Mathematical Models of the Arabidopsis Circadian Oscillator
Abstract
:1. Introduction
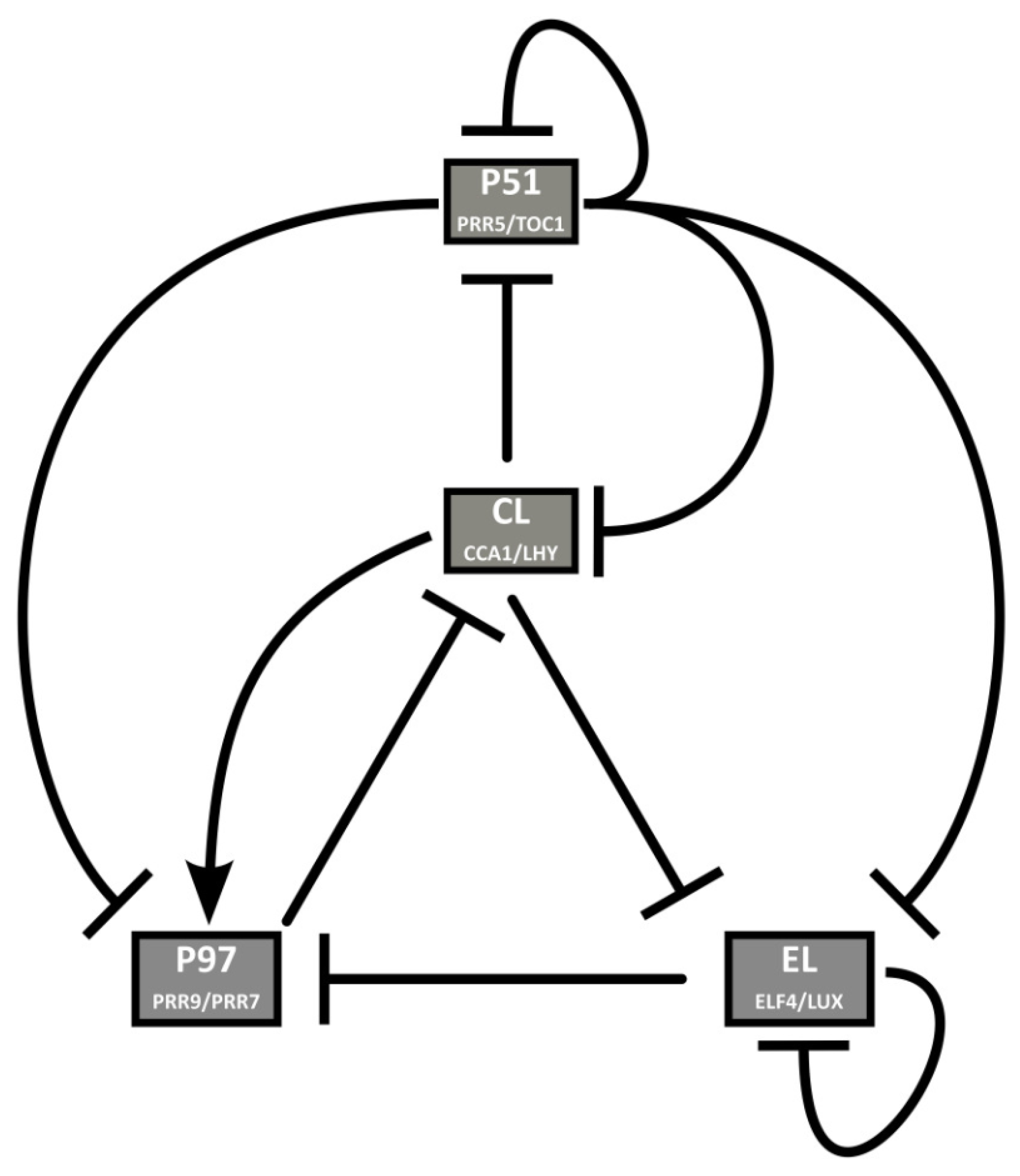
2. Chronological Development of Arabidopsis Circadian Clock Models: Expansion Phase
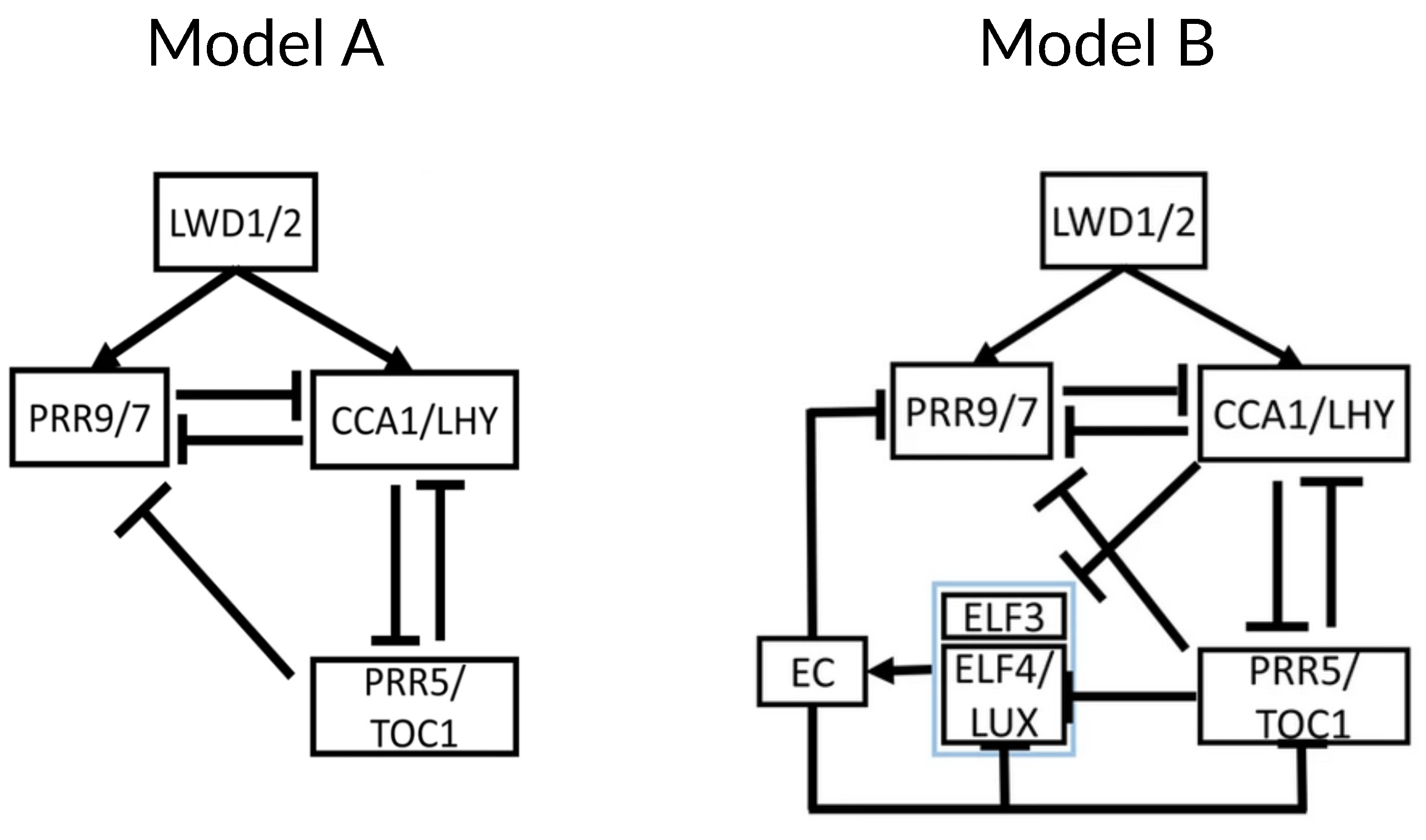
3. Recent Development of Arabidopsis Circadian Clock Models: Reduction Phase
4. Current Challenges and Overview
4.1. Spatial Models
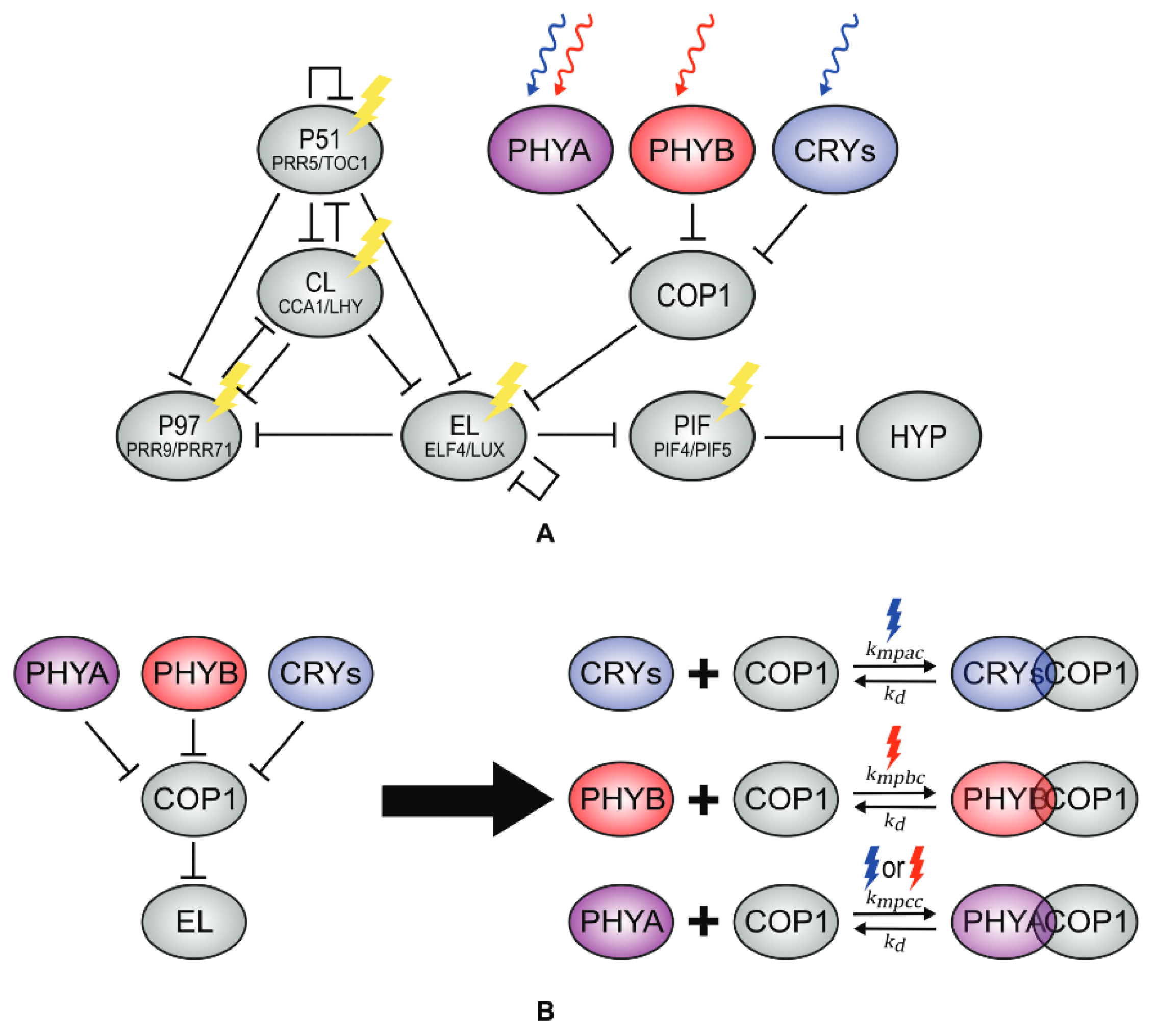
4.2. Effects of Light on the Clock
4.3. Effects of Temperature on the Clock
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bechtel, W.; Abrahamsen, A. Dynamic Mechanistic Explanation: Computational Modeling of Circadian Rhythms as an Exemplar for Cognitive Science. Stud. Hist. Philos. Sci. Part A 2010, 41, 321–333. [Google Scholar] [CrossRef]
- Kauffman, S.A. Articulation of Parts Explanation in Biology and the Rational Search for Them. PSA Proc. Bienn. Meet. Philos. Sci. Assoc. 1970, 1970, 257–272. [Google Scholar] [CrossRef]
- Alon, U. An Introduction to Systems Biology: Design Principles of Biological Circuits, 2nd ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2020. [Google Scholar]
- Jaeger, J.; Monk, N. Dynamical Modules in Metabolism, Cell and Developmental Biology. Interface Focus 2021, 11, 20210011. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Maroto, G.; Catalán, P.; Nieto, C.; Prat, S.; Ares, S. Mathematical Modeling of Photo-and Thermomorphogenesis in Plants. In Thermomorphogenesis: Methods and Protocols; Springer: New York, NY, USA, 2024; pp. 247–261. [Google Scholar]
- Bujdoso, N.; Davis, S.J. Mathematical Modeling of an Oscillating Gene Circuit to Unravel the Circadian Clock Network of Arabidopsis Thaliana. Front. Plant Sci. 2013, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Locke, J.C.W.; Millar, A.J.; Turner, M.S. Modelling Genetic Networks with Noisy and Varied Experimental Data: The Circadian Clock in Arabidopsis Thaliana. J. Theor. Biol. 2005, 234, 383–393. [Google Scholar] [CrossRef]
- Locke, J.C.W.; Southern, M.M.; Kozma-Bognár, L.; Hibberd, V.; Brown, P.E.; Turner, M.S.; Millar, A.J. Extension of a Genetic Network Model by Iterative Experimentation and Mathematical Analysis. Mol. Syst. Biol. 2005, 1, 2005.0013. [Google Scholar] [CrossRef]
- Zeilinger, M.N.; Farré, E.M.; Taylor, S.R.; Kay, S.A.; Doyle, F.J. A Novel Computational Model of the Circadian Clock in Arabidopsis That Incorporates PRR7 and PRR9. Mol. Syst. Biol. 2006, 2, 58. [Google Scholar] [CrossRef]
- Locke, J.C.W.; Kozma-Bognár, L.; Gould, P.D.; Fehér, B.; Kevei, É.; Nagy, F.; Turner, M.S.; Hall, A.; Millar, A.J. Experimental Validation of a Predicted Feedback Loop in the Multi-oscillator Clock of Arabidopsis thaliana. Mol. Syst. Biol. 2006, 2, 59. [Google Scholar] [CrossRef]
- Pokhilko, A.; Hodge, S.K.; Stratford, K.; Knox, K.; Edwards, K.D.; Thomson, A.W.; Mizuno, T.; Millar, A.J. Data Assimilation Constrains New Connections and Components in a Complex, Eukaryotic Circadian Clock Model. Mol. Syst. Biol. 2010, 6, 416. [Google Scholar] [CrossRef]
- Mangan, S.; Zaslaver, A.; Alon, U. The Coherent Feedforward Loop Serves as a Sign-sensitive Delay Element in Transcription Networks. J. Mol. Biol. 2003, 334, 197–204. [Google Scholar] [CrossRef]
- McWatters, H.G.; Kolmos, E.; Hall, A.; Doyle, M.R.; Amasino, R.M.; Gyula, P.; Nagy, F.; Millar, A.J.; Davis, S.J. ELF4 Is Required for Oscillatory Properties of the Circadian Clock. Plant Physiol. 2007, 144, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Kolmos, E.; Nowak, M.; Werner, M.; Fischer, K.; Schwarz, G.; Mathews, S.; Schoof, H.; Nagy, F.; Bujnicki, J.M.; Davis, S.J. Integrating ELF4 into the Circadian System through Combined Structural and Functional Studies. HFSP J. 2009, 3, 350–366. [Google Scholar] [CrossRef] [PubMed]
- Nusinow, D.A.; Helfer, A.; Hamilton, E.E.; King, J.J.; Imaizumi, T.; Schultz, T.F.; Farré, E.M.; Kay, S.A. The ELF4–ELF3–LUX Complex Links the Circadian Clock to Diurnal Control of Hypocotyl Growth. Nature 2011, 475, 398–402. [Google Scholar] [CrossRef]
- Herrero, E.; Kolmos, E.; Bujdoso, N.; Yuan, Y.; Wang, M.; Berns, M.C.; Uhlworm, H.; Coupland, G.; Saini, R.; Jaskolski, M.; et al. EARLY FLOWERING4 Recruitment of EARLY FLOWERING3 in the Nucleus Sustains the Arabidopsis Circadian Clock. Plant Cell 2012, 24, 428–443. [Google Scholar] [CrossRef]
- Pokhilko, A.; Fernández, A.P.; Edwards, K.D.; Southern, M.M.; Halliday, K.J.; Millar, A.J. The Clock Gene Circuit in Arabidopsis Includes a Repressilator with Additional Feedback Loops. Mol. Syst. Biol. 2012, 8, 574. [Google Scholar] [CrossRef]
- Nieto, C.; Catalán, P.; Luengo, L.M.; Legris, M.; López-Salmerón, V.; Davière, J.M.; Casal, J.J.; Ares, S.; Prat, S. COP1 Dynamics Integrate Conflicting Seasonal Light and Thermal Cues in the Control of Arabidopsis Elongation. Sci. Adv. 2022, 8, eabp8412. [Google Scholar] [CrossRef] [PubMed]
- Elowitz, M.B.; Leibler, S. A synthetic oscillatory network of transcriptional regulators. Nature 2000, 403, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Pokhilko, A.; Mas, P.; Millar, A.J. Modelling the Widespread Effects of TOC1 Signalling on the Plant Circadian Clock and Its Outputs. BMC Syst. Biol. 2013, 7, 23. [Google Scholar] [CrossRef]
- Fogelmark, K.; Troein, C. Rethinking Transcriptional Activation in the Arabidopsis Circadian Clock. PLoS Comput. Biol. 2014, 10, e1003705. [Google Scholar] [CrossRef]
- Kim, Y.; Han, S.; Yeom, M.; Kim, H.; Lim, J.; Cha, J.Y.; Kim, W.Y.; Somers, D.E.; Putterill, J.; Nam, H.G.; et al. Balanced Nucleocytosolic Partitioning Defines a Spatial Network to Coordinate Circadian Physiology in Plants. Dev. Cell 2013, 26, 73–85. [Google Scholar] [CrossRef]
- Mangan, S.; Alon, U. Structure and Function of the Feed-Forward Loop Network Motif. Proc. Natl. Acad. Sci. USA 2003, 100, 11980–11985. [Google Scholar] [CrossRef] [PubMed]
- Gutenkunst, R.N.; Waterfall, J.J.; Casey, F.P.; Brown, K.S.; Myers, C.R.; Sethna, J.P. Universally sloppy parameter sensitivities in systems biology models. PLoS Comput. Biol. 2007, 3, e189. [Google Scholar] [CrossRef] [PubMed]
- Transtrum, M.K.; Machta, B.B.; Brown, K.S.; Daniels, B.C.; Myers, C.R.; Sethna, J.P. Perspective: Sloppiness and emergent theories in physics, biology, and beyond. J. Chem. Phys. 2015, 143, 010901. [Google Scholar] [CrossRef] [PubMed]
- Foo, M.; Somers, D.E.; Kim, P.J. Kernel Architecture of the Genetic Circuitry of the Arabidopsis Circadian System. PLoS Comput. Biol. 2016, 12, e1004748. [Google Scholar] [CrossRef]
- Davidson, E.H.; Erwin, D.H. Gene Regulatory Networks and the Evolution of Animal Body Plans. Science 2006, 311, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, S.M.; Cho, K.H. Discovery of a Kernel for Controlling Biomolecular Regulatory Networks. Sci. Rep. 2013, 3, 2223. [Google Scholar] [CrossRef] [PubMed]
- Perez-Carrasco, R.; Barnes, C.P.; Schaerli, Y.; Isalan, M.; Briscoe, J.; Page, K.M. Combining a toggle switch and a repressilator within the AC-DC circuit generates distinct dynamical behaviors. Cell Syst. 2018, 6, 521–530. [Google Scholar] [CrossRef] [PubMed]
- De Caluwé, J.; Xiao, Q.; Hermans, C.; Verbruggen, N.; Leloup, J.C.; Gonze, D. A Compact Model for the Complex Plant Circadian Clock. Front. Plant Sci. 2016, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- De Melo, J.R.F.; Gutsch, A.; Caluwé, T.D.; Leloup, J.C.; Gonze, D.; Hermans, C.; Webb, A.A.R.; Verbruggen, N. Magnesium Maintains the Length of the Circadian Period in Arabidopsis. Plant Physiol. 2021, 185, 519–532. [Google Scholar] [CrossRef]
- Ohara, T.; Hearn, T.J.; Webb, A.A.; Satake, A. Gene Regulatory Network Models in Response to Sugars in the Plant Circadian System. J. Theor. Biol. 2018, 457, 137–151. [Google Scholar] [CrossRef]
- Ohara, T.; Satake, A. Photosynthetic Entrainment of the Circadian Clock Facilitates Plant Growth under Environmental Fluctuations: Perspectives from an Integrated Model of Phase Oscillator and Phloem Transportation. Front. Plant Sci. 2017, 8, 1859. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Gonze, D. Stochastic Simulation of a Model for Circadian Rhythms in Plants. J. Theor. Biol. 2021, 527, 110790. [Google Scholar] [CrossRef] [PubMed]
- Joanito, I.; Chu, J.W.; Wu, S.H.; Hsu, C.P. An Incoherent Feed-Forward Loop Switches the Arabidopsis Clock Rapidly between Two Hysteretic States. Sci. Rep. 2018, 8, 13944. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, I.T.; Akman, O.E.; Locke, J.C.W. Reducing the Complexity of Mathematical Models for the Plant Circadian Clock by Distributed Delays. J. Theor. Biol. 2019, 463, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Davis, W.; Endo, M.; Locke, J.C.W. Spatially Specific Mechanisms and Functions of the Plant Circadian Clock. Plant Physiol. 2022, 190, 938–951. [Google Scholar] [CrossRef] [PubMed]
- Nohales, M.A. Spatial Organization and Coordination of the Plant Circadian System. Genes 2021, 12, 442. [Google Scholar] [CrossRef] [PubMed]
- Gould, P.D.; Domijan, M.; Greenwood, M.; Tokuda, I.T.; Rees, H.; Kozma-Bognar, L.; Hall, A.J.; Locke, J.C. Coordination of Robust Single Cell Rhythms in the Arabidopsis Circadian Clock via Spatial Waves of Gene Expression. eLife 2018, 7, e31700. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, M.; Tokuda, I.T.; Locke, J.C.W. A Spatial Model of the Plant Circadian Clock Reveals Design Principles for Coordinated Timing. Mol. Syst. Biol. 2022, 18, e10140. [Google Scholar] [CrossRef] [PubMed]
- Juarrero, A. Context Changes Everything: How Constraints Create Coherence; The MIT Press: Cambridge, MA, USA, 2023. [Google Scholar] [CrossRef]
- Galvão, V.C.; Fankhauser, C. Sensing the light environment in plants: Photoreceptors and early signaling steps. Curr. Opin. Neurobiol. 2015, 34, 46–53. [Google Scholar] [CrossRef]
- Ohara, T.; Fukuda, H.; Tokuda, I.T. An Extended Mathematical Model for Reproducing the Phase Response of Arabidopsis Thaliana under Various Light Conditions. J. Theor. Biol. 2015, 382, 337–344. [Google Scholar] [CrossRef]
- Pay, M.L.; Kim, D.W.; Somers, D.E.; Kim, J.K.; Foo, M. Modelling of Plant Circadian Clock for Characterizing Hypocotyl Growth under Different Light Quality Conditions. In Silico Plants 2022, 4, diac001. [Google Scholar] [CrossRef] [PubMed]
- Pay, M.L.; Christensen, J.; He, F.; Roden, L.; Ahmed, H.; Foo, M. An Extended Plant Circadian Clock Model for Characterising Flowering Time under Different Light Quality Conditions. In Proceedings of the 2022 22nd International Conference on Control, Automation and Systems (ICCAS), Jeju, Republic of Korea, 27 November–1 December 2022; pp. 1848–1853. [Google Scholar] [CrossRef]
- Huang, T.; Shui, Y.; Wu, Y.; Hou, X.; You, X. Red Light Resets the Expression Pattern, Phase, and Period of the Circadian Clock in Plants: A Computational Approach. Biology 2022, 11, 1479. [Google Scholar] [CrossRef] [PubMed]
- De Caluwé, J.; De Melo, J.R.F.; Tosenberger, A.; Hermans, C.; Verbruggen, N.; Leloup, J.C.; Gonze, D. Modeling the Photoperiodic Entrainment of the Plant Circadian Clock. J. Theor. Biol. 2017, 420, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Schmal, C.; Myung, J.; Herzel, H.; Bordyugov, G. A Theoretical Study on Seasonality. Front. Neurol. 2015, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Gould, P.D.; Ugarte, N.; Domijan, M.; Costa, M.; Foreman, J.; MacGregor, D.; Rose, K.; Griffiths, J.; Millar, A.J.; Finkenstädt, B.; et al. Network Balance via CRY Signalling Controls the Arabidopsis Circadian Clock over Ambient Temperatures. Mol. Syst. Biol. 2013, 9, 650. [Google Scholar] [CrossRef]
- Avello, P.A.; Davis, S.J.; Ronald, J.; Pitchford, J.W. Heat the Clock: Entrainment and Compensation in Arabidopsis Circadian Rhythms. J. Circadian Rhythm. 2019, 17, 5. [Google Scholar] [CrossRef] [PubMed]
- Avello, P.; Davis, S.J.; Pitchford, J.W. Temperature Robustness in Arabidopsis Circadian Clock Models Is Facilitated by Repressive Interactions, Autoregulation, and Three-Node Feedbacks. J. Theor. Biol. 2021, 509, 110495. [Google Scholar] [CrossRef]
- Yuan, L.; Avello, P.; Zhu, Z.; Lock, S.C.; McCarthy, K.; Redmond, E.J.; Davis, A.M.; Song, Y.; Ezer, D.; Pitchford, J.W.; et al. Complex epistatic interactions between ELF3, PRR9, and PRR7 regulate the circadian clock and plant physiology. Genetics 2024, 226, iyad217. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henao, L.; Ares, S.; Catalán, P. Mathematical Models of the Arabidopsis Circadian Oscillator. Biophysica 2024, 4, 267-282. https://doi.org/10.3390/biophysica4020019
Henao L, Ares S, Catalán P. Mathematical Models of the Arabidopsis Circadian Oscillator. Biophysica. 2024; 4(2):267-282. https://doi.org/10.3390/biophysica4020019
Chicago/Turabian StyleHenao, Lucas, Saúl Ares, and Pablo Catalán. 2024. "Mathematical Models of the Arabidopsis Circadian Oscillator" Biophysica 4, no. 2: 267-282. https://doi.org/10.3390/biophysica4020019





