholar] [CrossRef]
- Cooper, J.B. Microelectrode studies without supporting electrolyte: Model and experimental comparison for singly and multiply charged ions. J. Electroanal. Chem. 1992, 331, 877–895. [Google Scholar] [CrossRef]
- Bond, A.M.; Fleischmann, M.; Robinson, J. Electrochemistry in organic solvents without supporting electrolyte using platinum microelectrodes. J. Electroanal. Chem. 1984, 168, 299–312. [Google Scholar] [CrossRef]
- Bradford, D.P.; Hector, D.A.; John, D.N.; Wendy, E.B.; Henry, S.W. Analysis of voltammetric half-wave potentials in low ionic strength solutions and voltammetric measurements of ion impurity concentrations. Anal. Chem. 1991, 63, 2766–2771. [Google Scholar] [CrossRef]
- David, O.W.; Wightman, R.M. Voltammetry with microvoltammetric electrodes in resistive solvents under linear diffusion conditions. Anal. Chem. 1990, 62, 98–102. [Google Scholar] [CrossRef]
- Bond, A.M.; Stephen, W.F.; Howard, B.G.; Peter, J.M.; Ray, C.; Tania, W. Instrumental, theoretical, and experimental aspects of determining thermodynamic and kinetic parameters from steady-state and non-steady-state cyclic voltammetry at microelectrodes in high-resistance solvents: Application to the fac/mer-[Cr(CO)3(η3−Ph2PCH2CH2P(Ph)CH2CH2PPh2)]+/0 square reaction scheme in dichloromethane. Anal. Chem. 1992, 64, 1014–1021. [Google Scholar]
- Oldham, K.B.; Terence, J.C.; Jose, H.S.; Bond, A.M. Effect of ion pairing on steady-state voltammetric limiting currents at microelectrodes. Part II. Experimental studies on charged (Br−, Ag+) and uncharged (copper diethyldithiocarbamate) species in toluene. J. Electroanal. Chem. 1997, 430, 39–46. [Google Scholar] [CrossRef]
- Darío, L.G.; Horacio, R.C. Electrochemistry in supercritical trifluoromethane. Electrochem. Commun. 2000, 2, 663–670. [Google Scholar] [CrossRef]
- Geng, L.; Andrew, G.E.; Jernigan, J.C.; Royce, W.M. Electrochemical reactions of solutes and of electroactive polymer films in low dielectric media: Toluene and heptane. Anal. Chem. 1986, 58, 852–860. [Google Scholar] [CrossRef]
- Bond, A.M.; Mann, T.F. Voltammetric measurements without ohmic and other forms of distortion in aromatic hydrocarbon solvents. Electrochim. Acta 1987, 32, 863–870. [Google Scholar] [CrossRef]
- Yun, F.; Johna, L. Voltammetry in gas phase environments. J. Electroanal. Chem. 1995, 384, 5–17. [Google Scholar] [CrossRef]
- Pamela, A.M.; Stephen, H.L. Feasibility studies for the detection of volatile organic compounds in the gas phase using microelectrode sensors. J. Electroanal. Chem. 1999, 460, 105–118. [Google Scholar] [CrossRef]
- John, R.L.S.; David, M. Amine oxidation. Part 13. Electrochemical oxidation of some substituted tertiary alkylamines. J. Chem. Soc. Perkin Trans. 1977, 2, 1732–1736. [Google Scholar]
- Teresa, L. Electrochemical behaviour of benzylamine, 2-phenylethylamine and 4-hydroxyphenylethylamine at gold. A comparative study. J. Appl. Electrochem. 2008, 38, 43–50. [Google Scholar]
- Teresa, L. Electrochemical oxidation of alkoholamines at gold. J. Appl. Electrochem. 2007, 37, 653–660. [Google Scholar]
- John, R.L.S.; David, M. Amine oxidation. Part IX. The electrochemical oxidation of some tertiary amines. The effect of structure on reactivity. J. Chem. Soc. Perkin Trans. 1976, 2, 47–51. [Google Scholar]
- Ashwin, K.V.M.; Angel, A.J.T. Mechanistic aspects of the electrochemical oxidation of aliphatic amines and aniline derivatives. Molecules 2023, 28, 471. [Google Scholar]
- Weinberg, N.L.; Brown, E.A. The anodic oxidation of organic compounds. II. The electrochemical alkoxylation of tertiary amines. J. Org. Chem. 1966, 31, 4058–4061. [Google Scholar] [CrossRef]
- Kiss, L.; Szabó, P. Acetic acid and ethyl acetate as solvents for electropolymerization reactions, considering 4-methoxyphenol and composition of solvent mixtures. Organics 2024, 5, 670–683. [Google Scholar] [CrossRef]
- Kiss, L. Studies on square wave and cyclic voltammetric behavior of 1,2- and 1,4-dihydroxybenzenes and their derivatives in acetic acid, ethyl acetate and mixtures of the two. Methods Protoc. 2024, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Agüí, L.; López-Guzmán, J.E.; González-Cortés, A.; Yánez-Sedeno, P.; Pingarrón, J.M. Analytical performance of cylindrical carbon fiber microelectrodes in low-permittivity organic solvents: Determination of vanillin in ethyl acetate. Anal. Chim. Acta 1999, 385, 241–248. [Google Scholar] [CrossRef]
- Hernández-Olmos, M.A.; Agüí, L.; Yánez-Sedeno, P.; Pingarrón, J.M. Analytical voltammetry in low-permittivity organic solvents using disk and cylindrical microelectrodes. Determination of thiram in ethyl acetate. Electrochim. Acta 2000, 46, 289–296. [Google Scholar] [CrossRef]
- Michalkiewicz, S.; Monika, T.; Maria, K.; Jan, M. Electrochemical behavior of tocopherols on microelectrodes in acetic acid medium. Electroanalysis 2002, 14, 297–302. [Google Scholar] [CrossRef]
- Michalkiewicz, S.; Monika, M.; Jan, M. Voltammetric study of some synthetic antioxidants on platinum microelectrodes in acetic acid medium. Electroanalysis 2004, 16, 588–595. [Google Scholar] [CrossRef]
- Michalkiewicz, S. Anodic oxidation of parabens in acetic acid–acetonitrile solutions. J. Appl. Electrochem. 2013, 43, 85–97. [Google Scholar] [CrossRef]
- Jan, M.; Monika, K. Electrochemical oxidation of trolox and α-tocopherol in acetic acid: A comparative study. J. Electroanal. Chem. 2006, 595, 136–144. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiss, L.; Kunsági-Máté, S. Microelectrode Studies of Tertiary Amines in Organic Solvents: Considering Triethanolamine to Estimate the Composition of Acetic Acid–Ethyl Acetate Mixtures. Eng 2025, 6, 280. https://doi.org/10.3390/eng6100280
Kiss L, Kunsági-Máté S. Microelectrode Studies of Tertiary Amines in Organic Solvents: Considering Triethanolamine to Estimate the Composition of Acetic Acid–Ethyl Acetate Mixtures. Eng. 2025; 6(10):280. https://doi.org/10.3390/eng6100280
Chicago/Turabian StyleKiss, László, and Sándor Kunsági-Máté. 2025. "Microelectrode Studies of Tertiary Amines in Organic Solvents: Considering Triethanolamine to Estimate the Composition of Acetic Acid–Ethyl Acetate Mixtures" Eng 6, no. 10: 280. https://doi.org/10.3390/eng6100280
APA StyleKiss, L., & Kunsági-Máté, S. (2025). Microelectrode Studies of Tertiary Amines in Organic Solvents: Considering Triethanolamine to Estimate the Composition of Acetic Acid–Ethyl Acetate Mixtures. Eng, 6(10), 280. https://doi.org/10.3390/eng6100280





