Recent Advances in Plasma-Based Cancer Treatments: Approaching Clinical Translation through an Intracellular View
Abstract
:1. Background and Motivation
2. Low Temperature Plasma Sources for Medical Applications
3. Mechanisms of Action of Plasma-Generated Species in Inhibition and Treatment of Cancer
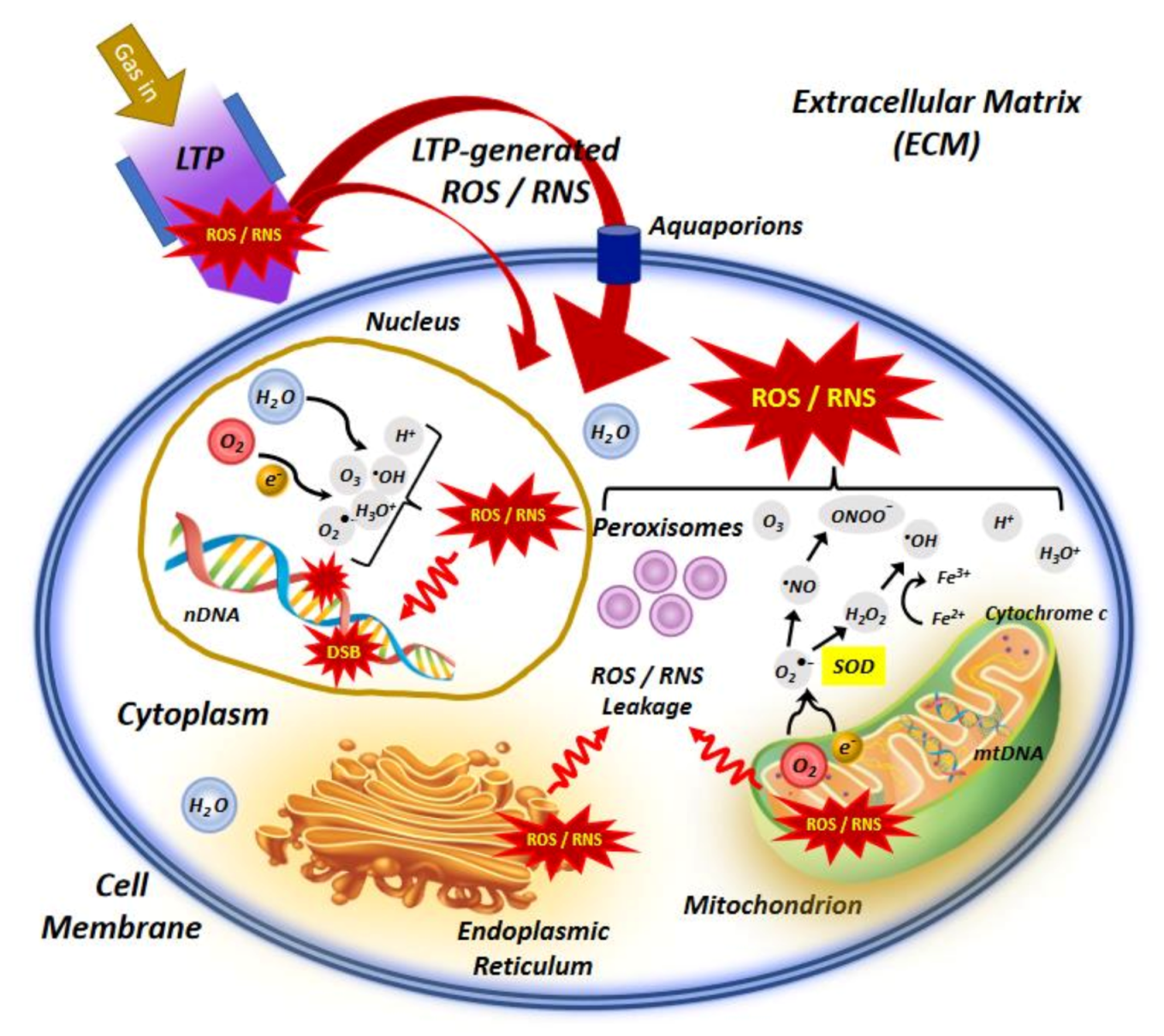
3.1. Production of Endogenous ROS without Plasma Exposure
3.2. Production of Mitochondria-Dependent ROS after Plasma Treatment
3.3. Oxidative Stress and Gene Expression
4. Challenges and Future Perspectives for Clinical Applications
Funding
Conflicts of Interest
Abbreviations
| °C | degree Celsius |
| Ar | Argon |
| ATP | adenosine triphosphate |
| Bcl-2 | B-cell lymphoma 2 |
| Ca2+ | Calcium ion |
| CCRT | concurrent chemoradiation therapy |
| Cdc2 | cyclin-dependent kinase 1 |
| CRT | chemoradiation therapy |
| DBD | dielectric barrier discharge |
| Drp1 | dynamin-related protein 1, also called dynamin-1-like protein |
| DSB | double strand break |
| ER | endoplasmic reticulum |
| ERO1 | endoplasmic reticulum oxidoreductin 1 |
| ETC | electron transport chain |
| FE-DBD | floating electrode dielectric barrier discharge |
| GPx | glutathione peroxidase |
| GSH | glutathione |
| H2O2 | hydrogen peroxide |
| He | helium |
| LTP | low temperature plasma |
| µs | microsecond |
| mtDNA | mitochondrial DNA |
| NADH | nicotinamide adenine dinucleotide |
| nDNA | nuclear DNA |
| NEAPP | non-equilibrium atmospheric pressure plasma |
| Ne | neon |
| ns | nanosecond |
| NO | nitric oxide |
| NO2− | nitrite |
| NTP | non-thermal plasma |
| O2 | molecular oxygen |
| O2•− | superoxide radical |
| OH | hydroxide |
| PAM | plasma-activated medium |
| PDI | protein disulfide isomerase |
| PS-MWM | microwave plasma source |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| rRNA | ribosomal ribonucleic acid |
| SMD | surface micro discharge |
| SOD2 | superoxide dismutase |
| TCA | tricarboxylic acid |
| tRNA | transfer ribonucleic acid |
| UV | ultraviolet |
| UV-Vis | ultraviolet-visible |
| VUV | vacuum ultraviolet |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2019, 69, 7–30. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Available online: https://www.who.int/health-topics/cancer#tab=tab_1 (accessed on 3 January 2021).
- Siva, S.; MacManus, M.P.; Martin, R.F.; Martin, O.A. Abscopal effects of radiation therapy: A clinical review for the radiobiologist. Cancer Lett. 2015, 356, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Ryan, E.J.; Doherty, G.A. Gastro-intestinal toxicity of chemotherapeutics in colorectal cancer: The role of inflammation. World J. Gastroenterol. 2014, 20, 3751–3761. [Google Scholar] [CrossRef]
- Trueb, R.M. Chemotherapy-induced hair loss. Skin Ther. Lett. 2010, 15, 5–7. [Google Scholar]
- Rezaee, M.; Alizadeh, E.; Cloutier, P.; Hunting, D.J.; Sanche, L. A single subexcitation-energy electron can induce a double-strand break in DNA modified by platinum chemotherapeutic drugs. Chem. Med. Chem. 2014, 9, 1145–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seiwert, T.; Salama, J.; Vokes, E. The concurrent chemoradiation paradigm—General principles. Nat. Rev. Clin. Oncol. 2007, 4, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Charest, G.; Tippayamontri, T.; Shi, M.; Wehbe, M.; Anantha, M.; Bally, M.; Sanche, L. Concomitant chemoradiation therapy with gold nanoparticles and platinum drugs co-encapsulated in liposomes. Int. J. Mol. Sci. 2020, 21, 4848. [Google Scholar] [CrossRef] [PubMed]
- Semmler, M.L.; Bekeschus, S.; Schäfer, M.; Bernhardt, T.; Fischer, T.; Witzke, K.; Seebauer, C.; Rebl, H.; Grambow, E.; Vollmar, B.; et al. Molecular mechanisms of the efficacy of cold atmospheric pressure plasma (CAP) in cancer treatment. Cancers 2020, 12, 269. [Google Scholar] [CrossRef] [Green Version]
- Gümbel, D.; Bekeschus, S.; Gelbrich, N.; Napp, M.; Ekkernkamp, A.; Kramer, A.; Stope, M.B. Cold atmospheric plasma in the treatment of osteosarcoma. Int. J. Mol. Sci. 2017, 18, 2004. [Google Scholar] [CrossRef] [Green Version]
- Saadati, F.; Mahdikia, H.; Abbaszadeh, H.; Khoramgah, M.S.; Shokri, B. Comparison of direct and indirect cold atmospheric-pressure plasma methods in the B16F10 melanoma cancer cells treatment. Sci. Rep. 2018, 8, 7689. [Google Scholar] [CrossRef] [Green Version]
- Laroussi, M.; Lu, X.; Keidar, M. Perspective: The physics, diagnostics, and applications of atmospheric pressure low temperature plasma sources used in plasma medicine. J. Appl. Phys. 2017, 122, 020901. [Google Scholar] [CrossRef]
- Lu, X.; Naidis, G.V.; Laroussi, M.; Reuter, S.; Graves, D.B.; Ostrikov, K. Reactive species in non-equilibrium atmospheric-pressure plasmas: Generation, transport, and biological effects. Phys. Rep. 2016, 630, 1–84. [Google Scholar] [CrossRef] [Green Version]
- Babaeva, N.Y.; Naidis, G.V.; Tereshonok, D.V.; Son, E.E.; Vasiliev, M.M.; Petrov, O.F.; Fortov, V.E. Production of active species in argon microwave plasma torch. J. Phys. D Appl. Phys. 2018, 51, 464004. [Google Scholar] [CrossRef]
- Schlegel, J.; Köritzer, J.; Boxhammer, V. Plasma in cancer treatment. Clin. Plasma Med. 2013, 1, 2–7. [Google Scholar] [CrossRef]
- Dobrynin, D.; Fridman, G.; Friedman, G.; Fridman, A. Physical and biological mechanisms of direct plasma interaction with living tissue. New J. Phys. 2009, 11, 115020. [Google Scholar] [CrossRef]
- Laroussi, M. Nonthermal decontamination of biological media by atmospheric-pressure plasmas: Review, analysis, and prospects. IEEE Trans. Plasma Sci. 2002, 30, 1409–1415. [Google Scholar] [CrossRef]
- Sakudo, A.; Misawa, T.; Shimizu, N.; Imanishi, Y. N2 gas plasma inactivates influenza virus mediated by oxidative stress. Front. Biosci. 2014, 6, 69–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weltmann, K.D.; von Woedtke, T. Plasma medicine—Current state of research and medical application. Plasma Phys. Control. Fusion 2017, 59, 014031. [Google Scholar] [CrossRef]
- Chizoba Ekezie, F.G.; Sun, D.W.; Cheng, J.H. A review on recent advances in cold plasma technology for the food industry: Current applications and future trends. Trends Food Sci. Technol. 2017, 69, 46–58. [Google Scholar] [CrossRef]
- Stoffels, E.; Sladek, R.E.J.; Kieft, I.E. Gas plasma effects on living cells. Phys. Scr. 2004, T107, 79–82. [Google Scholar] [CrossRef]
- Azzariti, A.; Iacobazzi, R.M.; Di Fonte, R.; Porcelli, L.; Gristina, R.; Favia, P.; Fracassi, F.; Trizio, I.; Silvestris, N.; Guida, G.; et al. Plasma-activated medium triggers cell death and the presentation of immune activating danger signals in melanoma and pancreatic cancer cells. Sci. Rep. 2019, 9, 4099. [Google Scholar] [CrossRef]
- Bruggeman, P.J.; Kushner, M.J.; Locke, B.R.; Gardeniers, J.G.E.; Graham, W.G.; Graves, D.B.; Hofman-Caris, R.C.H.M.; Maric, D.; Reid, J.P.; Ceriani, E.; et al. Plasma-liquid interactions: A review and roadmap. Plasma Sources Sci. Technol. 2016, 25, 053002. [Google Scholar] [CrossRef]
- Fridman, G.; Shereshevsky, A.; Jost, M.M.; Brooks, A.D.; Fridman, A.; Gutsol, A.; Vasilets, V.; Friedman, G. Floating electrode dielectric barrier discharge plasma in air promoting apoptotic behavior in melanoma skin cancer cell lines. Plasma Chem. Plasma Process. 2007, 27, 163–176. [Google Scholar] [CrossRef]
- Fridman, G.; Peddinghaus, M.; Ayan, H.; Fridman, A.; Balasubramanian, M.; Gutsol, A.; Brooks, A.; Friedman, G. Blood coagulation and living tissue sterilization by floating-electrode dielectric barrier discharge in air. Plasma Chem. Plasma Process 2006, 26, 425–442. [Google Scholar] [CrossRef]
- Fridman, G.; Friedman, G.; Gutsol, A.; Shekhter, A.B.; Vasilets, V.N.; Fridman, A. Applied plasma medicine. Plasma Process. Polym. 2008, 5, 503–533. [Google Scholar] [CrossRef]
- Keidar, M.; Walk, R.; Shashurin, A.; Srinivasan, P.; Sandler, A.; Dasgupta, S.; Ravi, R.; Guerrero-Preston, R.; Trink, B. Cold plasma selectivity and the possibility of a paradigm shift in cancer therapy. Br. J. Cancer 2011, 105, 1295–1301. [Google Scholar] [CrossRef]
- Gay-Mimbrera, J. Clinical and biological principles of cold atmospheric plasma application in skin cancer. Advances in therapy 2016, 33, 894–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitra, S.; Nguyen, L.N.; Akter, M.; Park, G.; Choi, E.H.; Kaushik, N.K. Impact of ROS generated by chemical, physical, and plasma techniques on cancer attenuation. Cancers 2019, 11, 1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuhvatulin, A.I.; Sysolyatina, E.V.; Scheblyakov, D.V.; Logunov, D.Y.; Vasiliev, M.M.; Yurova, M.A.; Danilova, M.A.; Petrov, O.F.; Naroditsky, B.S.; Morfill, G.E.; et al. Non-thermal plasma causes p53-dependent apoptosis in human colon carcinoma cells. Acta Nat. 2012, 4, 82–87. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.H.; Li, M.; Zhou, R.; Feng, K.; Yang, S. Ablation of liver cancer cells in vitro by a plasma needle. Appl. Phys. Lett. 2008, 93, 021502. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Shon, C.H.; Kim, Y.S.; Kim, S.; Kim, G.C.; Kong, M.G. Degradation of adhesion molecules of G361 melanoma cells by a non-thermal atmospheric pressure microplasma. New J. Phys. 2009, 11, 115026. [Google Scholar] [CrossRef]
- Kim, G.C.; Kim, G.J.; Park, S.R.; Jeon, S.M.; Seo, H.J.; Iza, F.; Lee, J.K. Air plasma coupled with antibody-conjugated nanoparticles: A new weapon against cancer. J. Phys. D Appl. Phys. 2009, 42, 032005. [Google Scholar] [CrossRef]
- Lupu, A.R.; Georgescu, N.; Calugaru, A.; Cremer, L.; Szegli, G.; Kerek, F. The effects of cold atmospheric plasma jets on B16 and COLO320 tumoral cells. Roum. Arch. Microbiol. Immunol. 2009, 68, 136–144. [Google Scholar]
- Georgescu, N.; Lupu, A.R. Tumoral and normal cells treatment with high-voltage pulsed cold atmospheric plasma jets. IEEE Trans. Plasma Sci. 2010, 38, 1949–1955. [Google Scholar] [CrossRef]
- Kim, C.H.; Bahn, J.H.; Lee, S.H.; Kim, G.Y.; Jun, S.I.; Lee, K.; Baek, S.J. Induction of cell growth arrest by atmospheric non-thermal plasma in colorectal cancer cells. J. Biotechnol. 2010, 150, 530–538. [Google Scholar] [CrossRef]
- Zirnheld, J.L.; Zucker, S.N.; DiSanto, T.M.; Berezney, R.; Etemadi, K. Nonthermal plasma needle: Development and targeting of melanoma cells. IEEE Trans. Plasma Sci. 2010, 38, 948–952. [Google Scholar] [CrossRef]
- Ahn, H.J.; Kim, K.I.; Kim, G.; Moon, E.; Yang, S.S.; Lee, J.S. Atmospheric-pressure plasma jet induces apoptosis involving mitochondria via generation of free radicals. PLoS ONE. 2011, 6, e28154. [Google Scholar] [CrossRef] [Green Version]
- Kalghatgi, S.; Kelly, C.M.; Cerchar, E.; Torabi, B.; Alekseev, O.; Fridman, A.; Friedman, G.; Azizkhan-Clifford, J. Effects of non-thermal plasma on mammalian cells. PLoS ONE 2011, 6, e16270. [Google Scholar] [CrossRef] [Green Version]
- Sensenig, R.; Kalghatgi, S.; Cerchar, E.; Fridman, G.; Shereshevsky, A.; Torabi, B.; Arjunan, K.P.; Podolsky, E.; Fridman, A.; Friedman, G.; et al. Non-thermal plasma induces apoptosis in melanoma cells via production of intracellular reactive oxygen species. Ann. Biomed. Eng. 2011, 39, 674–687. [Google Scholar] [CrossRef]
- Kim, J.Y.; Ballato, J.; Foy, P.; Hawkins, T.; Wei, Y.; Li, J.; Kim, S.O. Apoptosis of lung carcinoma cells induced by a flexible optical fiber-based cold microplasma. Biosens. Bioelectron. 2011, 28, 333–338. [Google Scholar] [CrossRef]
- Barekzi, N.; Laroussi, M. Dose-dependent killing of leukemia cells by low-temperature plasma. J. Phys. D Appl. Phys. 2012, 45, 422002. [Google Scholar] [CrossRef]
- Brulle, L.; Vandamme, M.; Ries, D.; Martel, E.; Robert, E.; Lerondel, S.; Trichet, V.; Richard, S.; Pouvesle, J.M.; Le Pape, A. Effects of a non thermal plasma treatment alone or in combination with gemcitabine in a MIA PaCa2-luc orthotopic pancreatic carcinoma model. PLoS ONE 2012, 7, e52653. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Uhm, H.; Choi, E.H. Micronucleus formation induced by dielectric barrier discharge plasma exposure in brain cancer cells. Appl. Phys. Lett. 2012, 100, 084102. [Google Scholar] [CrossRef]
- Iseki, S.; Nakamura, K.; Hayashi, M.; Tanaka, H.; Kondo, H.; Kajiyama, H.; Kano, H.; Kikkawa, F.; Hori, M. Selective killing of ovarian cancer cells through induction of apoptosis by nonequilibrium atmospheric pressure plasma. Appl. Phys. Lett. 2012, 100, 113702. [Google Scholar] [CrossRef]
- Partecke, L.I.; Evert, K.; Haugk, J.; Doering, F.; Normann, L.; Diedrich, S.; Weiss, F.U.; Evert, M.; Huebner, N.O.; Guenther, C.; et al. Tissue tolerable plasma (TTP) induces apoptosis in pancreatic cancer cells in vitro and in vivo. BMC Cancer 2012, 12, 473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandamme, M.; Robert, E.; Lerondel, S.; Sarron, V.; Ries, D.; Dozias, S.; Sobilo, J.; Gosset, D.; Kieda, C.; Legrain, B.; et al. ROS implication in a new antitumor strategy based on non-thermal plasma. Int. J. Cancer 2012, 130, 2185–2194. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Xiong, Z.; Zou, F.; Zhao, S.; Lu, X.; Yang, G.; He, G.; Ostrikov, K. Plasma-induced death of HepG2 cancer cells: Intracellular effects of reactive species. Plasma Process. Polym. 2012, 9, 59–66. [Google Scholar] [CrossRef]
- Arndt, S.; Wacker, E.; Li, Y.F.; Shimizu, T.; Thomas, H.M.; Morfill, G.E.; Karrer, S.; Zimmermann, J.L.; Bosserhoff, A.K. Cold atmospheric plasma, a new strategy to induce senescence in melanoma cells. Exp. Dermatol. 2013, 22, 284–289. [Google Scholar] [CrossRef]
- Han, X.; Klas, M.; Liu, Y.; Stack, M.S.; Ptasińska, S. DNA damage in oral cancer cells induced by nitrogen atmospheric pressure plasma jets. Appl. Phys. Lett. 2013, 102, 233703. [Google Scholar]
- Köritzer, J.; Boxhammer, V.; Schäfer, A.; Shimizu, T.; Klämpfl, T.G.; Li, Y.F.; Welz, C.; Schwenk-Zieger, S.; Morfill, G.E.; Zimmermann, J.L.; et al. Restoration of sensitivity in chemo-resistant glioma cells by cold atmospheric plasma. PLoS ONE 2013, 8, e64498. [Google Scholar] [CrossRef] [Green Version]
- Panngom, K.; Baik, K.Y.; Nam, M.K.; Han, J.H.; Rhim, H.; Choi, E.H. Preferential killing of human lung cancer cell lines with mitochondrial dysfunction by nonthermal dielectric barrier discharge plasma. Cell Death Dis. 2013, 4, e642. [Google Scholar] [CrossRef] [Green Version]
- Utsumi, F.; Kajiyama, H.; Nakamura, K.; Tanaka, H.; Mizuno, M.; Ishikawa, K.; Kondo, H.; Kano, H.; Hori, M.; Kikkawa, F. Effect of indirect nonequilibrium atmospheric pressure plasma on anti-proliferative activity against chronic chemo-resistant ovarian cancer cells in vitro and in vivo. PLoS ONE 2013, 8, e81576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, J.I. Effect of nonequilibrium atmospheric pressure plasma on cancer-initiating cells. Plasma Med. 2014, 4, 49–56. [Google Scholar] [CrossRef]
- Mirpour, S.; Ghomi, H.; Piroozmand, S.; Nikkhah, M.; Tavassoli, S.H.; Azad, S.Z. The selective characterization of nonthermal atmospheric pressure plasma jet on treatment of human breast cancer and normal cells. IEEE Trans. Plasma Sci. 2014, 42, 315–322. [Google Scholar] [CrossRef]
- Joseph-Marie, P.; Mohammed, Y.; Céline, F.; Olivier, E.; Bernard, D.; Nofel, M.; Valérie, L. Low-temperature plasma-induced antiproliferative effects on multi-cellular tumor spheroids. New J. Phys. 2014, 16, 043027. [Google Scholar]
- Utsumi, F.; Kajiyama, H.; Nakamura, K.; Tanaka, H.; Hori, M.; Kikkawa, F. Selective cytotoxicity of indirect nonequilibrium atmospheric pressure plasma against ovarian clear-cell carcinoma. SpringerPlus 2014, 3, 398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirst, A.M.; Simms, M.S.; Mann, V.M.; Maitland, N.J.; O’Connell, D.; Frame, F.M. Low-temperature plasma treatment induces DNA damage leading to necrotic cell death in primary prostate epithelial cells. Br. J. Cancer 2015, 112, 1536–1545. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, J.K.; Tsuruta, Y.; Nojima, S.; Sakakita, H.; Hori, M.; Ikehara, Y. Anti-cancer effects of nonequilibrium atmospheric pressure plasma on cancer-initiating cells in human endometrioid adenocarcinoma cells. Plasma Process. Polym. 2015, 12, 1370–1376. [Google Scholar] [CrossRef]
- Ishaq, M.; Evans, M.; Ostrikov, K. Effect of atmospheric gas plasmas on cancer cell signaling. Int. J. Cancer 2014, 134, 1517–1528. [Google Scholar] [CrossRef]
- Ishaq, M.; Han, Z.J.; Kumar, S.; Evans, M.; Ostrikov, K. Atmospheric-pressure plasma- and TRAIL-induced apoptosis in TRAIL-resistant colorectal cancer cells. Plasma Process. Polym. 2015, 12, 574–582. [Google Scholar] [CrossRef]
- Park, S.B.; Kim, B.; Bae, H.; Lee, H.; Lee, S.; Choi, E.H.; Kim, S.J. Differential epigenetic effects of atmospheric cold plasma on MCF-7 and MDA-MB-231 breast cancer cells. PLoS ONE 2015, 10, e0129931. [Google Scholar] [CrossRef] [Green Version]
- Lin, A.; Truong, B.; Pappas, A.; Kirifides, L.; Oubarri, A.; Chen, S.; Lin, S.; Dobrynin, D.; Fridman, G.; Fridman, A.; et al. Uniform nanosecond pulsed dielectric barrier discharge plasma enhances anti-tumor effects by induction of immunogenic cell death in tumors and stimulation of macrophages. Plasma Process. Polym. 2015, 12, 1392–1399. [Google Scholar] [CrossRef]
- Schmidt, A.; Bekeschus, S.; von Woedtke, T.; Hasse, S. Cell migration and adhesion of a human melanoma cell line is decreased by cold plasma treatment. Clin. Plasma Med. 2015, 3, 24–31. [Google Scholar] [CrossRef]
- Torii, K.; Yamada, S.; Nakamura, K.; Tanaka, H.; Kajiyama, H.; Tanahashi, K.; Iwata, N.; Kanda, M.; Kobayashi, D.; Tanaka, C.; et al. Effectiveness of plasma treatment on gastric cancer cells. Gastric Cancer 2015, 18, 635–643. [Google Scholar] [CrossRef]
- Hattori, N.; Yamada, S.; Torii, K.; Takeda, S.; Nakamura, K.; Tanaka, H.; Kajiyama, H.; Kanda, M.; Fujii, T.; Nakayama, G.; et al. Effectiveness of plasma treatment on pancreatic cancer cells. Int. J. Oncol. 2015, 47, 1655–1662. [Google Scholar] [CrossRef] [Green Version]
- Weiss, M.; Gumbel, D.; Hanschmann, E.M.; Mandelkow, R.; Gelbrich, N.; Zimmermann, U.; Walther, R.; Ekkernkamp, A.; Sckell, A.; Kramer, A.; et al. Cold atmospheric plasma treatment induces anti-proliferative effects in prostate cancer cells by redox and apoptotic signaling pathways. PLoS ONE 2015, 10, e0130350. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.; Gumbel, D.; Gelbrich, N.; Brandenburg, L.O.; Mandelkow, R.; Zimmermann, U.; Ziegler, P.; Burchardt, M.; Stope, M.B. Inhibition of cell growth of the prostate cancer cell model LNCaP by cold atmospheric plasma. In Vivo 2015, 29, 611–616. [Google Scholar]
- Akhlaghi, M.; Rajaei, H.; Mashayekh, A.S.; Shafiae, M.; Mahdikia, H.; Khani, M.; Hassan, Z.M.; Shokri, B. Determination of the optimum conditions for lung cancer cells treatment using cold atmospheric plasma. Phys. Plasmas 2016, 23, 103512. [Google Scholar] [CrossRef]
- Kajiyama, H.; Utsumi, F.; Nakamura, K.; Tanaka, H.; Mizuno, M.; Toyokuni, S.; Hori, M.; Kikkawa, F. Possible therapeutic option of aqueous plasma for refractory ovarian cancer. Clin. Plasma Med. 2015, 4, 14–18. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Kaushik, N.; Min, B.; Choi, K.H.; Hong, Y.J.; Miller, V.; Fridman, A.; Choi, E.H. Cytotoxic macrophage released tumor necrosis factor-alpha (TNF-α) as a killing mechanism for cancer cell death after cold plasma activation. J. Phys. D Appl. Phys. 2016, 49, 084001. [Google Scholar] [CrossRef]
- Hou, J.; Ma, J.; Yu, K.N.; Li, W.; Cheng, C.; Bao, L.; Han, W. Non-thermal plasma treatment altered gene expression profiling in non-small-cell lung cancer A549 cells. BMC Genom. 2015, 16, 435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirpour, S.; Piroozmand, S.; Soleimani, N.; Jalali Faharani, N.; Ghomi, H.; Fotovat Eskandari, H.; Sharifi, A.M.; Mirpour, S.; Eftekhari, M.; Nikkhah, M. Utilizing the micron sized non-thermal atmospheric pressure plasma inside the animal body for the tumor treatment application. Sci. Rep. 2016, 6, 29048. [Google Scholar] [CrossRef] [PubMed]
- Vermeylen, S.; de Waele, J.; Vanuytsel, S.; de Backer, J.; van der Paal, J.; Ramakers, M.; Leyssens, K.; Marcq, E.; van Audenaerde, J.; Dewilde, S.; et al. Cold atmospheric plasma treatment of melanoma and glioblastoma cancer cells. Plasma Process. Polym. 2016, 13, 1195–1205. [Google Scholar] [CrossRef]
- Xu, D.; Luo, X.; Xu, Y.; Cui, Q.; Yang, Y.; Liu, D.; Chen, H.; Kong, M.G. The effects of cold atmospheric plasma on cell adhesion, differentiation, migration, apoptosis and drug sensitivity of multiple myeloma. Biochem. Biophys. Res. Commun. 2016, 473, 1125–1132. [Google Scholar] [CrossRef]
- Zhu, W.; Lee, S.J.; Castro, N.J.; Yan, D.; Keidar, M.; Zhang, L.G. Synergistic effect of cold atmospheric plasma and drug loaded core-shell nanoparticles on inhibiting breast cancer cell growth. Sci. Rep. 2016, 6, 21974. [Google Scholar] [CrossRef] [Green Version]
- Binenbaum, Y.; Ben-David, G.; Gil, Z.; Slutsker, Y.Z.; Ryzhkov, M.A.; Felsteiner, J.; Krasik, Y.E.; Cohen, J.T. Cold atmospheric plasma, created at the tip of an elongated flexible capillary using low electric current, can slow the progression of melanoma. PLoS ONE 2017, 12, e0169457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Simonyan, H.; Cheng, X.; Gjika, E.; Lin, L.; Canady, J.; Sherman, J.H.; Young, C.; Keidar, M. A novel micro cold atmospheric plasma device for glioblastoma both in vitro and in vivo. Cancers 2017, 9, 61. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Zhang, S.; Levchenko, I.; Beilis, I.I.; Keidar, M. in vitro demonstration of cancer inhibiting properties from stratified self-organized plasma-liquid interface. Sci. Rep. 2017, 7, 12163. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yu, K.N.; Ma, J.; Shen, J.; Cheng, C.; Zhou, F.; Cai, Z.; Han, W. Non-thermal plasma induces mitochondria-mediated apoptotic signaling pathway via ROS generation in HeLa cells. Arch. Biochem. Biophys. 2017, 633, 68–77. [Google Scholar] [CrossRef]
- Yan, D.; Cui, H.; Zhu, W.; Nourmohammadi, N.; Milberg, J.; Zhang, L.G.; Sherman, J.H.; Keidar, M. The specific vulnerabilities of cancer cells to the cold atmospheric plasma-stimulated solutions. Sci. Rep. 2017, 7, 74479. [Google Scholar] [CrossRef]
- Lin, L.; Wang, L.; Liu, Y.; Xu, C.; Tu, Y.; Zhou, J. Non-thermal plasma inhibits tumor growth and proliferation and enhances the sensitivity to radiation in vitro and in vivo. Oncol. Rep. 2018, 40, 3405–3415. [Google Scholar] [CrossRef]
- Xu, X.; Dai, X.; Xiang, L.; Cai, D.; Xiao, S.; Ostrikov, K. Quantitative assessment of cold atmospheric plasma anti-cancer efficacy in triple-negative breast cancers. Plasma Process. Polym. 2018, 36, 1183–1198. [Google Scholar] [CrossRef]
- Smolková, B.; Lunova, M.; Lynnyk, A.; Uzhytchak, M.; Churpita, O.; Jirsa, M.; Kubinová, S.; Lunov, O.; Dejneka, A. Non-thermal plasma, as a new physicochemical source, to induce redox imbalance and subsequent cell death in liver cancer cell lines. Cell Physiol. Biochem. 2019, 52, 119–140. [Google Scholar]
- Adhikari, M.; Adhikari, B.; Ghimire, B.; Baboota, S.; Choi, E.H. Cold atmospheric plasma and silymarin nanoemulsion activate autophagy in human melanoma cells. Int. J. Mol. Sci. 2020, 21, 1939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurita, H.; Haruta, N.; Uchihashi, Y.; Seto, T.; Takashima, K. Strand breaks and chemical modification of intracellular DNA induced by cold atmospheric pressure plasma irradiation. PLoS ONE 2020, 15, e0232724. [Google Scholar] [CrossRef] [PubMed]
- Pasqual-Melo, G.; Sagwal, S.K.; Freund, E.; Gandhirajan, R.K.; Frey, B.; von Woedtke, T.; Gaipl, U.; Bekeschus, S. Combination of gas plasma and radiotherapy has immunostimulatory potential and additive toxicity in murine melanoma cells in vitro. Int. J. Mol. Sci. 2020, 21, 1379. [Google Scholar] [CrossRef] [Green Version]
- Pranda, M.A.; Murugesan, B.J.; Knoll, A.J.; Oehrlein, G.S.; Stroka, K.M. Sensitivity of tumor versus normal cell migration and morphology to cold atmospheric plasma-treated media in varying culture conditions. Plasma Process. Polym. 2020, 17, 1900103. [Google Scholar] [CrossRef]
- Zhou, X.; Cai, D.; Xiao, S.; Ning, M.; Zhou, R.; Zhang, S.; Chen, X.; Ostrikov, K.; Dai, X. Invivo Pen: A novel plasma source for in vivo cancer treatment. J. Cancer 2020, 11, 2273–2282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandamme, M.; Robert, E.; Pesnel, S.; Barbosa, E.; Dozias, S.; Sobilo, J.; Lerondel, S.; Le Pape, A.; Pouvesle, J.M. Antitumor effect of plasma treatment on U87 glioma xenografts: Preliminary results. Plasma Process Polym. 2010, 7, 264–273. [Google Scholar] [CrossRef]
- Vandamme, M.; Robert, E.; Dozias, S.; Sobilo, J.; Lerondel, S.; Le Pape, A.; Pouvesle, J.M. Response of human glioma U87 xenografted on mice to non thermal plasma treatment. Plasma Med. 2011, 1, 27–43. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Wei, Y.; Li, J.; Foy, P.; Hawkins, T.; Ballato, J.; Kim, S.O. Single-cell-level microplasma cancer therapy. Small 2011, 7, 2291–2295. [Google Scholar] [CrossRef] [PubMed]
- Daeschlein, G.; Scholz, S.; Lutze, S.; Arnold, A.; von Podewils, S.; Kiefer, T.; Tueting, T.; Hardt, O.; Haase, H.; Grisk, O.; et al. Comparison between cold plasma, electrochemotherapy and combined therapy in a melanoma mouse model. Exp. Dermatol. 2013, 22, 582–586. [Google Scholar] [CrossRef]
- Chernets, N.; Kurpad, D.S.; Alexeev, V.; Rodrigues, D.B.; Freeman, T.A. Reaction chemistry generated by nanosecond pulsed dielectric barrier discharge treatment is responsible for the tumor eradication in the B16 melanoma mouse model. Plasma Process. Polym. 2015, 12, 1400–1409. [Google Scholar] [CrossRef]
- Schuster, M.; Seebauer, C.; Rutkowski, R.; Hauschild, A.; Podmelle, F.; Metelmann, C.; Metelmann, B.; von Woedtke, T.; Hasse, S.; Weltmann, K.D.; et al. Visible tumor surface response to physical plasma and apoptotic cell kill in head and neck cancer. J. Craniomaxillofac. Surg. 2016, 44, 1445–1452. [Google Scholar] [CrossRef]
- Metelmann, H.R.; Nedrelow, D.S.; Seebauer, C.; Schuster, M.; von Woedtke, T.; Weltmann, K.D.; Kindler, S.; Metelmann, P.H.; Finkelstein, S.E.; Von Hoff, D.D.; et al. Head and neck cancer treatment and physical plasma. Clin. Plasma Med. 2015, 3, 17–23. [Google Scholar] [CrossRef]
- Schuster, M.; Rutkowski, R.; Hauschild, A.; Shojaei, R.K.; von Woedtke, T.; Rana, A.; Bauere, G.; Metelmanng, P.; Seebauer, C. Side Effects in Cold Plasma Treatment of Advanced Oral Cancer-Clinical Data and Biological Interpretation. Clin. Plasma Med. 2018, 10, 9–15. [Google Scholar] [CrossRef]
- Jablonowski, L.; Kocher, T.; Schindler, A.; Muller, K.; Dombrowski, F.; von Woedtke, T.; Arnold, T.; Lehmann, A.; Rupf, S.; Evert, M.; et al. Side effects by Oral application of atmospheric pressure plasma on the mucosa in mice. PLoS ONE 2019, 14, e0215099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helmke, A.; Gerling, T.; Weltmann, K.D. Plasma sources for biomedical applications. In Comprehensive Clinical Plasma Medicine; Metelmann, H.R., von Woedtke, T., Weltmann, K.D., Eds.; Springer: Cham, Switzerland, 2018; pp. 23–41. [Google Scholar]
- Laroussi, M.; Nie, L.L.; Lu, X. Cold atmospheric pressure plasma sources for cancer applications. In Plasma Cancer Therapy; Keidar, M., Ed.; Springer Series on Atomic, Optical, and Plasma Physics; Springer: Cham, Switzerland, 2020; Volume 115, pp. 15–51. [Google Scholar]
- Lee, T.; Keidar, M. Adaptive plasma and machine learning. In Plasma Cancer Therapy; Keidar, M., Ed.; Springer Series on Atomic, Optical, and Plasma Physics; Springer: Cham, Switzerland, 2020; Volume 115, pp. 223–250. [Google Scholar]
- von Woedtke, T.; Emmert, S.; Metelmann, H.R.; Rupf, S.; Weltmann, K.D. Perspectives on cold atmospheric plasma (CAP) applications in medicine. Phys. Plasmas 2020, 27, 070601. [Google Scholar] [CrossRef]
- Laroussi, M. Cold plasma in medicine and healthcare: The new frontier in low temperature plasma applications. Front. Phys. 2020, 8, 74. [Google Scholar] [CrossRef]
- Weltmann, K.D.; von Woedtke, T. Basic requirements for plasma sources in medicine. Eur. Phys. J. Appl. Phys. 2011, 55, 13807. [Google Scholar] [CrossRef]
- Dubuc, A.; Monsarrat, P.; Virard, F.; Merbahi, N.; Sarrette, J.F.; Laurencin-Dalicieux, S.; Cousty, S. Use of cold-atmospheric plasma in oncology: A concise systematic review. Ther. Adv. Med. Oncol. 2018, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Malyavko, A.; Yan, D.; Wang, Q.; Klein, A.L.; Patel, K.C.; Shermand, J.H.; Keidar, M. Cold atmospheric plasma cancer treatment, direct versus indirect approaches. Mater. Adv. 2020, 1, 1494–1505. [Google Scholar] [CrossRef]
- Alizadeh, E.; Sanche, L. The role of humidity and oxygen level on damage to DNA induced by soft X-rays and low-energy electrons. J. Phys. Chem. C 2013, 117, 22445–22453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harley, J.C.; Suchowerska, N.; McKenzie, D.R. Cancer treatment with gas plasma and with gas plasma-activated liquid: Positives, potentials and problems of clinical translation. Biophys. Rev. 2020, 12, 989–1006. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.S.; Szili, E.J.; Gaur, N.; Hong, S.H.; Furuta, H.; Kurita, H.; Mizuno, A.; Hatta, A.; Short, R.D. How to assess the plasma delivery of RONS into tissue fluid and tissue. J. Phys. D Appl. Phys. 2016, 49, 304005. [Google Scholar] [CrossRef]
- Szili, E.J.; Hong, S.H.; Oh, J.S.; Gaur, N.; Short, R.D. Tracking the penetration of plasma reactive species into tissue models. Plasma Biotech. 2018, 36, 594–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omran, A.V.; Busco, G.; Dozias, S.; Grillon, C.; Pouvesle, J.M.; Robert, E. Distribution and penetration of reactive oxygen and nitrogen species through a tissue phantom after plasma gun treatment. In Proceedings of the 24th International Symposium on Plasma Chemistry (ISPC24), Naples, Italy, 9–14 June 2019. [Google Scholar]
- Gjika, E.; Pal-Ghosh, S.; Tang, A.; Kirschner, M.; Tadvalkar, G.; Canady, J.; Stepp, M.A.; Keidar, M. Adaptation of operational parameters of cold atmospheric plasma for in vitro treatment of cancer cells. ACS Appl. Mater. Interfaces 2018, 10, 9269–9279. [Google Scholar] [CrossRef]
- Alizadeh, E.; Sanche, L. Precursors of solvated electrons in radiobiological physics and chemistry. Chem. Rev. 2012, 112, 5578–5602. [Google Scholar] [CrossRef]
- Alizadeh, E.; Orlando, T.M.; Sanche, L. Biomolecular damage induced by ionizing radiation: The direct and indirect effects of low-energy electrons on DNA. Annu. Rev. Phys. Chem. 2015, 66, 379–398. [Google Scholar] [CrossRef]
- Lehnert, S. Biomolecular Action of Ionizing Radiation, 1st ed.; Taylor & Francis: London, UK, 2008. [Google Scholar]
- Alizadeh, E.; Sanche, L. Induction of strand breaks in DNA by low energy electrons and soft X-Rays under nitrous oxide atmosphere. Radiat. Phys. Chem. 2012, 81, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Riley, P.A. Free radicals in biology: Oxidative stress and the effects of ionizing radiation. Int. J. Radiat. Biol. 1994, 65, 27–33. [Google Scholar] [CrossRef]
- Nuszkiewicz, J.; Woźniak, A.; Szewczyk-Golec, K. Ionizing radiation as a source of oxidative stress—The protective role of Melatonin and Vitamin D. Int. J. Mol. Sci. 2020, 21, 5804. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, E.; Sanz, A.G.; Garcia, G.; Sanche, L. Radiation damage to DNA: The indirect effect of low energy electrons. J. Phys. Chem. Lett. 2013, 4, 820–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alizadeh, E.; Sanche, L. Low-energy-electron interactions with DNA: Approaching cellular conditions with atmospheric experiments. Eur. Phys. J. D 2014, 68, 1–13. [Google Scholar] [CrossRef]
- Alizadeh, E.; Ptasińska, S.; Sanche, L. Transient anions in radiobiology and radiotherapy: From gaseous biomolecules to condensed organic and biomolecular solids. In Radiation Effects in Materials; Monteiro, W.A., Ed.; InTech: Rijeka, Croatia, 2016; pp. 179–230. [Google Scholar]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Ji, W.O.; Lee, M.H.; Kim, G.H.; Kim, E.H. Quantitation of the ROS production in plasma and radiation treatments of biotargets. Sci. Rep. 2019, 9, 19837. [Google Scholar] [CrossRef]
- Arjunan, K.P.; Sharma, V.K.; Ptasińska, P. Effects of atmospheric pressure plasmas on isolated and cellular DNA—A review. Int. J. Mol. Sci. 2015, 16, 2971–3016. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Kapaldo, J.; Lin, Y.; Sharon Stack, M.; Alizadeh, E.; Ptasińska, S. Large-scale image analysis for investigating spatio-temporal changes in cell DNA damage caused by nitrogen atmospheric pressure plasma jets. Int. J. Mol. Sci. Plasma Biol. 2020, 21, 4127. [Google Scholar] [CrossRef]
- Simon, H.U.; Haj-Yehia, A.; Levi-Schaffer, F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000, 5, 415–418. [Google Scholar] [CrossRef]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef] [Green Version]
- Barzilai, A.; Yamamoto, K.I. DNA damage responses to oxidative stress. DNA Repair 2004, 3, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Asai, T.; Fujiwara, K.; Sahara, J.; Koguchi, H.; Fukuda, N.; Suzuki-Karasaki, M.; Soma, M.; Suzuki-Karasaki, Y. Tumor-selective mitochondrial network collapse induced by atmospheric gas plasma-activated medium. Oncotarget 2016, 7, 19910–19927. [Google Scholar] [CrossRef]
- Gómez-Serrano, M.; Camafeita, E.; Loureiro, M.; Peral, B. Mitoproteomics: Tackling mitochondrial dysfunction in human disease. Oxid. Med. Cell Longev. 2018, 2018, 1435934. [Google Scholar] [CrossRef] [Green Version]
- Dezest, M.; Chavatte, L.; Bourdens, M.; Quinton, D.; Camus, M.; Garrigues, L.; Descargues, P.; Arbault, S.; Burlet-Schiltz, O.; Casteilla, L.; et al. Mechanistic insights into the impact of cold atmospheric pressure plasma on human epithelial cell lines. Sci. Rep. 2017, 7, 41163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickey, J.S.; Redon, C.E.; Nakamura, A.J.; Baird, B.J.; Sedelnikova, O.A.; Bonner, W.M. H2AX: Functional roles and potential applications. Chromosoma 2009, 118, 683–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dasgupta, A.; Wu, D.; Tian, L.; Xiong, P.Y.; Dunham-Snary, K.J.; Chen, K.H.; Alizadeh, E.; Motamed, M.; Potus, F.; Hindmarch, C.C.T.; et al. Mitochondria in the pulmonary vasculature in health and disease: Oxygen-sensing, metabolism, and dynamics. Compr. Physiol. 2020, 10, 713–765. [Google Scholar]
- Scheffler, I.E. Mitochondria, 2nd ed.; Wiley-Liss: Hoboken, NJ, USA, 2008. [Google Scholar]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS sources in physiological and pathological conditions. Oxid. Med. Cell Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.L.; Chen, C.L.; Yeh, S.T.; Zweier, J.L.; Chen, Y.R. Biphasic modulation of the mitochondrial electron transport chain in myocardial ischemia and reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1410–H1422. [Google Scholar] [CrossRef] [Green Version]
- Kalogeris, T.; Bao, Y.; Korthuis, R.J. Mitochondrial reactive oxygen species: A double edged sword in ischemia/reperfusion vs preconditioning. Redox Biol. 2014, 2, 702–714. [Google Scholar] [CrossRef] [Green Version]
- Sandalio, L.M.; Rodríguez-Serrano, M.; Romero-Puertas, M.C.; del Río, L.A. Role of peroxisomes as a source of reactive oxygen species (ROS) signaling molecules. Subcell Biochem. 2013, 69, 231–255. [Google Scholar]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Biochemistry, 6th ed.; W. H. Freeman and Company: New York, NY, USA, 2006. [Google Scholar]
- Leach, J.K.; Van Tuyle, G.; Lin, P.S.; Schmidt-Ullrich, R.; Mikkelsen, R.B. Ionizing radiation-induced, mitochondria-dependent generation of reactive oxygen/nitrogen. Cancer Res. 2001, 61, 3894–3901. [Google Scholar] [PubMed]
- Wu, D.; Dasgupta, A.; Read, A.D.; Bentley, R.E.T.; Motamed, M.; Chen, K.H.; Al-Qazazi, R.; Mewburn, J.D.; Dunham-Snary, K.J.; Alizadeh, E.; et al. Oxygen sensing, mitochondrial biology and experimental therapeutics for pulmonary hypertension and cancer. Free Radic. Biol. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Cadenas, E.; Davies, K.J. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000, 29, 222–230. [Google Scholar] [CrossRef]
- Wang, Q.; Goldstein, M.; Alexander, P.; Wakeman, T.P.; Sun, T.; Feng, J.; Lou, Z.; Kastan, M.B.; Wang, X.F. Rad17 recruits the MRE11-RAD50-NBS1 complex to regulate the cellular response to DNA double-strand breaks. EMBO J. 2014, 33, 862–877. [Google Scholar] [CrossRef] [Green Version]
- Azzam, E.I.; Jay-Gerin, J.P.; Pain, D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012, 327, 48–60. [Google Scholar] [CrossRef] [Green Version]
- Crooks, D.R.; Maio, N.; Lang, M.; Ricketts, C.J.; Vocke, C.D.; Gurram, S.; Turan, S.; Kim, Y.Y.; Cawthon, G.M.; Sohelian, F.; et al. Mitochondrial DNA alterations underlie an irreversible shift to aerobic glycolysis in fumarate hydratase–deficient renal cancer. Sci. Signal. 2021, 14, eabc4436. [Google Scholar] [CrossRef]
- Potter, M.; Newport, E.; Morten, K.J. The Warburg effect: 80 years on. Biochem. Soc. Trans. 2016, 44, 1499–1505. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.W.; Lee, S.; Lee, E.K. ROS and energy metabolism in cancer cells: Alliance for fast growth. Arch. Pharm. Res. 2015, 38, 338–345. [Google Scholar] [CrossRef]
- Uzhachenko, R.; Shanker, A.; Yarbrough, W.G.; Ivanova, A.V. Mitochondria, calcium, and tumor suppressor Fus1: At the crossroad of cancer, inflammation, and autoimmunity. Oncotarget 2015, 6, 20754–20772. [Google Scholar] [CrossRef] [Green Version]
- Li, B.Y.; Sun, J.; Wei, H.; Cheng, Y.-Z.; Xue, L.; Cheng, Z.-H.; Wam, J.-W.; Wang, A.-Q.; Hei, T.K.; Tong, J. Radon-induced reduced apoptosis in human bronchial epithelial cells with knockdown of mitochondria DNA. J. Toxicol. Environ. Health A 2012, 75, 1111–1119. [Google Scholar] [CrossRef] [Green Version]
- Dayal, D.; Martin, S.M.; Owens, K.M.; Aykin-Burns, N.; Zhu, Y.; Boominathan, A.; Pain, D.; Limoli, C.L.; Goswami, P.C.; Domann, F.E.; et al. Mitochondrial complex II dysfunction can contribute significantly to genomic instability after exposure to ionizing radiation. Radiat. Res. 2009, 172, 737–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, L.; Wang, H.; Huang, L.; Zhao, Y.; Wang, J. Crosstalk between autophagy and intracellular radiation response. Int. J. Oncol. 2016, 49, 2217–2226. [Google Scholar] [CrossRef] [PubMed]
- Twig, G.; Elorza, A.; Molina, A.J.; Mohamed, H.; Wikstrom, J.D.; Walzer, G.; Stiles, L.; Haigh, S.E.; Katz, S.; Las, G.; et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008, 27, 433–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, C.; Huang, Y.; Li, L. Drp1-dependent mitochondrial fission plays critical roles in physiological and pathological progresses in mammals. Int. J. Mol. Sci. 2017, 18, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimura, T.; Kobayashi, J.; Komatsu, K.; Kunugita, N. Severe mitochondrial damage associated with low-dose radiation sensitivity in ATM- and NBS1-deficient cells. Cell Cycle 2016, 15, 1099–1107. [Google Scholar] [CrossRef] [Green Version]
- Kawamura, K.; Qi, F.; Kobayashi, J. Potential relationship between the biological effects of low-dose irradiation and mitochondrial ROS production. J. Radiat. Res. 2018, 59, ii91–ii97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenhauer, P.; Chernets, N.; Song, Y.; Dobrynin, D.; Pleshko, N.; Steinbeck, M.J.; Freeman, T.A. Chemical modification of extracellular matrix by cold atmospheric plasma-generated reactive species affects chondrogenesis and bone formation. J. Tissue Eng. Regen. Med. 2016, 10, 772–782. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, X.; Gueydan, C.; Han, J. Plasma membrane changes during programmed cell deaths. Cell Res. 2018, 28, 9–21. [Google Scholar] [CrossRef]
- Poltavets, V.; Kochetkova, M.; Pitson, S.M.; Samuel, M.S. The role of the extracellular matrix and its molecular and cellular regulators in cancer cell plasticity. Front. Oncol. 2018, 8, 431. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Zou, F.; Zhao, S.; Lu, X. On the mechanism of plasma inducing cell apoptosis. IEEE Trans. Plasma Sci. 2010, 38, 2451–2457. [Google Scholar] [CrossRef]
- Kim, K.C.; Piao, M.J.; Madduma Hewage, S.R.K.; Han, X.; Kang, K.A.; Jo, J.O.; Mok, Y.S.; Shin, J.H.; Park, Y.; Yoo, S.J.; et al. Non-thermal dielectric-barrier discharge plasma damages human keratinocytes by inducing oxidative stress. Int. J. Mol. Med. 2016, 37, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Yan, D.; Talbot, A.; Nourmohammadi, N.; Sherman, J.H.; Cheng, X.; Keidar, M. Toward understanding the selective anticancer capacity of cold atmospheric plasma—A model based on aquaporins. Biointerphases 2015, 10, 040801. [Google Scholar] [CrossRef] [PubMed]
- Van der Paal, J.; Neyts, E.C.; Verlackt, C.C.W.; Bogaerts, A. Effect of lipid peroxidation on membrane permeability of cancer and normal cells subjected to oxidative stress. Chem. Sci. 2016, 7, 489–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCord, J.M. Superoxide dismutase, lipid peroxidation, and bell-shaped dose response curves. Dose Respons. 2008, 6, 223–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxid. Med. Cell Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef]
- Vallance, P.; Hingorani, A. Endothelial nitric oxide in humans in health and disease. Int. J. Exp. Pathol. 1999, 80, 291–303. [Google Scholar] [CrossRef]
- Mikkelsen, R.B.; Wardman, P. Biological chemistry of reactive oxygen and nitrogen and radiation- induced signal transduction mechanisms. Oncogene 2003, 22, 5734–5754. [Google Scholar] [CrossRef] [Green Version]
- Lorimore, S.A.; Mukherjee, D.; Robinson, J.I.; Chrystal, J.A.; Wright, E.G. Long-lived inflammatory signaling in irradiated bone marrow is genome dependent. Cancer Res. 2011, 71, 6485–6491. [Google Scholar] [CrossRef] [Green Version]
- Arndt, S.; Unger, P.; Wacker, E.; Shimizu, T.; Heinlin, J.; Li, Y.F.; Thomas, H.M.; Morfill, G.E.; Zimmermann, J.L.; Bosserhoff, A.K.; et al. Cold atmospheric plasma (CAP) changes gene expression of key molecules of the wound healing machinery and improves wound healing in vitro and in vivo. PLoS ONE 2013, 8, e79325. [Google Scholar] [CrossRef] [Green Version]
- Zhong, S.Y.; Dong, Y.Y.; Liu, D.X.; Xu, D.H.; Xiao, S.X.; Chen, H.L.; Kong, M.G. Surface air plasma induced cell death and cytokines release of human keratinocytes in the context of psoriasis. Br. J. Dermatol. 2016, 174, 542–552. [Google Scholar] [CrossRef]
- Schmidt, A.; von Woedtke, T.; Bekeschus, S. Periodic exposure of keratinocytes to cold physical plasma—An in vitro model for redox-related diseases of the skin. Oxid. Med. Cell Longev. 2016, 2016, 9816072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Woedtke, T.; Schmidt, A.; Bekeschus, S.; Wende, K.; Weltmann, K.D. Plasma Medicine: A field of applied Redox biology. In Vivo 2019, 33, 1011–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Year of Study | Study Group [Refs] | Plasma Device Type (Injected Gas) | Study Model (Cancer Cell Type) | Key Results |
|---|---|---|---|---|
| 2006–2007 | Fridman et al. [24,25] | FE-DBD plasma (Air) | Human melanoma skin cancer cells (A2058) | Promoting apoptotic behavior in cancer cells |
| 2008 | Zhang et al. [31] | Plasma jet (Ar) | Human hepatocellular liver cancer cells (BEL-7402) | Enhancing the apoptosis activity in cancer cells after adding oxygen to plasma |
| 2009 | Lee et al. [32] Kim et al. [33] | Radio-frequency plasma jet (He) | Human melanoma skin cancer cells (G361) | Inhibition of the malignant transformation and halt on cancer metastasis, death of melanoma cells |
| 2010 | Lupu et al. [34] Georgescu et al. [35] | Plasma jet (He) | Human colorectal cancer cells (COLO 320DM) Murine melanoma cells (B16) | Higher apoptotic behavior in cancer cells |
| 2010 | Kim et al. [36] | Spray torch NTP jet (He) | Human colorectal cancer cells (HCT116, SW480, LoVo) | Anti-proliferative activity and halt on cancer metastasis |
| 2010 | Zirnheld et al. [37] | NTP jet (He) | Human melanoma cells (1205Lu) | Significant death of melanoma cells |
| 2011 | Ahn et al. [38] | Micro-nozzle array plasma jet (Air and N2) | Human cervical carcinoma cells (HeLa) | Dysfunction of mitochondria and initiating mitochondria-mediated apoptosis |
| 2011 | Kalghatgi et al. [39] Sensenig et al. [40] | NTP-DBD (Air) |
Mammalian breast epithelial cells (MCF10A) Human melanoma cancer cells | Replication arrest or formation of single-stranded DNA breaks and induction of apoptosis |
| 2011 | Keidar et al. [27] | Plasma jet (He) |
Murine melanoma cells
(B16-F10) Normal human bronchial epithelial (NHBE) Lung cancer cell (SW900) | Higher apoptosis and decreasing cell migration velocity and metastasis |
| 2011 | Kim et al. [41] | Micro-size plasma jet (He) | Mouse lung carcinoma and fibroblast cells | Effective induction of apoptosis (but no necrosis) |
| 2011 | Barezki et al. [42] | Plasma jet (He) | Human acute lymphoblastic leukemia T-cells (CCRF-CEM and CCL-119) | Prevention of further progression and cell proliferation, dose-dependent cell death. |
| 2011 | Brulle et al. [43] | Plasma jet (He, Ne, Ar) | Human pancreatic carcinoma cancer cells (MIA PaCa2-luc) Human Embryonic Kidney cells (HEK293FT) | Inhibition of cancer cells proliferation, synergistic effect via association with radiosensitizer and chemotherapy medication |
| 2012 | Kaushik et al. [44] | DBD plasma (Ne) | Human glioblastoma cells (T98G) | Role of plasma exposure time in cell death and micronucleus formation rate, and inhibition of colony formation capacity of cancer cells |
| 2012 | Iseki et al. [45] | NEAPP jet (Ar) | Human ovarian cancer cells (SKOV3 and HRA) | Plasma-induced apoptosis and selectivity for cancer cells |
| 2012 | Partecke et al. [46] | Plasma jet (kINPen09) (Ar) | Human pancreatic cancer cells (Colo357 and PaTu8988T) Murine pancreatic cancer cells (6606PDA) | Reduction of microscopic residual disease in cancer resections |
| 2012 | Tuhvatulin et al. [30] | MicroPlaSter NTP (Ar) | Human colon cancer cells (HCT-116) | p53-dependent apoptosis in cancer cells |
| 2012 | Vandamme et al. [47] | FE-DBD (Air) | Human glioblastoma cells (U87MG) Human colon cancer cells (HCT-116) | Highly discrepancy of cell sensitivity between tumor and non-tumor cells and low proliferation rate |
| 2012 | Yan et al. [48] | Plasma jet (He) | Human hepatocellular carcinoma cells (HepG2) | Selectivity, inactivation and effective cell death in cancer cells |
| 2013 | Arndt et al. [49] | SMD-DBD plasma | Human melanoma cells (Mel Juso, Mel Ei, Mel Ho, Mel Im, Mel Ju, HTZ19) | Dose-dependent cell death |
| 2013 | Han et al. [50] | APP jet (N2) | Oral cancer cells (SCC-25) | Time-dependent DSB damage in DNA |
| 2013 | Köritzer et al. [51] | SMD-DBD plasma | Human glioblastoma cells (LN18, LN229, U87MG) | Synergistic effects of the combination of plasma and chemotherapeutic agent temozolomide on tumor growth and cell cycle distribution |
| 2013 | Panngom et al. [52] | NTP-DBD (Ne) | Human lung cancer cells (H460 and HCC1588) human lung normal cell lines (MRC5 and L132) | High efficiency in lung cancer therapy with mitochondrial dysfunction (morphological changes and reduction in mitochondrial metabolic activity) |
| 2013 | Utsumi et al. [53] | NEAPP-activated medium (PAM) (Ar) | Epithelial ovarian cancer cells including: NOS2, NOS3, NOS2TR and NOS3TR (paclitaxel resistant) NOS2CR and NOS3CR (cisplatin resistant) | Enhancing antitumor effect on chemo-resistant cancer cells |
| 2014 | Ikeda et al. [54] | NEAPP jet (He) | Human uterine endometrioid adenocarcinoma cells (HEC-1) Human gastric carcinoma cells (GCIY) | Decreased cell viability of ALDH-high cells in a comparable level to ALDH-low cells |
| 2014 | Mirpour et al. [55] | NEAPP jet (He) | Human breast cancer cells (MCF7) Non-tumorigenic epithelial cells (MCF10A) | Enhancing the apoptosis activity in cancer cells after adding oxygen to plasma |
| 2014 | Plewa et al. [56] | NTP-DBD (He) | Human colorectal cancer cells (HCT116) | Inhibition of colon carcinoma cell growth in a dose-dependent manner |
| 2014 | Utsumi et al. [57] | NEAPP jet (Ar) | Human ovarian cancer cells (TOV21G, ES-2, SKOV3 and NOS2) | Selective cytotoxicity against circulating cancer cells which are resistant to chemotherapy |
| 2015 | Hirst et al. [58] | NTP jet (He) | Human prostate cancer cells (BPH-1 and PC-3) | Induction of cytotoxic insult in primary prostate cells leading to rapid necrotic cell death |
| 2015 | Ikeda et al. [59] | NEAPP jet (He) | Human endometrioid cancer cells (HEC108 and HEC1) | NEAPP-induced cell apoptosis and more efficient anticancer effects in both ALDH-high and -low cells compared to anticancer drug |
| 2015 | Ishaq et al. [60,61] | Plasma jet (He) | Human colorectal cancer cells including: HT29 (TRAIL-resistant cells) and HCT116 | Synergistic effect of the plasma with TRAIL combination treatment in killing drug-resistant cancer cells by inducing apoptosis without toxicity to normal cells |
| 2015 | Park et al. [62] | DBD plasma (Ar) | Human breast cancer cells (MCF-7 and MDA-MB-231) Human normal breast cells (MCF-10A and MCF-12A) Human colon cancer cells (HCT-15) Human lung cancer cells (NCI-H1299) | Epigenetic dysregulation of crucial cancer-relevant molecules, including those pertinent to tumor development and apoptosis |
| 2015 | Lin et al. [63] | ns-Pulsed DBD plasma | Human nasopharyngeal radioresistant carcinoma cells (CNE1) Human acute monocytic leukemia cells (THP-1) | Enhancing macrophages antitumor effects resulting in stimulation of the immune system |
| 2015 | Schmidt et al. [64] | Plasma jet (Ar) | Human melanoma cells (SK-Mel-147) | Increasing anti-metastatic activity in melanoma cells |
| 2015 | Torii et al. [65] Hattori et al. [66] | NEAPP-activated medium (PAM) | Human gastric cancer cells (NUGC4, SC-2-NU, MKN28 and MKN45) Human fibroblast cells (WI-38) Human pancreatic cancer cells (PANC-1, Capan-2, BxPC-3 and MIA PaCa-2) | Cell apoptosis through ROS generation |
| 2015 | Weiss et al. [67,68] | Plasma jet (kINPen09) (Ar) | Prostatic cancer cells (PC-3 and LNCaP) | Significant inhibition of cancer proliferation, as observed for the first time in urogenital cancer |
| 2016 | Akhlaghi et al. [69] | NTP jet (He) | Human lung cancer (LL/2) and normal fibroblast cells (3T3) | Significant reduction of cancer cells viability |
| 2016 | Kajiyama et al. [70] | NEAPP-activated medium (PAM) (Ar | Human ovarian cancer cells including: K2 and K2R100 (paclitaxel resistant) and Control cells: TOV21G and ES-2 | Enhancing cancer chemosensitivity |
| 2016 | Kaushik et al. [71,72] | Micro-DBD plasma (N2) | Human glioblastoma cells (T98G) Human lung cancer (adenocarcinoma) cells (A549) | Cell mobility promotion in macrophages resulting in stimulation of the immune system |
| 2016 | Mirpour et al. [73] | Micro-DBD plasma (He) | Mouse metastatic breast cancer cells (4T1) | Inhibition of the cell migration and cancer metastasis |
| 2016 | Vermeylen et al. [74] | PAM and micro plasma jet (He) | Human melanoma cells including: Malme-3M and SK-MEL-28 Human glioblastoma cancer cells including: LN229 andU87 | Variations in sensitivity between different cell lines related to specific mutations; Role of plasma settings and experimental design in the plasma effect |
| 2016 | Xu et al. [75] | Plasma jet | Human myeloma cells (RPMI8226 and LP-1 MM) | Induction of myeloma cell apoptosis and enhancing cancer chemo-sensitivity (with bortezomib) |
| 2016 | Zhu et al. [76] | Plasma jet | Human breast adenocarcinoma cells (MDA-MB-231) | Synergetic inhibition of cancer cell growth and metastasis due to the combining of drug loaded nanoparticles |
| 2017 | Binenbaum et al. [77] | Plasma jet (Ne + Ar) | Murine squamous carcinoma cells (SCC-7) Colon cancer cells (DLD-1) Murine melanoma cells (B-16) | Significant reduction in proliferation of cancer cell lines |
| 2017 | Chen et al. [78,79] | Micro-size plasma jet (He) | Human glioblastoma cells (U87MG) | Synergetic treatment effect of short- and long-lived plasma-generated species on cancer cells |
| 2017 | Li et al. [80] | DBD plasma (Air) | Human cervical cancer (HeLa) | Induction of apoptosis in HeLa cells via activating ROS generation and mitochondria-mediated apoptotic signaling |
| 2017 | Yan et al. [81] | NEAPP-activated medium (PAM) (He) | Human pancreatic adenocarcinoma cells (PA-TU-8988T) Human glioblastoma cells (U87MG) Human breast adenocarcinoma cells (MDA-MB-231) | Significant killing of cancer cells using both plasma-stimulated medium (PSM) and plasma-stimulated buffered solution (PSB) |
| 2018 | Lin et al. [82] | NTP jet (Ar + O2) | Human non-small cell lung cancer cells (A549) Human cervical cancer (HeLa) Human hepatoblastoma (HepG2) Human skin fibroblasts (GM0637) | Synergies of plasma with radiotherapy on cancer cells owing to their combined cytotoxic effects by generating ROS, inducing cell cycle arrest and apoptosis in tumor cells |
| 2018 | Xu et al. [83] | NTP jet (He) | Human breast cancer cells (SUM149PT, SUM159PT, MDAMB231, MDAMB436, SKBR3) Human mammary gland epithelial cells (MCF10A) | Deterministic roles on the antitumor efficacy of plasma |
| 2019 | Azzariti et al. [22] | DBD plasma (Air + O2) | Human pancreatic ductal cell line (PANC-1) Human sporadic melanoma biopsy specimens Human breast carcinoma cells | Reduction in proliferation and an increase in calreticulin exposure and ATP release, induction of immunogenic cell death via activation of the innate immune system |
| 2019 | Smolkova et al. [84] | NTP jet (Air) | Human liver cancer cells (Huh7, Alexander and HepG2) | Induction of apoptotic death in Huh7 and Alexander liver cancer cells and resistance in HepG2 due to the Bcl-2 protein overexpression |
| 2020 | Adhikari et al. [85] | Micro-DBD Plasma (Air) | Human melanoma cells (G-361) | Cell apoptosis and autophagy activation due to the decrease in the extracellular pH, leading to a reduction in the intracellular glucose level via inhibition of mTOR and EGF survival pathways |
| 2020 | Kurita et al. [86] | NTP jet (He) | Human lung cancer cells (A549) | No induction of strand breaks but induction of 8-oxoG generation in DNA, and no notable reduction in cell viability |
| 2020 | Pasqual-Melo et al. [87] | Plasma jet (kINPen09) (Ar) | B16F10 murine melanoma cell | Additive effects of plasma and radiotherapy in cytotoxicity, cell cycle arrest and release of immune-stimulatory products in cancer cells |
| 2020 | Pranda et al. [88] | Plasma jet and SMD plasma (Ar) | Human breast adenocarcinoma cells (MDA-MB- 231) Human mammary gland epithelial cells (MCF10A) | Significant role of parameters (type of plasma source and media) in achieving selectivity of cancer cells |
| 2020 | Zhou et al. [89] | Two sources: In vivo Pen and PAM (He) | Human breast adenocarcinoma cells (MDA-MB- 231) | Similar efficacies in inducing tumor cell apoptosis and suppressing tumor migrative abilities in both sources |
| Year of Study | Study Group [Refs] | Plasma Device Type (Injected Gas) | Study Model (Cancer Cell Type) | Key Results |
|---|---|---|---|---|
| 2010 | Vandamme et al. [90] | µs-pulsed DBD jet | Human glioma (U87-luc) bearing mice | Reduction of tumor volume |
| 2011 | Keidar et al. [27] | Plasma jet (He) | B16 and subcutaneous bladder cancer tumors (SCaBER) xenografted in C57Bl6 mice | Reductions in tumor volumes and improving animal survival |
| 2011 | Kim et al. [92] | Micro-size plasma jet (He) | B16F0 melanoma tumor in C57BL/6J mouse | Inhibition of tumor growth in four-time treatment plan and no antitumor effect in one-time treatment |
| 2011 | Vandamme et al. [91] | NTP-DBD (Air) | Human glioma U87-MG (chemo-resistance) xenografted in mouse | Significant decrease of tumor volume and enhancement of life span |
| 2012 | Brulle et al. [43] | Plasma jet (He, Ne, Ar) | Human pancreatic carcinoma (MIA PaCa2-luc) xenografted in mouse | Reducing tumor proliferation and decreasing tumor weight |
| 2012 | Vandamme et al. [30] | NTP-DBD (Air) | Human glioma (U87-luc) grafted in mouse | Induction of apoptosis in whole tumor, significant reduction in tumor volume and accumulation of cells in S phase of cell cycle suggesting an arrest of tumor proliferation |
| 2013 | Daeschlein et al. [93] | Plasma jet (kINPen09) (Ar) | B16-F10 skin melanoma implantation in C57BL/6N mice | Significant delay in tumor growth |
| 2013 | Utsumi et al. [53] | NEAPP-activated medium (PAM) (Ar) | Epithelial ovarian cancer cells (NOS2 & NOS2TR) grafted in mouse | Enhancing cancer chemo-sensitivity |
| 2015 | Chernets et al. [94] | ns-pulsed DBD plasma | B16 orthotopic melanoma in C57BL/6 mouse | Tumor eradication |
| 2015 | Hattori et al. [66] | NEAPP-activated medium (PAM) | Human pancreatic cancer cells (Capan-2) tumor xenografted in nude mouse (BALB/c) | Significant decrease of pancreatic tumor volume |
| 2015–2016 | Schuster et al. [95] Metelmann et al. [96] | kINPen clinical plasma source (He) | 21 patients suffering head and neck cancer | No sign of an enhanced or stimulated tumor growth under influence of plasma treatment |
| 2016 | Mirpour et al. [73] | Micro-size plasma jet (He) | 4T1 grafted tumor in BALB/c mouse | Induction of apoptosis in the tumor cells and inhibition its growth |
| 2017 | Binenbaum et al. [77] | Plasma jet (Ne + Ar) | Human melanoma tumor in C57/bl mice | Significant reduction in tumor volume |
| 2018 | Schuster et al. [97] | kINPen plasma jet (He) | 20 patients suffering from locally advanced squamous cell carcinoma of the head and neck | Clinical point of view: no risk of severe side effects of applying plasma in cancer patients for palliation |
| 2019 | Jablonowski et al. [98] | Two sources: kINPen09 and PS-MWM | Oral Mucosa B6C3F1 mouse | More overt macroscopical and histological lesions, losing more weight in mice, more efficiency of high-temperature PS-MWM than kINPen09 |
| 2020 | Zhou et al. [89] | Two sources: InvivoPen and PAM (He) | Human breast cancer grafted tumor (MDA-MB- 231) in BALB/c mouse | Comparison of two different treatments in preserving mice viability and suppressing tumor growth |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alizadeh, E.; Ptasińska, S. Recent Advances in Plasma-Based Cancer Treatments: Approaching Clinical Translation through an Intracellular View. Biophysica 2021, 1, 48-72. https://doi.org/10.3390/biophysica1010005
Alizadeh E, Ptasińska S. Recent Advances in Plasma-Based Cancer Treatments: Approaching Clinical Translation through an Intracellular View. Biophysica. 2021; 1(1):48-72. https://doi.org/10.3390/biophysica1010005
Chicago/Turabian StyleAlizadeh, Elahe, and Sylwia Ptasińska. 2021. "Recent Advances in Plasma-Based Cancer Treatments: Approaching Clinical Translation through an Intracellular View" Biophysica 1, no. 1: 48-72. https://doi.org/10.3390/biophysica1010005
APA StyleAlizadeh, E., & Ptasińska, S. (2021). Recent Advances in Plasma-Based Cancer Treatments: Approaching Clinical Translation through an Intracellular View. Biophysica, 1(1), 48-72. https://doi.org/10.3390/biophysica1010005






