Cholesterol Significantly Affects the Interactions between Pirfenidone and DPPC Liposomes: Spectroscopic Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Liposome Preparation
2.3. Liposomal Form of Pirfenidone Preparation
2.4. DLS Measurements
2.5. UV-Vis Spectroscopy
2.6. ATR-FTIR Spectroscopy
3. Results
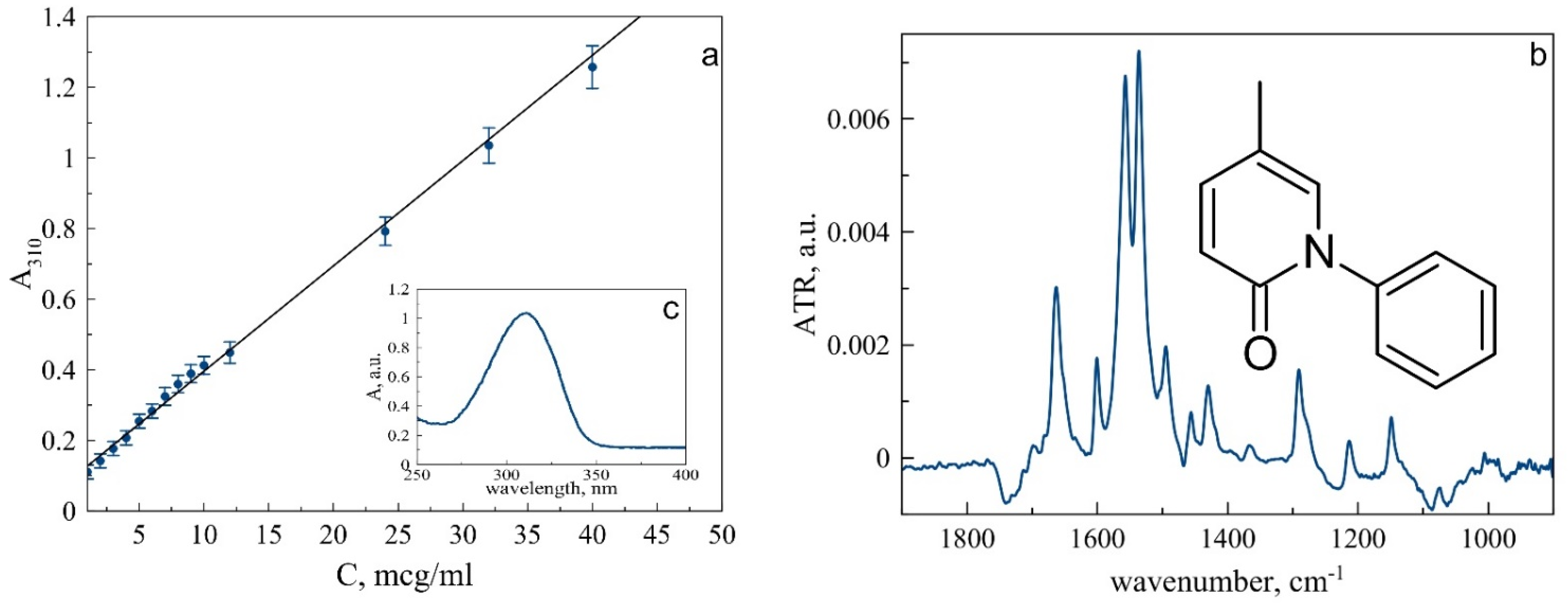
3.1. Pirfenidone Characterization
3.2. Physico-Chemical Characterization of Liposomal Form of Pirfenidone
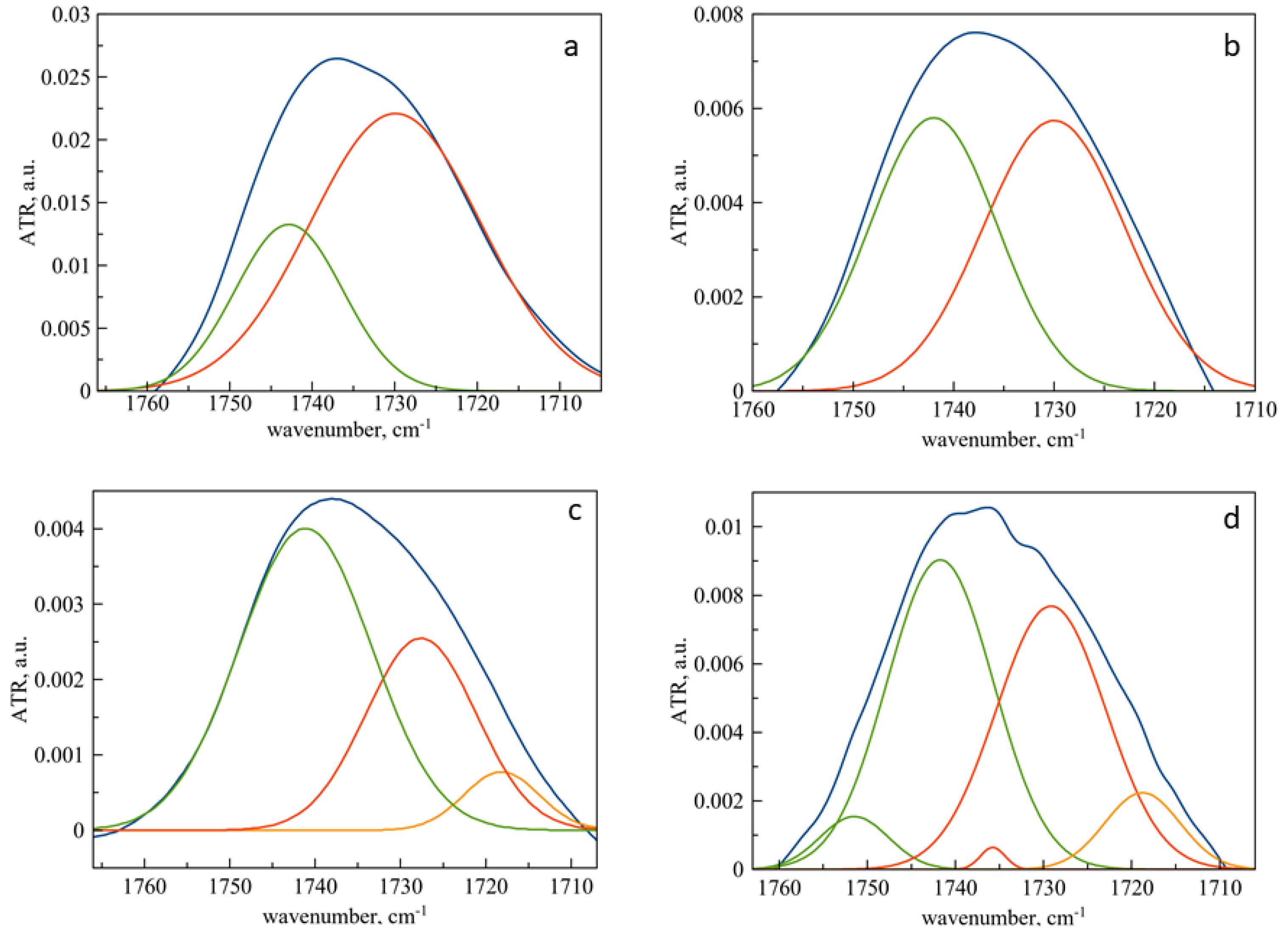
3.3. Mechanism of Interaction of Pirfenidone with Liposomes as Revealed by ATR-FTIR Spectroscopy
3.4. Changes in the Microenvironment of Pirfenidone upon Loading into Liposomes as Revealed by ATR-FTIR Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Oronsky, B.; Larson, C.; Hammond, T.C.; Oronsky, A.; Kesari, S.; Lybeck, M.; Reid, T.R. A Review of Persistent Post-COVID Syndrome (PPCS). Clin. Rev. Allergy Immunol. 2021, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Maltezou, H.; Pavli, A.; Tsakris, A. Post-COVID Syndrome: An Insight on Its Pathogenesis. Vaccines 2021, 9, 497. [Google Scholar] [CrossRef] [PubMed]
- Udwadia, Z.; Koul, P.; Richeldi, L. Post-COVID lung fibrosis: The tsunami that will follow the earthquake. Lung India 2021, 38, S41–S47. [Google Scholar] [CrossRef] [PubMed]
- Scelfo, C.; Fontana, M.; Casalini, E.; Menzella, F.; Piro, R.; Zerbini, A.; Spaggiari, L.; Ghidorsi, L.; Ghidoni, G.; Facciolongo, N.C. A Dangerous Consequence of the Recent Pandemic: Early Lung Fibrosis Following COVID-19 Pneumonia—Case Reports. Ther. Clin. Risk Manag. 2020, 16, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- McGroder, C.F.; Zhang, D.; Choudhury, M.A.; Salvatore, M.M.; D’Souza, B.M.; Hoffman, E.A.; Wei, Y.; Baldwin, M.R.; Garcia, C.K. Pulmonary fibrosis 4 months after COVID-19 is associated with severity of illness and blood leucocyte telomere length. Thorax 2021, 76, 1242–1245. [Google Scholar] [CrossRef]
- Hughes, G.; Toellner, H.; Morris, H.; Leonard, C.; Chaudhuri, N. Real World Experiences: Pirfenidone and Nintedanib are Effective and Well Tolerated Treatments for Idiopathic Pulmonary Fibrosis. J. Clin. Med. 2016, 5, 78. [Google Scholar] [CrossRef] [Green Version]
- Richeldi, L.; Du Bois, R.M.; Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y.; et al. Efficacy and Safety of Nintedanib in Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2014, 370, 2071–2082. [Google Scholar] [CrossRef] [Green Version]
- Nkadi, P.O.; Merritt, T.A.; Pillers, D.-A.M. An overview of pulmonary surfactant in the neonate: Genetics, metabolism, and the role of surfactant in health and disease. Mol. Genet. Metab. 2009, 97, 95–101. [Google Scholar] [CrossRef] [Green Version]
- Onoue, S.; Seto, Y.; Kato, M.; Aoki, Y.; Kojo, Y.; Yamada, S. Inhalable Powder Formulation of Pirfenidone with Reduced Phototoxic Risk for Treatment of Pulmonary Fibrosis. Pharm. Res. 2013, 30, 1586–1596. [Google Scholar] [CrossRef]
- Kaminskas, L.M.; Landersdorfer, C.B.; Bischof, R.J.; Leong, N.; Ibrahim, J.; Davies, A.N.; Pham, S.; Beck, S.; Montgomery, A.B.; Surber, M.W. Aerosol Pirfenidone Pharmacokinetics after Inhaled Delivery in Sheep: A Viable Approach to Treating Idiopathic Pulmonary Fibrosis. Pharm. Res. 2019, 37, 3. [Google Scholar] [CrossRef]
- Clancy, J.P.; Dupont, L.; Konstan, M.W.; Billings, J.; Fustik, S.; Goss, C.H.; Lymp, J.; Minic, P.; Quittner, A.L.; Rubenstein, R.C.; et al. Phase II studies of nebulised Arikace in CF patients with Pseudomonas aeruginosa infection. Thorax 2013, 68, 818–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parvathaneni, V.; Kulkarni, N.S.; Shukla, S.K.; Farrales, P.T.; Kunda, N.K.; Muth, A.; Gupta, V. Systematic Development and Optimization of Inhalable Pirfenidone Liposomes for Non-Small Cell Lung Cancer Treatment. Pharmaceutics 2020, 12, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, L.D.; Tai, L.C.; Ko, S.C.; Masin, D.; Ginsberg, R.S.; Cullis, P.R.; Bally, M.B. Influence of Vesicle Size, Lipid Composition, and Drug-to-Lipid Ratio on the Biological Activity of Liposomal Doxorubicin in Mice. Cancer Res. 1989, 49, 5922–5930. [Google Scholar] [PubMed]
- Szoka, F.C.; Milholland, D.; Barza, M. Effect of lipid composition and liposome size on toxicity and in vitro fungicidal activity of liposome-intercalated amphotericin B. Antimicrob. Agents Chemother. 1987, 31, 421–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Large, D.E.; Abdelmessih, R.G.; Fink, E.A.; Auguste, D.T. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv. Drug Deliv. Rev. 2021, 176, 113851. [Google Scholar] [CrossRef]
- Kellaway, I.W.; Farr, S.J. Liposomes as drug delivery systems to the lung. Adv. Drug Deliv. Rev. 1990, 5, 149–161. [Google Scholar] [CrossRef]
- Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.C. Liposomes as biomembrane models: Biophysical techniques for drug-membrane interaction studies. J. Mol. Liq. 2021, 334, 116141. [Google Scholar] [CrossRef]
- Efimova, A.A.; Trosheva, K.S.; Krasnikov, E.A.; Krivtsov, G.G.; Yaroslavov, A.A. Complexes of Anionic Cholesterol-Containing Liposomes and Cationic Chitosan Microparticles. Polym. Sci. Ser. A 2019, 61, 737–742. [Google Scholar] [CrossRef]
- Stuart, B. FTIR of Biomolecules. In Encyclopedia of Molecular Cell Biology and Molecular Medicine; Wiley-Blackwell: Hoboken, NJ, USA, 2006; pp. 651–683. [Google Scholar] [CrossRef]
- Le-Deygen, I.M.; Skuredina, A.A.; Safronova, A.S.; Yakimov, I.D.; Kolmogorov, I.M.; Deygen, D.M.; Burova, T.V.; Grinberg, N.V.; Grinberg, V.Y.; Kudryashova, E.V. Moxifloxacin interacts with lipid bilayer, causing dramatic changes in its structure and phase transitions. Chem. Phys. Lipids 2020, 228, 104891. [Google Scholar] [CrossRef]
- Deygen, I.M.; Seidl, C.; Kölmel, D.K.; Bednarek, C.; Heissler, S.; Kudryashova, E.V.; Bräse, S.; Schepers, U. Novel Prodrug of Doxorubicin Modified by Stearoylspermine Encapsulated into PEG-Chitosan-Stabilized Liposomes. Langmuir 2016, 32, 10861–10869. [Google Scholar] [CrossRef]
- Deygen, I.; Kudryashova, E.V. Structure and stability of anionic liposomes complexes with PEG-chitosan branched copolymer. Russ. J. Bioorg. Chem. 2014, 40, 547–557. [Google Scholar] [CrossRef]
- Soni, S.R.; Bhunia, B.K.; Kumari, N.; Dan, S.; Mukherjee, S.; Mandal, B.B.; Ghosh, A. Therapeutically Effective Controlled Release Formulation of Pirfenidone from Nontoxic Biocompatible Carboxymethyl Pullulan-Poly(vinyl alcohol) Interpenetrating Polymer Networks. ACS Omega 2018, 3, 11993–12009. [Google Scholar] [CrossRef] [PubMed]
- Manjusha, P.; Prasana, J.C.; Muthu, S.; Raajaraman, B. Density functional studies and spectroscopic analysis (FT-IR, FT-Raman, UV–visible, and NMR) with molecular docking approach on an antifibrotic drug Pirfenidone. J. Mol. Struct. 2020, 1203, 127394. [Google Scholar] [CrossRef]
- Abnoos, M.; Mohseni, M.; Mousavi, S.A.J.; Ashtari, K.; Ilka, R.; Mehravi, B. Chitosan-alginate nano-carrier for transdermal delivery of pirfenidone in idiopathic pulmonary fibrosis. Int. J. Biol. Macromol. 2018, 118, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, S.; Patil, P.; Rajput, R.; Mujumdar, A.; Naik, J. Preparation and characterization of sustained release pirfenidone loaded microparticles for pulmonary drug delivery: Spray drying approach. Dry. Technol. 2021, 39, 337–347. [Google Scholar] [CrossRef]
- Bensikaddour, H.; Snoussi, K.; Lins, L.; Van Bambeke, F.; Tulkens, P.M.; Brasseur, R.; Goormaghtigh, E.; Mingeot-Leclercq, M.-P. Interactions of ciprofloxacin with DPPC and DPPG: Fluorescence anisotropy, ATR-FTIR and 31P NMR spectroscopies and conformational analysis. Biochim. Biophys. Acta (BBA)-Biomembr. 2008, 1778, 2535–2543. [Google Scholar] [CrossRef] [PubMed]
- Manrique-Moreno, M.; Garidel, P.; Suwalsky, M.; Howe, J.; Brandenburg, K. The membrane-activity of Ibuprofen, Diclofenac, and Naproxen: A physico-chemical study with lecithin phospholipids. Biochim. Biophys. Acta (BBA)-Biomembr. 2009, 1788, 1296–1303. [Google Scholar] [CrossRef] [Green Version]
- Tretiakova, D.; Le-Deigen, I.; Onishchenko, N.; Kuntsche, J.; Kudryashova, E.; Vodovozova, E. Phosphatidylinositol Stabilizes Fluid-Phase Liposomes Loaded with a Melphalan Lipophilic Prodrug. Pharmaceutics 2021, 13, 473. [Google Scholar] [CrossRef]
- Sugár, I.P.; Chong, P.L.-G. A Statistical Mechanical Model of Cholesterol/Phospholipid Mixtures: Linking Condensed Complexes, Superlattices, and the Phase Diagram. J. Am. Chem. Soc. 2011, 134, 1164–1171. [Google Scholar] [CrossRef] [Green Version]
- Disalvo, E.A.; Frias, M.A. Water State and Carbonyl Distribution Populations in Confined Regions of Lipid Bilayers Observed by FTIR Spectroscopy. Langmuir 2013, 29, 6969–6974. [Google Scholar] [CrossRef]
- Arsov, Z.; Quaroni, L. Direct interaction between cholesterol and phosphatidylcholines in hydrated membranes revealed by ATR-FTIR spectroscopy. Chem. Phys. Lipids 2007, 150, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Vernooij, E.A.A.M.; Bosch, J.J.K.-V.D.; Crommelin, D.J.A. Fourier Transform Infrared Spectroscopic Determination of the Hydrolysis of Poly(ethylene glycol)—Phosphatidylethanolamine-Containing Liposomes. Langmuir 2002, 18, 3466–3470. [Google Scholar] [CrossRef]
- Wei, T.-T.; Sun, H.-Y.; Deng, G.; Gu, J.-Y.; Guo, H.-Y.; Xu, J.; Wu, R.-G. The interaction of paeonol with DPPC liposomes. J. Therm. Anal. 2018, 132, 685–692. [Google Scholar] [CrossRef]
- Beck, Z.; Matyas, G.R.; Alving, C.R. Detection of liposomal cholesterol and monophosphoryl lipid A by QS-21 saponin and Limulus polyphemus amebocyte lysate. Biochim. Biophys. Acta (BBA)-Biomembr. 2015, 1848, 775–780. [Google Scholar] [CrossRef] [Green Version]
- Briuglia, M.-L.; Rotella, C.M.; McFarlane, A.; Lamprou, D.A. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv. Transl. Res. 2015, 5, 231–242. [Google Scholar] [CrossRef] [Green Version]
| Functional Group | PF, cm−1 | PF DPPC, cm−1 | PF DPPC:Chol 90:10, cm−1 |
|---|---|---|---|
| C=O stretch [25] | 1663 | 1665 | 1665 |
| C=C aromatic [23,24,26] | 1557 | 1557 | 1555 |
| 1536 | 1536 | 1535 | |
| βHCH [24] | 1495 | 1492 | 1490 |
| Sample | Encapsulation Efficacy, % | ζ-Potential, mV | Dh, nm |
|---|---|---|---|
| DPPC control | −10.1 ± 0.5 | 103 ± 2 | |
| DPPC PF | 32 ± 4 | −13.1 ± 0.6 | 107 ± 6 |
| DPPC:Chol 90:10 | −12.5 ± 0.7 | 100 ± 3 | |
| DPPC:Chol 90:10 PF | 20 ± 3 | −10.9 ± 0.5 | 98 ± 2 |
| Sample | νCH2 as, cm−1 | νCH2 as, cm−1 | νCO, cm−1 | νPO2− as, cm−1 |
|---|---|---|---|---|
| DPPC control | 2918 | 2850 | 1737 | 1223 |
| DPPC PF | 2918 | 2850 | 1736 | 1223, 1243 shoulder |
| DPPC:Chol 90:10 | 2918 | 2850 | 1738 | 1224 |
| DPPC:Chol 90:10 PF | 2918 | 2850 | 1740 | 1223, 1243 shoulder |
| Sample | Low Hydrated, % | Medium Hydrated, % | High Hydrated |
|---|---|---|---|
| DPPC control | 27 | 73 | - |
| DPPC PF | 48 | 52 | - |
| DPPC:Chol 90:10 | 61 | 32 | 7 |
| DPPC:Chol 90:10 PF | 51 | 41 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le-Deygen, I.M.; Safronova, A.S.; Mamaeva, P.V.; Skuredina, A.A.; Kudryashova, E.V. Cholesterol Significantly Affects the Interactions between Pirfenidone and DPPC Liposomes: Spectroscopic Studies. Biophysica 2022, 2, 79-88. https://doi.org/10.3390/biophysica2010008
Le-Deygen IM, Safronova AS, Mamaeva PV, Skuredina AA, Kudryashova EV. Cholesterol Significantly Affects the Interactions between Pirfenidone and DPPC Liposomes: Spectroscopic Studies. Biophysica. 2022; 2(1):79-88. https://doi.org/10.3390/biophysica2010008
Chicago/Turabian StyleLe-Deygen, Irina M., Anastasia S. Safronova, Polina V. Mamaeva, Anna A. Skuredina, and Elena V. Kudryashova. 2022. "Cholesterol Significantly Affects the Interactions between Pirfenidone and DPPC Liposomes: Spectroscopic Studies" Biophysica 2, no. 1: 79-88. https://doi.org/10.3390/biophysica2010008
APA StyleLe-Deygen, I. M., Safronova, A. S., Mamaeva, P. V., Skuredina, A. A., & Kudryashova, E. V. (2022). Cholesterol Significantly Affects the Interactions between Pirfenidone and DPPC Liposomes: Spectroscopic Studies. Biophysica, 2(1), 79-88. https://doi.org/10.3390/biophysica2010008








