The Signature of Fluctuations of the Hydrogen Bond Network Formed by Water Molecules in the Interfacial Layer of Anionic Lipids
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Suspension Preparations
2.2. FT-IR Spectroscopy
2.2.1. FT-IR Spectra Acquisition
2.2.2. FT-IR Spectra Analysis
2.3. Molecular Dynamics Simulations
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamenac, A.; Obser, T.; Wixforth, A.; Schneider, M.F.; Westerhausen, C. The Activity of the Intrinsically Water-Soluble Enzyme ADAMTS13 Correlates with the Membrane State When Bound to a Phospholipid Bilayer. Sci. Rep. 2021, 11, 24476. [Google Scholar] [CrossRef] [PubMed]
- Damodaran, S. Water at Biological Phase Boundaries: Lts Role in Interfacial Activation of Enzymes and Metabolic Pathways. In Membrane Hydration; Disalvo, E.A., Ed.; Springer: Cham, Switzerland, 2015; Volume 71, pp. 233–261. ISBN 9783319190600. [Google Scholar]
- Schneck, E.; Sedlmeier, F.; Netz, R.R. Hydration Repulsion between Biomembranes Results from an Interplay of Dehydration and Depolarization. Proc. Natl. Acad. Sci. USA 2012, 109, 14405–14409. [Google Scholar] [CrossRef] [PubMed]
- Kasson, P.M.; Lindahl, E.; Pande, V.S. Water Ordering at Membrane Interfaces Controls Fusion Dynamics. J. Am. Chem. Soc. 2011, 133, 3812–3815. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, M.; Krok, E.; Orlikowska, H.; Schwille, P.; Franquelim, H.G.; Piatkowski, L. Hydration Layer of Only a Few Molecules Controls Lipid Mobility in Biomimetic Membranes. J. Am. Chem. Soc. 2021, 143, 14551–14562. [Google Scholar] [CrossRef]
- Saak, C.M.; Dreier, L.B.; Machel, K.; Bonn, M.; Backus, E.H.G. Biological Lipid Hydration: Distinct Mechanisms of Interfacial Water Alignment and Charge Screening for Model Lipid Membranes. Faraday Discuss. 2024, 249, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Dreier, L.B.; Nagata, Y.; Lutz, H.; Gonella, G.; Hunger, J.; Backus, E.H.G.; Bonn, M. Saturation of Charge-Induced Water Alignment at Model Membrane Surfaces. Sci. Adv. 2018, 4, eaap7415. [Google Scholar] [CrossRef]
- Laage, D.; Hynes, J.T. A Molecular Jump Mechanism of Water Reorientation. Science 2006, 311, 832–835. [Google Scholar] [CrossRef]
- Stirnemann, G.; Rossky, P.J.; Hynes, J.T.; Laage, D. Water Reorientation, Hydrogen-Bond Dynamics and 2D-IR Spectroscopy next to an Extended Hydrophobic Surface. Faraday Discuss. 2010, 146, 263–281. [Google Scholar] [CrossRef]
- Re, S.; Nishima, W.; Tahara, T.; Sugita, Y. Mosaic of Water Orientation Structures at a Neutral Zwitterionic Lipid/Water Interface Revealed by Molecular Dynamics Simulations. J. Phys. Chem. Lett. 2014, 5, 4343–4348. [Google Scholar] [CrossRef]
- Kundu, A.; Kwak, K.; Cho, M. Water Structure at the Lipid Multibilayer Surface: Anionic Versus Cationic Head Group Effects. J. Phys. Chem. B 2016, 120, 5002–5007. [Google Scholar] [CrossRef]
- Singh, P.C.; Inoue, K.I.; Nihonyanagi, S.; Yamaguchi, S.; Tahara, T. Femtosecond Hydrogen Bond Dynamics of Bulk-like and Bound Water at Positively and Negatively Charged Lipid Interfaces Revealed by 2D HD-VSFG Spectroscopy. Angew. Chem. Int. Ed. 2016, 55, 10621–10625. [Google Scholar] [CrossRef]
- Flanagan, J.C.; Valentine, M.L.; Baiz, C.R. Ultrafast Dynamics at Lipid-Water Interfaces. Acc. Chem. Res. 2020, 53, 1860–1868. [Google Scholar] [CrossRef]
- Livingstone, R.A.; Zhang, Z.; Piatkowski, L.; Bakker, H.J.; Hunger, J.; Bonn, M.; Backus, E.H.G. Water in Contact with a Cationic Lipid Exhibits Bulklike Vibrational Dynamics. J. Phys. Chem. B 2016, 120, 10069–10078. [Google Scholar] [CrossRef] [PubMed]
- Ohto, T.; Backus, E.H.G.; Hsieh, C.S.; Sulpizi, M.; Bonn, M.; Nagata, Y. Lipid Carbonyl Groups Terminate the Hydrogen Bond Network of Membrane-Bound Water. J. Phys. Chem. Lett. 2015, 6, 4499–4503. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, R.A.; Nagata, Y.; Bonn, M.; Backus, E.H.G. Two Types of Water at the Water-Surfactant Interface Revealed by Time-Resolved Vibrational Spectroscopy. J. Am. Chem. Soc. 2015, 137, 14912–14919. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.F.; Nielsen, S.O.; Klein, M.L.; Moore, P.B. Hydrogen Bonding Structure and Dynamics of Water at the Dimyristoylphosphatidylcholine Lipid Bilayer Surface from a Molecular Dynamics Simulation. J. Phys. Chem. B 2004, 108, 6603–6610. [Google Scholar] [CrossRef]
- Shen, H.; Wu, Z.; Zou, X. Interfacial Water Structure at Zwitterionic Membrane/Water Interface: The Importance of Interactions between Water and Lipid Carbonyl Groups. ACS Omega 2020, 5, 18080–18090. [Google Scholar] [CrossRef] [PubMed]
- Karathanou, K.; Bondar, A.N. Dynamic Water Hydrogen-Bond Networks at the Interface of a Lipid Membrane Containing Palmitoyl-Oleoyl Phosphatidylglycerol. J. Membr. Biol. 2018, 251, 461–473. [Google Scholar] [CrossRef]
- Inoue, K.I.; Singh, P.C.; Nihonyanagi, S.; Yamaguchi, S.; Tahara, T. Cooperative Hydrogen-Bond Dynamics at a Zwitterionic Lipid/Water Interface Revealed by 2D HD-VSFG Spectroscopy. J. Phys. Chem. Lett. 2017, 8, 5160–5165. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Malik, S.; Debnath, A. Heterogeneity in Structure and Dynamics of Water near Bilayers Using TIP3P and TIP4P/2005 Water Models. Chem. Phys. 2019, 525, 110396. [Google Scholar] [CrossRef]
- Calero, C.; Stanley, H.E.; Franzese, G. Structural Interpretation of the Large Slowdown of Water Dynamics at Stacked Phospholipid Membranes for Decreasing Hydration Level: All-Atom Molecular Dynamics. Materials 2016, 9, 319. [Google Scholar] [CrossRef]
- Zhao, L.; Ma, K.; Yang, Z. Changes of Water Hydrogen Bond Network with Different Externalities. Int. J. Mol. Sci. 2015, 16, 8454–8489. [Google Scholar] [CrossRef]
- Fayer, M.D. Dynamics of Water Interacting with Interfaces, Molecules, and Ions. Acc. Chem. Res. 2012, 45, 3–14. [Google Scholar] [CrossRef]
- Piskulich, Z.A.; Laage, D.; Thompson, W.H. On the Role of Hydrogen-Bond Exchanges in the Spectral Diffusion of Water. J. Chem. Phys. 2021, 154, 064501. [Google Scholar] [CrossRef]
- Gruenbaum, S.M.; Skinner, J.L. Vibrational Spectroscopy of Water in Hydrated Lipid Multi-Bilayers. I. Infrared Spectra and Ultrafast Pump-Probe Observables. J. Chem. Phys. 2011, 135, 075101. [Google Scholar] [CrossRef]
- Valentine, M.L.; Waterland, M.K.; Fathizadeh, A.; Elber, R.; Baiz, C.R. Interfacial Dynamics in Lipid Membranes: The Effects of Headgroup Structures. J. Phys. Chem. B 2021, 125, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, J.C.; Cardenas, A.E.; Baiz, C.R. Ultrafast Spectroscopy of Lipid-Water Interfaces: Transmembrane Crowding Drives H-Bond Dynamics. J. Phys. Chem. Lett. 2020, 11, 4093–4098. [Google Scholar] [CrossRef] [PubMed]
- Maréchal, Y. The H2O Molecules in Liquid Water. In The Hydrogen Bond and the Water Molecule; Elsevier Ltd.: Amsterdam, The Netherlands, 2007; pp. 215–248. [Google Scholar]
- Verma, P.K.; Kundu, A.; Puretz, M.S.; Dhoonmoon, C.; Chegwidden, O.S.; Londergan, C.H.; Cho, M. The Bend+Libration Combination Band Is an Intrinsic, Collective, and Strongly Solute-Dependent Reporter on the Hydrogen Bonding Network of Liquid Water. J. Phys. Chem. B 2018, 122, 2587–2599. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Kampfrath, T.; Campen, R.K. Experimentally Probing the Libration of Interfacial Water: The Rotational Potential of Water Is Stiffer at the Air/Water Interface than in Bulk Liquid. Phys. Chem. Chem. Phys. 2016, 18, 18424–18430. [Google Scholar] [CrossRef] [PubMed]
- Brkljača, Z.; Butumović, M.; Bakarić, D. Water Does Not Dance as Ions Sing: A New Approach in Elucidation of Ion-Invariant Water Fluctuation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 271, 120907. [Google Scholar] [CrossRef] [PubMed]
- Libnau, F.O.; Toft, J.; Christy, A.A.; Kvalheim, O.M. Structure of Liquid Water Determined from Infrared Temperature Profiling and Evolutionary Curve Resolution. J. Am. Chem. Soc. 1994, 116, 8311–8316. [Google Scholar] [CrossRef]
- Czarnik-Matusewicz, B.; Pilorz, S.; Hawranek, J.P. Temperature-Dependent Water Structural Transitions Examined by near-IR and Mid-IR Spectra Analyzed by Multivariate Curve Resolution and Two-Dimensional Correlation Spectroscopy. Anal. Chim. Acta 2005, 544, 15–25. [Google Scholar] [CrossRef]
- Chen, Y.; Dupertuis, N.; Okur, H.I.; Roke, S. Temperature Dependence of Water-Water and Ion-Water Correlations in Bulk Water and Electrolyte Solutions Probed by Femtosecond Elastic Second Harmonic Scattering. J. Chem. Phys. 2018, 148, 222835. [Google Scholar] [CrossRef] [PubMed]
- Schönfeldová, T.; Piller, P.; Kovacik, F.; Pabst, G.; Okur, H.I.; Roke, S. Lipid Melting Transitions Involve Structural Redistribution of Interfacial Water. J. Phys. Chem. B 2021, 125, 12457–12465. [Google Scholar] [CrossRef] [PubMed]
- Bach, D.; Miller, I.R. Hydration of Phospholipid Bilayers in the Presence and Absence of Cholesterol. Biochim. Biophys. Acta 1998, 1368, 216–224. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Heimburg, T. Thermal Biophysics of Membranes; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2007; ISBN 9783527404711. [Google Scholar]
- May, S. Curvature Elasticity and Thermodynamic Stability of Electrically Charged Membranes. J. Chem. Phys. 1996, 105, 8314–8323. [Google Scholar] [CrossRef]
- Pašalić, L.; Pem, B.; Bakarić, D. Lamellarity-Driven Differences in Surface Structural Features of DPPS Lipids: Spectroscopic, Calorimetric and Computational Study. Membranes 2023, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Sadžak, A.; Brkljača, Z.; Crnolatac, I.; Baranović, G.; Šegota, S. Flavonol Clustering in Model Lipid Membranes: DSC, AFM, Force Spectroscopy and MD Simulations Study. Colloids Surf. B Biointerfaces 2020, 193, 111147. [Google Scholar] [CrossRef]
- Dale Keefe, C. Computer Programs for the Determination of Optical Constants from Transmission Spectra and the Study of Absolute Absorption Intensities. J. Mol. Struct. 2002, 641, 165–173. [Google Scholar] [CrossRef]
- Menges, F. Spectragryph—Optical Spectroscopy Software. Available online: https://www.effemm2.de/spectragryph/ (accessed on 3 January 2024).
- Cevc, G.; Watts, A.; Marsh, D. Titration of the Phase Transition of Phosphatidylserine Bilayer Membranes. Effects of pH, Surface Electrostatics, Ion Binding, and Head-Group Hydration. Biochemistry 1981, 20, 4955–4965. [Google Scholar] [CrossRef]
- Lewis, R.N.A.H.; McElhaney, R.N. Calorimetric and Spectroscopic Studies of the Thermotropic Phase Behavior of Lipid Bilayer Model Membranes Composed of a Homologous Series of Linear Saturated Phosphatidylserines. Biophys. J. 2000, 79, 2043–2055. [Google Scholar] [CrossRef]
- Maeder, M.; de Juan, A. Two-Way Data Analysis: Evolving Factor Analysis. Compr. Chemom. 2009, 2, 261–274. [Google Scholar] [CrossRef]
- Keller, H.R.; Massart, D.L. Evolving Factor Analysis. Chemom. Intell. Lab. Syst. 1991, 12, 209–224. [Google Scholar] [CrossRef]
- Maleš, P.; Brkljača, Z.; Crnolatac, I.; Bakarić, D. Application of MCR-ALS with EFA on FT-IR Spectra of Lipid Bilayers in the Assessment of Phase Transition Temperatures: Potential for Discernment of Coupled Events. Colloids Surf. B Biointerfaces 2021, 201, 111645. [Google Scholar] [CrossRef] [PubMed]
- Jaumot, J.; Gargallo, R.; De Juan, A.; Tauler, R. A Graphical User-Friendly Interface for MCR-ALS: A New Tool for Multivariate Curve Resolution in MATLAB. Chemom. Intell. Lab. Syst. 2005, 76, 101–110. [Google Scholar] [CrossRef]
- De Juan, A.; Jaumot, J.; Tauler, R. Multivariate Curve Resolution (MCR). Solving the Mixture Analysis Problem. Anal. Methods 2014, 6, 4964–4976. [Google Scholar] [CrossRef]
- Fega, K.R.; Wilcox, D.S.; Ben-Amotz, D. Application of Raman Multivariate Curve Resolution to Solvation-Shell Spectroscopy. Appl. Spectrosc. 2012, 66, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A Web-Based Graphical User Interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindah, E. Gromacs: High Performance Molecular Simulations through Multi-Level Parallelism from Laptops to Supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Klauda, J.B.; Venable, R.M.; Freites, J.A.; O’Connor, J.W.; Tobias, D.J.; Mondragon-Ramirez, C.; Vorobyov, I.; MacKerell, A.D., Jr.; Pastor, R.W. Update of the CHARMM All-Atom Additive Force Field for Lipids: Validation on Six Lipid Types. J. Phys. Chem. B 2010, 114, 7830–7843. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of Simple Potential Functions for Simulating Liquid Water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. CHARMM General Force Field: A Force Field for Drug-Like Molecules Compatible with the CHARMM All-Atom Additive Biological Force Fields. J. Comput. Chem. 2010, 31, 671–690. [Google Scholar] [CrossRef] [PubMed]
- MacKerell Lab. Available online: http://mackerell.umaryland.edu/charmm_ff.shtml#gromacs (accessed on 12 February 2024).
- Nosé, S. A Molecular Dynamics Method for Simulations in the Canonical Ensemble. Mol. Phys. Int. J. Interface Between Chem. Phys. 1984, 52, 255–268. [Google Scholar] [CrossRef]
- Parrinello, M.; Rahman, A. Polymorphic Transitions in Single Crystals: A New Molecular Dynamics Method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A Smooth Particle Mesh Ewald Method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Lewis, R.N.A.H.; McElhaney, R.N. Membrane Lipid Phase Transitions and Phase Organization Studied by Fourier Transform Infrared Spectroscopy. Biochim. Biophys. Acta-Biomembr. 2013, 1828, 2347–2358. [Google Scholar] [CrossRef]
- Browning, J.L.; Seelig, J. Bilayers of Phosphatidylserine: A Deuterium and Phosphorus Nuclear Magnetic Resonance Study. Biochemistry 1980, 19, 1262–1270. [Google Scholar] [CrossRef]
- Ge, M.; Freed, J.H. Hydration, Structure, and Molecular Interactions in the Headgroup Region of Dioleoylphosphatidylcholine Bilayers: An Electron Spin Resonance Study. Biophys. J. 2003, 85, 4023–4040. [Google Scholar] [CrossRef]
- Šegota, S.; Vojta, D.; Kendziora, D.; Ahmed, I.; Fruk, L.; Baranović, G. Ligand-Dependent Nanoparticle Clustering within Lipid Membranes Induced by Surrounding Medium. J. Phys. Chem. B 2015, 119, 5208–5219. [Google Scholar] [CrossRef]
- Khakbaz, P.; Klauda, J.B. Investigation of Phase Transitions of Saturated Phosphocholine Lipid Bilayers via Molecular Dynamics Simulations. Biochim. Biophys. Acta-Biomembr. 2018, 1860, 1489–1501. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.; Nowroozi, A.; Shahlaei, M. Shedding Light on the Structural Properties of Lipid Bilayers Using Molecular Dynamics Simulation: A Review Study. RSC Adv. 2019, 9, 4644–4658. [Google Scholar] [CrossRef]
- Hishida, M.; Endo, A.; Nakazawa, K.; Yamamura, Y.; Saito, K. Effect of N-Alkanes on Lipid Bilayers Depending on Headgroups. Chem. Phys. Lipids 2015, 188, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Korchowiec, B.; Gorczyca, M.; Rogalska, E.; Regnouf-De-Vains, J.B.; Mourer, M.; Korchowiec, J. The Selective Interactions of Cationic Tetra-p-Guanidinoethylcalix[4]Arene with Lipid Membranes: Theoretical and Experimental Model Studies. Soft Matter 2015, 12, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Yousefpour, A.; Amjad-Iranagh, S.; Goharpey, F.; Modarress, H. Effect of Drug Amlodipine on the Charged Lipid Bilayer Cell Membranes DMPS and DMPS + DMPC: A Molecular Dynamics Simulation Study. Eur. Biophys. J. 2018, 47, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Hauser, H.; Paltauf, F.; Shipley, G.G. Structure and Thermotropic Behavior of Phosphatidylserine Bilayer Membranes. Biochemistry 1982, 21, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Eid, J.; Jraij, A.; Greige-Gerges, H.; Monticelli, L. Effect of Quercetin on Lipid Membrane Rigidity: Assessment by Atomic Force Microscopy and Molecular Dynamics Simulations. BBA Adv. 2021, 1, 100018. [Google Scholar] [CrossRef] [PubMed]
- Raghunathan, M.; Zubovski, Y.; Venable, R.M.; Pastor, R.W.; Nagle, J.F.; Tristram-Nagle, S. Structure and Elasticity of Lipid Membranes with Genistein and Daidzein Bioflavinoids Using X-Ray Scattering and MD Simulations. J. Phys. Chem. B 2012, 116, 3918–3927. [Google Scholar] [CrossRef]
- Selvaraj, S.; Krishnaswamy, S.; Devashya, V.; Sethuraman, S.; Krishnan, U.M. Influence of Membrane Lipid Composition on Flavonoid-Membrane Interactions: Implications on Their Biological Activity. Prog. Lipid Res. 2015, 58, 1–13. [Google Scholar] [CrossRef]
- Abram, V.; Berlec, B.; Ota, A.; Šentjurc, M.; Blatnik, P.; Ulrih, N.P. Effect of Flavonoid Structure on the Fluidity of Model Lipid Membranes. Food Chem. 2013, 139, 804–813. [Google Scholar] [CrossRef]
- Altunayar-Unsalan, C.; Unsalan, O.; Mavromoustakos, T. Insights into Molecular Mechanism of Action of Citrus Flavonoids Hesperidin and Naringin on Lipid Bilayers Using Spectroscopic, Calorimetric, Microscopic and Theoretical Studies. J. Mol. Liq. 2022, 347, 118411. [Google Scholar] [CrossRef]
- Srivastava, A.; Debnath, A. Hydration Dynamics of a Lipid Membrane: Hydrogen Bond Networks and Lipid-Lipid Associations. J. Chem. Phys. 2018, 148, 094901. [Google Scholar] [CrossRef]
- Boughter, C.T.; Monje-Galvan, V.; Im, W.; Klauda, J.B. Influence of Cholesterol on Phospholipid Bilayer Structure and Dynamics. J. Phys. Chem. B 2016, 120, 11761–11772. [Google Scholar] [CrossRef] [PubMed]


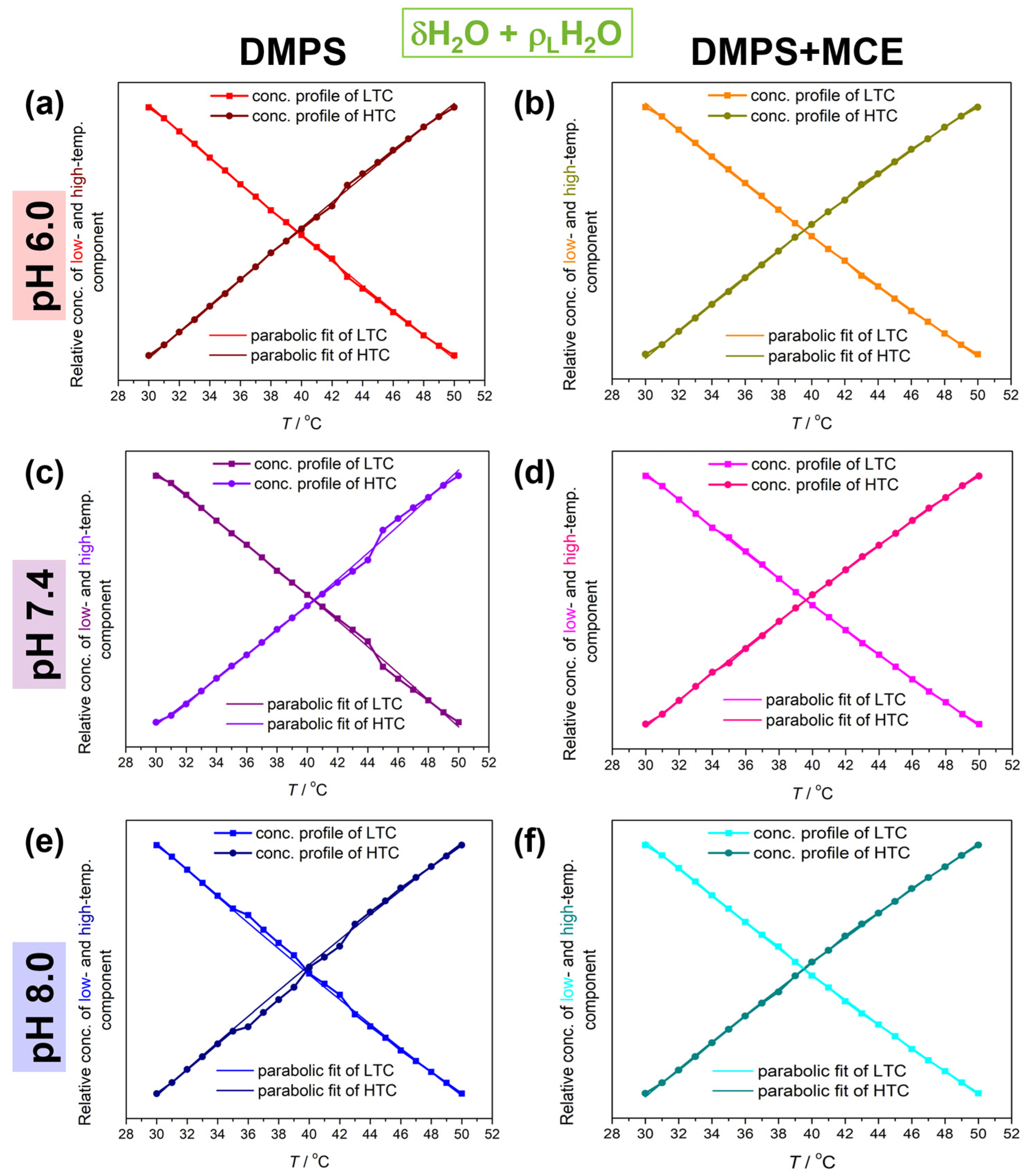
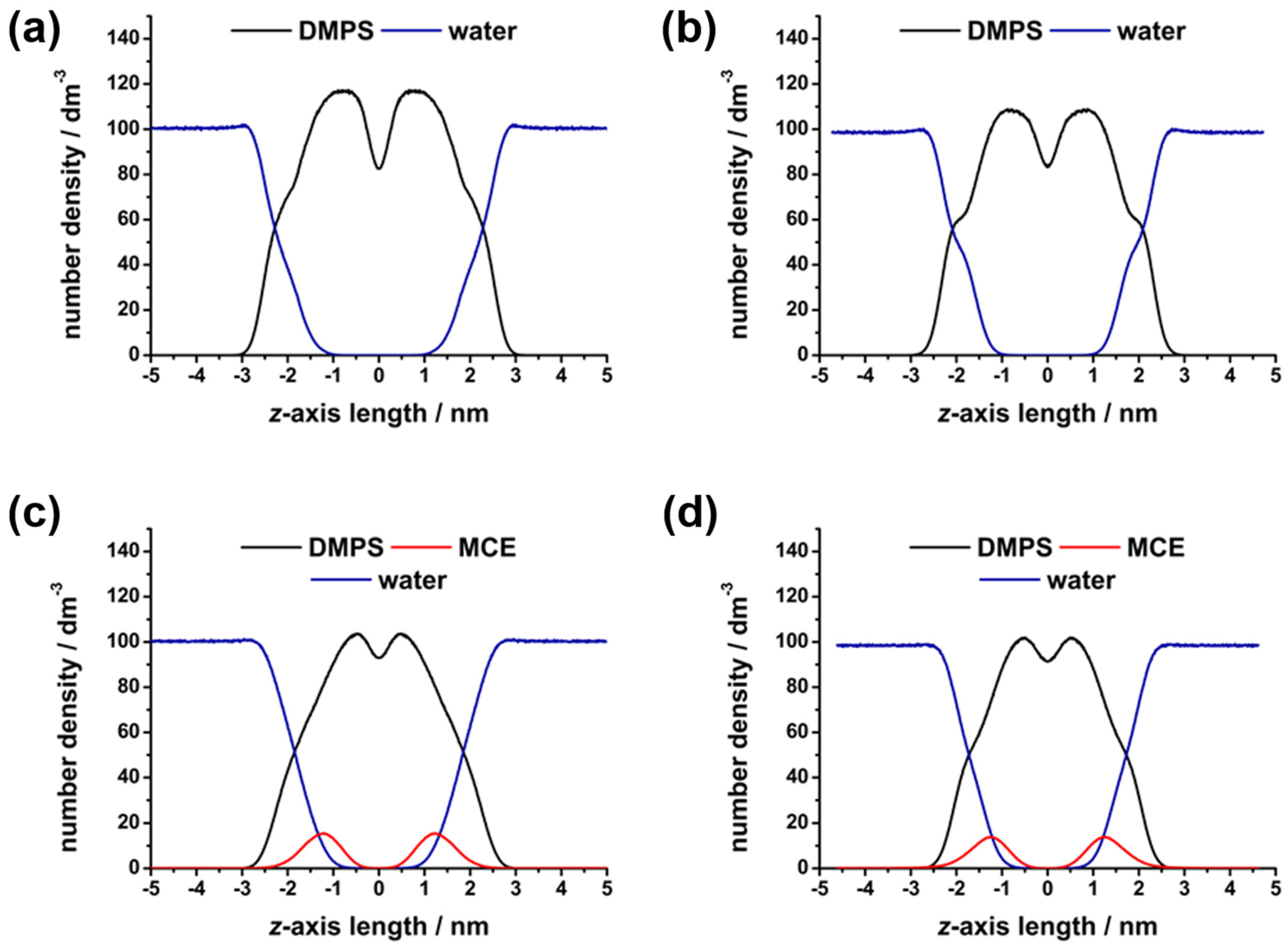
| System/pH | νmax (30° C/50 °C) a | B0 b | B1 c | B2 d · 104 | ~Δ|B0| b | ~Δ|B1| c | ~Δ|B2| d · 104 |
|---|---|---|---|---|---|---|---|
| DMPS/6.0 | 2131/2118 | 2.80 ± 0.07 −1.8 ± 0.1 | −0.065 ± 0.004 0.062 ± 0.005 | 1.8 ± 0.5 −1.3 ± 0.6 | 1 | 0.003 | 0.3–0.5 |
| DMPS/7.4 | 2130/2114 | 2.3 ± 0.1 −1.1 ± 0.2 | −0.036 ± 0.007 0.027 ± 0.009 | 1.9 ± 0.9 −3 ± 1 | 1 | 0.009 | 1 |
| DMPS/8.0 | 2132/2119 | 2.83 ± 0.08 −1.8 ± 0.1 | −0.067 ± 0.004 0.065 ± 0.006 | 1.9 ± 0.5 −1.8 ± 0.7 | 1 | 0.002 | 0.1 |
| DMPS + MCE/6.0 | 2131/2117 | 2.91 ± 0.06 −1.96 ± 0.07 | −0.070 ± 0.003 0.073 ± 0.004 | 2.4 ± 0.4 −2.7 ± 0.4 | 1 | 0.003 | 0.3 |
| DMPS + MCE/7.4 | 2130/2118 | 2.83 ± 0.05 −1.83 ± 0.06 | −0.067 ± 0.003 0.066 ± 0.003 | 2.0 ± 0.3 −1.9 ± 0.4 | 1 | 0.001 | 0.1 |
| DMPS + MCE/8.0 | 2132/2117 | 2.92 ± 0.06 −1.93 ± 0.06 | −0.071 ± 0.003 0.073 ± 0.003 | 2.5 ± 0.4 −2.8 ± 0.4 | 1 | 0.002 | 0.3 |
| System | Area per Lipid a | Membrane Thickness b | |
|---|---|---|---|
| DMPS | 30 °C | 0.467 ± 0.005 | 4.255 ± 0.021 |
| 50 °C | 0.553 ± 0.009 | 3.867 ± 0.009 | |
| DMPS + MCE | 30 °C | 0.623 ± 0.012 | 3.820 ± 0.010 |
| 50 °C | 0.659 ± 0.010 | 3.465 ± 0.009 | |
| HBs | DMPS | DMPS + MCE | ||
|---|---|---|---|---|
| 30 °C | 50 °C | 30 °C | 50 °C | |
| DMPS–water | 2165 ± 32 | 2257 ± 38 | 2355 ± 41 | 2334 ± 41 |
| DMPS–MCE | / | / | 124 ± 10 | 119 ± 10 |
| COO−–water | 910 ± 16 | 883 ± 20 | 929 ± 18 | 910 ± 18 |
| NH3+–water | 227 ± 9 | 242 ± 11 | 255 ± 14 | 266 ± 12 |
| PO2−–water | 574 ± 18 | 621 ± 19 | 647 ± 18 | 634 ± 19 |
| C=O–water | 225 ± 10 | 274 ± 12 | 299 ± 14 | 307 ± 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlek, A.-M.; Pem, B.; Bakarić, D. The Signature of Fluctuations of the Hydrogen Bond Network Formed by Water Molecules in the Interfacial Layer of Anionic Lipids. Biophysica 2024, 4, 92-106. https://doi.org/10.3390/biophysica4010007
Pavlek A-M, Pem B, Bakarić D. The Signature of Fluctuations of the Hydrogen Bond Network Formed by Water Molecules in the Interfacial Layer of Anionic Lipids. Biophysica. 2024; 4(1):92-106. https://doi.org/10.3390/biophysica4010007
Chicago/Turabian StylePavlek, Ana-Marija, Barbara Pem, and Danijela Bakarić. 2024. "The Signature of Fluctuations of the Hydrogen Bond Network Formed by Water Molecules in the Interfacial Layer of Anionic Lipids" Biophysica 4, no. 1: 92-106. https://doi.org/10.3390/biophysica4010007
APA StylePavlek, A.-M., Pem, B., & Bakarić, D. (2024). The Signature of Fluctuations of the Hydrogen Bond Network Formed by Water Molecules in the Interfacial Layer of Anionic Lipids. Biophysica, 4(1), 92-106. https://doi.org/10.3390/biophysica4010007






