Abstract
ATP analogues are essential tools in enzymology and structural biology, but the structural and functional implications of their chemical modifications on nucleotide-binding proteins are often underappreciated. To address this, we evaluated a panel of ATP analogues, focusing on thiosubstituted and fluorinated molecules, using the AAA+ ATPase p97 as a benchmark system. Hydrolysis stability and impact on protein conformation, binding modes, and kinetics of enzymatic catalysis were assessed by protein-detected methyl NMR and ligand-detected 19F NMR in solution, as well as 31P solid-state NMR of nucleotides within protein sediments. ATPγS and AMP-PNP emerged as the most suitable analogues for preserving pre-hydrolysis states over extended periods, despite undergoing gradual hydrolysis. In contrast, both AMP-PCP and α/β-thiosubstituted analogues failed to induce native protein conformations in p97. Notably, we demonstrate a novel real-time NMR setup to explore the effect of nucleotide mixtures on cooperativity and the regulation of enzymes. Additionally, aromatic fluorine TROSY-based 19F NMR shows promise for direct ligand detection in solution, even in the context of large macromolecular complexes. These findings provide critical guidance for selecting ATP analogues in functional and structural studies of nucleotide-binding proteins.
1. Introduction
1.1. Why Nucleotide Analogues?
Adenosine triphosphate (ATP) is the central metabolite cells use when they require the rapid release of chemical energy into molecular motion to power diverse classes of enzymes, such as transporters, chaperones, helicases, or motor proteins. Typical cellular ATP concentrations are in the low millimolar range (~5 mM) [1], mostly complexed by intracellular Mg2+ ions (~10 mM) with an affinity in the medium micromolar range [2,3]. The turnover of ATP is one of the most prevalent chemical reactions in living systems, and there is a fundamental interest in how this reaction is enzymatically catalysed. Mechanistic investigations in structural biology and biophysics often require maintaining some functionalities of ATP, e.g., affinity for a protein, while altering others, e.g., sensitivity to hydrolysis, or the availability of a fluorophore or luminophore.
The synthetic exploration of ATP analogues with modified stability, reactivity, or spectroscopic properties has witnessed intense research efforts from the 1970s [4,5,6], in parallel with groundwork in enzyme kinetics and the charting of fundamental protein folds. Today, a vast array of ATP analogues [7] is commercially available (https://www.biolog.de/ (accessed on 23 February 2025) and https://www.jenabioscience.com/ (accessed on 23 February 2025)). Analogues can be categorised in terms of chemical modification, e.g., fluorination [8] or thiosubstitution [4]; in terms of their modification site: phosphate [4], ribose [9], or base [10]; or focusing on their reactivity as fully, non-, or slowly hydrolysable (Figure 1). Specifically for NMR spectroscopy, ATP and analogues with natural (1H, 31P) or synthetically incorporated (15N, 19F, 13C) NMR-active nuclei are available. ATP analogues find applications in the preparation of uniform pre-hydrolysis states of enzymes for single-particle cryo-electron microscopy, crystallisation, NMR and EPR spectroscopy [11], competition experiments in enzyme kinetics, and single-molecule studies performed in nucleotide mixtures to unravel the operation mode and cooperativity of multimeric enzymes [12].
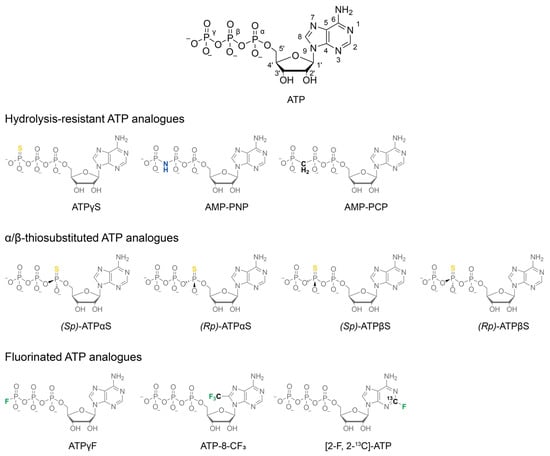
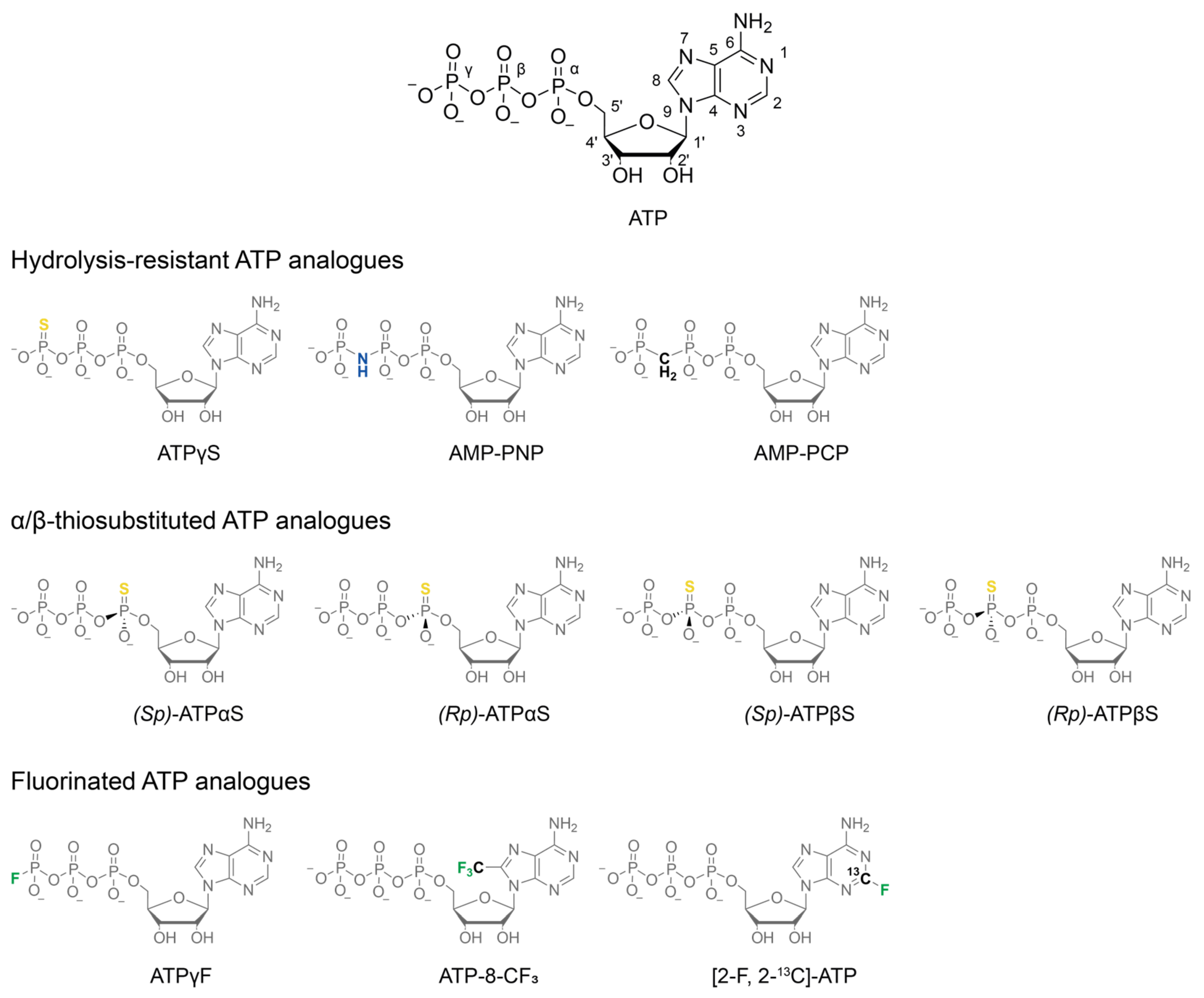
Figure 1.
Chemical structures of nucleotide analogue classes discussed in this work. Native ATP is shown for comparison with conventional atom numbering. Hydrolysis-resistant analogues are employed where a stable pre-hydrolysis state is required. Their actual resistance to spontaneous and catalysed hydrolysis is investigated below. α- and β-thiosubstitutions impose P-chirality on the respective thiophosphate, resulting in different binding modes and affinities of the diastereomers. Fluorinated analogues enable 19F NMR spectroscopy, harnessing the bio-orthogonality and high NMR sensitivity of this nucleus.
Distinct stages of ATP processing are mimicked using various ATP analogues. Pre-hydrolysis mimics, such as AMP-PNP, AMP-PCP, and ATPγS, are designed for slow or no hydrolysis, while transition-state mimics like ADP·AlFx, ADP·MgFx, and ADP·Vi replicate the pentavalent transition state of the γ-phosphate during the nucleophilic attack by water [11]. The post-hydrolysis state is characterised by the binding of ADP and inorganic phosphate (ADP·Pi) at the active site and can be observed natively in the presence of ATP and a regeneration system [13,14] if phosphate release is the rate-limiting step of the enzymatic cycle.
1.2. Why Scrutinise Results Obtained Using Analogues?
The assumption that ATP analogues faithfully preserve the natural ATP-bound protein state is challenged by at least four potential concerns:
- At the active site, the binding sites and poses of ATP and Mg2+ ions can be altered, even by subtle synthetic modifications, such as thiophosphate substitution [15]. Changes in nucleotide–ion or nucleotide–protein contacts can affect binding affinity.
- Due to non-native binding modes at the active site, long-range conformational changes associated with ATP binding and hydrolysis may not be accurately replicated, leading to misrepresentations of the overall protein conformational space.
- The nucleotide bound at the active site can be different to the one supplied in solution. Indeed, not only ATP but also slowly hydrolysable analogues may inadvertently trap enzymes in post-hydrolysis states in which product release, not hydrolysis, is rate-limiting.
- Analogues differ from ATP in thermal and chemical stability and compatibility with ATP-regeneration systems for multiple-turnover experiments.
1.3. Setup and Goals of This Study
NMR spectroscopy provides atomic-level insight into the chemical environment of NMR-active nuclei in a sample through its most fundamental observable, the chemical shift. This capability makes it a powerful tool for simultaneously identifying bound nucleotides, monitoring small-molecule composition, and assessing structural states of enzymes. These strengths were leveraged to detect subtle structural transitions along the trajectory of enzymatic ATP hydrolysis, capturing static snapshots of multiple conformational states [11]. Importantly, NMR experiments can also be conducted in a time-resolved manner to trace in situ the progression of enzymatic reactions and to detect associated protein conformational changes. For example, by employing ATP in conjunction with a regeneration system fuelled by phosphoenolpyruvate (PEP), the activity of ATPase enzymes can be observed over extended periods of time. This system uses pyruvate kinase (PK) to continuously reform ATP from PEP and ADP [13,14]. The experiment’s duration is constrained by PK activity and PEP availability, as highly active ATPases can deplete PEP rapidly or outpace the regeneration process.
Many ATPases are multimeric complexes, and with molecular weights nearing or exceeding 100 kDa, the choice of spin systems that remain detectable by solution NMR becomes limited. In magic-angle spinning solid-state NMR (MAS ssNMR), the protein is sedimented into a rotor and becomes immobilised but remains surrounded by the reaction buffer with freely diffusing small molecules. Under these conditions, dynamic spectral editing separates molecules from different motional regimes [16,17,18,19]: Dipolar-based magnetisation transfers, being mediated via orientation-dependent spin interactions through space, are only efficient for rigid elements, notably for immobilised enzymes along with bound nucleotides. A simple dipolar-based filtering element is cross polarisation (CP); it becomes ineffective in the presence of molecular motions. In contrast, scalar-coupling-based transfers through covalent bonds or direct excitation via pulses combined with short relaxation delays select for unbound small molecules retaining rotational mobility in solution and for immobilised molecules with high internal mobility. Dynamic editing thus allows to spectroscopically separate bound from free nucleotides. The readout of protein conformational state is possible by recording fingerprint spectra of amide or methyl moieties, either in solution or in the protein sediment under MAS.
Producing protein sediments for MAS ssNMR is a resource-intensive process, requiring milligram quantities of protein, and the necessary setups are not readily available in all laboratories. Therefore, to characterise bound nucleotides in solution-state NMR, we also explored the potential of fluorinated ATP analogues in 19F NMR as an alternative, offering a potential solution to overcome this limitation.
ATPases are functionally diverse, yet their nucleotide binding pockets often originate from a common fold. Those belonging to the evolutionary lineage of the P-loop NTPase enzymes share the name-giving loop, which is flanked by conserved Walker A and B motifs responsible for nucleotide binding and hydrolysis, respectively (Figure 2a,b). Within this lineage, the AAA+ superfamily (ATPases associated with various cellular activities) furthermore features sensor motifs—where sensor 1 is essential, while sensor 2 is absent from some enzymes—as well as arginine fingers that act in trans (i.e., from the neighbouring subunits) within symmetric ring-shaped homo-oligomeric assemblies [20,21,22,23]. Given the general topology of the protein-nucleotide interface [21], findings from one prototypical enzyme can exemplify common principles of ATP analogue interactions.
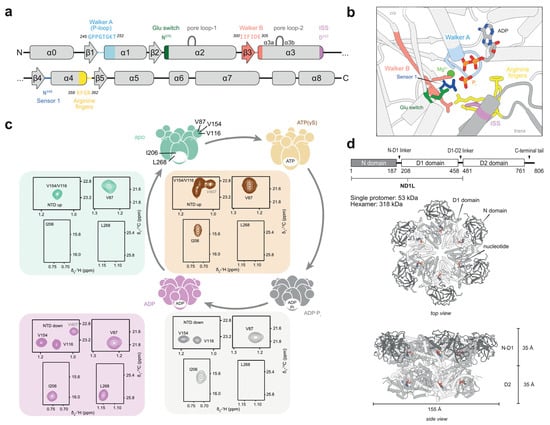
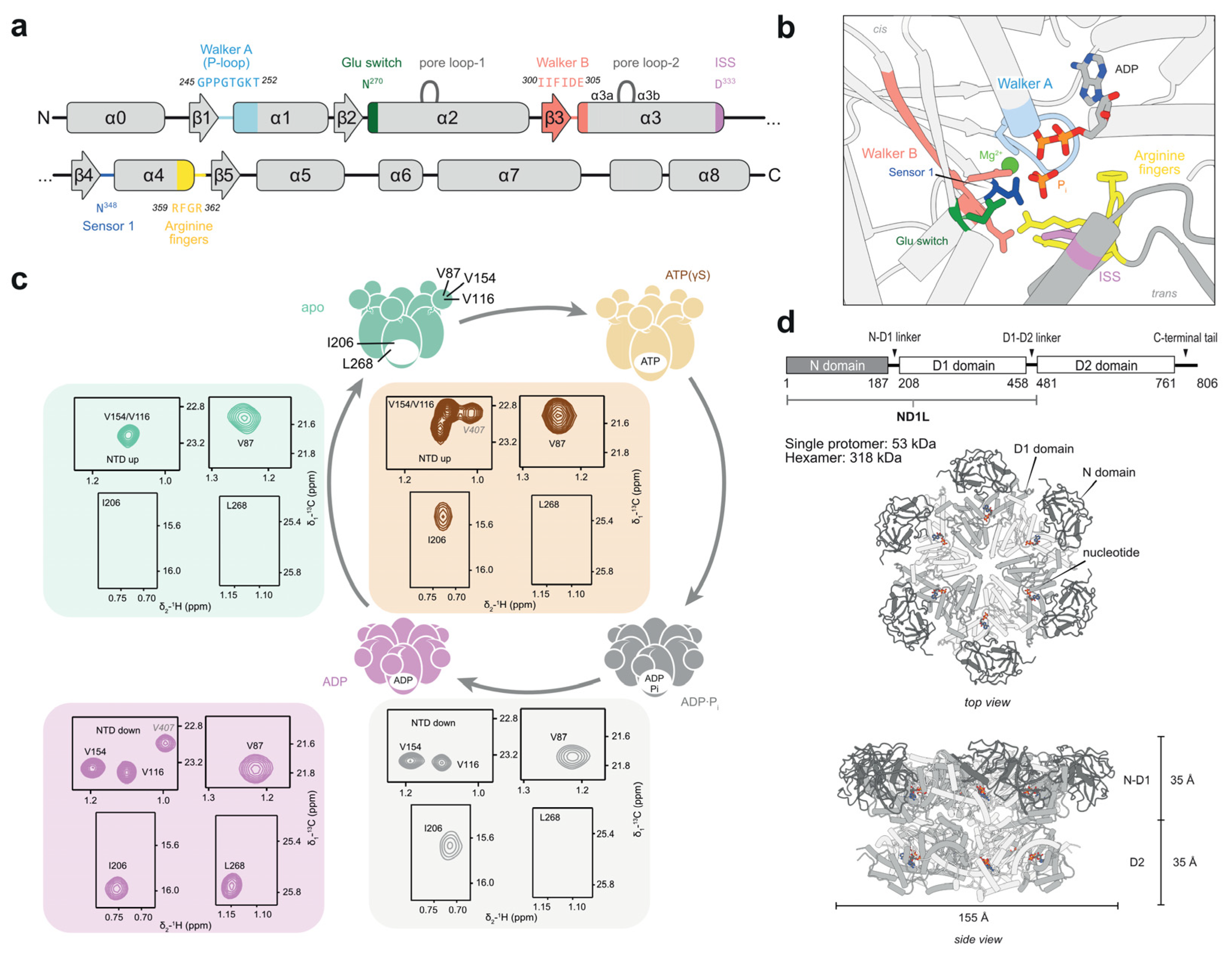
Figure 2.
The p97 protein as prototypical AAA+-ATPase. (a) Secondary structure with the position of conserved motifs of the D1 domain. (b) Structure of the nucleotide-binding pocket at the interface between two protomers, in a post-hydrolysis (ADP·Pi) state (PDB: 8OOI). The Walker A motif is involved in nucleotide binding and Mg2+ coordination, which is essential for functional ATP binding. Walker B enables ATP hydrolysis through the activation of water; mutations thereof are associated with deficient hydrolysis. Trans-acting elements, the arginine finger and intersubunit signalling residues (ISS), enable nucleotide sensing. (c) Schematic ATP-hydrolysis cycle of p97-ND1L, shown in (d). In the apo or ATP-bound states, the N-terminal domain (NTD) adopts an upward-pointing conformation. In the ADP·Pi- or ADP-bound state, the NTD is coplanar with D1. Methyl correlations of residues V116, V154, and V87 appear at distinct chemical shifts, serving as probes for NTD conformation, while the identity of the bound nucleotide is sensed by I206 and L268. The assignment of the unrelated residue V407 is indicated for completeness. Due to the high temperature stability of p97, which is associated with the heat-shock response [24], methyl-TROSY spectra can be recorded at 50 °C or 37 °C. Altogether, methyl probes allow to discriminate between all four nucleotide states.
The AAA+ family member p97 is a highly abundant human protein [25] involved in diverse cellular processes, ranging from protein homeostasis and degradation to organelle assembly and DNA repair [26]. It has ATP-dependent segregase and unfoldase activity, whereby it pulls substrates from protein or DNA complexes and processes them for downstream actors, notably the ubiquitin–proteasome system [27]. It is composed of two homologous ATPase-active domains [28], with the first, D1, being sufficient to assemble into hexamers and replicate the hydrolysis-induced large-scale motion of the N-terminal domain (NTD), which in turn binds to cofactors and substrates. Neither D1 nor D2 comprises a sensor 2 motif. The minimal p97-ND1L construct, comprising NTD, D1, and the D1-D2-linker region, was used throughout this study (Figure 2c,d).
The p97 protein is an intensely investigated member of the AAA+ family with a large pool of cryo-EM [29,30] and crystal [28,31] structures in complex with ADP, ATP, and analogues thereof, which firmly establish the conformational transitions induced throughout the ATP-hydrolysis cycle. Four well-established nucleotide states in D1 can be distinguished: apo (nucleotide-free), pre-hydrolysis bound to ATPγS+Mg2+, post-hydrolysis ADP·Pi, and ADP. Furthermore, the transition state analogue ADP·AlFx was employed previously in a small-angle X-ray scattering (SAXS) study [32]. The motion of the NTD from an elevated to a coplanar position with respect to D1 upon ATP hydrolysis is readily detected in the NMR spectra of methyl groups [33]. To investigate the ADP·Pi state, p97 is measured under constant ATP turnover, i.e., in the presence of an ATP regeneration system [13,14]. The NMR spectra of ADP and ADP·Pi-bound p97 are overall similar with clear chemical shift perturbations (CSPs) for residues near the active site and peak broadening for the ADP·Pi state, indicative of conformational exchange within this short-lived reaction intermediate. In the apo state, the NTD is flexible and poorly resolved in cryo-EM [34], while the residues located in the NTD still show a distinctive NMR fingerprint (Figure 2c). Introducing the Walker B mutation (E305Q) into the active site reduces the rate of ATP turnover, but the rate-limiting step of the enzymatic cycle remains product release from the ADP·Pi state [13].
Using p97-ND1L as a benchmark system, we investigated eight commercially available ATP analogues, depicted in Figure 1, with respect to: resistance to thermal and enzymatic hydrolysis, compatibility with the regeneration of the triphosphate moiety by PK, capacity to mimic the pre-hydrolysis nucleotide state of p97-ND1L with Mg2+-dependent ATP binding, and ability to replicate long-range conformational changes associated with ATP processing that are essential to p97-ND1L function. Enzyme conformation was assessed from methyl fingerprint NMR spectra in solution, while the nucleotide state was inferred from 31P spectra acquired in a MAS ssNMR setup. Towards the straightforward detection of protein-bound nucleotides in solution, we explored the potential of fluorinated ATP analogues for 19F NMR.
2. Materials and Methods
2.1. Source of Nucleotide Analogues
Nucleotide analogues were obtained either in their ATP form ((Sp)-ATPαS: Biolog LSI, Bremen, Germany; ATPγS, ATPγF, [2-F, 2-13C]-ATP, AMP-PNP, AMP-PCP: Jena Bioscience, Jena, Germany) or in their ADP form (ADPβS: Biolog LSI, Bremen, Germany; 8-Trifluoromethyl-ADP: Jena Bioscience, Jena, Germany). ADP analogues were enzymatically regenerated using rabbit muscle PK (Merck, Darmstadt, Germany) at room temperature for 2 days in a reaction containing 8 mM analogue, 4 mM ribose-5-phosphate, 30 U PK, 30 mM PEP, 4 mM MgCl2, 50 mM KCl, and 5 mM TCEP. All analogues were lyophilised and dissolved in D2O prior to use in NMR spectroscopy.
2.2. Protein Expression and Purification
p97-ND1L protein samples were prepared as described previously [14,33]. Briefly, His6-TEV-p97-ND1L (residues 1–480, wild-type or E305Q mutation) was expressed in E. coli BL21(DE3) cells from a pET28a(+) vector. For methyl-TROSY experiments, perdeuterated and selectively [1H, 13C]-labelled samples were produced with I-δ1-[13CH3], V/L-γ1/δ1(proR)-[13CH3,12CD3] and M-ε1-[13CH3] (NMRBio, Grenoble, France) following the established procedure [33].
Protein purification involved nickel-immobilised affinity chromatography (Ni-IMAC), TEV protease cleavage, and dialysis. The sample was further purified by size-exclusion chromatography (Superdex 200 16/600 column) against SEC buffer (20 mM HEPES pH 7.5, 250 mM KCl, 1 mM MgCl2). Purified protein samples were either directly prepared for NMR spectroscopy or flash-frozen with 1 mM DTT and 5% glycerol.
2.3. NMR Sample Preparation
For solution-state NMR experiments, selectively labelled p97-ND1L samples were buffer-exchanged into 25 mM HEPES (pD 7.5), 25 mM NaCl, and 5 mM TCEP in 100% D2O, and then concentrated to 100–200 µM. For reference state measurements, no nucleotide was added for the apo state, while 5 mM ADP were used for the ADP state, and 5 mM ATP analogues with 4 mM MgCl2 were added for ATP analogue states. Regeneration system measurements were prepared with 5 mM ATP or ATP analogue, 4 mM MgCl2, 30–200 mM PEP, 4 mM ribose-5-phosphate, 50 mM KCl, and 10–50 U pyruvate kinase from Bacillus stearothermophilus (Merck, Darmstadt, Germany).
For decay series measurements, a solution containing 1 mM nucleotide analogue and 4 mM MgCl2 in the same buffer was prepared. Optionally, 100 µM p97-ND1L was added, and the mixture was incubated at 37 °C.
For solid-state NMR measurements under MAS, p97-ND1L-E305Q was buffer-exchanged into 25 mM HEPES (pH 7.4), 50 mM KCl, 5 mM TCEP, and 1 mM DSS in H2O and concentrated to 0.5–1 mM. Nucleotides were added identically to the solution state; however, rabbit muscle PK was used instead to benefit from its higher activity at low temperatures. Protein samples were filled into 1.3 mm or 2.5 mm rotors through ultracentrifugation at 130,000× g for 12–24 h using commercial filling tools (Giotto Biotech, Florence, Italy).
2.4. NMR Measurements
Protein spectra were acquired on Bruker Avance III spectrometers operating at 800, 900, or 950 MHz and equipped with cryogenically cooled TCI probes. Measurements in the apo state were conducted at 310 K, while all other states were measured at 323 K. Two-dimensional methyl-TROSY spectra for protein state assessment and 1D 1H spectra for reaction monitoring were recorded interleaved over a total acquisition time of 12–30 h.
For measurements on protein sediments, experiments were performed on Bruker 700 or 800 MHz spectrometers operating Avance III HD or Avance Neo consoles with MAS probes in the HPC mode. Sample temperatures were calibrated to 280 K using the water chemical shift with respect to DSS. MAS rates were set at 45 kHz for 1.3 mm rotors and 30 kHz for 2.5 mm rotors. Directly pulsed 1H-decoupled 1D 31P spectra and 1H-decoupled 1D 1H-31P cross-polarisation (CP) spectra were recorded interleaved over 72–160 h, with identical spectra accumulated. Detailed acquisition and processing parameters for protein and solid-state NMR have been described previously [14].
1H 1D spectra for stability measurements were recorded on a Bruker 400 MHz spectrometer equipped with a broadband BBO probe or on an 800 MHz spectrometer with a TCI cryoprobe. Spectra were acquired using a 30° flip angle at 310 K. Spectra were apodised with an exponential window function (0.2 Hz line broadening) and zero-filled twice before Fourier transform. Processing was performed using TopSpin 4.4, and spectra were converted for further analysis using Nmrglue [35]. Reference peaks were fitted using SciPy, and integrals were calculated from these fits. Exponential decay curves were also fitted using SciPy.
Solution 31P 1D and 19F 1D spectra were recorded on a Bruker 400 MHz spectrometer with a broadband BBO probe or on a Bruker 500 MHz spectrometer equipped with an Avance Neo console and a Prodigy nitrogen-cooled probe. Aromatic 13C-19F-TROSY spectra were acquired on the 500 MHz spectrometer at 318 K in D2O buffer (as described above). Protein-bound spectra were recorded in the presence of deuterated, otherwise unlabelled p97-ND1L. Experiments were performed using the pulse sequence from [36], using Echo-AntiEcho in the indirect dimension. The 13C signal was encoded in the direct dimension with 4096 points, corresponding 81.9 ms acquisition time and a 199 ppm spectral window centred at 100 ppm. The 19F signal was encoded in the indirect dimension with 64 points and 17 ms acquisition time, with the 4 ppm spectrum centred at −52.5 ppm. Spectra were apodised with sine-squared window functions in both dimensions. Experimental durations were 10 min for the free compound and 8 to 12 h for the protein-bound state.
All spectra were processed using Topspin 3.7 and analysed using CcpNmr v3 [37] or Nmrglue [35] if not indicated otherwise.
3. Results
3.1. Stability of Nucleotide Analogues in Solution
Long-term experiments at physiological temperatures require nucleotide analogues with high stability against spontaneous hydrolysis. The nucleotide present in solution can be monitored using ¹H-1D NMR spectroscopy, where reporter peaks of the ribose protons (H2′/H3′) and the adenine base proton (H8) are observed at approximately 4.9 ppm and 8.7 ppm, respectively. These peaks shift upfield as hydrolysis progresses from an ATP to an ADP analogue (Figure 3). While the H2′/H3′ signal can be obscured by interference from the water signal, the H8 signal is generally well-resolved and more reliable for analysis. To evaluate the stability of nucleotide analogues and distinguish between spontaneous and p97-catalysed hydrolysis, we recorded ¹H-1D NMR spectra over several days, both in the presence and absence of p97-ND1L (Figure 4).
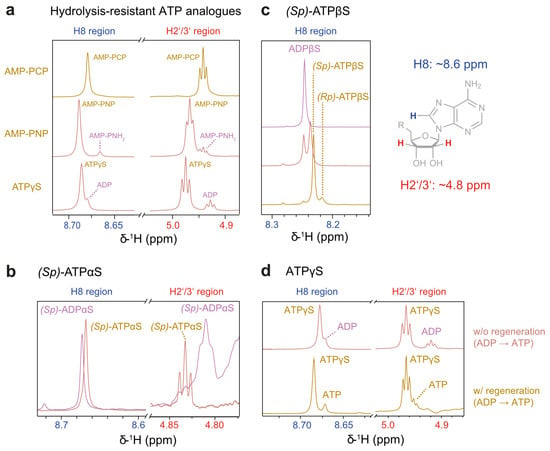
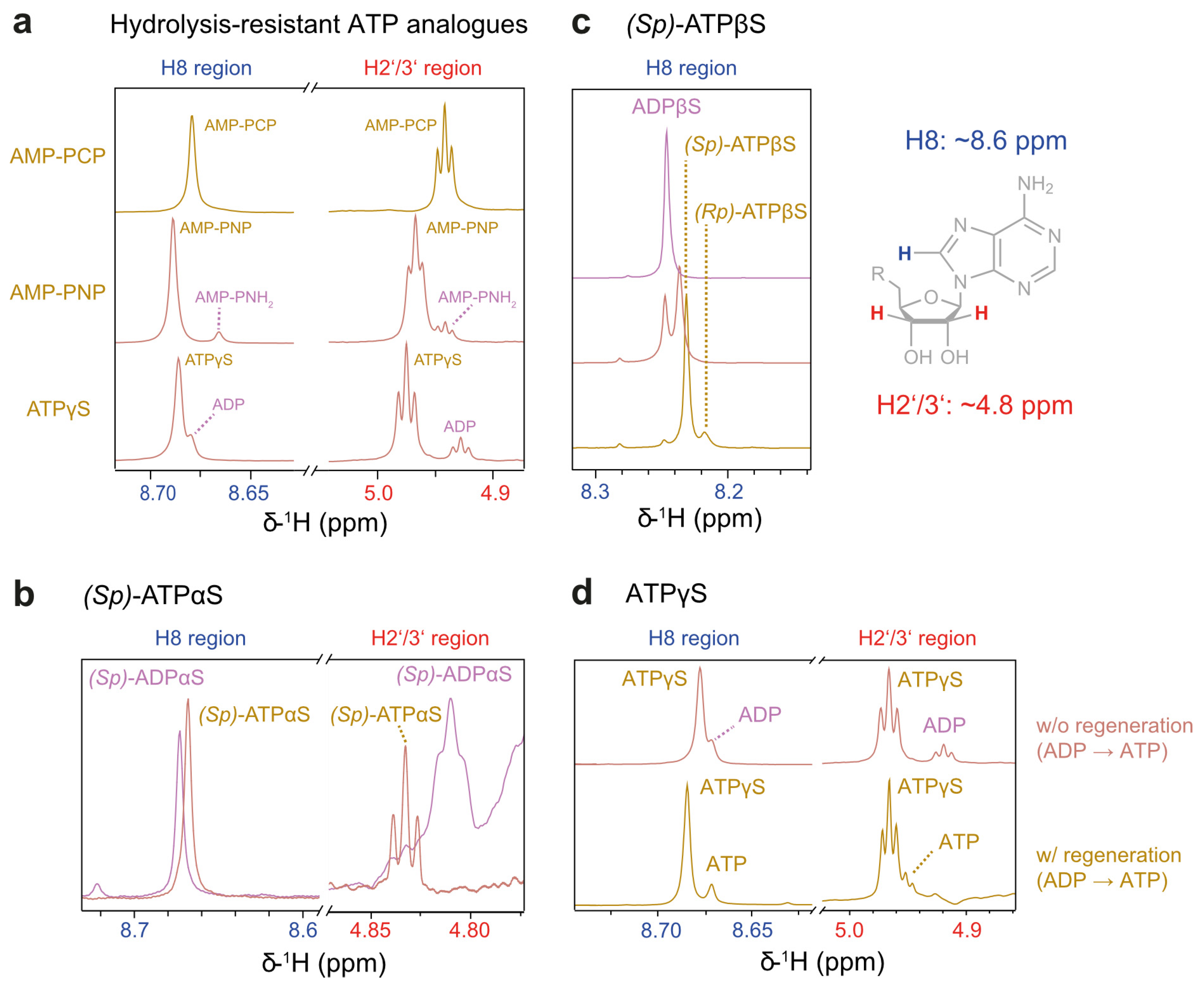
Figure 3.
Reporter signals for nucleotide analogue hydrolysis with and without a regeneration system at 37 °C. All analogues can be identified in the 1H NMR spectra by signals corresponding to adenine H8 (approx. 8.6 ppm) and ribose H2′/3′ (approx. 4.8 ppm). Respective spectra are shown for (a) hydrolysis-resistant analogues, (b) (Sp)-ATPαS, and (c) (Sp)-ATPβS. The latter was produced by regeneration from ADPβS; the spectrum of the intermediate stage is shown as well. Peak assignments are derived from reported product preference of PK [38]. The reaction stalled after converting approx. 50% (4 mM) of ADPβS but resumed upon a four-fold dilution. Due to the pronounced water signal, no spectrum of H2′/3′ is shown herein. (d) Differences in nucleotide composition for ATPγS in presence or absence of the regeneration system: Without regeneration, ADP build-up is observed, while with regeneration, both ATPγS and ATP are present.
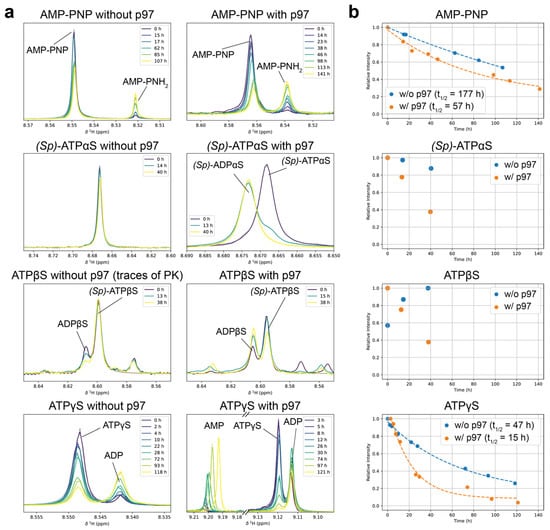
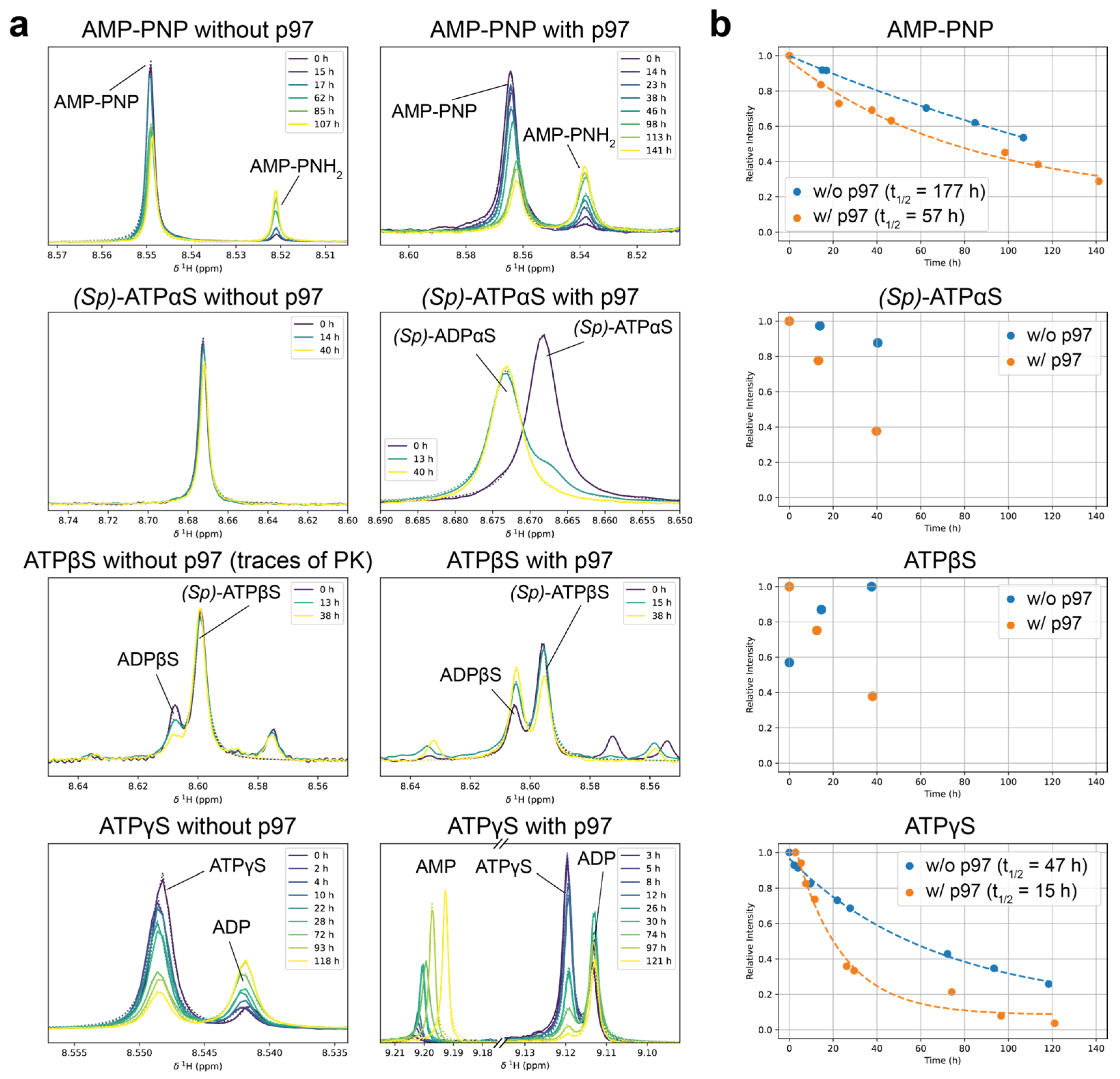
Figure 4.
Hydrolysis stability of nucleotide analogues in the presence and absence of p97-ND1L. (a) Nucleotide analogues were incubated at 37 °C as 1 mM solutions with or without 100 µM p97. Periodically recorded 1H 1D NMR spectra indicate hydrolysis over time, derived from the resonances introduced in Figure 3. Lorentzian fit curves are superimposed in dashed lines. With (Sp)-ATPβS, an initial concentration increase is observed due to the regeneration from the starting molecule ADPβS from residual components of the regeneration system. In the presence of p97-ND1L, ATPγS undergoes further hydrolysis from ADP to AMP. (b) Time evolution of relative intensities from peaks in (a). Exponential decay was fitted where feasible, and the approximate half-life is provided.
3.1.1. Hydrolysis-Resistant ATP Analogues
Allegedly hydrolysis-resistant ATP analogues are used to induce conformations mimicking the ATP-bound (pre-hydrolysis) state. Still, they undergo both spontaneous and enzyme-catalysed hydrolysis to varying extents. In initial trials, AMP-PCP proved stable, displaying a single peak for both the H2′/H3′ and H8 atoms, confirming its resistance to hydrolysis. Consequently, it was excluded from further decay series experiments. In contrast, AMP-PNP showed the accumulation of its hydrolysis product, AMP-PNH2, while ATPγS hydrolysis was evident from the appearance of ADP signals (Figure 3a).
The stability of the ATP analogues AMP-PNP and ATPγS (each 1 mM) was assessed by monitoring the intensities of their respective peaks over several days at 37 °C. In both cases, the decay of peak intensities followed an exponential trend, enabling the determination of half-life values. For AMP-PNP, the half-life decreased significantly from 7.4 days in the absence of p97-ND1L to 2.4 days in its presence at 100 µM, indicating partial catalysed hydrolysis. Similarly, ATPγS exhibited a marked reduction in half-life from 2.0 days without p97-ND1L to just 0.6 days with the protein, demonstrating a much greater susceptibility to hydrolysis (Figure 4). These findings highlight that AMP-PNP is considerably more stable than ATPγS under the tested conditions. However, it is important to note that ATPγS exhibits a higher binding affinity for p97-ND1L compared to ADP and ATP (36.7 nM vs. 132 nM and 131 nM) [39]. This property allows for the characterisation of ATPγS-bound states of p97-ND1L, even when a substantial portion of ATPγS has undergone hydrolysis. In summary, ATPγS, AMP-PNP and AMP-PCP all show the stability necessary to run experiments over the course of at least two days at physiological temperatures.
3.1.2. α/β-Thiosubstituted ATP Analogues
We hypothesised that the α- or β-thiosubstituted ATP analogues would exhibit reduced hydrolysis rates while remaining compatible with the regeneration system. This, in turn, would extend the system’s lifetime while preserving native-like effects on protein structure.
In contrast to ATPγS, the thiosubstitution in ATPαS and ATPβS introduces a new P-chirality centre at the αP or βP positions. This modification alters metal ion interactions, as sulphur—a soft Lewis base—interacts differently with metal ions compared to oxygen, a hard Lewis base [40,41]. The distinct substrate preferences for the (Sp)- and (Rp)-diastereomers have been leveraged to structurally characterise nucleotide-binding sites, including metal ion coordination in ATP-processing enzymes such as hexokinase [38] and adenylate kinase [42]. Regarding the substrate specificity of PK, (Rp)-ADPαS functions unequivocally as an inhibitor, whereas (Sp)-ADPαS is reported to act either as a poor substrate or as a non-competitive inhibitor [38,41,43,44]. This prompted the selection of the (Sp)-diastereomer for further investigation. Neither diastereomer of ATPβS is commercially available; so, we obtained ATPβS via the regeneration of its ADP analogue. Hereby, the nucleotide composition becomes more complex due to PK’s preference for generating (Sp)-ATPβS over (Rp)-ATPβS, resulting in a reported ratio of 80:20 [38], compatible with our own observation (Figure 3c).
Over a 40 h period, ATPαS displayed minimal decay (approximately 10%) in the absence of p97-ND1L. However, in the presence of p97-ND1L, the 1H-1D spectra suggest near-complete hydrolysis within the same timeframe. When p97-ND1L was introduced, ATPβS was hydrolysed at a rate comparable to that of ATPγS, reinforcing its reduced but still significant susceptibility to p97-ND1L-catalysed hydrolysis. Overall, ATPαS and ATPβS exhibit lower stability compared to AMP-PNP but similar to ATPγS, with both analogues showing specific hydrolysis catalysed by p97-ND1L (Figure 4). The hydrolysis rate observed still enables robust measurements over a time span of at least one day at physiological temperatures.
3.2. Protein Conformation Induced by Nucleotide Analogues
3.2.1. ATPγS
The slowly hydrolysable analogue ATPγS has been widely employed to induce a pre-hydrolysis state in p97-ND1L. This state is characterised by the NTDs adopting an upward-pointing conformation, as evidenced through X-ray crystallography [31,45], NMR [33], and cryo-EM [29]. As a structurally faithful ATP analogue, ATPγS binds to p97-ND1L in a native and Mg2+-dependent manner. The characteristic NMR fingerprint of ATPγS-bound p97-ND1L serves as a reference for a stable pre-hydrolysis conformation.
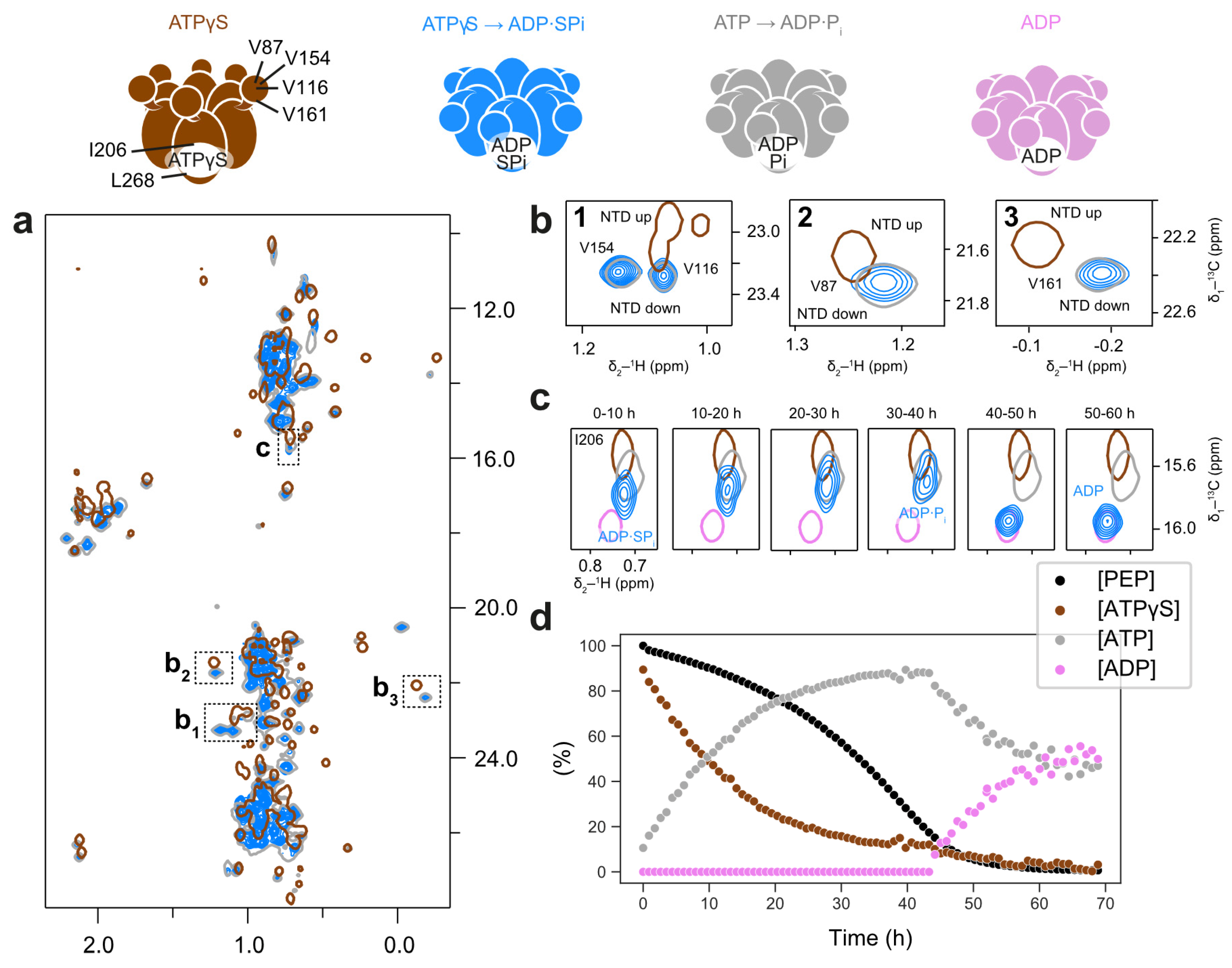
Unexpectedly, the protein conformation changes drastically when combining ATPγS with the regeneration system: methyl spectra from both p97-ND1L-wt and the Walker B mutant E305Q reveal a post-hydrolysis conformation best described as the ADP·SPi state (Figure 5a,b). The I206 peak features a minor downfield shift relative to ADP·Pi (Figure 5c). The regeneration system gradually introduces native ATP into the reaction mixture, starting with an initial [ATPγS]:[ATP] ratio of 9:1, which reverses to 1:9 as the reaction proceeds (Figure 5d). Correspondingly, the I206 peak shifts incrementally from its initial ADP·SPi position to the native ADP·Pi position as ATP concentrations increase. Once ATP is fully depleted, p97-ND1L adopts the ADP state (Figure 5c).
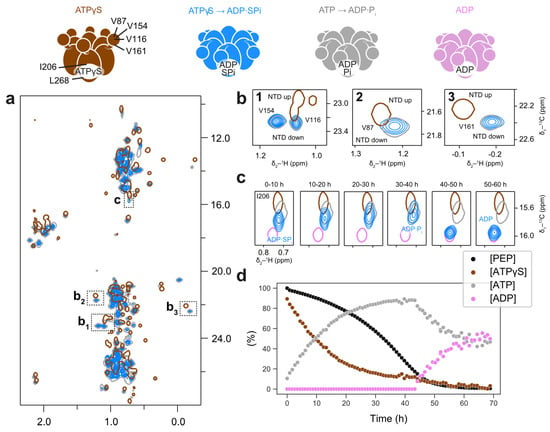
Figure 5.
In the presence of the regeneration system, ATPγS induces a post-hydrolysis conformation in the D1 domain of p97. Methyl spectra of wt p97-ND1L in the presence of ATPγS and a regeneration system at 50 °C. The colour scheme of contour lines is indicated by the schematic representations of p97 nucleotide states shown above. (a) The overall spectrum (blue) largely resembles the ADP·Pi state (grey), not the ATPγS state (brown). (b) Enlargements of NTD peaks V116, V154, V87, and V161, corresponding to inlets b1-b3 in panel (a), indicate pure NTD down-conformation. (c) Bound nucleotide reporter I206, located close to the binding pocket, shows an ADP·Pi-like peak position with a minor chemical shift perturbation, likely due to the presence of inorganic thiophosphate instead of phosphate. As ATPγS transforms into ATP, a bona fide ADP·Pi state is formed. After PEP is depleted, the ADP state is observed, indicated by comparison to the ADP-bound state (pink). (d) Evolution of the relative amounts of ATPγS, ATP, PEP, and ADP present in the reaction over time corresponding to the timeline shown in (c). The quantities were measured from peak intensities in the 1H-1D spectra interleaved with the methyl spectra in (c).
The observed post-hydrolysis state suggests that ATPase activity in p97-ND1L is stimulated in the presence of ATP to such an extent that ATPγS becomes an efficient substrate. We attribute this phenomenon to cooperativity effects in p97-ND1L: ATP hydrolysis propagates around the hexamer in a counterclockwise fashion, with adjacent subunits stimulating ATP hydrolysis in trans [20]. We hypothesise that, as a few ATP molecules in the mixture bind to protomers and become hydrolysed, the activity of adjacent ATPγS-bound protomers is stimulated. The rate-limiting step then becomes thiophosphate release from a persistent ADP·SPi state. ATPγS thus undergoes hydrolysis at a slower initial rate compared to native ATP under regeneration conditions, thereby extending the lifetime of the observed post-hydrolysis state.
3.2.2. α/β-Thiosubstituted ATP Analogues
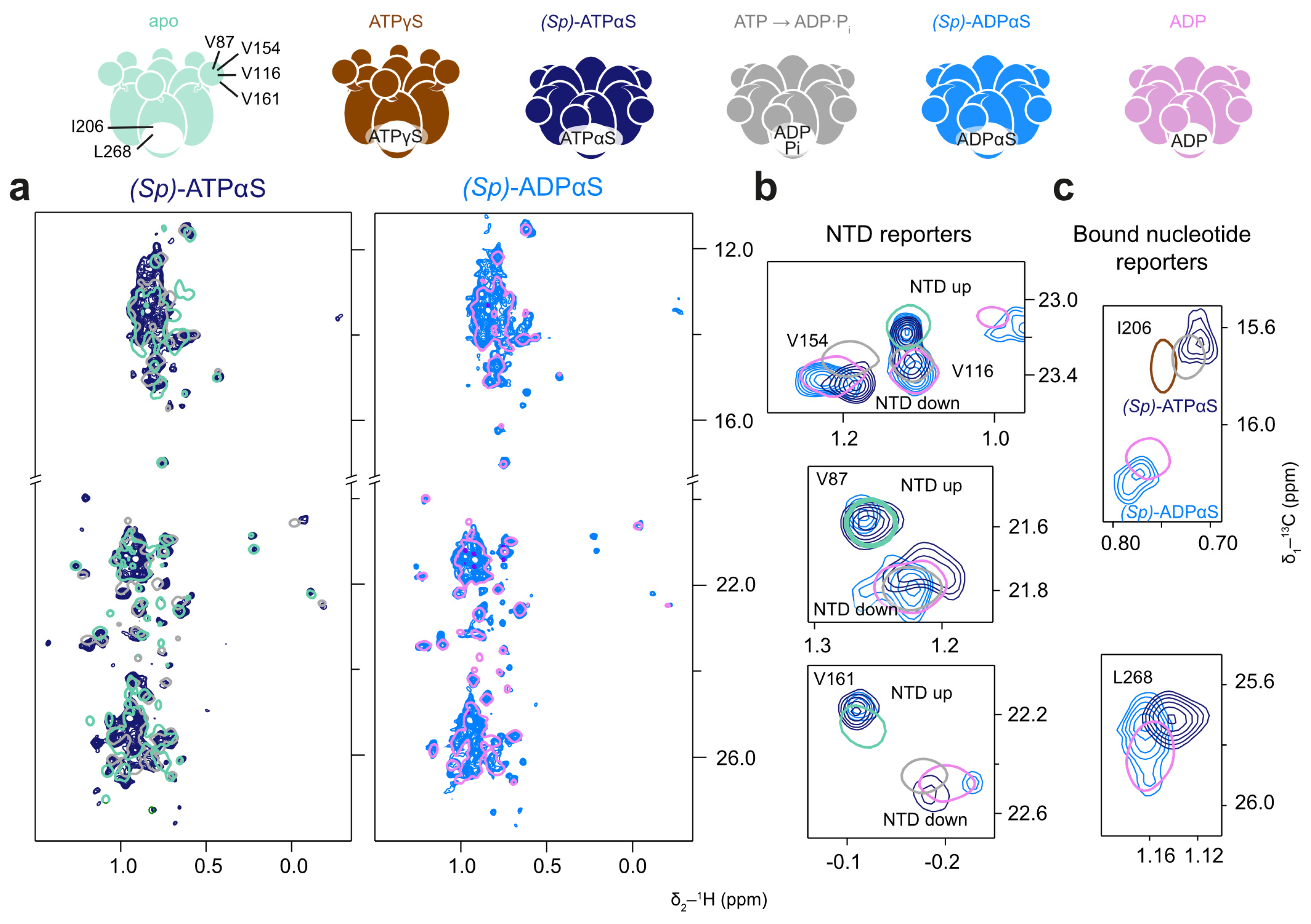
The effects of (Sp)-ATPαS and (Sp)-ATPβS in the presence of the regeneration system were assessed using the Walker B (E305Q) mutant to slow down hydrolysis rates. Under these conditions, (Sp)-ATPαS produces a methyl spectrum indicative of a mixed NTD up/down-conformation (Figure 6b). This could result from the slow conformational exchange of the NTD or from a mixture of fixed conformations within the p97-ND1L hexamer, with some NTDs locked in the up-conformation and others in the down-conformation. Additionally, methyl probes reporting on the nucleotide state yield ambiguous results: the chemical shifts of I206 resemble the ADP·Pi or ATPγS state, while L268 resembles an ADP-like state (Figure 6c).
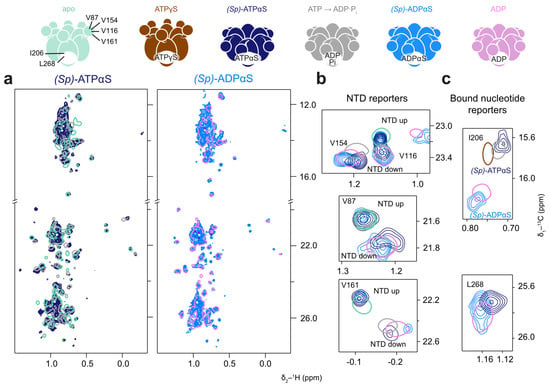
Figure 6.
p97-ND1L adopts a non-native conformation in the presence of (Sp)-ATPαS. Methyl spectra of p97-ND1L-E305Q in the presence of (Sp)-ATPαS and a regeneration system, and (Sp)-ADPαS at 50 °C. The colour scheme of contour lines is indicated by the schematic representations of p97 above. (a) (Sp)-ATPαS (navy) induces a non-uniform conformation (apo and ADP·Pi shown in cyan and grey for reference, respectively), while (Sp)-ADPαS (light blue) leads to an ADP-like state (reference shown in pink). (b) Enlargements of NTD peaks V116, V154, V87, and V161 indicate a mixture of NTD up- and down-conformational states for both nucleotides, while (c) methyl groups reporting on the bound nucleotide yield ambiguous results, with I206 indicating an ATPγS or ADP·Pi-like state and L268 showing an ADP-like state. (Sp)-ADPαS shows mostly an ADP-like state.
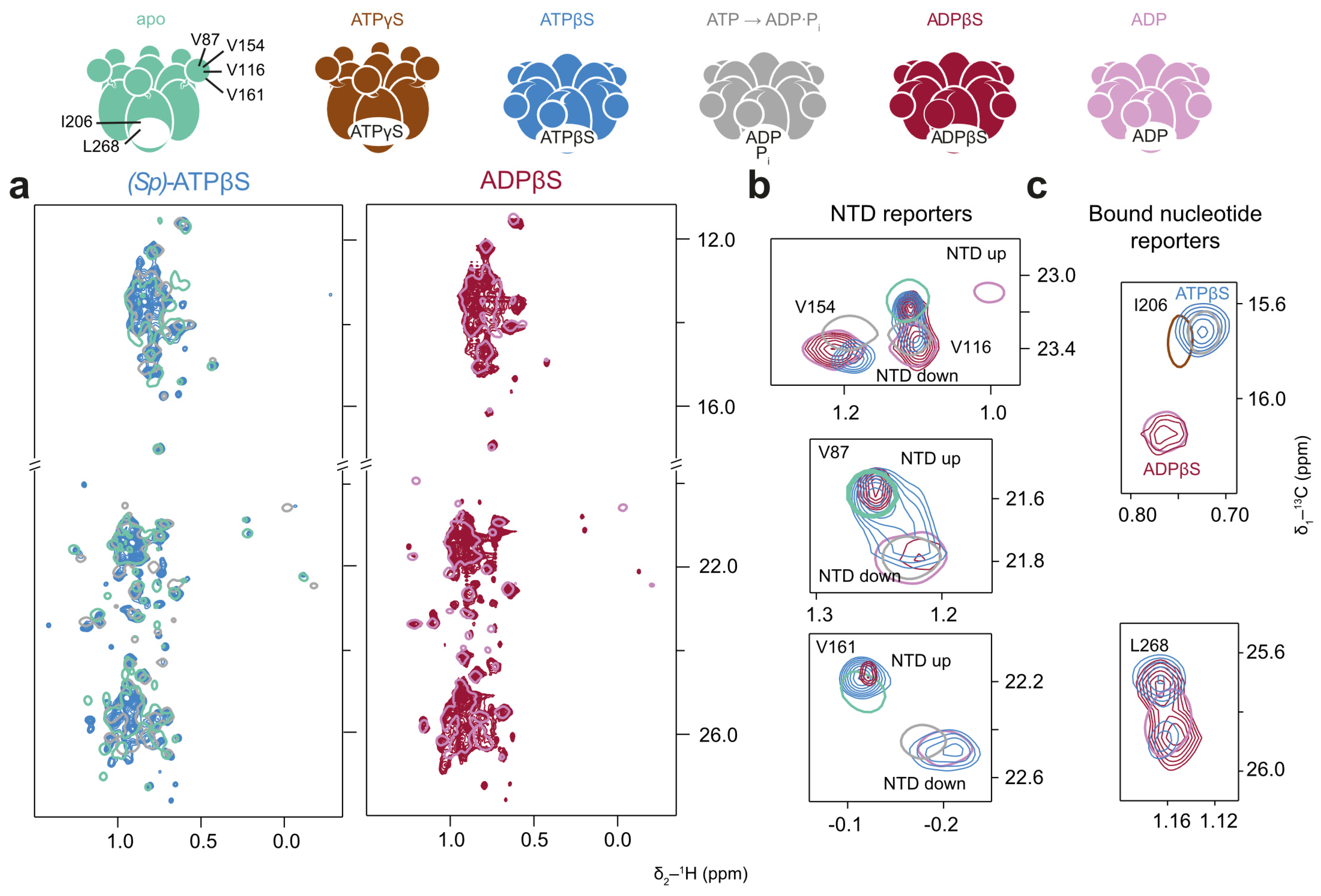
The spectra recorded in the presence of (Sp)-ATPβS exhibit similar features (Figure 7). Interestingly, the peak of L268 is split, indicating two distinct conformations of the binding site in slow exchange (Figure 7c). By comparing these spectra with those recorded in the presence of the achiral ADPβS, which shows the same splitting pattern, we excluded the possibility that the signals arise from the (Rp)- and (Sp)-diastereomers of ATPβS. Only slow PEP consumption was observed in the presence of (Sp)-ATPβS. The overall p97-ND1L conformation could thus arise due to a non-physiological pre-hydrolysis state or a post-hydrolysis state, from which the protein is unable to release the resulting product.
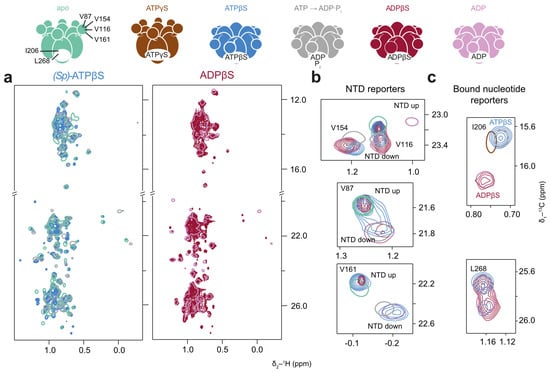
Figure 7.
p97-ND1L adopts a non-native conformation in the presence of (Sp)-ATPβS. Methyl spectra of p97-ND1L-E305Q in the presence of (Sp)-ATPβS plus a regeneration system, and of ADPβS at 50 °C. The colour scheme of contour lines is indicated by the schematic representations of p97 above. (a) (Sp)-ATPβS (light blue) induces a non-uniform conformation (apo and ADP·Pi shown in cyan and grey for reference, respectively), while ADPβS (crimson) leads to a uniform ADP-like state (shown in pink for reference). (b) Enlargements of NTD peaks V116, V154, V87, and V161 indicate a mixture of NTD up- and down-conformational states for both nucleotides resembling the situation seen with (Sp)-ATPαS, while (c) methyl groups reporting on the bound nucleotide yield ambiguous results, with I206 indicating an ADP·Pi-like state and L268 showing a split state. ADPβS shows a pure ADP-like state.
After the complete depletion of PEP, when all (Sp)-ATPαS is converted to (Sp)-ADPαS, the majority of p97-ND1L adopts an unambiguous ADP-like state. This is also true for the spectra recorded in the presence of ADPβS, indicating uniform nucleotide binding across most protomers within the hexamer.
These findings suggest that both (Sp)-ATPαS and (Sp)-ATPβS likely adopt an atypical binding mode, resulting in a non-native NTD conformation and altered binding pocket structure. Alternatively, the observed behaviour could stem from a lower affinity for these nucleotide analogues, leaving a significant proportion of the hexamer in the apo state. Nevertheless, the overall consumption of both analogues indicates that they can still be processed by p97-ND1L, albeit slower than native ATP.
3.2.3. Hydrolysis-Resistant ATP Analogues
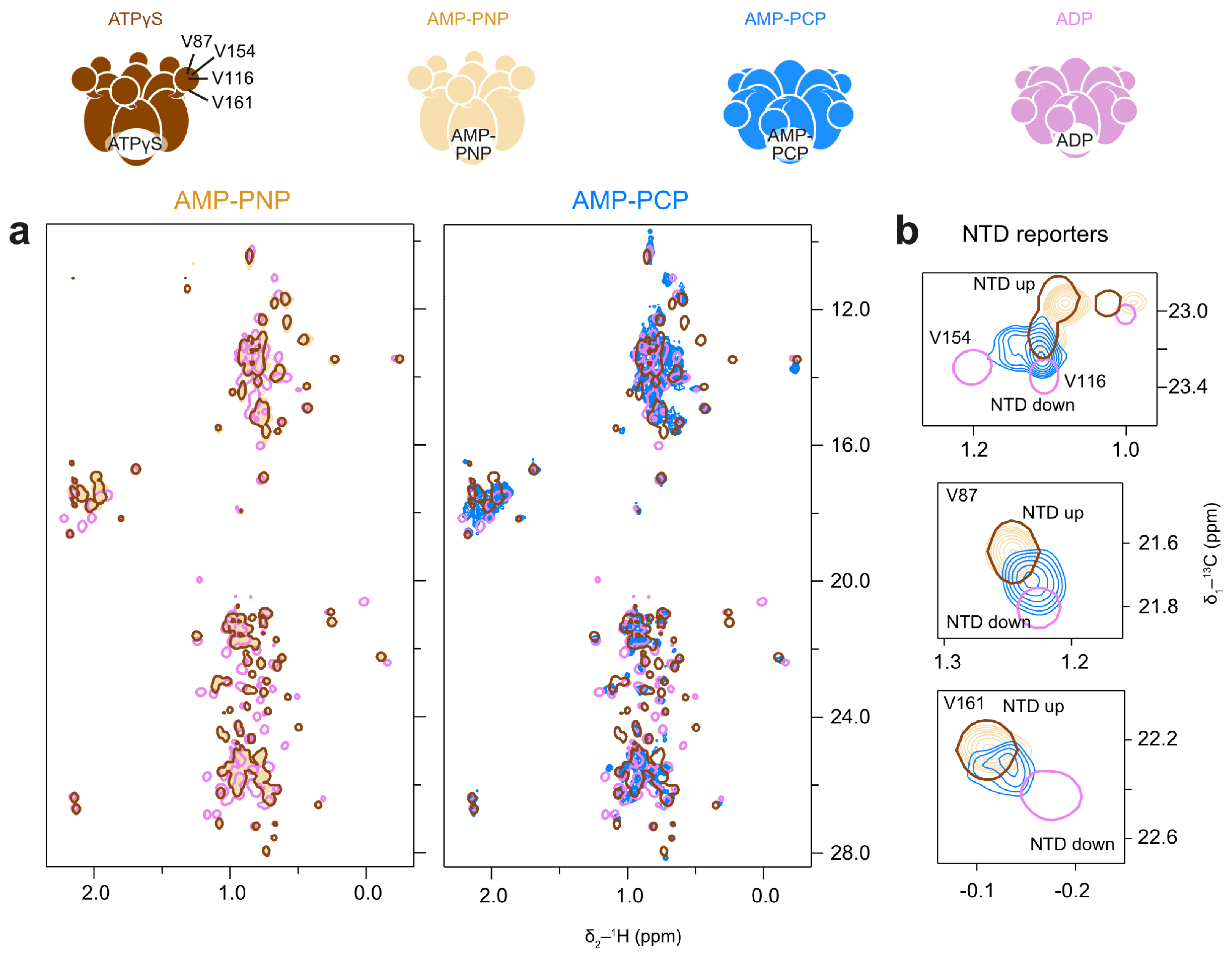
The spectrum of AMP-PNP-bound p97-ND1L-wt closely resembles the spectrum recorded in the presence of ATPγS, indicating that AMP-PNP induces a similar pre-hydrolysis state (Figure 8a), with an upward conformation of NTD. In contrast, the AMP-PCP spectrum is more comparable to an ADP-bound state (Figure 8a). Reporters of NTD conformation reveal increased mobility of the NTD, reflected in peak positions that are averaged between the up- and down-conformations, indicating fast exchange on the NMR time scale (Figure 8b). Thus, while AMP-PNP and ATPγS effectively mimic ATP, AMP-PCP does not reliably induce the native pre-hydrolysis conformation in p97-ND1L.
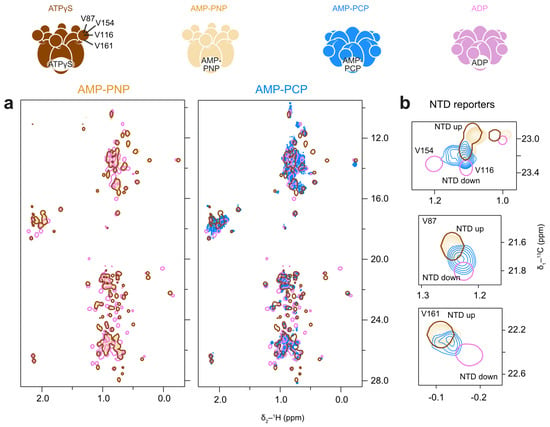
Figure 8.
AMP-PNP stabilises the pre-hydrolysis state of p97-ND1L; AMP-PCP induces a mix of conformations in fast exchange. (a) Methyl spectra at 50 °C of p97-ND1L bound to AMP-PNP but not to AMP-PCP highly resemble those measured in the presence of ATPγS. (b) Enlargement of NTD residues V87, V116, V154, and V161. In the AMP-PCP-bound state (blue contours), peak positions are averaged between the up and down positions, indicating fast exchange between the two states.
3.3. Nucleotide Detection by 31P NMR in the Protein Sediment
To monitor nucleotide turnover and evaluate the activity of the regeneration system in a MAS ssNMR setup, directly pulsed 31P NMR spectra can be recorded to detect unbound nucleotides in a solution-state like manner. This approach enables the identification of distinct species in complex mixtures of native and modified, e.g., thiosubstituted, nucleotides plus the components of the regeneration system.
To measure ssNMR on protein sediments, several prerequisites must be addressed. A key challenge arises from the high protein concentration in the sediment: Assuming a spherical shape and considering the protein density ρprot of 1430 kg/m3, the protein concentration in a sediment is approximately ρprot · 0.74 = 1060 mg/mL [46]. For p97-ND1L, this translates to a molarity of 20 mM—a value two orders of magnitude higher than in solution-state NMR experiments. As a result, the PEP-to-protein concentration ratio shifts from 300–1000:1 in solution to 1.5–10:1 in the sediment. These conditions are further exacerbated by the extended sample preparation and measurement times: sedimentation to fill the ssNMR rotor requires 12–24 h, while the experiments themselves span several days.
To mitigate this challenge, all MAS ssNMR experiments were performed using the hydrolysis-deficient Walker B mutant. Furthermore, experiments were conducted at 7 °C, reducing enzymatic rates. It should be mentioned that the high viscosity in the sediment restricts diffusion [47], and the slowed conformational dynamics [48,49] could limit the internal motions required for nucleotide processing, extending the lifetime of the regeneration system further.
3.3.1. Detection of Unbound Nucleotides via Directly Pulsed 31P NMR
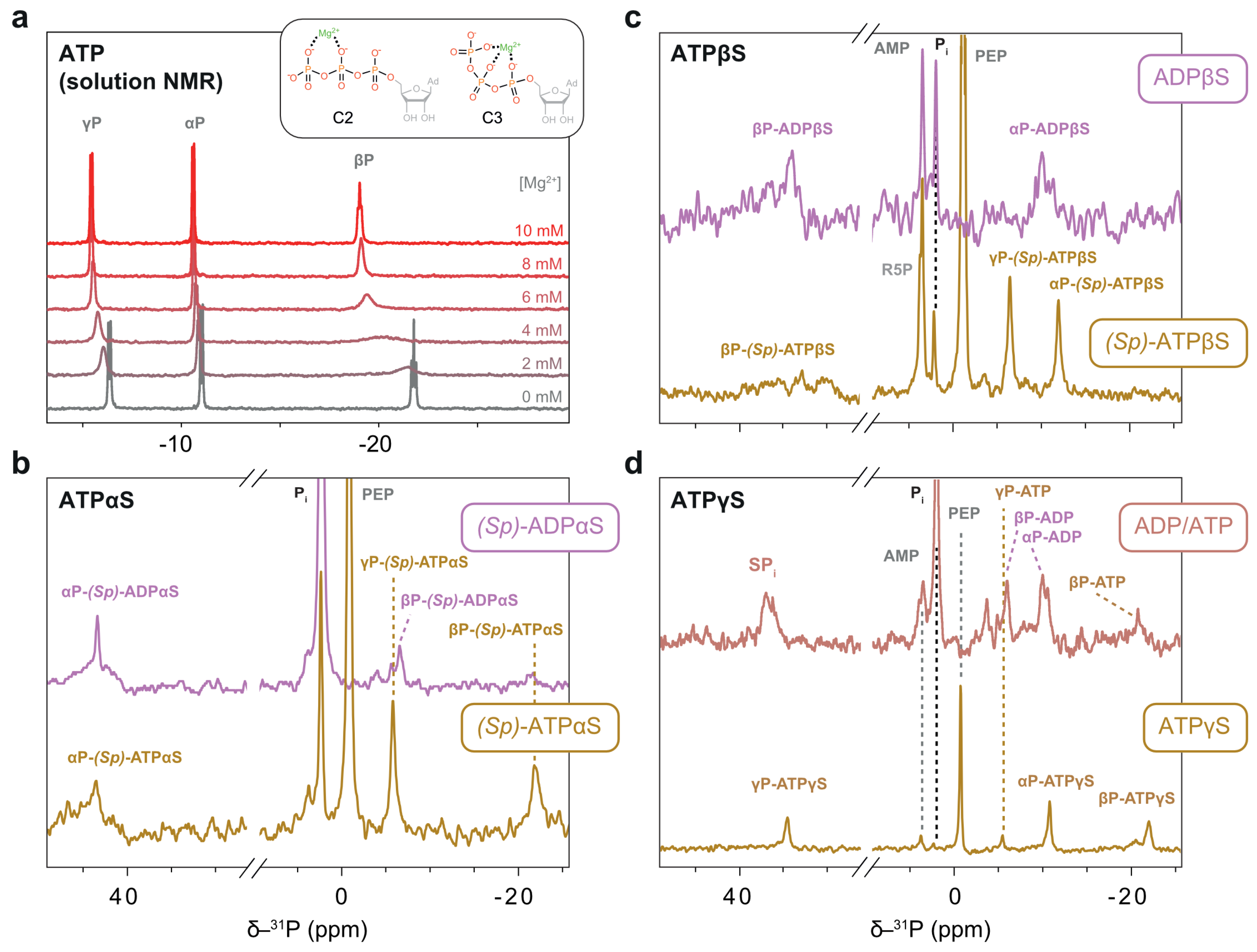
The distinctive downfield chemical shifts of thiophosphates distinguish them from orthophosphate moieties and enable their unambiguous NMR assignment. For (Sp)-ATPαS, shown in Figure 9b, the αSP resonance was assigned from its downfield shift, βP from its upfield shift, and, by elimination, γP. Additional signals were attributed to PEP and free phosphate. Only upon the complete depletion of PEP did the γP resonance vanish, and a new signal appeared assigned to (Sp)-ADPαS. The γP-(Sp)-ATPαS and βP-(Sp)-ADPαS resonances overlapped, rendering γP-(Sp)-ATPαS unsuitable as a reaction control marker. The disappearance of the βP-(Sp)-ATPαS signal reliably tracked the progression of hydrolysis. The build-up of (Sp)-ADPαS occurred only after the depletion of PEP, confirming the regeneration system’s compatibility with α-thiosubstituted ATP analogues.
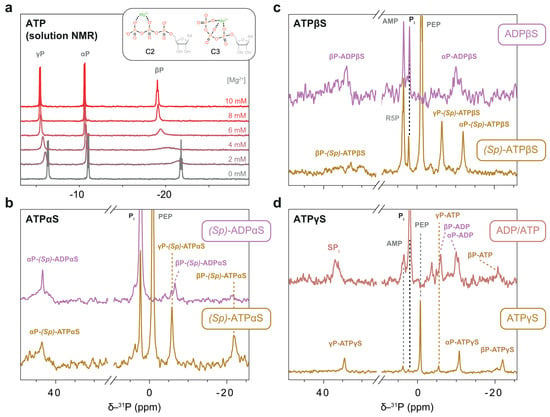
Figure 9.
31P NMR measurements of thiosubstituted ATP analogues in the soluble fraction of protein sediments. (a) Directly pulsed 31P 1D spectra of ATP with various Mg2+ concentrations at 25 °C in a solution-state NMR setup. 31P chemical shifts of the βP group are highly sensitive to Mg2+ concentration. Structures of the bidentate (C2) and tridentate (C3) coordination modes are shown in the inlay. (b–d) Directly pulsed 31P 1D spectra of the respective nucleotides in a regeneration system at 7 °C. (b) (Sp)-ATPαS is present while there is PEP to fuel the regeneration. (c) No PEP turnover was seen for (Sp)-ATPβS. The spectrum of ADPβS was recorded separately without a regeneration system. The broad distribution of βSP signals is discussed in the main text. (d) Regeneration of hydrolysed ATPγS to ATP occurs while PEP is present; after its depletion, both are hydrolysed to ADP.
For (Sp)-ATPβS, we observed no significant PEP consumption during the experiment. The αP and γP phosphate resonances were nearly identical to those of native ATP, while the βP thiophosphate displayed signals extending from 30 to 39 ppm (Figure 9c). We attribute this complexity not to a mixture of nucleotides, but to the formation of multiple Mg2+ complexes, likely in intermediate exchange regimes that broaden the βP signals. In contrast, chemical shift differences between the nucleotides present—(Sp)-ATPβS, (Rp)-ATPβS, and ADPβS—do not explain this distribution, as their literature values differ by a mere 0.2–0.4 ppm [50]. In comparison, the spectra of ADPβS displayed a less broad distribution of βSP signals, suggesting no or a more uniform Mg2+ complexation.
The argument that Mg2+ complexation introduces a distribution of βP chemical shifts is experimentally supported. Solution-state titration experiments with ATP in the same buffer reveal a downfield shift of all three resonances upon Mg2+ addition, with the βP peak showing the most pronounced effect (Figure 9a). At a stoichiometric ratio of ATP:Mg2+ of 1:1, the system enters intermediate exchange, evidenced by significant line broadening (Figure 9a). These observations highlight the strong influence of Mg2+ on the ³¹P chemical shifts in ATP [51]. Two geometries of Mg2+ coordination were postulated to exist in solution: bidentate (C2) and tridentate (C3) (Figure 9a) [52]. However, these geometries remain indistinguishable in solution, suggesting a low energy barrier between the two states [53]. Notably, the majority of P-loop NTPase structures demonstrate Mg2+ binding in the C2 geometry [53]. However, non-native nucleotides like (Sp)-ATPβS may deviate from this norm [50]. Sulphur, with a larger van der Waals radius of 1.80 Å compared to oxygen (1.52 Å) in native ATP, is a softer Lewis base and could therefore influence the coordination properties. Consequently, Mg2+ complexes with (Sp)-ATPβS are likely less stable than those with ATP, reflecting the nuanced interplay between the nucleotide’s chemical structure and Mg2+ coordination.
The behaviour of ATPγS aligns with our findings from solution-state NMR. At the outset of the reaction, small amounts of ADP present in commercial ATPγS are regenerated into native ATP. The progress of ATPγS hydrolysis can be tracked through the build-up of the free thiophosphate signal at 36.9 ppm (Figure 9d).
3.3.2. Detection of Protein-Bound Nucleotides Through 1H-31P-CP
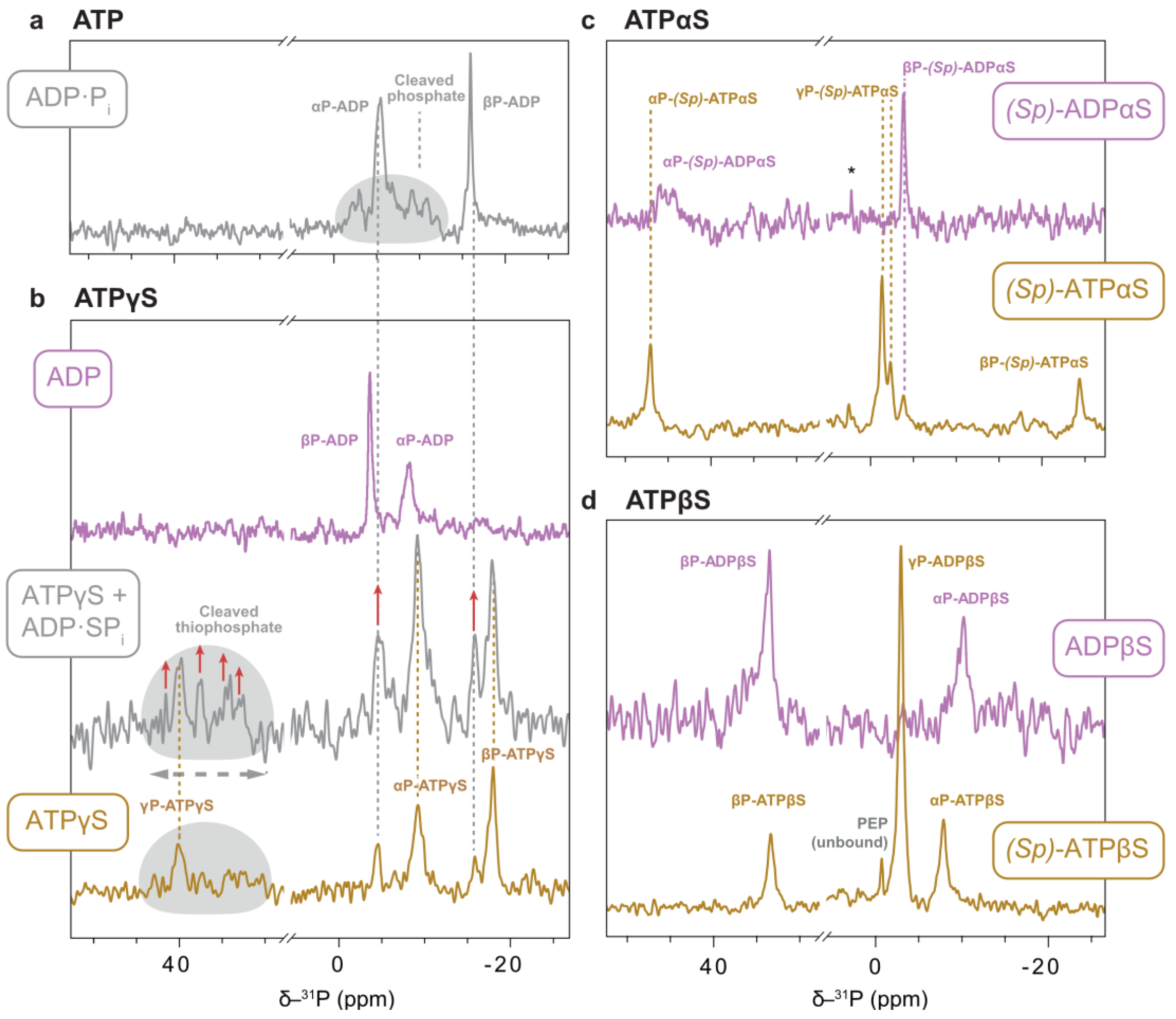
So far, we have only inventoried the nucleotides present in solution in directly pulsed spectra. These can be interleaved with ¹H-³¹P-CP experiments under MAS, which selectively detect molecules with little internal and slow overall motion. The measurement of nucleotides co-sedimented with the protein thus enable the direct determination of the enzyme state [13,14].
Protein-detected spectra established that, between the presence of ATPγS and ATP, p97-ND1L switches between pre-hydrolysis and post-hydrolysis states with the NTD in up- and down-conformation, respectively. The ¹H-³¹P-CP spectrum of the ADP·Pi state displays two sharp signals for the αP and βP units (Figure 10a) and a characteristic heterogeneous signal pattern assigned to the inorganic phosphate. In contrast, the ¹H-³¹P-CP spectra of ATPγS bound to p97-ND1L in the absence of a regeneration system reveals, predominantly, a pre-hydrolysis state with one sharp resonance from uncleaved γSP. Additionally, a minor fraction of the post-hydrolysis state is observed, in which the heterogenous signals, corresponding here to cleaved thiophosphate, are shifted downfield relative to the ATP counterpart (Figure 10b). When a regeneration system is included, the system evolves as a time-dependent mixture of ATP and ATPγS. Under these conditions, signals from the post-hydrolysis state become more pronounced—a finding that aligns with the corresponding protein-detected experiments of p97-ND1L (Figure 5) and confirms the interpretation that ATPγS hydrolysis is stimulated in the presence of ATP. ATPγS is thus a suitable analogue for studying the pre-hydrolysis state of p97-ND1L, though not in mixtures with ATP.
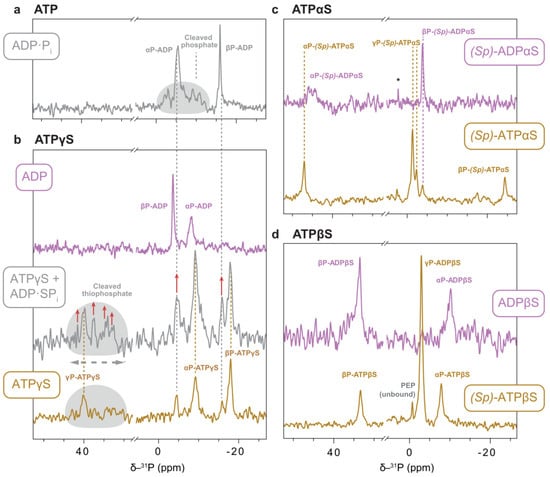
Figure 10.
NMR spectra of thiosubstituted ATP analogues bound to p97-ND1L. 1H-31P CP experiments recorded on sedimented p97-ND1L-E305Q in a MAS ssNMR setup in the presence of the respective nucleotide and a regeneration system at 7 °C, unless indicated otherwise. The experiments report on phosphorus species that are immobilised on the CP time scale, which is the case for molecules in the nucleotide binding pocket of the protein. The respective presumable bound nucleotide is indicated in boxes. (a) Reference in the presence of ATP, where p97-ND1L forms a post-hydrolysis (ADP·Pi) state with the cleaved phosphate in a heterogenous chemical environments [14]. (b) Nucleotide state ratios shift from predominantly pre-hydrolysis (ATPγS-bound) towards post-hydrolysis state (ADP·SPi) as the ratio of ATP to ATPγS increases; increased signals of ADP·SPi state are highlighted with red arrows. A separately recorded ADP-bound spectrum is presented for reference. (c) The spectrum of (Sp)-ATPαS indicates a pre-hydrolysis state while the regeneration system is active. Afterwards, ADPαS remains bound. The asterisk denotes unbound Pi. (d) (Sp)-ATPβS is found in a single nucleotide state. Again, the ADPβS-state was recorded separately because of the lack of nucleotide turnover.
For (Sp)-ATPαS in the presence of an ATP-regeneration system, three phosphate peaks are observed, consistent with a pre-hydrolysis state of bound (Sp)-ATPαS. Notably, the central peak, attributed to the γP, is split into one major and two minor signals (Figure 10c). Spectra recorded with (Sp)-ADPαS identify the upfield signal (−3.96 ppm) as originating from this hydrolysis product. This finding suggests that a minor fraction of (Sp)-ADPαS-bound protein is present at the start of the reaction. However, protein-detected spectra (Figure 6) show no evidence of (Sp)-ADPαS binding in the presence of (Sp)-ATPαS. The γP splitting likely reflects two distinct binding modes, possibly due to the presence or absence of a Mg2+ ion, consistent with split methyl signals in solution spectra indicating two NTD conformations—up and down—in slow exchange. Alternatively, the cleaved phosphate might associate with (Sp)-ADPαS to form a uniform post-hydrolysis state. However, the sharpness and clarity of the putative γP or Pi signal contrast sharply with the diffuse signals of loosely associated Pi typically seen in the native ADP·Pi state (Figure 10a). In the absence of direct evidence for a post-hydrolysis state, we infer that hydrolysis, rather than phosphate release, is likely the rate-limiting step in the enzymatic turnover of this ATP analogue. The non-native behaviour observed, in terms of non-uniform protein conformation and nucleotide state, renders (Sp)-ATPαS an unsuitable candidate as a functional ATP analogue for p97-ND1L.
The ¹H-³¹P-CP spectra of (Sp)-ATPβS bound to p97-ND1L in the presence of the regeneration system exhibit some features resembling an ATP-like state (Figure 10d). Three prominent peaks are observed and indicate a single conformation of the bound nucleotide. The resonance at 33.4 ppm is assigned to the βSP thiophosphate, and the remaining ones tentatively to αP and γP, based on comparison with the ADPβS-bound state. Notably, the chemical shifts of αP and βP are remarkably similar between the two nucleotide states, and the terminal phosphate signal appears exceptionally intense—a pattern that is unlike all other spectra of nucleotides bound to p97-ND1L (Figure 10d).
The quantitative interpretation of signal intensities in CP spectra is not feasible without atomic-level structure: transfer efficiencies depend on factors such as the distance between involved nuclei, internal dynamics scaling dipolar couplings, and spin relaxation during CP contact time. The signal with an exceptionally high intensity could represent an inorganic phosphate coordinated directly to a 1H-containing moiety. In stark contrast to the ADP·Pi state, in which multiple NMR signals and cryo-EM densities are assigned to the phosphate in multiple environments, it is possible that (Sp)-ATPβS stabilises a later stage of the phosphate dissociation trajectory, with the Mg2+-Pi ion pair being dissociated from the thiosubstituted βP of ADP [50]. However, this remains speculative in the absence of a cryo-EM structure. The observation of three sharp signals in the 31P spectrum establishes that either (Sp)-ATPβS or its hydrolysis product ADPβS·Pi bind p97-ND1L in a single pose. However, this binding mode is non-native, as it fails to induce a defined protein conformation. The broadening and doubling of NTD signals in methyl spectra in the presence of both (Sp)-ATPβS and ADPβS suggests that β-thiosubstitution is not suitable for structural and functional studies of p97-ND1L.
3.4. Nucleotide Detection by 19F NMR in Solution
Given the challenges of directly observing p97-ND1L-bound nucleotides in solution NMR, we investigated the potential of 19F NMR as an alternative to 31P, in the hope of circumventing the technical effort of the MAS ssNMR setup. 19F is a highly NMR-sensitive nucleus with 100% natural abundance. Its high sensitivity to changes in the chemical environment finds many applications in drug screening and structural studies [54]. Especially, trifluoromethyl groups enhance sensitivity due to the presence of three equivalent 19F nuclei. Their utility as probes of molecular dynamics stems from sensitivity to both rotational motion about the internal symmetry axis and movements of the moiety to which they are attached [55,56]. Therefore, 8-trifluoromethyl-ATP (ATP-8-CF3) was developed as a nucleotide analogue [57].
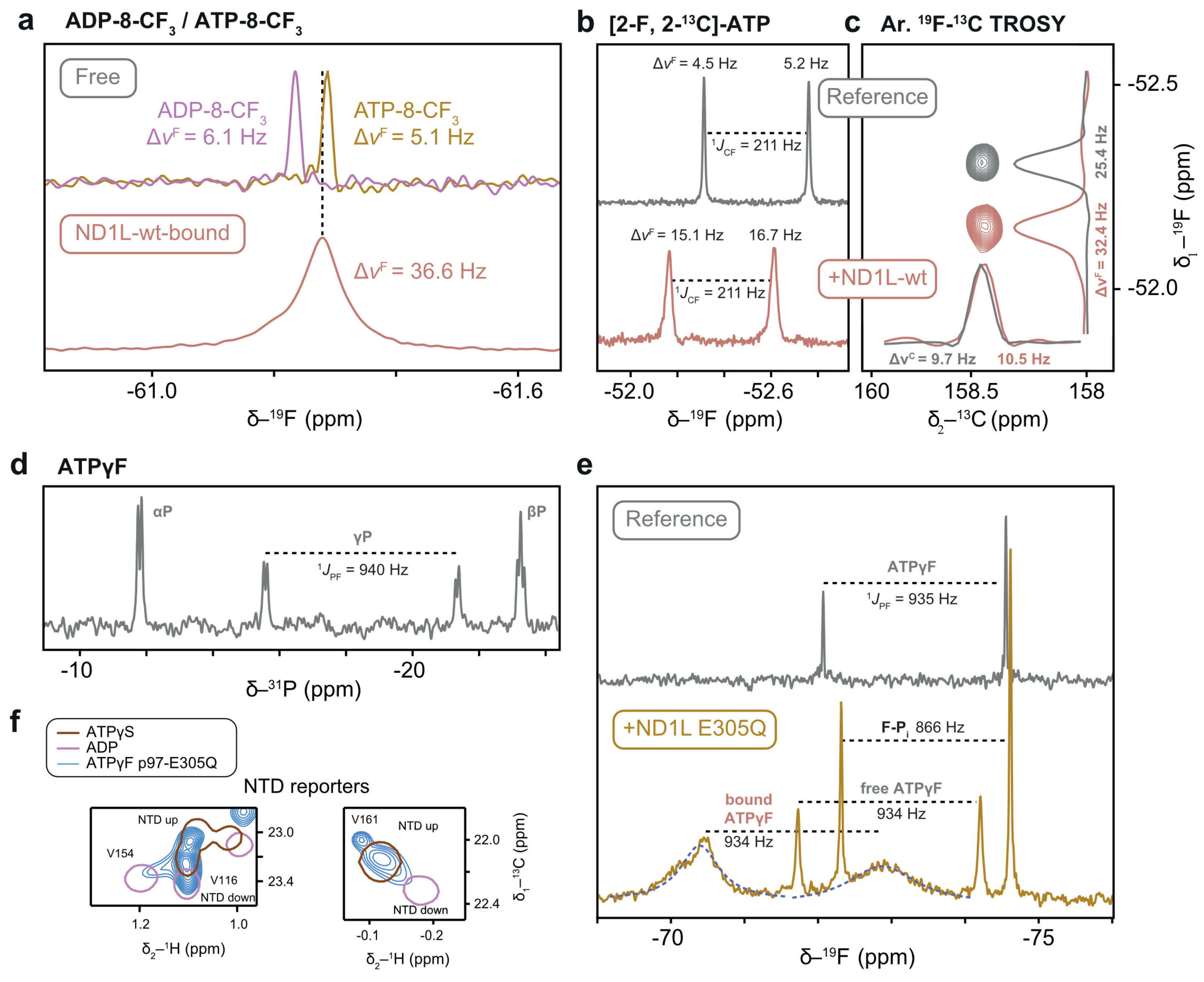
The regeneration of ATP-8-CF3 from its ADP equivalent was monitored using 19F-NMR, yielding a minute yet detectable upfield peak shift. When p97-ND1L-wt was subjected to ATP-8-CF3 at sub-stoichiometric concentrations in the presence of the regeneration system, severe line-broadening was observed in the 19F spectrum (Figure 11a) and unequivocally establishes the binding of the analogue. The trifluoro-methyl group is a potential candidate for direct detection of bound nucleotides, but not ideal due to its relaxation properties and the significant structural modification of the base moiety.
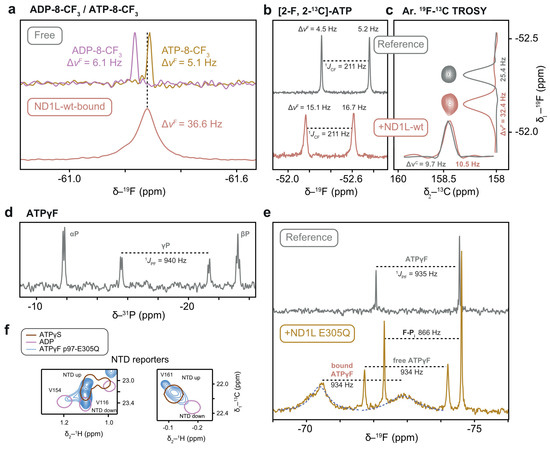
Figure 11.
NMR spectra of fluorinated ATP analogues. (a) Regeneration of ATP-8-CF3 from its ADP equivalent results in a 6.7 ppb change in 19F chemical shift. Binding to the protein manifests in significant line broadening. (b) 19F chemical shift and linewidth of [2-19F, 2-13C]-ATP change upon binding to p97-ND1L-wt. (c) Aromatic 13CF-TROSY experiment maintains the original 13C linewidth. The linewidth in the 19F dimension was limited by the indirect acquisition time. (d) The 31P-1D NMR spectrum of ATPγF shows a doublet for the γP due to the strong 1JPF coupling. (e) 19F-1D NMR spectra of ATPγF in free and bound form. In the presence of p97-ND1L-E305Q, additional signals from protein-bound nucleotide and from cleaved fluorophosphate (F-Pi) appear. The size of the 1JPF coupling constant (fit curve in blue) enables the assignment of different fluorophosphate species. A shoulder on the downfield peak of the bound form hints at an additional, unassigned species. (f) Methyl groups in NTD indicate an up-conformation of p97-ND1L-E305Q in complex with ATPγF.
Despite its attractivity as a bio-orthogonal isotope, 19F-detected spectroscopy thus poses challenges for large molecular systems like p97-ND1L due to the substantial chemical shift anisotropy (CSA) of 19F, which leads to an additional transverse relaxation pathway and causes severe line broadening. However, 19F-13C spin pairs in aromatic moieties mitigate this issue and produce sharp 13C signals, an effect known as aromatic 13CF-TROSY [36]. This phenomenon inspired the development of [2-19F, 2-13C]-ATP, which was successfully applied in RNA structure determination [10].
Given that van der Waals radii of hydrogen (1.2 Å) and fluorine (1.47 Å) are similar, a single fluorination site on the adenine base is a subtle chemical modification [58]. The binding of [2-19F, 2-13C]-ATP to p97-ND1L-wt at sub-stoichiometric concentrations under regeneration conditions caused a noticeable shift in 19F chemical shift and the expected significant line broadening between the free and bound forms (Figure 11b). We recorded a 2D aromatic 13CF-TROSY experiment using an out-and-back approach, encoding 19F in the indirect dimension and detecting 13C in the direct dimension [36]. As expected, a single peak was observed with both samples. Surprisingly, the 13C dimension, which benefits from the TROSY effect, showed nearly identical linewidths for free and bound forms, highlighting the efficiency of this approach for the detection of nucleotides bound to macromolecular complexes by solution-state NMR (Figure 11c). The sensitivity of the 19F probe to assess the hydrolysis state at the remote phosphate moieties at the opposite site of the ribose remains to be evaluated.
A fluorine atom attached to the γ-P position provides the most direct probe of the hydrolysis state of ATP, as it is converted into a free fluorophosphate upon hydrolysis, in contrast to fluorine substitutions at other nucleotide positions. In the 19F and 31P 1D spectra of ATPγF, the large one-bond scalar 31P-19F coupling yields a doublet (Figure 11d). The linewidths of the two doublet lines can be attributed to a differential contribution of the cross-correlation term between CSA and dipolar relaxation [59]. The temperature stability of ATPγF was remarkable, with no sign of hydrolysis over the course of four days at 37 °C in the absence of p97-ND1L. In its presence, cleavage product accumulation was only detectable in 19F 1D spectra after two days, with a half-life that exceeds four days. In the presence of the hydrolysis-inhibited Walker B mutant, the 19F spectra revealed a broadened doublet, attributed to the protein-bound nucleotide, alongside sharp signals from free ATPγF and its hydrolysis product, fluorophosphate (F-Pi). The coupling constant 1JPF is characteristic of each phosphate species [8], enabling the assignment of ATPγF (1JPF = 932 Hz) and F-Pi (1JPF = 866 Hz) (Figure 11e). Notably, the coupling constant for the protein-bound nucleotide matches that of free ATPγF, indicating that the nucleotide remains in a pre-hydrolysis state. Consistently, protein-detected spectra clearly indicate an up state of the NTD in the Walker B mutant in complex with ATPγF, which was not observed with ATP [13] (Figure 11f). The substitution of a single oxygen atom with fluorine is thus sufficient to induce a dramatic shift in the protein conformational space, underscoring the importance of scrutinising the structural effects of ATP analogues. Nevertheless, this experiment represents a proof-of-principle that the coupling constant 1JPF in ATPγF is an NMR observable from which the hydrolysis state can be directly deduced even when the nucleotide is bound to the 320 kDa p97-ND1L complex.
4. Discussion
ATP analogues are common tools in enzyme research, yet their effects on local protein–nucleotide interactions in the binding pocket and broader implications on the overall protein conformational space are not routinely evaluated. Assessing the compatibility of different ATP analogues is particularly important, given that structural variations among P-loop NTPases can impact nucleotide binding kinetics, hydrolysis rates, and phosphate release, as demonstrated in various systems, including myosin [60] and dynein [61]. These differences, in turn, affect the functional relevance of a given analogue. In the context of kinetic studies, the compatibility of analogues with ATP regeneration systems must be evaluated on a case-to-case basis. While this study focuses on a single domain of p97, it delineates an experimental protocol for scrutinising protein–nucleotide interactions across diverse P-loop NTPase systems.
The potential impact of investigating non-physiological protein states in structural biology and functional studies can be severe. NMR spectroscopy provides ample possibilities to shed light on such properties. To showcase this, we examined the interactions of several commercially available ATP analogues with the prototypical AAA+ ATPase p97-ND1L, specifically its first nucleotide-binding domain, D1. Using protein-detected ‘fingerprint’ methyl NMR spectra, we were able to rapidly classify protein conformations at high throughput. We assessed functional ATP binding and the resulting long-range conformational shift of the substrate-binding domain from characteristic reporter methyl probes.
We evaluated five ATP analogues designed to slow enzymatic hydrolysis through 1H and 31P NMR spectroscopy, examining their thermal stability, susceptibility to enzyme-mediated hydrolysis, effects on protein conformation, and compatibility with a pyruvate kinase-driven ATP regeneration system.
During the conception of this study outlined in the Introduction, we identified four key concerns (i–iv) when working with nucleotide analogues: (i) potential local structural alterations at the binding site, which could (ii) lead to long-range allosteric effects, thereby influencing the overall protein conformation. Our findings show that ATPγS and AMP-PNP are the most effective analogues in preserving both the local and global conformations of p97-ND1L in a pre-hydrolysis state. In contrast, AMP-PCP binds to p97-ND1L in a non-native manner, with rapid interconversion between multiple enzyme conformations. ATPαS and ATPβS are no ideal analogues: thio-substitution introduces a chirality center, generating two diastereomers. Both molecules, along with their respective ADP derivatives, bind to p97-ND1L in a non-native and non-uniform manner.
Furthermore, (iii) we anticipated that some nucleotide analogues might exist in a different form when bound at the active site compared to when unbound in solution. Indeed, nucleotide hydrolysis without phosphate release could potentially trap the enzyme in a post-hydrolysis conformation. Methyl NMR spectra confirm that this is not the case with AMP-PNP. In the absence of ATP, ATPγS induces a true pre-hydrolysis state as evidenced by protein-detected methyl NMR spectra and ligand-detected 1H 31P-CP-MAS NMR spectra. When ATP is present in mixtures with ATPγS, the efficient hydrolysis of ATP stimulates the hydrolysis of ATPγS, shifting the nucleotide state toward the native post-hydrolysis state. ATPαS and ATPβS likely adopt a pre-hydrolysis state, according to 1H 31P-CP-MAS, though the exact nature of the bound nucleotide remains uncertain for ATPβS and heterogeneous for ATPαS, rendering both analogues unsuitable for structural studies.
Finally, (iv) long-term observations of enzymes in the presence of ATP analogues require both thermal stability and compatibility with pyruvate kinase for regeneration. Both AMP-PNP and ATPγS are prone to thermal hydrolysis and are also hydrolysed by p97-ND1L, albeit at approx. 20x slower rate than ATP. At 37 °C, AMP-PNP’s half-life is one week, but it decreases to two days in the presence of 0.1 molar equivalents of p97-ND1L. Similarly, ATPγS’s half-life drops from two days to half a day under the same conditions. Notably, ATPγS displays a 3.7-fold higher binding affinity to p97-ND1L than ADP, which helps to mitigate its high hydrolysis rate: Even in the presence of 50% ADP, ATPγS-bound p97-ND1L is predominantly observed. ATPαS and ATPβS do not display superior stability against enzyme-catalysed hydrolysis compared to ATPγS. Only ATPαS and ATPβS can be restored from their hydrolysis product by pyruvate, whereas ATPγS turnover in the presence of PEP and pyruvate kinase gradually converts ATPγS to ATP. The resulting nucleotide mixtures allow the observation of a native post-hydrolysis conformation.
The detection of nucleotides bound to large enzyme complexes is enabled by 31P NMR only if a MAS ssNMR setup is available. To overcome such technical hurdles, we explored three fluorinated ATP analogues for the feasibility of 19F detection of enzyme-bound nucleotides in solution. The sensitivity and resolution in spectra of protein-bound CF3 groups suffered from CSA-mediated relaxation. Molecules with built-in 13CF-TROSY effects, notably [2-19F, 2-13C]-ATP, yielded high-resolution spectra even when bound to the >300 kDa p97-ND1L complex. Finally, even within the same macromolecular complex, the strong 1JPF scalar coupling in fluorophosphate remains NMR-observable and directly informs on the chemical environment of the moiety—cleaved or part of a polyphosphate chain—and hence on enzyme-catalysed hydrolysis.
Author Contributions
Conceptualisation, methodology, and analysis, M.A.D., M.S. and A.K.S.; writing—original draft preparation, M.A.D. and A.K.S.; writing—review and editing, M.A.D., M.S. and A.K.S.; visualisation, M.A.D. and M.S.; supervision, project administration, and funding acquisition, A.K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by DFG (project no. 394455587; 201302640 project B15) and supported by the Rise up! programme of the Boehringer Ingelheim Foundation (BIS).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Acknowledgments
We thank Julian Holzinger and the NMR facility at the Central Analytics of LMU Munich for their excellent technical support.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| ATP | Adenosine 5′-triphosphate |
| ADP | Adenosine 5′-diphosphate |
| AMP | Adenosine 5′-monophosphate |
| PEP | 2-Phosphoenolpyruvate |
| PK | Pyruvate kinase |
| MAS | Magic-angle spinning |
| NMR | Nuclear magnetic resonance |
| ssNMR | Solid-state NMR |
| AAA+ | ATPases Associated with diverse cellular activities |
| TCEP | Tris(2-carboxyethyl)phosphine |
| HEPES | (4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid) |
| NTD | N-terminal domain |
| CP | Cross-polarisation |
| CSA | Chemical shift anisotropy |
| HMQC | Heteronuclear multiple quantum coherence |
| TROSY | Transverse relaxation-optimised spectroscopy |
References
- Beis, I.; Newsholme, E.A. The contents of adenine nucleotides, phosphagens and some glycolytic intermediates in resting muscles from vertebrates and invertebrates. Biochem. J. 1975, 152, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.M. Cellular magnesium homeostasis. Arch. Biochem. Biophys. 2011, 512, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Mudryk, K.; Lee, C.; Tomanik, L.; Malerz, S.; Trinter, F.; Hergenhahn, U.; Neumark, D.M.; Slavicek, P.; Bradforth, S.; Winter, B. How Does Mg2+(aq) Interact with ATP(aq)? Biomolecular Structure through the Lens of Liquid-Jet Photoemission Spectroscopy. J. Am. Chem. Soc. 2024, 146, 16062–16075. [Google Scholar] [CrossRef] [PubMed]
- Goody, R.S.; Eckstein, F. Thiophosphate analogs of nucleoside di- and triphosphates. J. Am. Chem. Soc. 1971, 93, 6252–6257. [Google Scholar] [CrossRef]
- Yount, R.G.; Babcock, D.; Ballantyne, W.; Ojala, D. Adenylyl imidodiphosphate, an adenosine triphosphate analog containing a P--N--P linkage. Biochemistry 1971, 10, 2484–2489. [Google Scholar] [CrossRef]
- Eckstein, F. Investigation of enzyme mechanisms with nucleoside phosphorothioates. Angew. Chem. Int. Ed. Engl. 1975, 14, 160–166. [Google Scholar] [CrossRef]
- Bagshaw, C. ATP analogues at a glance. J. Cell Sci. 2001, 114, 459–460. [Google Scholar] [CrossRef]
- Baranowski, M.R.; Nowicka, A.; Rydzik, A.M.; Warminski, M.; Kasprzyk, R.; Wojtczak, B.A.; Wojcik, J.; Claridge, T.D.; Kowalska, J.; Jemielity, J. Synthesis of fluorophosphate nucleotide analogues and their characterization as tools for 19F NMR studies. J. Org. Chem. 2015, 80, 3982–3997. [Google Scholar] [CrossRef]
- Ono, T.; Scalf, M.; Smith, L.M. 2′-Fluoro modified nucleic acids: Polymerase-directed synthesis, properties and stability to analysis by matrix-assisted laser desorption/ionization mass spectrometry. Nucleic Acids Res. 1997, 25, 4581–4588. [Google Scholar] [CrossRef]
- Juen, F.; Glanzer, D.; Plangger, R.; Kugler, V.; Fleischmann, J.; Stefan, E.; Case, D.A.; Kovacs, H.; Kwaku Dayie, T.; Kreutz, C. Enhanced TROSY Effect in [2-19F, 2-13C] Adenosine and ATP Analogs Facilitates NMR Spectroscopy of Very Large Biological RNAs in Solution. Angew. Chem. Int. Ed. Engl. 2024, 63, e202316273. [Google Scholar] [CrossRef]
- Lacabanne, D.; Wiegand, T.; Wili, N.; Kozlova, M.I.; Cadalbert, R.; Klose, D.; Mulkidjanian, A.Y.; Meier, B.H.; Bockmann, A. ATP Analogues for Structural Investigations: Case Studies of a DnaB Helicase and an ABC Transporter. Molecules 2020, 25, 5268. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Aliaga, P.; Ramirez, L.; Kim, F.; Bustamante, C.; Martin, A. Substrate-translocating loops regulate mechanochemical coupling and power production in AAA+ protease ClpXP. Nat. Struct. Mol. Biol. 2016, 23, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Rydzek, S.; Shein, M.; Bielytskyi, P.; Schutz, A.K. Observation of a Transient Reaction Intermediate Illuminates the Mechanochemical Cycle of the AAA-ATPase p97. J. Am. Chem. Soc. 2020, 142, 14472–14480. [Google Scholar] [CrossRef] [PubMed]
- Shein, M.; Hitzenberger, M.; Cheng, T.C.; Rout, S.R.; Leitl, K.D.; Sato, Y.; Zacharias, M.; Sakata, E.; Schutz, A.K. Characterizing ATP processing by the AAA+ protein p97 at the atomic level. Nat. Chem. 2024, 16, 363–372. [Google Scholar] [CrossRef]
- Jaffe, E.K.; Cohn, M. Divalent cation-dependent stereospecificity of adenosine 5‘-O-(2-thiotriphosphate) in the hexokinase and pyruvate kinase reactions. The absolute stereochemistry of the diastereoisomers of adenosine 5‘-O-(2-thiotriphosphate). J. Biol. Chem. 1978, 253, 4823–4825. [Google Scholar] [CrossRef]
- Matlahov, I.; van der Wel, P.C.A. Hidden motions and motion-induced invisibility: Dynamics-based spectral editing in solid-state NMR. Methods 2018, 148, 123–135. [Google Scholar] [CrossRef]
- Siemer, A.B. Advances in studying protein disorder with solid-state NMR. Solid. State Nucl. Magn. Reson. 2020, 106, 101643. [Google Scholar] [CrossRef]
- Schutz, A.K. Solid-state NMR approaches to investigate large enzymes in complex with substrates and inhibitors. Biochem. Soc. Trans. 2021, 49, 131–144. [Google Scholar] [CrossRef]
- Harati Taji, Z.; Bielytskyi, P.; Shein, M.; Sani, M.A.; Seitz, S.; Schutz, A.K. Transient RNA Interactions Leave a Covalent Imprint on a Viral Capsid Protein. J. Am. Chem. Soc. 2022, 144, 8536–8550. [Google Scholar] [CrossRef]
- Song, C.; Wang, Q.; Li, C.C. ATPase activity of p97-valosin-containing protein (VCP). D2 mediates the major enzyme activity, and D1 contributes to the heat-induced activity. J. Biol. Chem. 2003, 278, 3648–3655. [Google Scholar] [CrossRef]
- Puchades, C.; Sandate, C.R.; Lander, G.C. The molecular principles governing the activity and functional diversity of AAA+ proteins. Nat. Rev. Mol. Cell Biol. 2020, 21, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Wendler, P.; Ciniawsky, S.; Kock, M.; Kube, S. Structure and function of the AAA+ nucleotide binding pocket. Biochim. Biophys. Acta 2012, 1823, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Seraphim, T.V.; Houry, W.A. AAA+ proteins. Curr. Biol. 2020, 30, R251–R257. [Google Scholar] [CrossRef] [PubMed]
- Gates, S.N.; Martin, A. Stairway to translocation: AAA+ motor structures reveal the mechanisms of ATP-dependent substrate translocation. Protein Sci. 2020, 29, 407–419. [Google Scholar] [CrossRef]
- Beck, M.; Schmidt, A.; Malmstroem, J.; Claassen, M.; Ori, A.; Szymborska, A.; Herzog, F.; Rinner, O.; Ellenberg, J.; Aebersold, R. The quantitative proteome of a human cell line. Mol. Syst. Biol. 2011, 7, 549. [Google Scholar] [CrossRef]
- Chu, S.; Xie, X.; Payan, C.; Stochaj, U. Valosin containing protein (VCP): Initiator, modifier, and potential drug target for neurodegenerative diseases. Mol. Neurodegener. 2023, 18, 52. [Google Scholar] [CrossRef]
- van den Boom, J.; Meyer, H. VCP/p97-Mediated Unfolding as a Principle in Protein Homeostasis and Signaling. Mol. Cell 2018, 69, 182–194. [Google Scholar] [CrossRef]
- DeLaBarre, B.; Brunger, A.T. Complete structure of p97/valosin-containing protein reveals communication between nucleotide domains. Nat. Struct. Biol. 2003, 10, 856–863. [Google Scholar] [CrossRef]
- Banerjee, S.; Bartesaghi, A.; Merk, A.; Rao, P.; Bulfer, S.L.; Yan, Y.; Green, N.; Mroczkowski, B.; Neitz, R.J.; Wipf, P.; et al. 2.3 A resolution cryo-EM structure of human p97 and mechanism of allosteric inhibition. Science 2016, 351, 871–875. [Google Scholar] [CrossRef]
- Cooney, I.; Han, H.; Stewart, M.G.; Carson, R.H.; Hansen, D.T.; Iwasa, J.H.; Price, J.C.; Hill, C.P.; Shen, P.S. Structure of the Cdc48 segregase in the act of unfolding an authentic substrate. Science 2019, 365, 502–505. [Google Scholar] [CrossRef]
- Tang, W.K.; Li, D.; Li, C.C.; Esser, L.; Dai, R.; Guo, L.; Xia, D. A novel ATP-dependent conformation in p97 N-D1 fragment revealed by crystal structures of disease-related mutants. EMBO J. 2010, 29, 2217–2229. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.M.; Tsuruta, H.; May, A.P.; Weis, W.I. Conformational changes of p97 during nucleotide hydrolysis determined by small-angle X-Ray scattering. Structure 2005, 13, 183–195. [Google Scholar] [CrossRef]
- Schuetz, A.K.; Kay, L.E. A Dynamic molecular basis for malfunction in disease mutants of p97/VCP. Elife 2016, 5, e20143. [Google Scholar] [CrossRef]
- Yu, G.; Bai, Y.; Li, K.; Amarasinghe, O.; Jiang, W.; Zhang, Z.Y. Cryo-electron microscopy structures of VCP/p97 reveal a new mechanism of oligomerization regulation. iScience 2021, 24, 103310. [Google Scholar] [CrossRef] [PubMed]
- Helmus, J.J.; Jaroniec, C.P. Nmrglue: An open source Python package for the analysis of multidimensional NMR data. J. Biomol. NMR 2013, 55, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Boeszoermenyi, A.; Chhabra, S.; Dubey, A.; Radeva, D.L.; Burdzhiev, N.T.; Chanev, C.D.; Petrov, O.I.; Gelev, V.M.; Zhang, M.; Anklin, C.; et al. Aromatic 19F-13C TROSY: A background-free approach to probe biomolecular structure, function, and dynamics. Nat. Methods 2019, 16, 333–340. [Google Scholar] [CrossRef]
- Skinner, S.P.; Fogh, R.H.; Boucher, W.; Ragan, T.J.; Mureddu, L.G.; Vuister, G.W. CcpNmr AnalysisAssign: A flexible platform for integrated NMR analysis. J. Biomol. NMR 2016, 66, 111–124. [Google Scholar] [CrossRef]
- Jaffe, E.K.; Cohn, M. Diastereomers of the nucleoside phosphorothioates as probes of the structure of the metal nucleotide substrates and of the nucleotide binding site of yeast hexokinase. J. Biol. Chem. 1979, 254, 10839–10845. [Google Scholar] [CrossRef]
- Chou, T.F.; Bulfer, S.L.; Weihl, C.C.; Li, K.; Lis, L.G.; Walters, M.A.; Schoenen, F.J.; Lin, H.J.; Deshaies, R.J.; Arkin, M.R. Specific inhibition of p97/VCP ATPase and kinetic analysis demonstrate interaction between D1 and D2 ATPase domains. J. Mol. Biol. 2014, 426, 2886–2899. [Google Scholar] [CrossRef]
- Lassila, J.K.; Zalatan, J.G.; Herschlag, D. Biological phosphoryl-transfer reactions: Understanding mechanism and catalysis. Annu. Rev. Biochem. 2011, 80, 669–702. [Google Scholar] [CrossRef]
- Eckstein, F. Nucleoside phosphorothioates. Annu. Rev. Biochem. 1985, 54, 367–402. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Byeon, I.J.; Jiang, R.T.; Tsai, M.D. Mechanism of adenylate kinase. What can be learned from a mutant enzyme with minor perturbation in kinetic parameters? Biochemistry 1993, 32, 6450–6458. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, F.; Goody, R.S. Synthesis and properties of diastereoisomers of adenosine 5′-(O-1-thiotriphosphate) and adenosine 5′-(O-2-thiotriphosphate). Biochemistry 1976, 15, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Wennefors, C.K.; Dobrikov, M.I.; Xu, Z.; Li, P.; Shaw, B.R. Stereospecificity, substrate, and inhibitory properties of nucleoside diphosphate analogs for creatine and pyruvate kinases. Bioorg. Chem. 2008, 36, 169–177. [Google Scholar] [CrossRef]
- Tang, W.K.; Xia, D. Altered intersubunit communication is the molecular basis for functional defects of pathogenic p97 mutants. J. Biol. Chem. 2013, 288, 36624–36635. [Google Scholar] [CrossRef]
- Sarkar, R.; Mainz, A.; Busi, B.; Barbet-Massin, E.; Kranz, M.; Hofmann, T.; Reif, B. Immobilization of soluble protein complexes in MAS solid-state NMR: Sedimentation versus viscosity. Solid. State Nucl. Magn. Reson. 2016, 76, 7–14. [Google Scholar] [CrossRef][Green Version]
- Bell, D.; Lindemann, F.; Gerland, L.; Aucharova, H.; Klein, A.; Friedrich, D.; Hiller, M.; Grohe, K.; Meier, T.; van Rossum, B.; et al. Sedimentation of large, soluble proteins up to 140 kDa for 1H-detected MAS NMR and 13C DNP NMR—Practical aspects. J. Biomol. NMR 2024, 78, 179–192. [Google Scholar] [CrossRef]
- Tollinger, M.; Sivertsen, A.C.; Meier, B.H.; Ernst, M.; Schanda, P. Site-resolved measurement of microsecond-to-millisecond conformational-exchange processes in proteins by solid-state NMR spectroscopy. J. Am. Chem. Soc. 2012, 134, 14800–14807. [Google Scholar] [CrossRef]
- Ma, P.; Haller, J.D.; Zajakala, J.; Macek, P.; Sivertsen, A.C.; Willbold, D.; Boisbouvier, J.; Schanda, P. Probing transient conformational states of proteins by solid-state R(1rho) relaxation-dispersion NMR spectroscopy. Angew. Chem. Int. Ed. Engl. 2014, 53, 4312–4317. [Google Scholar] [CrossRef]
- Jaffe, E.K.; Cohn, M. 31P nuclear magnetic resonance spectra of the thiophosphate analogues of adenine nucleotides; effects of pH and Mg2+ binding. Biochemistry 1978, 17, 652–657. [Google Scholar] [CrossRef]
- Glonek, T. 31P NMR of Mg-ATP in dilute solutions: Complexation and exchange. Int. J. Biochem. 1992, 24, 1533–1559. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.L.; Tsai, M.D. Does the magnesium(II) ion interact with the alpha-phosphate of adenosine triphosphate? An investigation by oxygen-17 nuclear magnetic resonance. Biochemistry 1982, 21, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Buelens, F.P.; Leonov, H.; de Groot, B.L.; Grubmuller, H. ATP-Magnesium Coordination: Protein Structure-Based Force Field Evaluation and Corrections. J. Chem. Theory Comput. 2021, 17, 1922–1930. [Google Scholar] [CrossRef] [PubMed]
- Gimenez, D.; Phelan, A.; Murphy, C.D.; Cobb, S.L. 19F NMR as a tool in chemical biology. Beilstein J. Org. Chem. 2021, 17, 293–318. [Google Scholar] [CrossRef]
- Ye, L.; Larda, S.T.; Frank Li, Y.F.; Manglik, A.; Prosser, R.S. A comparison of chemical shift sensitivity of trifluoromethyl tags: Optimizing resolution in 19F NMR studies of proteins. J. Biomol. NMR 2015, 62, 97–103. [Google Scholar] [CrossRef]
- Rashid, S.; Lee, B.L.; Wajda, B.; Spyracopoulos, L. Side-Chain Dynamics of the Trifluoroacetone Cysteine Derivative Characterized by 19F NMR Relaxation and Molecular Dynamics Simulations. J. Phys. Chem. B 2019, 123, 3665–3671. [Google Scholar] [CrossRef]
- Chrominski, M.; Baranowski, M.R.; Chmielinski, S.; Kowalska, J.; Jemielity, J. Synthesis of Trifluoromethylated Purine Ribonucleotides and Their Evaluation as 19F NMR Probes. J. Org. Chem. 2020, 85, 3440–3453. [Google Scholar] [CrossRef]
- Gronenborn, A.M. Small, but powerful and attractive: 19F in biomolecular NMR. Structure 2022, 30, 6–14. [Google Scholar] [CrossRef]
- Withers, S.G.; Madsen, N.B.; Sykes, B.D. Relaxation of individual transitions in an AX spectrum. Use of interference terms to separate the dipolar and chemical-shift anisotropy contributions to the relaxation of 31P and 19F nuclei in macromolecules. J. Magn. Reson. (1969) 1985, 61, 545–549. [Google Scholar] [CrossRef]
- Kiani, F.A.; Fischer, S. ATP-dependent interplay between local and global conformational changes in the myosin motor. Cytoskelet 2016, 73, 643–651. [Google Scholar] [CrossRef]
- Schmidt, H.; Carter, A.P. Review: Structure and mechanism of the dynein motor ATPase. Biopolymers 2016, 105, 557–567. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).